Abstract
Neurones which are excited by CO2 or H+ are present in a number of brainstem structures in addition to the ventrolateral region of the medulla, the site at which the respiratory response to hypercapnia is traditionally believed to originate. In this review we examine recent work concerned with establishing the relationship between these chemosensitive neurones and respiration, the emphasis being placed on the use for this purpose of in vitro preparations of the mammalian brainstem.
Under normoxic conditions CO2 is the most powerful natural stimulus to respiration in man and other air breathing vertebrates (Dejours, 1988). In the steady state much of the resulting ventilatory response (up to 80 % during hyperoxia; Heeringa et al. 1979) depends on the action of CO2 (or H+) at sites located within the lower brainstem. These sites, usually held to be neurones, are thus sometimes referred to as central (respiratory) chemoreceptors, though the term itself is little more than a useful fictional device for structures which have proved to be very difficult to identify with any confidence. The purpose of this article is to review recent work concerned with one specific aspect of this problem, namely with the question whether brainstem neurones which belong to the respiratory network are themselves CO2 and/or pH chemosensitive.
A commonly held view is that the ventilatory response to hypercapnia or acidosis is mediated by structures (chemoreceptors) located at, or close to, the ventrolateral surface of the medulla (reviewed by Loeschke, 1982). Nothing which has emerged from more recent experimental work has diminished what appears to be the particular importance of this region in stimulating ventilation, and indeed has tended to reinforce it by adding previously unavailable detail. First, by directly demonstrating the presence within the ventrolateral medulla of chemosensitive neurones (Jarolimek et al. 1990; Kawai et al. 1996), some of which are located immediately adjacent to, and others within a few hundred micrometres of, the surface (Richerson, 1995). Second, by showing that even where chemosensitive neurones in this region are located at some distance from the surface they may nevertheless be morphologically specialized to detect pH changes occurring in the vicinity of the surface (see Pilowsky et al. 1993; Kawai et al. 1996). And third, by showing that, at least in the non-awake animal, the functional integrity of a specific, superficially located group of (putative) chemosensitive cells within the rostral ventrolateral medulla (retrotrapezoid nucleus) is required for the generation of a respiratory response to systemic hypercapnia (Nattie et al. 1991; Nattie & Li, 1994; Erlichman et al. 1998).
That said, what has changed in the last few years is the belief that the ventrolateral medulla is uniquely chemosensitive. The picture which is now beginning to emerge is one in which CO2/pH chemosensitivity is a property shared by a significantly large number of neurones belonging to several quite distinct brainstem networks. The experimental background to this revised view is largely the result of two developments. First, improvements to technique have made it possible in in vivo preparations to produce localized areas of tissue acidosis (300-500 μm in extent) within the brainstem, including deep-lying structures. So it is possible to demonstrate that focal acidification at a number of individual, widely distributed sites stimulates the respiratory motor (phrenic) output (Coates et al. 1993; Nattie & Li, 1996; Bernard et al. 1996; Li & Nattie, 1997; Erlichman et al. 1998). Second, the introduction of a variety of in vitro preparations – some of which show a rudimentary, but still recognizably respiratory, motor output – has made it possible to extend the search for chemosensitive neurones beyond the traditionally chemosensitive region of the ventrolateral medulla and to include identified respiratory neurones in this search (for references see below). The encouraging feature of these developments is that, at least on a regional basis, there is a large measure of agreement between in vitro and in vivo work as to the distribution of chemosensitive structures, but in all this, the problem remains of linking chemosensitive neurones directly to the control of respiration, and it is with this issue that we are concerned here. In the remainder of this article we will use the term ‘CO2’ in referring to the stimulus because its concentration is the most commonly employed experimental variable; the nature and site of action of the adequate stimulus are still very much subjects of debate (e.g. Voipio & Ballanyi, 1997; Ritucci et al. 1997).
Chemosensitive neurones are present in several anatomically distinct brainstem structures
From work on in vitro preparations it is clear that significantly large numbers of brainstem neurones, present in several anatomically distinct structures and exhibiting very different patterns of activity, can be classified as chemosensitive. This is conventionally understood to mean that they exhibit an excitatory response to an increase in CO2 or H+ concentration which they retain under conditions in which chemical synaptic transmission is minimal or absent. Within the lower brainstem the currently known distribution of such neurones falls into four main groups: the pontine locus coeruleus (LC; Pineda & Aghajanian, 1997; Oyamada et al. 1998); the nucleus tractus solitarii (Dean et al. 1989, 1990; Huang et al. 1997); the midline (raphe) nuclei of the ventral medulla (Richerson, 1995; Wang et al. 1998) and the ventrolateral quadrant of the medulla (VLM; Jarolimek et al. 1990; Richerson, 1995) where they are distributed over a fairly extensive column along the rostro-caudal axis (Kawai et al. 1996).
The actual incidence of such neurones in these various structures is difficult to assess accurately because of differences in recording technique and in the preparations which have been employed, but it ranges from in excess of 90 % in the LC, which is a homogeneous structure in the rat (Pineda & Aghajanian, 1997; Oyamada et al. 1998) to about 14 % in the ventral raphe and adjacent rostral VLM where CO2-excited neurones constitute about half of the responsive population (Richerson, 1995).
We will consider later two classes of chemosensitive neurone for which it is possible to infer a link with respiratory control because they form part of a synaptically connected network of brainstem neurones showing respiratory rhythm. However, beyond this specific relationship the evidence provided by trans-synaptic (pseudorabies virus) tracing of connections back from the phrenic nucleus reveals that each of those structures (see above) now known to contain chemosensitive neurones forms part of a substantially larger network involved in controlling the input to this nucleus, and therefore the contraction of the diaphragm (Dobbins & Feldman, 1994). For example, the ventral respiratory group of neurones, located within the VLM, represents the main source of rhythmic respiratory input to the phrenic nucleus (in the rat), but it is also a point of convergence for numerous additional pathways, including those originating in nuclei outside the VLM and which contain chemosensitive neurones. Thus, there are reasonable grounds for suspecting that these various chemosensitive neurones are involved in some way in controlling the diaphragm, whether for its primary purpose in ventilating the lungs or as part of a wider range of homoeostatic adjustments.
