Abstract
Information about the prevalence of antibiotic resistance in commensal enteric bacteria is of interest because these bacteria are potential indicators of selection pressure on enteric bacteria and represent a reservoir of resistance genes in potentially pathogenic bacteria. This study reports changes in the prevalence of resistance to antibiotics in commensal Escherichia coli from cattle receiving either subcutaneously injectable oxytetracycline in addition to in-feed chlortetracycline or only in-feed chlortetracycline. Resistance to 19 antibiotics was examined. The use of injectable oxytetracycline in addition to in-feed chlortetracycline was significantly associated (P < 0.05) with an increase in the prevalence of resistance only to chloramphenicol and sulfisoxazole.
Introduction
Until recently, the principal food safety concern associated with antibiotic use in food animals was the presence of residues in edible tissues. Worldwide concern about increased antibiotic resistance in organisms infecting humans is rapidly changing this situation. Much greater scrutiny is now placed on antibiotic use and the development of resistance in commensal and pathogenic bacteria that may be transferred to humans from contact with food or food-producing animals. Many research papers, reviews and lay commentaries have discussed the effects of using antibiotics at subtherapeutic levels, as with antimicrobial performance enhancers; however, little attention has been paid to the impact of therapeutic use of antimicrobials on selection for resistance in Escherichia coli (1,2). Stabler, Fagerberg and Quarles (3) reported that the use of subtherapeutic doses of injectable oxytetracycline [0.05 mg/lb of body weight (BW)] was associated with a transient increase in the prevalence of resistant E. coli in cattle. We compared changes in the prevalence of resistance to antibiotics in E. coli from cattle receiving subcutaneously (SC) injectable oxytetracycline in addition to in-feed chlortetracycline with E. coli from cattle receiving only in-feed chlortetracycline.
Materials and methods
The study population consisted of 139 purebred yearling bulls of various breeds housed in an open-sided barn at the University of Guelph bull test station between October 1998 and February 1999. While at the facility, all cattle received the same diet. The cattle were grouped in 8 pens with various numbers of cattle per pen. The cattle were from a variety of sources and had started entering the facility in late September 1998. Their history of exposure to antibiotics prior to arrival was not available.
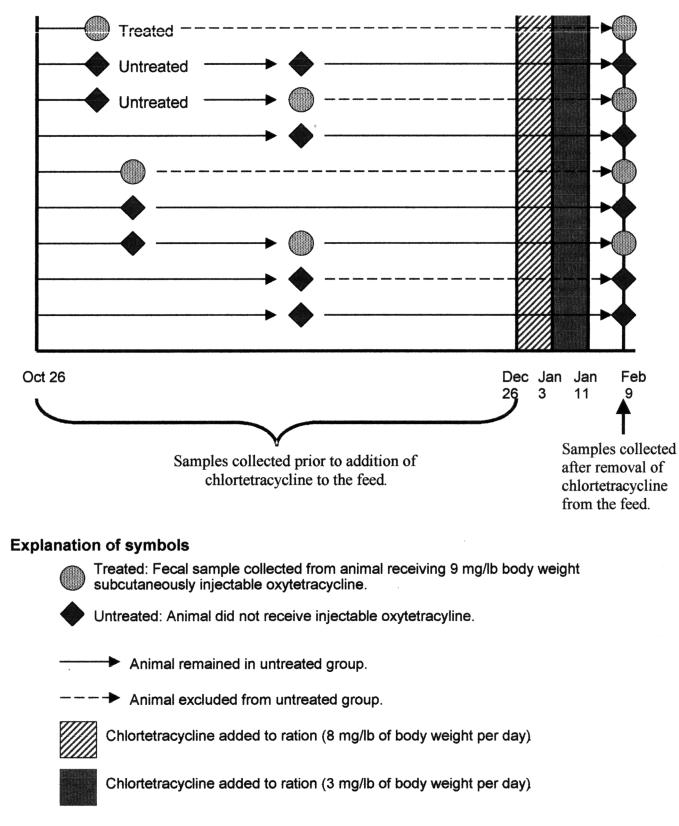
The incidence-density-based study design (4) is outlined in Figure 1. At the outset of the study, a list of animals in each pen was randomly generated. Each time an animal was treated for undifferentiated fever/depression or infectious interdigital dermatitis, the next 2 animals on the untreated list were selected to act as controls, and the treated animal was removed from the list. Since the untreated animals were selected from the same pen as the treated animals, some pens were not sampled, because no animals became sick. Owing to the longitudinal nature of the study, untreated animals could subsequently be treated. Several times both controls were subsequently treated.
