Abstract
In this study, the effect of shear stress on the expression of genes of the human endothelin-1 system was examined. Primary cultures of human umbilical vein endothelial cells (HUVEC) were exposed to laminar shear stress of 1, 15 or 30 dyn cm−2 (i.e. 0.1, 1.5 or 3 N m−2) (venous and two different arterial levels of shear stress) in a cone-and-plate viscometer.
Laminar shear stress transiently upregulates preproendothelin-1 (ppET-1) mRNA, reaching its maximum after 30 min (approx 1.7-fold increase). In contrast, long-term application of shear stress (24 h) causes downregulation of ppET-1 mRNA in a dose-dependent manner.
Arterial levels of shear stress result in downregulation of endothelin-converting enzyme-1 isoform ECE-1a (predominating in HUVEC) to 36.2 ± 8.5%, and isoform ECE-1b mRNA to 72.3 ± 1.9% of static control level.
The endothelin-1 (ET-1) release is downregulated by laminar shear stress in a dose-dependent manner.
This downregulation of ppET-1 mRNA and ET-1 release is not affected by inhibition of protein kinase C (PKC), or tyrosine kinase. Inhibition of endothelial NO synthase (L-NAME, 500 μm) prevents downregulation of ppET-1 mRNA by shear stress.
In contrast, increasing degrees of long-term shear stress upregulate endothelin receptor type B (ETB) mRNA by a NO- and PKC-, but not tyrosine kinase-dependent mechanism.
In conclusion, our data suggest the downregulation of human endothelin synthesis, and an upregulation of the ETB receptor by long-term arterial laminar shear stress. These effects might contribute to the vasoprotective and anti-arteriosclerotic potential of arterial laminar shear stress.
The endothelial cells in situ are permanently exposed to shear stress, the dragging frictional force created by flowing blood. Physiological degrees of shear stress are involved in the regulation of vascular tone, and are considered as possible pathophysiological mechanisms for the localization of arteriosclerotic plaques (Glagov et al. 1988; Traub & Berk, 1998). Shear stress acting on endothelial cells is higher in arterial vessels (∼15-30 dyn cm−2 (∼1.5-3 N m−2)), compared with shear stress in venous vessels (∼1 dyn cm−2). These differences in shear stress may contribute to altered endothelial gene expression.
A variety of genes have been described to be regulated by shear stress. These include genes encoding transcription factors, factors affecting coagulation, migration of leucocytes, smooth muscle proliferation, lipoprotein uptake and metabolism, cytoskeletal structure, apoptosis and release of vasoactive substances (Davies et al. 1997). Arterial laminar shear stress was shown to induce the endothelial generation of vasodilators nitric oxide (NO) and prostacyclin (Frangos et al. 1985; Uematsu et al. 1995). Interestingly, the vasoconstrictor angiotensin II generating angiotensin-converting enzyme is downregulated by arterial laminar shear stress (Rieder et al. 1997). However, the effect of shear stress on the expression of genes of the endothelin-1 system in human endothelial cells is less clearly understood.
The endothelin family includes peptides which are the most potent vasoconstrictors known to date (Yanagisawa et al. 1988). Three endothelin isoforms have been identified: endothelin-1 (ET-1), ET-2 and ET-3 (Inoue et al. 1989a). Of these three isoforms, ET-1 was the first to be cloned, is the most abundant in circulation, and is the one studied in most detail. The biosynthesis of human ET-1 includes: (1) the expression of the 212 amino acid preproendothelin-1 (ppET-1) (Itoh et al. 1988; Inoue et al. 1989b); (2) the proteolytic cleavage to form big-ET-1 (39 amino acids) by a furin convertase (Denault et al. 1995); and (3) in the final key step, the cleavage of big-ET-1 into the active ET-1 peptide of 21 amino acids by the recently cloned metalloprotease endothelin-converting enzyme-1 (ECE-1) (Xu et al. 1994; Turner & Murphy, 1996). ET-1 binds to endothelin type A (ETA) and type B (ETB) receptors (Huggins et al. 1993). ETA receptors are present on vascular smooth muscle cells and induce ET-1-mediated vasoconstriction (Hosoda et al. 1991). On the other hand, most probably two ETB receptor subtypes are present on endothelial and vascular smooth muscle cells. The endothelial subtype mediates vasodilatation and is sensitive to the ETA and/or ETB non-selective antagonist PD142893 (Ogawa et al. 1991), while the smooth muscle cell-specific subtype causes vasoconstriction and is resistant to this antagonist (Warner et al. 1993).
The data regarding the regulation of endothelin synthesis and release by shear forces in endothelial cells are controversial. Initial reports described a shear stress-dependent induction of endothelin production (Yoshizumi et al. 1989; Morita et al. 1993). Other groups found no significant changes in ET-1 release (Noris et al. 1995), or a downregulation of ppET-1 mRNA and endothelin release by shear stress in human and bovine endothelial cells (Sharefkin et al. 1991; Malek & Izumo, 1992; Kuchan & Frangos, 1993). Therefore, the time- and dose-dependent regulation of ppET-1 mRNA as well as endothelin peptide release by shear stress in human umbilical vein endothelial cells (HUVEC) was studied. In addition, the effect of shear stress on ECE-1 isoforms and the endothelial ETB receptor was analysed. Finally, we investigated the involvement of NO and protein kinases in shear stress-dependent regulation of the endothelin system in human endothelial cells.
METHODS
Cell culture and application of shear stress
All cell culture reagents and chemicals were purchased from Sigma Chemicals (St Louis, MO, USA) except when specified. Primary cultures of human umbilical vein endothelial cells (HUVEC) were isolated using collagenase IV as described previously (Morawietz et al. 1999b). In order to minimize variations of primary cultures, each day after collagenase IV treatment the endothelial cells isolated from different umbilical veins were pooled in medium M199 with 1.25 g l−1 sodium bicarbonate, 100 mg l−1 L-glutamine (Life Technologies, Paisley, UK), 20 % calf serum, 15 mm Hepes, 100 000 IU l−1 penicillin, 100 mg l−1 streptomycin, 250 mg l−1 fungizone (Life Technologies), and 16.7 μg l−1 endothelial cell growth supplement (C. C. Pro, Neustadt, Germany) and subsequently separated on 94 × 16 mm cell culture dishes coated with 1 % gelatin. The cell cultures reached confluency after 5–7 days. The average number of cells was 106 endothelial cells per culture dish.