One approach to looking for connections between chemosensitive neurones and the neurones controlling ventilation is to use the respiratory rhythm itself as an indication of connectivity, and to ask whether neurones showing this rhythm are also chemosensitive. A start has been made in this direction by using in vitro preparations of the brainstem and spinal cord, and this is described next.
Respiratory chemosensitivity in vitro
A number of in vitro preparations of the brainstem show the property that they spontaneously generate a rhythmic output on respiratory nerve roots which is sustained for more or less long periods (hours) after isolation. Only a few have been specifically examined from the point of view of chemosensitivity (Suzue, 1984; Morin-Surun et al. 1995; Eugenin & Nicholls, 1997) and the most commonly used in work on single neurones is the isolated and superfused brainstem- spinal cord of the neonatal rat. In this preparation the rhythm is invariably present on the phrenic (inspiratory) nerve roots, but when stimulated by lowering the bath pH it also appears with the appropriate phasing in expiratory intercostal nerves (Iizuka, 1999). The two important experimental features of this preparation are as follows.
First, it exhibits a reproducible response to increasing the CO2 concentration of the superfusate (hypercapnic acidosis). The most prominent feature of this response is an increase in phrenic burst (= respiratory) frequency with (typically) little change in burst structure and just a slight shortening in burst duration (see phrenic trace in Figs 2A–C and 4A; Suzue, 1984; Okada et al. 1993; Brockhaus et al. 1993; Kawai et al. 1996; Voipio & Ballanyi, 1997; Oyamada et al. 1998; Iizuka, 1999; but see also Harada et al. 1985). Neither the pons nor the dorsal half of the medulla is required for the response, while microinjection of a small volume of CO2-tonometered saline at numerous individual sites just below the ventrolateral surface of the medulla elicits at short latency a transient increase in respiratory frequency (Issa & Remmers, 1992), i.e. the VLM contains all that is necessary to generate a CO2-sensitive rhythm.
Figure 2. Chemosensitivity in respiration-modulated LC neurones in vitro.
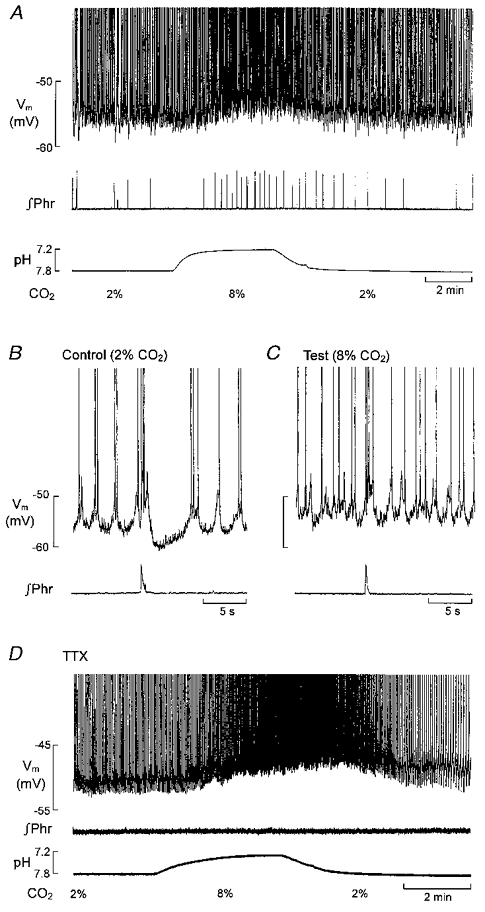
A, the acid shift in bath pH which follows switching from 2 to 8 % CO2-equilibrated superfusate, the subsequent increase in phrenic burst frequency (∞Phr), the depolarizing shift in membrane potential (Vm) and increase in discharge frequency in an LC neurone. B and C, short episodes on a fast time base taken from the record in A. D (different LC neurone), the depolarizing response and increase in discharge frequency are retained after blocking phrenic discharge and respiratory modulation of membrane potential with TTX (0.2 μM). (Modified after Oyamada et al. (1998) and reproduced with permission.)
Figure 4. Chemosensitivity in an inspiratory VLM neurone in vitro.

A and B, the same neurone recorded in perforated patch configuration. A, switching from 2 to 8 % CO2 resulted in an acid shift in bath pH, an increase in phrenic burst (∞Phr) frequency and membrane depolarization. B, TTX suppressed phrenic discharge and correspondingly phased synaptic input to the neurone, but left the depolarizing response to CO2 intact (A. Kawai, D. Ballantyne, K. Mückenhoff & P. Scheid, unpublished data shown with permission).
Second, with extracellular or whole-cell recording techniques it has been possible to identify respiratory neurones within the VLM, to map their distribution and to classify individual neurones according to the timing of their activity in relation to phrenic discharge (Smith et al. 1990, 1992; Onimaru & Homma, 1992; Brockhaus et al. 1993). For present purposes the details of this classification are less important than the fact that the existence of various sub-types of respiratory neurone is evidence both of a substantial synaptic circuitry and of differences, experimentally verified in a few cases (Onimaru et al. 1996; Rekling et al. 1996; Koshiya & Smith, 1999), in their intrinsic membrane properties.
Together these two features – a reproducible response to CO2 and identifiable respiratory neurones – would seem to make the brainstem preparation well suited to asking whether the latter function as chemoreceptors. However, there are also ‘problems’ with the in vitro rhythm: it is slow, the inspiratory burst is brief and decrementing in pattern rather than incrementing as in eupnoeic respiration. Most importantly, the way it changes in response to CO2 is almost exactly the reverse of the response to hypercapnia seen in the vagotomized (adult) animal in vivo where respiratory frequency remains largely unchanged at increased tidal volume. Some of these differences have been variously attributed to the effects of (low) temperature, de-afferentation, tissue acidosis and hypoxia in the in vitro preparation (see St John, 1998, and Rekling & Feldman, 1998, for opposing views as to their origin). However they arise, it is not unreasonable to ask whether it is worthwhile studying the chemosensitivity of what would certainly be an abnormal respiratory rhythm in a brain with intact blood supply. We believe that it is because it allows questions to be asked about chemosensitivity which cannot at present be readily answered either in less reduced preparations showing a normal eupnoeic rhythm or in conventional slice preparations lacking an identifiable respiratory rhythm.