Figure 1. Study timeline and animal selection process.
Between October 26 and December 26, 1998, animals with a diagnosis by the facility staff of either undifferentiated fever/depression or infectious interdigital dermatitis were treated with SC long-acting oxytetracycline, used according to the manufacturer's label instructions (9 mg/lb BW). On December 26, the facility veterinarian diagnosed chronic respiratory disease in several animals and prescribed in-feed chlortetracycline for all the animals. Chlortetracycline (Aureomycin; Roche Animal Nutrition and Health, Paramus, New Jersey, USA) was added to the ration at a daily dose of 8 mg/lb BW for approximately 8 d and then 3 mg/lb BW for approximately 8 d.
At the time of treatment, a fecal sample was collected per rectum from the treated animal and from the 2 untreated controls. On February 9, 1999, 30 d after the cessation of chlortetracycline treatment, fecal samples were collected from all the bulls in the facility at the same time as they underwent routine monthly weighing. All samples were transported with ice packs in Styrofoam containers to the laboratory for culture, isolation, and identification of E. coli and sensitivity testing.
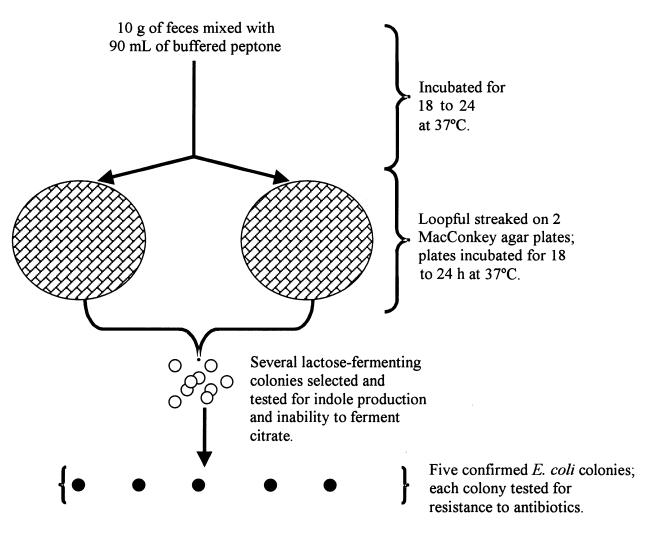
As illustrated in Figure 2, 10 g of feces was taken with a sterile tongue depressor from each sample and deposited in a Stomacher bag (Seward Medical Stomacher “400” Bags; Seward, London, England); 90 mL of buffered peptone water (Difco Laboratories, Detroit, Michigan, USA) was added to each bag. The mixture was incubated at 37°C for 18 to 24 h, then a loopful was streaked onto 2 MacConkey agar plates, which were incubated at 37°C for 18 to 24 h. Lactose-fermenting purple colonies with a well in the centre from different streak lines were examined to determine if they were indole producers and unable to ferment citrate as a sole carbon source and, therefore, E. coli. From among these confirmed E. coli colonies, 5 isolates were selected. Each was inoculated into 3 mL of Mueller–Hinton (MH) broth (Difco Laboratories), which was incubated for 1 h to a density of 0.5 McFarland standard and examined for resistance to antibiotics by the agar dilution method.