Cells were subjected to laminar shear stress 1 day after reaching confluence in a cone-and-plate viscometer (Sdougos et al. 1984) with minor modifications. Briefly, this viscometer consists of a cone with an angle of 0.5 deg rotating on top of a 94 × 16 mm cell culture dish. Laminar shear stress of 1 dyn cm−2 (venous level of shear stress), 15 or 30 dyn cm−2 (two different arterial levels of shear stress) was applied by a constant angular velocity in a humidified environment with 5 % CO2 at 37°C. The cell culture medium has a viscosity of 0.007 dyn s cm−2. For application of arterial levels of shear stress (15 or 30 dyn cm−2), 5 % dextran (MW 71 400) was added to the cell culture medium to increase the viscosity of the medium 2.95-fold to 0.02065 dyn s cm−2. The angular velocity of the cone was in the case of 1 dyn cm−2 (cell culture medium without dextran): 1.25 s−1, of 15 dyn cm−2 (medium supplemented with dextran): 6.34 s−1, and of 30 dyn cm−2 (medium supplemented with dextran): 12.68 s−1. In these experiments each cell culture dish was accompanied by two controls from the same HUVEC preparation incubated with cell culture medium supplemented with or without 5 % dextran for 24 h without application of shear stress.
RNA isolation and Northern analysis
Total RNA from endothelial cells was isolated by guanidinium thiocyanate-caesium chloride centrifugation (Chirgwin et al. 1979). Northern analysis was done as described previously (Morawietz et al. 1999a), using total cellular RNA from endothelial cells. The cDNA fragments of human preproendothelin-1 (ppET-1, position 409-870) (Itoh et al. 1988) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, position 278-721) (Arcari et al. 1984) were cloned after reverse transcriptase polymerase chain reaction (RT-PCR) from HUVEC RNA. Their identity was then confirmed by cycle sequencing with the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction Kit (Perkin Elmer, Foster City, CA, USA) on an automated ABI 373A DNA Sequencer (ABI/Perkin Elmer). The DNA sequence was analysed using Gene Runner software (Hastings Software, Inc.). Database searches of GenBank were performed using BLASTN (Altschul et al. 1990). The cDNA probes were labelled using the Oligolabelling Kit and [α-32P]dCTP (Amersham Pharmacia Biotech, Bucks, UK). Oligonucleotides specific for human endothelin-converting enzyme-1 isoform ECE-1a (5′-GCA GGG AAG GAG GCA GGA GGG G-3′, position 140-161) (Shimada et al. 1995), and ECE-1b (5′-GCC GTT GGG GTA TGC GTC GCC-3′, position 121-141) (Schmidt et al. 1994) were labelled at the 5′-end with [γ-32P]ATP (Amersham Pharmacia Biotech). The labelled probes were hybridized with membranes in hybridization solution at 65°C for 16 h (Sambrook et al. 1989), washed and exposed at -80°C to Kodak BIOMAX MS films. Subsequently, Northern filters were stripped in 0.1 × saline-sodium citrate buffer (SSC), 0.1 % SDS at 80°C, and hybridized with the human GAPDH cDNA probe.
Standard-calibrated competitive RT-PCR
In order to determine the number of human ppET-1 mRNA molecules in response to shear stress, a standard-calibrated competitive RT-PCR was established. Firstly, a human ppET-1-specific cDNA fragment of 461 bp (position 409-870) (Itoh et al. 1988) was amplified by PCR using the following primers: ET-1 sense primer: 5′-TGC TCC TGC TCG TCC CTG ATG GAT AAA GAG-3′, and ET-1 antisense primer: 5′-GGT CAC ATA ACG CTC TCT GGA GGG CTT-3′. The ET-1-specific cDNA fragment was subsequently cloned into the pCR-Script Amp SK(+) Cloning Vector (Stratagene, La Jolla, CA, USA) and its identity confirmed by DNA sequencing as described. Secondly, an internally deleted cRNA standard was constructed by modification of a linker primer method (Forster, 1994). In brief, a ppET-1-specific cDNA fragment was amplified with a 30-mer linker primer and the antisense primer. The linker primer includes at the 5′ end 10 bp of the 3′ end of the ET-1 sense primer, followed at the 3′ end by 20 bp of ppET-1-specific cDNA sequence (Itoh et al. 1988), located 136 bp in the 3′ direction from the sense primer (position 545-564, ET-1 linker primer: 5′-GGA TAA AGA GTT CCC ACA AAG GCA ACA GAC-3′). The subsequently isolated cDNA fragment was reamplified with the initially used ET-1-specific sense and antisense primers. This method resulted in an internally deleted ppET-1 cDNA standard bound by the sense and antisense primer of the first PCR amplification step (length of ppET-1 standard: 355 bp). The identity of the standard was confirmed by cloning and DNA sequencing. Thirdly, internally deleted cDNA standard was in vitro transcribed into cRNA (RNA Transcription Kit, Stratagene), and standard cRNA was quantified spectrophotometrically (Sambrook et al. 1989).
In competitive RT-PCR experiments, equal amounts of total RNA (100 ng) were incubated in separate reactions with defined amounts of ppET-1 standard cRNA (e.g. 1 × 108, 3 × 107, 1 × 107, 3 × 106, and 1 × 106 cRNA molecules) for 3 min at 70°C, and subsequently reverse transcribed into cDNA using random hexamer primers and SuperScript II RNase H− reverse transcriptase (Life Technologies) for 1 h at 42°C. Afterwards, 20 % of each reverse transcription reaction was amplified in separate reactions with 20 pm ET-1 sense and antisense primers by the following PCR protocol: 30 s 95°C, 30 s 65°C, 1 min 72°C (40 cycles). PCR primers compete for sample-specific and standard molecules in the amplification reaction. The PCR reactions were separated by standard agarose gel electrophoresis, stained with ethidium bromide and documented by photography using Polaroid film type 665. The optical density of standard and sample-specific PCR fragments was estimated by a Personal Densitometer (Molecular Dynamics, Sunnyvale, CA, USA). Optical density of standard PCR fragments was normalized with a correction coefficient (ET-1: 461 bp/355 bp = 1.298), and the logarithm of the quotient of normalized standard and sample-specific PCR fragment density was graphically plotted vs. amount of standard RNA molecules, using the SigmaPlot scientific graphing software (Jandel Corp.). In the graph, equal amounts of RNA molecules in the sample and standard were present at the equivalence point.
The mRNA expression of human endothelin receptor type B (ETB) (Ogawa et al. 1991) was quantified as attomolar per microgram RNA (am μg−1 RNA) by standard-calibrated, competitive RT-PCR as described previously (Heinroth-Hoffmann et al. 1998).