Chemosensitivity within the rhythmically active brainstem respiratory network
In the neonatal rat brainstem in vitro both LC neurones and some VLM neurones have been shown both to be responsive to CO2 and to exhibit a rhythmic synaptic input which is synchronized to the burst discharge of phrenic motoneurones (Fig. 1A and B; Kawai et al. 1996; Oyamada et al. 1998). Since there is no peripheral afferent feedback in this preparation this input must ultimately be attributed to the way these neurones are connected to the respiratory rhythm generator which, in this preparation, is located within the ventral medulla. Most current evidence places this location immediately rostral to the ventral respiratory group of neurones (defined using the classification of Dobbins & Feldman, 1994) within a functionally identified region referred to as the ‘pre-Bötzinger complex’ (PBC) (Smith et al. 1991; Johnson et al. 1994; Rekling & Feldman, 1998; Koshiya & Smith, 1999). However, there is also evidence for endogenously rhythmic (pre-inspiratory) neurones outside the PBC at a more rostral location within the medulla (RVL; Onimaru et al. 1988). Since these may be important in generating respiratory rhythm in vitro (reviewed by Ballanyi et al. 1999), we have included both the PBC and RVL as possibilities in the schematic rhythm generator of Fig. 1C. The issue is of little consequence for the present discussion since both LC neurones and those medullary respiratory neurones which have been examined in any detail from the point of view of their chemosensitivity are located ‘downstream’ of the rhythm generator, i.e. their respiratory-phased activity is dependent upon intact chemical synaptic transmission. Since the most prominent feature of the response to CO2in vitro is an increase in respiratory frequency, the obvious question to ask is what purpose is served by the chemosensitivity of ‘downstream’ neurones. To answer this it is first necessary to examine the form which their chemosensitivity takes.
Figure 1. Respiratory-phased innervation of neurones in the locus coeruleus (LC) and ventrolateral medulla in the neonatal rat brainstem in vitro.

A and B, spontaneous activity (membrane potential, Vm) recorded with a whole-cell pipette from a respiration-modulated LC neurone (A) and an inspiratory neurone of the ventral respiratory group (VRG) (B). Inspiratory discharge was recorded with a suction pipette from a phrenic nerve root and is shown in integrated form (∞Phr). The large difference in respiratory frequency between A and B is typical of preparations with (A) and without (B) the pons. C shows schematically the relationship of these synaptically driven respiratory neurones to the respiratory-rhythm generator. Since there is still uncertainty as to whether the essential rhythm generating neurones are located within the pre-Bötzinger complex (PBC) or the rostroventrolateral medulla (RVL), both possibilities are shown. VRG neurones are shown with dendritic projections to the ventrolateral surface. The existence of gap junction coupling of LC neurones within the pericoerulear dendritic network (PCD) is based on inference (see text). The + and – symbols refer to excitatory and inhibitory synaptic input, respectively.
The LC as a chemosensory nucleus
The respiratory input to LC neurones generates a biphasic pattern of excitation and inhibition, the former occurring approximately synchronously with the phrenic burst, and the latter occupying the early stage of the expiratory interval between bursts (Fig. 1A). The effect of this input is to modulate at respiratory frequency the on-going discharge of LC neurones which may take the form of a regular rhythm of single spikes, but more commonly in the neonate (in vitro) is patterned into spike doublets or triplets by an underlying subthreshold oscillation of the membrane potential. Since this non-respiratory component of spike discharge continues in the absence of chemical synaptic input (Williams et al. 1984) it can be regarded as being endogenously generated.
Bath application of glutamate antagonists suppresses both components of the respiratory input, while the inhibitory component alone is suppressed by the specific α2-adrenoceptor antagonist, idazoxan. In Fig. 1C we have indicated two possibilities, for both of which there is evidence, which may determine the timing of the inhibitory input. The first is to suppose that LC neurones are connected via collateral axonal or dendritic noradrenaline-releasing inhibitory pathways (Aghajanian et al. 1977; Egan et al. 1983; Ennis & Aston-Jones, 1986; Harris et al. 1992) which are activated by the preceding phase of excitation, the latter deriving from the medulla. Such an intracoerulear inhibitory mechanism would be consistent with the fact that in the brainstem preparation exposure to α2-adrenoceptor antagonists not only suppresses respiratory-phased inhibition with no effect on the preceding excitation, but also results in an increase in frequency of on-going spike discharge. A second possibility is that the sequence reflects the coordinated activation of excitatory and inhibitory afferents to the LC. If this is the case these afferents are likely to originate in the nucleus paragigantocellularis (located within the RVL) since this is the main medullary source of both excitatory amino acid-mediated excitation and adrenergically mediated inhibition of the LC, and is itself a point of convergence for a variety of afferent inputs involved in the control of the LC (reviewed by Aston-Jones et al. 1991). One reason why it will become important in future to clarify the source of respiratory-phased inhibition is that its suppression contributes significantly to the excitatory response of the LC to increased CO2 (see below).
What makes the respiratory innervation of the LC of interest is the very extensive nature of the noradrenergic output projections from this nucleus (Foote et al. 1983). Since, on the evidence of trans-synaptic tracing, these targets appear to include (functionally unidentified) elements of the medullary network of phrenic premotoneurones (Dobbins & Feldman, 1994) the LC may feed back directly to the respiratory network. We will consider the possible significance of this later. At this stage we note that if the respiratory-phased burst is regarded as an output ‘signal’ transmitted by the LC, and the on-going spike activity as ‘noise’, then the signal-to-noise ratio becomes smaller as the CO2 concentration is increased. Two factors contribute to this. The first is that, while the excitatory component of the respiratory input is essentially unaffected by an increase in CO2 (from 2 to 8 %), the inhibitory component becomes weaker so that the corresponding period becomes occupied by action potentials (Fig. 2A–C). The second is that increasing the CO2 concentration depolarizes the neurones and results in an increase in the frequency of the non-respiratory component of their spike discharge (Pineda & Aghajanian, 1997; Oyamada et al. 1998). Thus, the modulating effect of the respiratory input, prominent at a low CO2 concentration, is diminished at a higher concentration. These effects are reversible, they follow the same time course as the CO2-induced increase in respiratory frequency recorded on the phrenic roots, and so, after a short delay, they also follow the time course of the change in bath pH. That the overall response is physiologically relevant is suggested by the fact that under in vivo conditions systemic hypercapnia results in an increase in LC discharge frequency which parallels the increase in sympathetic outflow (Elam et al. 1981) and phrenic activity.
What is the significance of the respiratory-phased burst discharge of LC neurones? A clue is suggested by the fact that the intensity of this burst (measured as spike frequency) is virtually unchanged over a range of CO2 concentrations which result in a fairly large (about 50 %) change in the frequency of spontaneous spike discharge, i.e. the response to CO2 shows selectivity. There is nothing unique about CO2 in this regard. For example, bath application of noradrenaline suppresses the on-going spike activity and subthreshold oscillation of LC neurones, but leaves their respiratory-phased (presumed glutamatergic) excitation intact (Oyamada et al. 1998) and their burst response to iontophoretically pulsed glutamate almost unaffected (Aston-Jones et al. 1991). This suggests that transmission of the respiratory-phased burst may continue under conditions in which background activity in the LC is negligible. This in turn may reflect a more general requirement for the LC, perhaps connected with its arousal function, to remain responsive to afferent inputs under such conditions. If the LC does innervate the medullary respiratory network then the continued phasic release of noradrenaline may be an important factor in controlling the average level of excitability of the network or certain elements of it (see e.g. Arata et al. 1998) even if the phasic character of this input is subsequently filtered out postsynaptically. In any event, it is clear that the relatively selective nature of the effect of CO2 on LC activity raises questions as to the mechanism and site of action of this stimulus, and we examine these aspects next.
Are LC neurones intrinsically chemosensitive?
In their study of adult LC neurones (pontine slice) Pineda & Aghajanian (1997) showed that the CO2-induced increase in tonic discharge frequency was dependent on intracellular acidification and that this was associated with inhibition of the outward current component through a potassium inward rectifier. In addition they reported that the response was retained in the presence of idazoxan, a result which makes it very unlikely that the response is mediated indirectly via depression of noradrenaline release, in effect disinhibiting the synaptically coupled network. Other evidence favouring a mechanism intrinsic to LC neurones is the fact that in the in vitro neonatal brainstem exposure to TTX, at a concentration sufficient to suppress phrenic activity and the corresponding respiratory synaptic input to the LC, leaves both the depolarizing component of the response to CO2 and the increase in the frequency of the endogenously generated rhythm of (Cd2+-blockable) spikes intact (Fig. 2D). The point is important because LC neurones are equipped with a variety of receptors which act to modulate discharge frequency via inwardly rectifying potassium channels, though it must be said there is no direct evidence that TTX-sensitive afferent inputs to the LC are completely blocked under these conditions.
One possibility which should be further investigated follows the finding that LC neurones are equipped with P2 purinoceptors which, when stimulated by (enzymatically stable) ATP, generate a depolarizing response and an increase in spike frequency (Harms et al. 1992). The co-release of ATP from noradrenergic LC neurones into an acidic extracellular space – which may be expected to potentiate the purinergic (P2 X2) response (King et al. 1996) – may contribute to the TTX-resistant CO2 response (see Thomas et al. 1999 for a discussion of this point in relation to ventral medullary chemosensitivity). How significant purinergic transmission may be in chemosensitivity is difficult to assess at present because LC neurones – as indeed VRG inspiratory neurones – remain responsive to CO2 in a low Ca2+-high Mg2+ medium (Kawai et al. 1996; Oyamada et al. 1999), which certainly depresses Ca2+-dependent synaptic transmission, and which in the LC also suppresses the excitatory response to exogenously applied methylene-ATP (Harms et al. 1992).
Electrical coupling and synchronization of LC neurones is retained during their chemosensory response
Since dendritic mechanisms have been implicated in the timing of spontaneous discharge in LC neurones (Williams et al. 1984; Williams & Marshall, 1987) the dendritic tree may also represent a site at which the CO2 sensitivity of the membrane potential acts to control the output from the nucleus. An important aspect of this control in the neonate arises from the fact that LC neurones form a dye- and electrically coupled network with the coupling apparently mediated by dendro-dendritic interactions (Christie et al. 1989; Christie & Jelinek, 1993; Travagli et al. 1995; Oyamada et al. 1999). Electrical coupling may also be present under some circumstances in the adult (Travagli et al. 1995), for in the adult slice, the capacity of the nucleus to synchronize its output depends on the physical presence of a dense pericoerulear network of dendrites (PCD, Fig. 1C) (Ishimatsu & Williams, 1996). This network, extending for several hundred micrometres outside the compact cell soma region of the nucleus proper (Shipley et al. 1996), may be presumed therefore to represent a major site at which electrically mediated interactions occur.
The most prominent manifestation of such electrotonic coupling in the neonatal LC is the synchronized occurrence of spontaneous subthreshold oscillations of membrane potential in paired recordings. These persist in the absence of chemical synaptic transmission and after pharmacological blockade of a variety of potential transmitters (Christie et al. 1989; Travagli et al. 1995). The frequency of oscillation is sensitive to CO2, approximately doubling when the concentration is raised from 2 to 8 % (Oyamada et al. 1998). Since the effect of this presumed dendritically located oscillation (Williams & Marshall, 1987) is to trigger spike discharge (Fig. 3A) the mechanism of oscillation becomes crucial to understanding the action of CO2. Although little is known about the mechanism, some of the conditions influencing its occurrence are beginning to emerge.