ET-1 peptide release
The release of ET-1 peptide into the cell culture medium was measured with the Endothelin-1 ELISA system (Amersham Pharmacia Biotech) and normalized vs. protein concentration determined with the BCA Protein Assay Reagent (Pierce, Rockford, IL, USA).
Inhibitor studies
After preincubation with inhibitor for 1 h, arterial levels of shear stress were applied on HUVEC with or without inhibitor. Each experiment was accompanied by two controls from the same HUVEC preparation incubated for 24 h without application of shear stress: (1) without 5 % dextran and without inhibitor and (2) with 5 % dextran and the identical inhibitor concentration. The effect of inhibitors of endothelial constitutive NO synthase (ecNOS), Nω-nitro-L-arginine methyl ester (L-NAME) and Nω-nitro-L-arginine (L-NNA) (up to 500 μM), of protein kinase C inhibitors (RO-31-8220, up to 1 μM, Calbiochem, San Diego, CA, USA), or of tyrosine kinase inhibitors (herbimycin A, 1 μM, Calbiochem) was tested. From these cultures ppET-1 and ETB mRNA expression was determined by competitive RT-PCR, and ET-1 peptide release by ET-ELISA.
Statistics
In experiments with dimensionless quantities, band densities or peptide release from multiple similar experiments were combined by calculation of the fold increase or decrease vs. control under each experimental condition. Data are given as means ±s.e.m. (n≥ 3 in all cases, where n is the number of independent experiments). Statistical analysis was performed with the ANOVA procedure followed by Dunn's test (multiple comparison) or Student's t test (SigmaStat software, Jandel Corp.). Differences were taken as statistically significant at P < 0.05.
RESULTS
Dose-dependent downregulation of human prepro-endothelin-1 mRNA by shear stress
Cultures of human umbilical vein endothelial cells (HUVEC) constantly generate and release endothelin-1 (ET-1) into the cell culture medium. In initial studies, mRNA expression of preproendothelin-1 (ppET-1) was quantified by Northern analysis (Fig. 1A). Application of laminar shear stress to HUVEC for 24 h caused a dose-dependent downregulation of ppET-1 mRNA. As the Northern blot method is limited in the quantitative detection of mRNA species expressed at a low level, a standard-calibrated, competitive reverse transcriptase PCR (RT-PCR) for human ppET-1 mRNA was established (Fig. 1B), and the dose-dependent downregulation of ppET-1 mRNA by long-term (24 h) laminar shear stress was quantified (Fig. 1C). For application of 15 or 30 dyn cm−2, control medium was supplemented with 5 % dextran without effect on basal ppET-1 expression.
Figure 1. Downregulation of preproendothelin-1 mRNA by laminar shear stress in human endothelial cells.
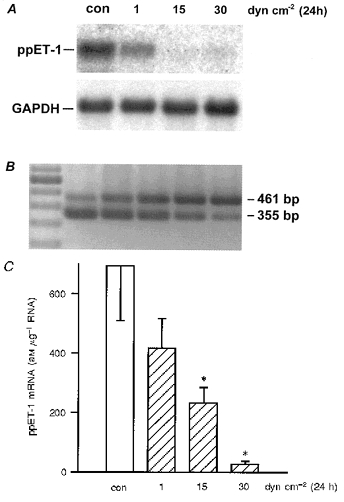
In A, human umbilical vein endothelial cells (HUVEC) were exposed to long-term (24 h) laminar shear stress, and the expression of preproendothelin-1 (ppET-1) mRNA in these cells was determined by Northern analysis. The filter was subsequently stripped and hybridized with a human GAPDH cDNA probe as a control. Results shown are representative of three independent experiments. Laminar shear stress (24 h) caused dose-dependent downregulation of ppET-1 mRNA, compared with control (for 15 and 30 dyn cm−2, control was supplemented with 5 % dextran medium). B shows the method of quantification of mRNA levels of the human preproendothelin-1 (ppET-1) gene by competitive RT-PCR. The method compares the amplification of a ppET-1 cDNA fragment from reverse transcribed total RNA of HUVEC (461 bp) vs. different concentrations of an internally deleted and reverse transcribed cRNA standard (355 bp) by PCR. The PCR fragments were separated on agarose gels and stained with ethidium bromide. Lane 1: molecular weight marker (100 bp ladder). Amount of standard molecules per reaction: 1 × 108 (lane 2), 3 × 107 (lane 3), 1 × 107 (lane 4), 3 × 106 (lane 5), and 1 × 106 (lane 6). C, downregulation of ppET-1 mRNA by laminar shear stress determined using standard-calibrated, competitive RT-PCR (static condition: open bar, shear stress: dashed bars). Values are given as means ±s.e.m. (aM μg−1 RNA); n= 5, where n is the number of independent experiments; * P < 0.05 vs. static control (con).
In parallel experiments, the time course of ppET-1 mRNA expression in response to arterial shear stress was determined (Fig. 2). Application of arterial shear stress resulted in transient induction of ppET-1 mRNA reaching its maximum after 30 min (∼1.7-fold increase). In contrast, long-term application of arterial shear stress for 24 h resulted in significant downregulation of ppET-1 mRNA. Since long-term application of shear stress resembles the in vivo situation more closely, in all subsequent experiments shear stress was applied for 24 h.
Figure 2. Time course of ppET-1 mRNA expression in human endothelial cells in response to arterial levels of laminar shear stress.
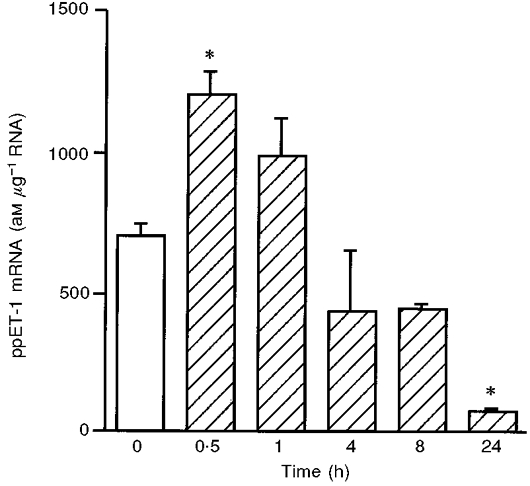
HUVEC were subjected to shear stress of 30 dyn cm−2. ppET-1 mRNA expression was determined using standard-calibrated, competitive RT-PCR. Values are given as means ±s.e.m. (am μg−1 RNA); n= 3. * P < 0.05 vs. static control with dextran medium (static condition: open bar, shear stress: hatched bars).