Figure 3. Synchronization of the electrically coupled rhythm within the LC network remains effective during the chemosensory response.

A–C, perforated patch recording (Vm) from the same LC neurone under control conditions (A) and during exposure to a medium of reduced Ca2+ (0.2 mM) and increased Mg2+ (5 mM) concentration (B and C). The frequency of this rhythm was increased and intraburst discharge frequency reduced on switching from 2 to 8 % CO2. Note suppression of phrenic discharge (∞Phr) and respiratory synaptic input in low Ca2+-high Mg2+. D shows rhythmic activity in low Ca2+-high Mg2+ as a control. E (same neurone), loss of membrane potential oscillation in low Ca2+-high Mg2+ plus carbenoxolone (300 μm, 25 min), but retention of the capacity to discharge spikes in response to current injection. F, an extracellular recording showing multi-unit synchronization in low Ca2+-high Mg2+ as a control. G (same recording as F), desynchronization after 15 min exposure to carbenoxolone. (Modified after Oyamada et al. (1999) and reproduced with permission from Respiration Physiology, Elsevier Science.)
In the brainstem preparation exposed to a low Ca2+-high Mg2+ medium all LC neurones exhibit a regular rhythm of large amplitude, slow oscillations of membrane potential (Fig. 3B). The effect of each cycle of depolarization is to trigger a rapid train of smaller oscillations – resembling those seen in normal medium and which we will refer to as dendritic spikes – and these, in turn, trigger full-sized spikes. Extracellular recordings show that this rhythm is synchronized throughout the nucleus. The large oscillation is blocked by Cd2+. The entire rhythm is suppressed by TTX, but the large oscillation is restored by addition of Ba2+ and TEA. Thus, in low Ca2+-high Mg2+ LC neurones exhibit a synchronized pattern of burst discharge which is dependent on an external source of Ca2+ or a source of depolarizing current via Cd2+-sensitive channels. If voltage-sensitive Na+ channels are normally involved in sustaining the large oscillation, they are resistant or inaccessible to somatic injection of QX314. The emergence of such synchronization is somewhat similar to the situation shown by hippocampus CA1 neurones in a zero Ca2+ medium where the effect appears to be due to enhanced gap junction coupling (Perez-Velazquez et al. 1994). There is, however, an important difference between these neurones. In the LC the effect of increasing the CO2 concentration (from 2 to 8 %), and therefore probably producing a significant intracellular acidification (Ritucci et al. 1997; Huang et al. 1997), is not to suppress the rhythm as in the hippocampus, but to increase its frequency (on average by 35 %), i.e. the rhythm remains synchronized and the only obvious effect which might be due to pH-induced gap junction uncoupling is a (reversible) reduction in intraburst discharge frequency (Fig. 3B and C). Exposure to the gap junction uncoupler carbenoxolone (or to very high concentrations (> 20 %) of CO2) suppresses the oscillation of the membrane potential, but has no effect on the capacity of LC neurones to discharge full-sized spikes (Fig. 3D and E; see also Travagli et al. 1995). Under these conditions the discharge becomes desynchronized (Fig. 3F and G) and individual neurones discharge at a frequency determined by the prevailing membrane potential (Fig. 3E; see also Travagli et al. 1995). This, in turn, depends on the CO2 concentration (Oyamada et al. 1999).
There are several reasons why this low Ca2+-high Mg2+-induced rhythm is of interest. First, it reinforces the notion, previously proposed for the LC (Ishimatsu & Williams, 1996) and some other networks (e.g. Draguhn et al. 1998), that electrical coupling may be a requirement for the generation of some forms of synchronized oscillatory activity. Second, it reveals evidence of a CO2-sensitive oscillation of potential which appears to trigger dendritic spikes. Third, it suggests that electrical coupling within the LC network is retained at CO2 levels which are unlikely to be exceeded in vivo (see also Huang et al. 1997). Finally, the behaviour of LC neurones in low Ca2+-high Mg2+ reveals a remarkable similarity to the behaviour of certain classes of VLM respiratory neurone which have been implicated in respiratory rhythmogenesis in vitro (see below).
Chemosensitivity in respiratory rhythmogenic and non-rhythmogenic regions of the VLM
There is direct evidence for the existence within the PBC of identifiable inspiratory neurones in which the rhythm shows voltage-sensitive properties, i.e. the occurrence of rhythm is conditional on depolarization to an appropriate range of membrane potentials (Koshiya & Smith, 1999). However, one very striking feature of some PBC respiratory neurones (Johnson et al. 1994) and also of pre-inspiratory neurones of the RVL (Onimaru et al. 1989, 1995) is that when they are exposed to a low Ca2+-high Mg2+ medium they lose their type-specific pattern of discharge and exhibit instead a rhythmic burst discharge which resembles that shown by LC neurones in this medium. This similarity is reinforced by the finding that in low Ca2+-high Mg2+ the burst frequency of pre-inspiratory neurones is increased when the CO2 concentration is raised (Onimaru et al. 1989). Although dye (biocytin) and electrical coupling have been demonstrated between inspiratory neurones of the nucleus ambiguus (Rekling & Feldman, 1997) and coupling is a prominent feature of dorsal medullary chemosensitive neurones (Huang et al. 1997), there is, as yet, no evidence to our knowledge for its existence within the pre-inspiratory network or among the variety of neurone types within the PBC. The possibility nevertheless remains that, at least in low Ca2+-high Mg2+, these neurones form one or more gap-junction coupled network(s) and that the rhythm is the product of the interaction of this coupling with the intrinsic conductances of these neurones (see also Butera et al. 1999). If this putative coupling occurs at the dendro-dendritic level, then it may provide a means of synchronizing slow CO2-induced changes in membrane potential within the network.
There are at present no reports of the membrane potential response of rhythmogenic respiratory neurones to a change in CO2 concentration. However, this has been examined in non-rhythmogenic inspiratory neurones of the VRG, and here the membrane potential is clearly sensitive to CO2 (Kawai et al. 1996). In neurones of this kind the phasic character of their synaptically driven inspiratory discharge is retained at increased CO2 concentrations when the neurone depolarizes (Fig. 4A), and eliminating voltage-sensitive Na+ currents (with TTX) results in a flat membrane potential with a level dependent on the prevailing CO2 concentration (Fig. 4B). These neurones do not show rhythmic activity in low Ca2+-high Mg2+, though they do retain their depolarizing response to CO2.
The behaviour shown by inspiratory neurones of this kind might be explained if the CO2-induced depolarization, which is accompanied by a modest increase in input resistance, is of remote dendritic origin, perhaps even originating in other cells electrically coupled to the recorded cell. In this case, when viewed from the cell soma, remotely located membrane chemosensors may function as a current source which passively displaces the membrane potential. This might account for the fact that in these neurones the only obvious consequence of such depolarization is to reduce the amplitude of their respiratory-phased excitatory synaptic potential, typically leaving intraburst discharge frequency unaffected. There is no direct evidence for such a remotely located chemosensor, which remains therefore no more than conjecture, but there is one piece of morphological evidence which would be at least consistent with this general idea. This consists in the finding that chemosensitive neurones in this region, which are not confined to respiratory neurones, exhibit dendritic projections to within a short distance (< 50 μm) of the ventrolateral surface, and that this pattern of projections is independent of the depth of the parent cell soma which may vary over several hundred micrometres (Fig. 1C; Kawai et al. 1996), i.e. they project into the ‘traditional’ site of respiratory chemosensitivity. If it is the function of these dendrites to detect pH changes at the surface and to transmit the response to the deeper lying somata (but see Voipio & Ballanyi, 1997), then this function is presumably dependent on the typically low frequency character of physiologically occurring changes in pH and PCO2. The chemosensor itself could be located within the dendrite, within some associated cell connected by gap junctions, or perhaps in one releasing transmitter with low or no Ca2+ sensitivity and not dependent on TTX-sensitive Na+ conductances.
Finding a respiratory function for chemosensitive neurones
As pointed out earlier the structure of the inspiratory burst in VRG neurones is almost unchanged by CO2-induced depolarization. In essence this resembles the situation in LC neurones, but with the important difference that the latter are equipped with conductance mechanisms which convert the depolarizing response to an increased frequency of tonic spiking, i.e. it is their endogenously generated rhythm which is sensitive to CO2. In other words in both classes of respiratory-innervated neurone the action of CO2 appears to be relatively selective. Are there any grounds for believing that this kind of chemosensitivity is at all relevant to the control of respiration? In the case of the LC a plausible case can be made for supposing that the increased activity which accompanies hypercapnia may feed back at some stage to the medullary respiratory network, and may exert a stimulating action on some endogenous bursters (Arata et al. 1998). It is very much more difficult to suggest a function for chemosensitive VRG inspiratory neurones in relation to the in vitro respiratory rhythm, but this may simply reflect the way this rhythm is generated. Chemosensitivity at the VRG level may have (or acquire) a function when the rhythm is of the normal eupnoeic kind, and therefore requiring a different preparation for its study.
Acknowledgments
Work in the authors’ laboratory is supported by the Deutsche Forschungsgemeinschaft.
References
- Aghajanian GK, Cedarbaum JM, Wang RY. Evidence for norepinephrine-mediated collateral inhibition of locus coeruleus neurons. Brain Research. 1977;136:570–577. doi: 10.1016/0006-8993(77)90083-x. [DOI] [PubMed] [Google Scholar]
- Arata A, Onimaru H, Homma I. The adrenergic modulation of firings of respiratory rhythm-generating neurons in medulla-spinal cord preparation from newborn rat. Experimental Brain Research. 1998;119:399–408. doi: 10.1007/s002210050355. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, Van Brockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B, Charléty P, Valentino RJ, Williams JT. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Progress in Brain Research. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Progress in Neurobiology. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Bernard DG, Li A, Nattie EE. Evidence for central chemoreception in the medullary raphe. Journal of Applied Physiology. 1996;52:131–140. doi: 10.1152/jappl.1996.80.1.108. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K, Smith JC, Richter DW. Microenvironment of respiratory neurones in the in vitro brainstem-spinal cord of neonatal rats. The Journal of Physiology. 1993;462:421–445. doi: 10.1113/jphysiol.1993.sp019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butera RJ, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex. II. Populations of coupled pacemaker neurons. Journal of Neurophysiology. 1999;81:398–415. doi: 10.1152/jn.1999.82.1.398. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Jelinek HF. Dye-coupling among neurons of the rat locus coeruleus during postnatal development. Neuroscience. 1993;56:129–137. doi: 10.1016/0306-4522(93)90568-z. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Williams JT, North RA. Electrical coupling synchronizes subthreshold activity in locus coeruleus neurons in vitro from neonatal rats. Journal of Neuroscience. 1989;9:3584–3589. doi: 10.1523/JNEUROSCI.09-10-03584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates EL, Li A, Nattie EE. Widspread sites of brainstem ventilatory chemoreceptors. Journal of Applied Physiology. 1993;75:5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- Dean JB, Bayliss DA, Erickson JT, Lawing WL, Millhorn DL. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neuroscience. 1990;36:207–216. doi: 10.1016/0306-4522(90)90363-9. [DOI] [PubMed] [Google Scholar]