Shear stress-dependent downregulation of human endothelin-converting enzyme-1 isoforms
In HUVEC exposed to shear stress, endothelin-converting enzyme-1 (ECE-1) isoform mRNA expression was determined by Northern analysis (Fig. 3). Long-term laminar shear stress (24 h) caused downregulation of ECE-1 isoforms (Fig. 3A). The most abundant ECE-1 isoform in HUVEC, ECE-1a, is downregulated by laminar shear stress (24 h) in a dose-dependent manner (Fig. 3B). The isoform ECE-1b is downregulated by long-term arterial levels of shear stress as well (Fig. 3C).
Figure 3. Downregulation of endothelin-converting enzyme-1 isoforms by shear stress in human endothelial cells.
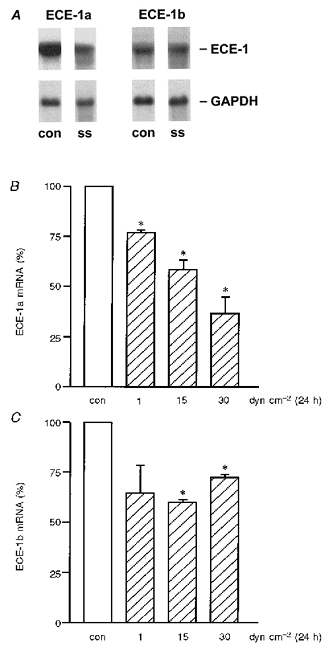
HUVEC were cultured under static conditions (con) or exposed to shear stress (ss). Endothelin-converting enzyme-1 (ECE-1) isoform mRNA expression was quantified by Northern analysis (A). Long-term laminar shear stress (24 h) causes downregulation of ECE-1 isoforms ECE-1a (B) and ECE-1b (C);n= 3; * P < 0.05 vs. static control (static condition: open bars, shear stress: hatched bars).
Downregulation of ET-1 peptide release in response to shear stress
The effect of shear stress on ET-1 protein expression was quantified as ET-1 peptide release into the cell culture medium. The basal release of ET-1 in stationary cultures of HUVEC varied considerably (range: 0.3-1.9 pm ET-1 (mg protein)−1 day−1; mean: 0.8 ± 0.1 pm ET-1 (mg protein)−1 day−1, n= 14). Therefore, each shear stress experiment was accompanied by an internal stationary control and ET-1 released into the medium was quantified as a percentage of the internal control without shear stress. Release of ET-1 from HUVEC was downregulated by long-term (24 h) shear stress in a dose-dependent manner (Fig. 4; static control: 100 %; 1 dyn cm−2: 59.1 ± 4.8 %*, n= 5; 15 dyn cm−2: 35.6 ± 5.3 %*, n= 3; 30 dyn cm−2: 30.8 ± 5.7 %*, n= 6; *P < 0.05vs. static control). In the case of experiments applying 15 or 30 dyn cm−2, control medium was supplemented with 5 % dextran as described. Dextran had no effect on basal ET-1 release from HUVEC.
Figure 4. Shear stress-dependent downregulation of endothelin-1 (ET-1) peptide release.
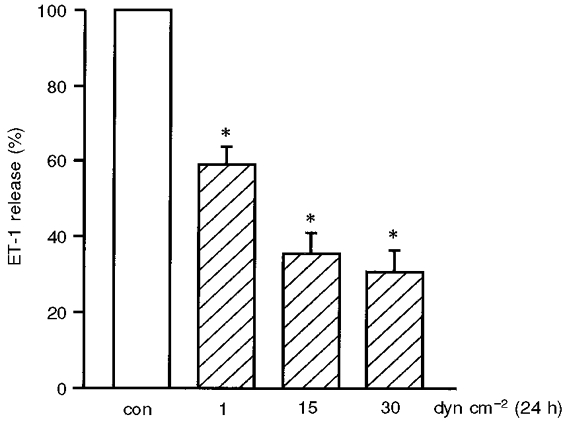
The effect of shear stress on ET-1 protein expression was quantified as ET-1 peptide release into the cell culture medium (in percentage of internal control without shear stress). The ET-1 release of HUVEC is downregulated by long-term (24 h) shear stress in a dose-dependent manner. Values are given as means ±s.e.m.; n= 6; * P < 0.05 vs. static control (con) (static condition: open bar, shear stress: hatched bars).
Effect of inhibition of protein kinases and ecNOS on shear stress-dependent ET-1 expression
In order to investigate the underlying signal transduction mechanism of ppET-1 mRNA downregulation by shear stress, experiments in the presence of inhibitors of protein kinase C (PKC) or tyrosine kinase were carried out (Fig. 5). Each experiment was accompanied by two controls from the same HUVEC preparation incubated for 24 h without application of shear stress: (1) medium without 5 % dextran and without inhibitor, and (2) medium with 5 % dextran and the identical inhibitor concentration. The downregulation of ppET-1 mRNA and ET-1 peptide release by an arterial level of shear stress (30 dyn cm−2, 24 h) was not modulated by inhibition of PKC or tyrosine kinase (Fig. 5).
Figure 5. Downregulation of ppET-1 mRNA and ET-1 peptide release by arterial levels of shear stress is not modulated by inhibition of protein kinase C or of tyrosine kinase in HUVEC.
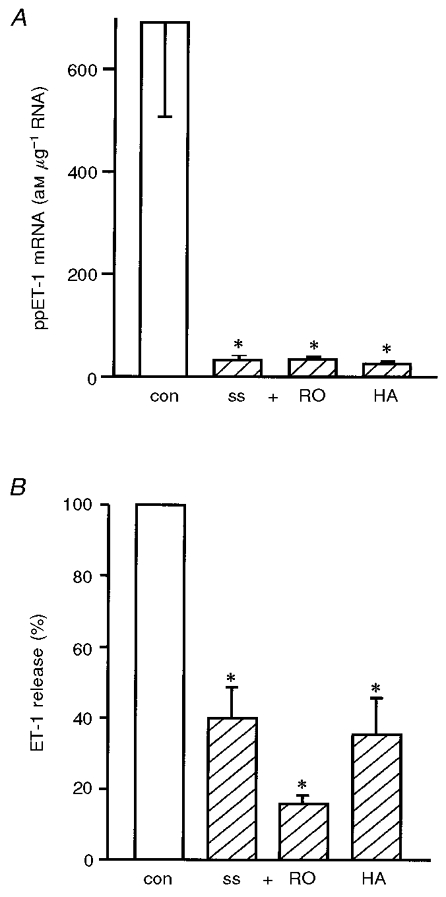
An arterial level of laminar shear stress (30 dyn cm−2, 24 h) was applied on HUVEC with or without inhibitors of protein kinase C (RO: 1 μM RO-31-8220) or of tyrosine kinase (HA: 1 μM herbimycin A). The ppET-1 mRNA expression was determined by standard-calibrated, competitive RT-PCR (am μg−1 RNA; A) and ET-1 peptide release by ET-ELISA (percentage of control without shear stress; B);n= 3; * P < 0.05 vs. control without shear stress (con) (static condition: open bars, shear stress: hatched bars).