- Dean JB, Lawing WL, Millhorn DE. CO2 decreases membrane conductance and depolarizes neurons in the nucleus tractus solitarius. Experimental Brain Research. 1989;76:656–661. doi: 10.1007/BF00248922. [DOI] [PubMed] [Google Scholar]
- Dejours P. Respiration in Water and Air. Amsterdam: Elsevier Science Publishers; 1988. [Google Scholar]
- Dobbins E, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. Journal of Comparative Neurology. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Traub RD, Schmitz D, Jefferys JGR. Electrical coupling underlies high frequency oscillations in the hippocampus in vitro. Nature. 1998;394:189–192. doi: 10.1038/28184. [DOI] [PubMed] [Google Scholar]
- Egan TM, Henderson G, North RA, Wiliams JT. Noradrenaline-mediated synaptic inhibition in rat locus coeruleus neurones. The Journal of Physiology. 1983;345:477–488. doi: 10.1113/jphysiol.1983.sp014990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam M, Yao T, Thorén P, Svensson TH. Hypercapnia and hypoxia: chemoreceptor-mediated control of locus coeruleus neurons and splanchnic sympathatic nerves. Brain Research. 1981;222:373–381. doi: 10.1016/0006-8993(81)91040-4. [DOI] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G. Evidence for self- and neighbor-mediated postactivation inhibition of locus coeruleus neurons. Brain Research. 1986;324:299–305. doi: 10.1016/0006-8993(86)90424-5. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Li A, Nattie EE. Ventilatory effects of glial dysfunction in a rat brain stem chemoreceptor region. Journal of Applied Physiology. 1998;85:1599–1604. doi: 10.1152/jappl.1998.85.5.1599. [DOI] [PubMed] [Google Scholar]
- Eugenin J, Nicholls JG. Chemosensory and cholinergic stimulation of fictive respiration in the isolated CNS of neonatal opossum. The Journal of Physiology. 1997;501:425–437. doi: 10.1111/j.1469-7793.1997.425bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Bloom EF, Aston-Jones G. Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiological Reviews. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Harada Y, Kuno M, Wang YZ. Differential effects of carbon dioxide and pH on central chemoreception in the rat in vitro. The Journal of Physiology. 1985;368:679–693. doi: 10.1113/jphysiol.1985.sp015883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms L, Finta M, Tschöpl M, Illes P. Depolarization of rat locus coeruleus neurons by adenosine 5′-triphosphate. Neuroscience. 1992;48:941–952. doi: 10.1016/0306-4522(92)90282-7. [DOI] [PubMed] [Google Scholar]
- Harris GC, Hausken ZE, Williams JT. Cocaine induced synchronous oscillations in central noradrenergic neurons in vitro. Neuroscience. 1992;50:253–257. doi: 10.1016/0306-4522(92)90420-7. [DOI] [PubMed] [Google Scholar]
- Heeringa J, Berkenbosch A, DeGoede J, Olievier CN. Relative contribution of central and peripheral chemoreceptors to the ventilatory response to CO2 during hyperoxia. Respiration Physiology. 1979;37:365–379. doi: 10.1016/0034-5687(79)90082-3. [DOI] [PubMed] [Google Scholar]
- Huang R-Q, Erlichman JS, Dean JB. Cell-cell coupling between CO2-excited neurons in the dorsal medulla oblongata. Neuroscience. 1997;80:41–57. doi: 10.1016/s0306-4522(97)00017-1. [DOI] [PubMed] [Google Scholar]
- Iizuka M. Intercostal expiratory activity in an in vitro brainstem-spinal cord-rib preparation from the neonatal rat. The Journal of Physiology. 1999;520:293–302. doi: 10.1111/j.1469-7793.1999.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimatsu M, Williams JT. Synchronous activity in locus coeruleus results from dendritic interactions in pericoerulear regions. Journal of Neuroscience. 1996;16:5196–5204. doi: 10.1523/JNEUROSCI.16-16-05196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa FG, Remmers JE. Identification of a subsurface area in the ventral medulla sensitive to local changes in PCO2. Journal of Applied Physiology. 1992;72:439–446. doi: 10.1152/jappl.1992.72.2.439. [DOI] [PubMed] [Google Scholar]
- Jarolimek W, Misgeld U, Lux HD. Neurons sensitive to pH in slices of the rat ventral medulla. Pflügers Archiv. 1990;416:247–253. doi: 10.1007/BF00392060. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Funk G, Feldman JL. Pacemaker behavior of respiratory neurons in medullary slices from neonatal rat. Journal of Neurophysiology. 1994;72:2598–2608. doi: 10.1152/jn.1994.72.6.2598. [DOI] [PubMed] [Google Scholar]
- Kawai A, Ballantyne D, Mückenhoff K, Scheid P. Chemosensitive medullary neurones in the brainstem-spinal cord preparation of the neonatal rat. The Journal of Physiology. 1996;492:277–292. doi: 10.1113/jphysiol.1996.sp021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Ziganshina LE, Pintor J, Burnstock G. Full sensitivity of P2X purinoceptor to ATP revealed by changing extracellular pH. British Journal of Pharmacology. 1996;117:1371–1373. doi: 10.1111/j.1476-5381.1996.tb15293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature. 1999;400:360–363. doi: 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- Li A, Nattie EE. Focal central chemoreceptor sensitivity in the RTN studied with a CO2 difffusion pipette in vivo. Journal of Applied Physiology. 1997;83:420–428. doi: 10.1152/jappl.1997.83.2.420. [DOI] [PubMed] [Google Scholar]
- Loeschke HH. Central chemosensitivity and the reaction theory. The Journal of Physiology. 1982;322:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Surun MP, Boudinot E, Schäfer T, Denavit-Saubié M. Localization of chemosensitive strctures in the isolated brainstem of adult guinea-pig. The Journal of Physiology. 1995;485:203–212. doi: 10.1113/jphysiol.1995.sp020724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A. Retrotrapezpoid nucleus lesions decrease phrenic activity and CO2 sensitivity in rats. Respiration Physiology. 1994;97:63–77. doi: 10.1016/0034-5687(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Central chemoreception in the region of the ventral respiratory group of the rat. Journal of Applied Physiology. 1996;81:1987–1995. doi: 10.1152/jappl.1996.81.5.1987. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A, St John W. Lesions in retrotrapezoid nucleus decrease ventilatory output in anaesthetized or decerebrate cats. Journal of Applied Physiology. 1991;71:1364–1375. doi: 10.1152/jappl.1991.71.4.1364. [DOI] [PubMed] [Google Scholar]