Since Kuchan & Frangos (1993) found an effect of ecNOS inhibitors on flow-mediated suppression of ET-1 peptide release, we performed experiments applying an arterial level of shear stress similar to their studies (15 dyn cm−2, 24 h) on HUVEC with increasing doses of ecNOS inhibitor (up to 500 μM L-NAME) (Fig. 6). At high doses (500 μM) L-NAME caused inhibition of shear stress-dependent downregulation of ppET-1 mRNA (Fig. 6A). A similar trend was seen in the case of ET-1 peptide release which did not reach the level of statistical significance (Fig. 6B). Similar results were observed with ecNOS inhibitor L-NNA (data not shown).
Figure 6. Effect of ecNOS inhibitors on shear stress-dependent downregulation of ET-1 expression.
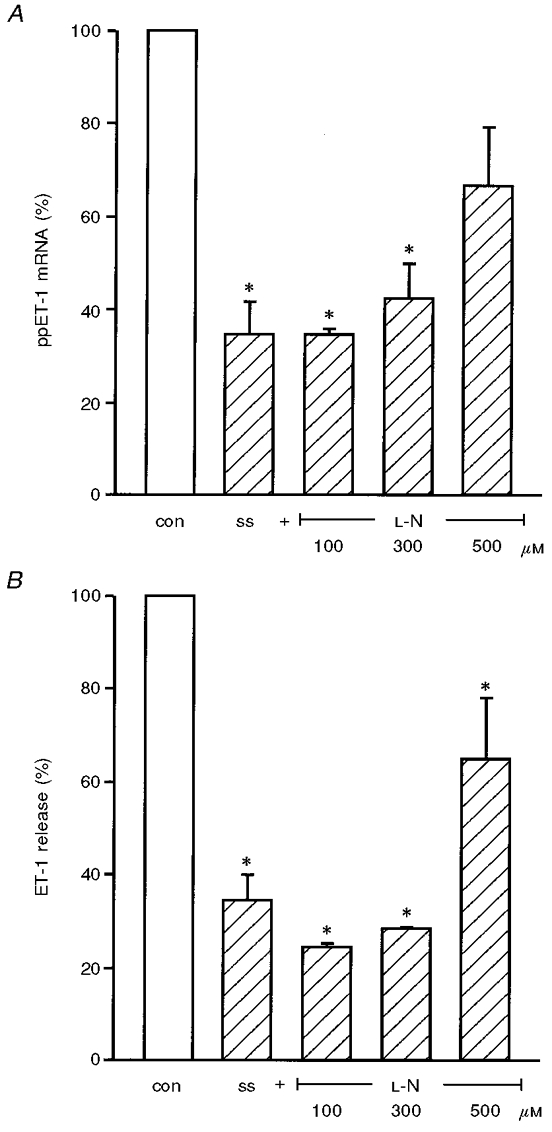
HUVEC were exposed to an arterial level of shear stress (ss, 15 dyn cm−2, 24 h) without or with increasing concentrations of ecNOS inhibitor (up to 500 μM L-NAME, L-N). From these cultures ppET-1 mRNA expression was determined by competitive RT-PCR (A), and ET-1 peptide release by ET-ELISA (B). Values are expressed as percentage of internal control with inhibitor and dextran, but without shear stress, and given as means ±s.e.m.; n= 3; * P < 0.05 vs. control without shear stress (con) (static condition: open bars, shear stress: hatched bars).
Upregulation of endothelin receptor B by shear stress
We have previously shown that HUVEC almost exclusively express endothelin receptor type B (ETB) (Heinroth-Hoffmann et al. 1998). Long-term application of shear stress increased ETB receptor mRNA expression in a dose-dependent manner (Fig. 7A). The upregulation of ETB receptor mRNA expression by arterial levels of shear stress is mediated by NO and PKC, but not by tyrosine kinases (Fig. 7B). In summary, these data suggest a shear stress-dependent interaction between the endothelin and the NO system on the level of ETB expression as well.
Figure 7. The upregulation of ETB mRNA expression by arterial levels of shear stress is mediated by NO and PKC, but not by tyrosine kinases.
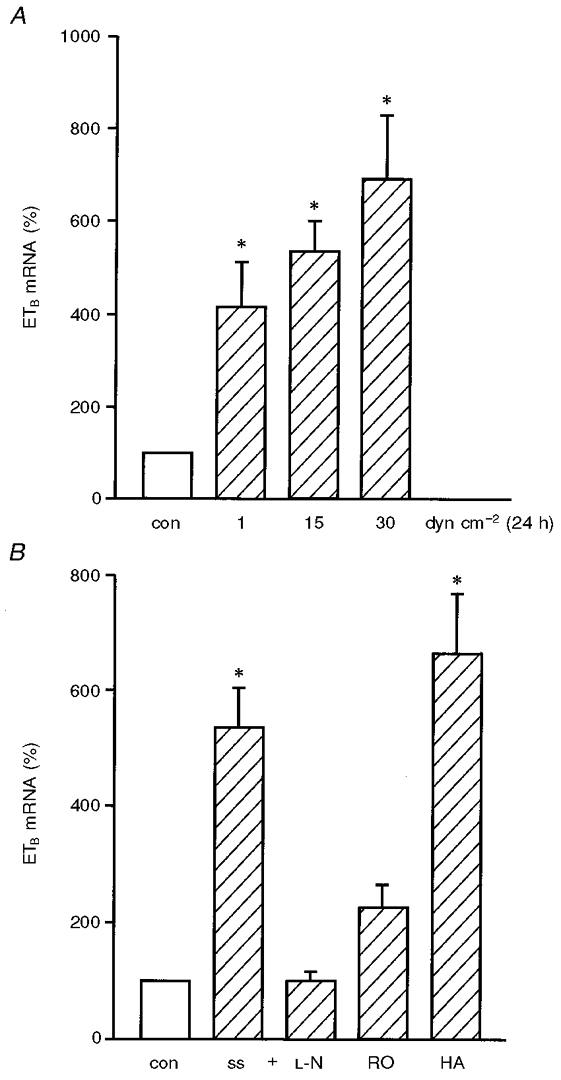
In HUVEC exposed to long-term (24 h) laminar shear stress, endothelin receptor type B (ETB) mRNA expression was determined by standard-calibrated, competitive RT-PCR. Application of shear stress increased ETB receptor mRNA expression in a dose-dependent manner (A). In further studies, HUVEC were exposed to arterial levels of shear stress (ss) for 24 h in the presence or absence of inhibitors of ecNOS (L-N: 100 μM L-NAME, similar results with up to 500 μM L-NAME, not shown), of protein kinase C (RO: 1 μM RO-31-8220), or of tyrosine kinase (HA: 1 μM herbimycin A) (B). Values are expressed as percentage of control without shear stress; n= 4; * P < 0.05 vs. control without shear stress (con) (static condition: open bar, shear stress: hatched bars). ETB mRNA expression was determined using standard-calibrated, competitive RT-PCR. Values are given as percentage of static control (for L-N, RO and HA: control with inhibitor and dextran, but without shear stress), as means ±s.e.m.; n= 3; * P < 0.05 vs. control without shear stress (con) (static condition: open bar, shear stress: hatched bars).