- Okada Y, Mückenhoff K, Scheid P. Hypercapnia and medullary neurons in the isolated brainstem-spinal cord of the rat. Respiration Physiology. 1993;93:327–336. doi: 10.1016/0034-5687(93)90078-o. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Primary respiratory rhythm generator in the medulla of brainstem-spinal cord preparations from newborn rats. Brain Research. 1988;445:314–324. doi: 10.1016/0006-8993(88)91194-8. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Firing properties of respiratory rhythm generating neurons in the absence of synaptic transmission in rat medulla in vitro. Experimental Brain Research. 1989;76:530–536. doi: 10.1007/BF00248909. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Intrinsic burst generation of preinspiratory neurons in the medulla of brainstem-spinal cord preparations isolated from newborn rats. Experimental Brain Research. 1995;106:57–68. doi: 10.1007/BF00241356. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ballanyi K, Richter DW. Calcium-dependent responses in neurons of the isolated respiratory network of newborn rats. The Journal of Physiology. 1996;491:677–695. doi: 10.1113/jphysiol.1996.sp021249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Whole cell recording from respiratory neurons in the medulla of brainstem-spinal cord preparations isolated from newborn rats. Pflügers Archiv. 1992;420:399–406. doi: 10.1007/BF00374476. [DOI] [PubMed] [Google Scholar]
- Oyamada Y, Andrzejewski M, Mückenhoff K, Scheid P, Ballantyne D. Locus coeruleus neurones in vitro: pH-sensitive oscillations of membrane potential in an electrically coupled network. Respiration Physiology. 1999;118:131–147. doi: 10.1016/s0034-5687(99)00088-2. [DOI] [PubMed] [Google Scholar]
- Oyamada Y, Ballantyne D, Mückenhoff K, Scheid P. Respiration-modulated membrane potential and chemosensitivity of locus coeruleus neurones in the in vitro brainstem-spinal cord of the neonatal rat. The Journal of Physiology. 1998;513:381–398. doi: 10.1111/j.1469-7793.1998.381bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Velazquez JL, Valiante TA, Carlen PL. Modulation of gap junctional mechanisms during calcium-induced field burst activity: a possible role for electrotonic coupling in epileptogenesis. Journal of Neuroscience. 1994;14:4308–4317. doi: 10.1523/JNEUROSCI.14-07-04308.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky O, Llewellynsmith IJ, Arnolda L, Lipski J, Minson J, Chalmers J. Are the ventrally projecting dendrites of respiratory neurons a neuroanatomical basis for the chemosensitivity of the ventral medulla oblongata? Sleep. 1993;16:S53–55. [PubMed] [Google Scholar]
- Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton and polyamine-sensitive inward rectifier potassium current. Neuroscience. 1997;77:519–528. doi: 10.1016/s0306-4522(96)00485-x. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Champagnat J, Denavit-Saubié M. Electroresponsive properties and membrane potential trajectories of three types of inspiratory neurons in the newborn mouse brain stem in vitro. Journal of Neurophysiology. 1996;75:795–810. doi: 10.1152/jn.1996.75.2.795. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. Bidirectional electrical coupling between inspiratory motoneurons in the newborn mouse nucleus ambiguus. Journal of Neurophysiology. 1997;78:3508–3510. doi: 10.1152/jn.1997.78.6.3508. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. Pre-Bötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annual Review of Physiology. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. Journal of Neurophysiology. 1995;73:933–944. doi: 10.1152/jn.1995.73.3.933. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Dean JB, Putnam RW. Intracellular pH response to hypercapnia in neurons from chemosensitive areas of the medulla. American Journal of Physiology. 1997;273:R433–441. doi: 10.1152/ajpregu.1997.273.1.R433. [DOI] [PubMed] [Google Scholar]
- St John WM. Neurogenesis of patterns of automatic ventilatory activity. Progress in Neurobiology. 1998;56:97–117. doi: 10.1016/s0301-0082(98)00031-8. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Fu L, Ennis M, Liu W-L, Aston-Jones G. Dendrites of locus coeruleus neurons extend preferentially into two pericoerulear zones. Journal of Comparative Neurology. 1996;365:56–68. doi: 10.1002/(SICI)1096-9861(19960129)365:1<56::AID-CNE5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ballanyi K, Richter DW. Whole cell patch clamp recordings from respiratory neurons in neonatal rat brainstem in vitro. Neuroscience Letters. 1992;134:153–156. doi: 10.1016/0304-3940(92)90504-z. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Greer JJ, Liu G, Feldman JL. Neural mechanisms generating respiratory pattern in mammalian brain-stem-spinal cord in vitro. I. Spatiotemporal patterns of motor and medullary neuron activity. Journal of Neurophysiology. 1990;64:1149–1169. doi: 10.1152/jn.1990.64.4.1149. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brainstem-spinal cord preparation of the neonatal rat. The Journal of Physiology. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Ralevic V, Gadd CA, Spyer KM. Central CO2 chemoreception: a mechanism involving P2 purinoceptors localized in the ventrolateral medulla of the anaesthetized rat. The Journal of Physiology. 1999;517:899–905. doi: 10.1111/j.1469-7793.1999.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Dunwiddie TV, Williams JT. Opioid inhibition in locus coeruleus. Journal of Neurophysiology. 1995;74:519–528. doi: 10.1152/jn.1995.74.2.519. [DOI] [PubMed] [Google Scholar]
- Voipio J, Ballanyi K. Interstitial PCO2 and pH, and their role as chemostimulants in the isolated respiratory network of neonatal rats. The Journal of Physiology. 1997;499:527–542. doi: 10.1113/jphysiol.1997.sp021946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of medullary raphe neurones in primary tissue culture. The Journal of Physiology. 1998;511:433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Marshall KC. Membrane properties and adrenergic responses in locus coeruleus neurons of young rats. Journal of Neuroscience. 1987;7:3687–3694. doi: 10.1523/JNEUROSCI.07-11-03687.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, North RA, Shefner SA, Nishi S, Egan TM. Membrane properties of rat locus coeruleus neurons. Neuroscience. 1984;13:137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]