DISCUSSION
In this study, we demonstrate a dose-dependent downregulation of human endothelin synthesis by exposure of HUVEC to long-term arterial levels of laminar shear stress. These data confirm previous studies showing a similar shear stress-dependent downregulation of ppET-1 mRNA in bovine endothelial cells (Malek & Izumo, 1992). Sharefkin et al. (1991) found a downregulation of ppET-1 mRNA utilizing PCR amplification and subsequent Southern blotting in HUVEC exposed to arterial shear stress. Using the method of standard calibrated competitive reverse transcriptase PCR, it was possible to quantify the degree of downregulation of ppET-1 mRNA in HUVEC. The ppET-1 mRNA expression found at 15 dyn cm−2 is comparable with the degree of downregulation in bovine endothelial cells (Malek & Izumo, 1992). In contrast to the study in bovine cells, a further downregulation of ppET-1 mRNA at 30 dyn cm−2 in HUVEC was found. This might reflect a species difference.
In addition, downregulation of endothelin-converting enzyme-1 (ECE-1) mRNA and of endothelin peptide release into the medium was shown. These data suggest that endothelial cells can regulate endothelin release at multiple sites. Arterial levels of shear stress downregulate the most prominent ECE-1 isoform (ECE-1a) and endothelin release to similar amounts. This is consistent with the promoter structure of both isoforms, suggesting ECE-1a as the more transcriptionally regulated compared with the rather constitutively expressed isoform ECE-1b (Valdenaire et al. 1995). ECE-1-like immunoreactivity has been detected on the cell surface directed to the luminal site of human umbilical vein and coronary artery endothelial cells (Russell et al. 1998). A part of ECE-1 enzymatic activity is therefore directed to the blood stream and exposed to shear forces itself. An attractive hypothesis is that ECE-1 might process circulating big-endothelin-1 in a shear stress-dependent manner. In this way endothelial cells could adapt to flow-dependent changes by regulation of endothelin synthesis.
The downregulation of ppET-1 mRNA was not PKC dependent. This is in agreement with experiments using bovine endothelial cells, showing that arterial levels of shear stress were unable to induce PKC translocation and enzymatic PKC activity (Malek et al. 1993). In addition, no involvement of tyrosine kinases could be proven. Arterial levels of shear stress induce the generation and release of nitric oxide (Uematsu et al. 1995). Furthermore, endothelin release from porcine aorta has been shown to be inhibited by NO (Boulanger & Luscher, 1990), and flow-induced suppression of ET-1 peptide release is mediated by NO (Kuchan & Frangos, 1993). We extended the studies of Kuchan & Frangos (1993) on the level of ppET-1 mRNA expression and found a similar NO-mediated inhibition of ET-1 downregulation by arterial levels of shear stress. The shear stress-dependent downregulation of ET-1 peptide release in our experiments was partially inhibited by high doses of ecNOS inhibitors, but did not reach the level of statistical significance. These data suggest that NO is involved in the downregulation of ET-1 synthesis at the ppET-1 mRNA level.
Surprisingly, the predominant endothelin receptor of endothelial cells, ETB, is dose dependently upregulated by shear stress. This is in agreement with the original observation, that ETB is inconsistently expressed in different preparations of primary cultures of HUVEC, but expressed at a high level in primary cultures of bovine aortic endothelial cells isolated from vessels previously exposed to arterial shear stress in vivo (Ogawa et al. 1991). A similar upregulation of ETB binding sites was found in canine arterial smooth muscle in response to chronic increases in blood flow (Barber et al. 1996). An inverse relationship of ET-1 and ETB receptors has been described in other cell types. Increasing ET-1 peptide was found to downregulate ETB receptor mRNA (Asada et al. 1995). The shear stress-dependent downregulation of ET-1 peptide and upregulation of ETB mRNA might represent an opposite mechanism. However, our pharmacological studies reveal that upregulation of ETB receptor mRNA by shear stress is PKC dependent, while downregulation of ppET-1 mRNA and ET-1 release is not. These data suggest additional PKC-dependent pathways involved in shear stress-dependent upregulation of ETB.
The endothelial ETB receptor has been reported to mediate NO release (Boulanger & Luscher, 1990) and ET-1-mediated endothelial cell survival (Shichiri et al. 1997). In addition, it has been proposed that ETB serves as a clearance receptor; therefore shear stress-dependent upregulation of ETB might reflect an anti-atherosclerotic mechanism by increasing the clearance of circulating ET-1. Increased ET-1 serum concentration and increased ET-1 immunoreactivity in the vessel wall correlate with the extent of coronary endothelial dysfunction and atherosclerosis (Lerman et al. 1991, 1995; Barton et al. 1998). Increased ET-1 serum concentrations may be a result of spill-over from the vessel wall (Ando et al. 1989). Also, increased ET-1 immunoreactivity in the early stages of atherosclerosis may result from locally reduced or disturbed flow (e.g. in the bifurcations, regions shown to be prone to the development of atherosclerotic plaques) (Glagov et al. 1988). These findings suggest that locally increased ET-1 concentration may mediate pro-atherosclerotic mechanisms, including neointima formation due to stimulated growth of vascular smooth muscle cells, in an autocrine and/or paracrine fashion (Komuro et al. 1988). Thus, the endothelial ETB receptor might mediate anti-atherosclerotic mechanisms in a shear stress-dependent manner. The findings presented in this paper show a flow-induced upregulation of ETB that suggests an anti-atherosclerotic role. These results raise a question regarding the use of non-selective ETA/ETB endothelin receptor blockade, and emphasize the need for selective endothelin receptor antagonists (Packer et al. 1998; Webb et al. 1998).
In summary, the downregulation of ET-1 might contribute to the vasoprotective and anti-arteriosclerotic potential of high laminar shear stress. Additionally, the upregulation of the ETB receptor, whose activation causes endothelial NO release, might represent an atheroprotective effect of laminar shear stress. These effects might contribute to the vasoprotective and anti-arteriosclerotic potential of arterial laminar shear stress.
Acknowledgments
We are grateful to E. Wilson (Texas A & M University Health Science Center, College Station, TX, USA) for critically reading the manuscript, and E. Heinke, R. Gall and R. Busath for excellent technical assistance. This study was supported by the Novartis foundation for therapeutic research (to H.M.).
References
- Altschul SF, Gish W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ando K, Hirata Y, Shichiri M, Emori T, Marumo F. Presence of immunoreactive endothelin in human plasma. FEBS Letters. 1989;245:164–166. doi: 10.1016/0014-5793(89)80213-3. [DOI] [PubMed] [Google Scholar]
- Arcari P, Martinelli R, Salvatore F. The complete sequence of a full length cDNA for human liver glyceraldehyde-3-phosphate dehydrogenase: evidence for multiple mRNA species. Nucleic Acids Research. 1984;12:9179–9189. doi: 10.1093/nar/12.23.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada S, Kasuya Y, Sakurai T, Masaki T, Goto K. Endothelin-1-induced downregulation of ETB receptor mRNA: participation of cAMP. Journal of Cardiovascular Pharmacology. 1995;26:S272–275. [PubMed] [Google Scholar]
- Barber DA, Michener SR, Ziesmer SC, Miller VM. Chronic increases in blood flow upregulate endothelin-B receptors in arterial smooth muscle. American Journal of Physiology. 1996;270:H65–71. doi: 10.1152/ajpheart.1996.270.1.H65. [DOI] [PubMed] [Google Scholar]
- Barton M, Haudenschild CC, d'Uscio LV, Shaw S, Munter K, Luscher TF. Endothelin ETA receptor blockade restores NO-mediated endothelial function and inhibits atherosclerosis in apolipoprotein E-deficient mice. Proceedings of the National Academy of Sciences of the USA. 1998;95:14367–14372. doi: 10.1073/pnas.95.24.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger C, Luscher TF. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. Journal of Clinical Investigation. 1990;85:587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Davies PF, Barbee KA, Volin MV, Robotewskyj A, Chen J, Joseph L, Griem ML, Wernick MN, Jacobs E, Polacek DC, dePaola N, Barakat AI. Spatial relationships in early signaling events of flow-mediated endothelial mechanotransduction. Annual Review of Physiology. 1997;59:527–549. doi: 10.1146/annurev.physiol.59.1.527. [DOI] [PubMed] [Google Scholar]
- Denault JB, Claing A, D'Orleans-Juste P, Sawamura T, Kido T, Masaki T, Leduc R. Processing of proendothelin-1 by human furin convertase. FEBS Letters. 1995;362:276–280. doi: 10.1016/0014-5793(95)00249-9. [DOI] [PubMed] [Google Scholar]
- Forster E. An improved general method to generate internal standards for competitive PCR. Biotechniques. 1994;16:18–20. [PubMed] [Google Scholar]
- Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Archives of Pathology and Laboratory Medicine. 1988;112:1018–1031. [PubMed] [Google Scholar]
- Heinroth-Hoffmann I, Vogelsang M, Schiewe P, Morawietz H, Holtz J, Ponicke K, Brodde OE. Mechanism of ET(A)-receptor stimulation-induced increases in intracellular Ca2+ in SK-N-MC cells. British Journal of Pharmacology. 1998;125:1202–1211. doi: 10.1038/sj.bjp.0702208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda K, Nakao K, Hiroshi A, Suga S, Ogawa Y, Mukoyama M, Shirakami G, Saito Y, Nakanishi S, Imura H. Cloning and expression of human endothelin-1 receptor cDNA. FEBS Letters. 1991;287:23–26. doi: 10.1016/0014-5793(91)80007-p. [DOI] [PubMed] [Google Scholar]
- Huggins JP, Pelton JT, Miller RC. The structure and specificity of endothelin receptors: their importance in physiology and medicine. Pharmacology and Therapeutics. 1993;59:55–123. doi: 10.1016/0163-7258(93)90041-b. [DOI] [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proceedings of the National Academy of Sciences of the USA. 1989a;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Takuwa Y, Mitsui Y, Kobayashi M, Masaki T. The human preproendothelin-1 gene. Complete nucleotide sequence and regulation of expression. Journal of Biological Chemistry. 1989b;264:14954–14959. [PubMed] [Google Scholar]
- Itoh Y, Yanagisawa M, Ohkubo S, Kimura C, Kosaka T, Inoue A, Ishida N, Mitsui Y, Onda H, Fujino M, Masaki T. Cloning and sequence analysis of cDNA encoding the precursor of a human endothelium-derived vasoconstrictor peptide, endothelin: identity of human and porcine endothelin. FEBS Letters. 1988;231:440–444. doi: 10.1016/0014-5793(88)80867-6. [DOI] [PubMed] [Google Scholar]
- Komuro I, Kurihara H, Sugiyama T, Yoshizumi M, Takaku F, Yazaki Y. Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS Letters. 1988;238:249–252. doi: 10.1016/0014-5793(88)80489-7. [DOI] [PubMed] [Google Scholar]
- Kuchan MJ, Frangos JA. Shear stress regulates endothelin-1 release via protein kinase C and cGMP in cultured endothelial cells. American Journal of Physiology. 1993;264:H150–156. doi: 10.1152/ajpheart.1993.264.1.H150. [DOI] [PubMed] [Google Scholar]
- Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC., Jr Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. New England Journal of Medicine. 1991;325:997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- Lerman A, Holmes DR, Jr, Bell MR, Garratt KN, Nishimura RA, Burnett JC., Jr Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation. 1995;92:2426–2431. doi: 10.1161/01.cir.92.9.2426. [DOI] [PubMed] [Google Scholar]
- Malek A, Izumo S. Physiological fluid shear stress causes downregulation of endothelin-1 mRNA in bovine aortic endothelium. American Journal of Physiology. 1992;263:C389–396. doi: 10.1152/ajpcell.1992.263.2.C389. [DOI] [PubMed] [Google Scholar]
- Malek AM, Greene AL, Izumo S. Regulation of endothelin 1 gene by fluid shear stress is transcriptionally mediated and independent of protein kinase C and cAMP. Proceedings of the National Academy of Sciences of the USA. 1993;90:5999–6003. doi: 10.1073/pnas.90.13.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawietz H, Ma YH, Vives F, Wilson E, Sukhatme VP, Holtz J, Ives HE. Rapid induction and translocation of Egr-1 in response to mechanical strain in vascular smooth muscle cells. Circulation Research. 1999a;84:678–687. doi: 10.1161/01.res.84.6.678. [DOI] [PubMed] [Google Scholar]
- Morawietz H, Rueckschloss U, Niemann B, Duerrschmidt N, Galle J, Hakim K, Zerkowski HR, Sawamura T, Holtz J. Angiotensin II induces LOX-1, the human endothelial receptor for oxidized low-density lipoprotein. Circulation. 1999b;100:899–902. doi: 10.1161/01.cir.100.9.899. [DOI] [PubMed] [Google Scholar]
- Morita T, Kurihara H, Maemura K, Yoshizumi M, Yazaki Y. Disruption of cytoskeletal structures mediates shear stress-induced endothelin-1 gene expression in cultured porcine aortic endothelial cells. Journal of Clinical Investigation. 1993;92:1706–1712. doi: 10.1172/JCI116757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noris M, Morigi M, Donadelli R, Aiello S, Foppolo M, Todeschini M, Orisio S, Remuzzi G, Remuzzi A. Nitric oxide synthesis by cultured endothelial cells is modulated by flow conditions. Circulation Research. 1995;76:536–543. doi: 10.1161/01.res.76.4.536. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Nakao K, Arai H, Nakagawa O, Hosoda K, Suga S, Nakanishi S, Imura H. Molecular cloning of a non-isopeptide-selective human endothelin receptor. Biochemical and Biophysical Research Communications. 1991;178:248–255. doi: 10.1016/0006-291x(91)91806-n. [DOI] [PubMed] [Google Scholar]
- Packer M, Caspi A, Charlon V, Cohen-Solal A, Kiowski W, Kostuk W, Krum H, Levine B, Massie B, McMurray J, Rizzon P, Swedberg K. Multicenter, double-blind, placebo-controlled study of long-term endothelin blockade with bosentan in chronic heart failure – results of the REACH-1 trial. Circulation. 1998;98(suppl.):I–3. [Google Scholar]
- Rieder MJ, Carmona R, Krieger JE, Pritchard KA, Jr, Greene AS. Suppression of angiotensin-converting enzyme expression and activity by shear stress. Circulation Research. 1997;80:312–319. doi: 10.1161/01.res.80.3.312. [DOI] [PubMed] [Google Scholar]
- Russell FD, Skepper JN, Davenport AP. Human endothelial cell storage granules: a novel intracellular site for isoforms of the endothelin-converting enzyme. Circulation Research. 1998;83:314–321. doi: 10.1161/01.res.83.3.314. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Schmidt M, Kroger B, Jacob E, Seulberger H, Subkowski T, Otter R, Meyer T, Schmalzing G, Hillen H. Molecular characterization of human and bovine endothelin converting enzyme (ECE-1) FEBS Letters. 1994;356:238–243. doi: 10.1016/0014-5793(94)01277-6. [DOI] [PubMed] [Google Scholar]
- Sdougos HP, Bussolari SR, Dewey CFJ. Secondary flow and turbulence in a cone-plate device. Journal of Fluid Mechanics. 1984;138:379–404. [Google Scholar]
- Sharefkin JB, Diamond SL, Eskin SG, McIntire LV, Dieffenbach CW. Fluid flow decreases preproendothelin mRNA levels and suppresses endothelin-1 peptide release in cultured human endothelial cells. Journal of Vascular Surgery. 1991;14:1–9. [PubMed] [Google Scholar]
- Shichiri M, Kato H, Marumo F, Hirata Y. Endothelin-1 as an autocrine/paracrine apoptosis survival factor for endothelial cells. Hypertension. 1997;30:1198–1203. doi: 10.1161/01.hyp.30.5.1198. [DOI] [PubMed] [Google Scholar]
- Shimada K, Matsushita Y, Wakabayashi K, Takahashi M, Matsubara A, Iijima Y, Tanzawa K. Cloning and functional expression of human endothelin-converting enzyme cDNA. Biochemical and Biophysical Research Communications. 1995;207:807–812. doi: 10.1006/bbrc.1995.1258. [DOI] [PubMed] [Google Scholar]
- Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- Turner AJ, Murphy LJ. Molecular Pharmacology of endothelin converting enzymes. Biochemical Pharmacology. 1996;51:91–102. doi: 10.1016/0006-2952(95)02036-5. [DOI] [PubMed] [Google Scholar]
- Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ, Alexander RW, Nerem RM, Harrison DG. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. American Journal of Physiology. 1995;269:C1371–1378. doi: 10.1152/ajpcell.1995.269.6.C1371. [DOI] [PubMed] [Google Scholar]
- Valdenaire O, Rohrbacher E, Mattei MG. Organization of the gene encoding the human endothelin-converting enzyme (ECE-1) Journal of Biological Chemistry. 1995;270:29794–29798. doi: 10.1074/jbc.270.50.29794. [DOI] [PubMed] [Google Scholar]
- Warner TD, Allcock GH, Corder R, Vane JR. Use of the endothelin antagonists BQ-123 and PD 142893 to reveal three endothelin receptors mediating smooth muscle contraction and the release of EDRF. British Journal of Pharmacology. 1993;110:777–782. doi: 10.1111/j.1476-5381.1993.tb13879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DJ, Monge JC, Rabelink TJ, Yanagisawa M. Endothelin: new discoveries and rapid progress in the clinic. Trends in Pharmacological Sciences. 1998;19:5–8. doi: 10.1016/s0165-6147(97)01144-9. [DOI] [PubMed] [Google Scholar]
- Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, deWit D, Yanagisawa M. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994;78:473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yoshizumi M, Kurihara H, Sugiyama T, Takaku F, Yanagisawa M, Masaki T, Yazaki Y. Hemodynamic shear stress stimulates endothelin production by cultured endothelial cells. Biochemical and Biophysical Research Communications. 1989;161:859–864. doi: 10.1016/0006-291x(89)92679-x. [DOI] [PubMed] [Google Scholar]