Figure 2. Method of isolation of Escherichia coli from fecal samples obtained from cattle at an Ontario bull test station.
Bacteria from each isolate grown in broth were deposited in wells of a Steers replicator multi-well plate and replicated onto an MH agar plate (Difco Laboratories) without antibiotics and MH agar plates containing the following antibiotics: amikacin, 32 μg/mL; ampicillin, 32 μg/mL; apramycin, 16 μg/mL; ceftiofur, 8 μg/mL; ceftriaxone, 32 μg/mL; cephalothin, 32 μg/mL; chloramphenicol, 32 μg/mL; ciprofloxacin, 0.125 μg/mL; gentamicin, 16 μg/mL; kanamycin, 64 μg/mL; nalidixic acid, 32 μg/mL; neomycin, 16 μg/mL; polymyxin B, 16 μg/mL; spectinomycin, 64 μg/mL; streptomycin, 64 μg/mL; sulfisoxazole, 512 μg/mL; tetracycline, 16 μg/mL; tobramycin, 16 μg/mL; and trimethoprim/ sulfamethoxazole, 4/76 μg/mL. After incubation for 18 to 24 h at 37°C the plates were examined for growth of E. coli at the site of inoculation, which determined the minimum inhibitory concentration (MIC) at the resistance breakpoints (the specified concentrations).
The resistance breakpoints and the reference isolates were those described by the National Committee for Clinical Laboratory Standards (NCCLS) (5). Exceptions were as follows: Intermediate-resistance breakpoints were determined for amikacin, 32 μg/mL (6), apramycin, 16 μg/mL (7), and ceftriaxone, 32 μg/mL (5). The resistance breakpoint for streptomycin, 64 μg/mL, was that of the US Food and Drug Administration, US Department of Agriculture, and Centers for Disease Control standard (7). Similarly, isolates were considered resistant to spectinomycin if growing on MH agar with 64 μg/mL of the antibiotic (no resistance breakpoint available). NCCLS guidelines for ceftiofur are available for pathogens associated with bovine respiratory disease (Mannhaemia haemolytica, Pasteurella multocida, and Haemophilus somnus), and in the absence of other guidelines these breakpoints were used (NCCLS standard M31-A). Because treatment failures with ciprofloxacin have occurred in humans infected with Enterobacteriaceae growing in the presence of an MIC of 0.125 μg/mL, reduced sensitivity to ciprofloxacin was determined at that level: 600 μL of E. coli grown in MH broth was added to 600 μL of a 50/50 mixture of glycerol/MH broth in 1.25-mL Sarstedt tubes (Sarstedt, Numbrecht, Germany), and the tubes were deposited in a −70°C freezer.
Statistical analyses
All statistical procedures were performed using SAS software, version 8.1 (SAS Institute, Cary, North Carolina, USA). Data were analyzed only for animals from which samples had been collected prior to and after addition of chlortetracycline to the diet. The analysis, therefore, was considered a matched-pairs analysis, the bull being the unit of matching.
For each bull, the prevalence of resistance (p) among the E. coli isolates selected for testing was estimated by dividing the number of resistant isolates (r) by the total number of isolates (n). The prevalence was calculated for both sampling periods and for each antibiotic. As animals could be selected several times between October 26 and December 26, for this period the denominator (n) varied among the animals (Figure 1). The prevalence of resistant E. coli in the ith bull for the samples collected between October 26 and December 26, before chlortetracycline was added to the diet, was denoted as pi before. The prevalence of resistant E. coli in the ith bull for the samples collected February 9, after chlortetracycline had been removed from the ration, was denoted as pi after. The change in the prevalence of resistance for each bull was therefore pi after − pi before. The null hypothesis was that the distribution of the ranked values for pi after − pi before in the cattle that received both injectable oxytetracycline and in-feed chlortetracycline did not differ from the distribution of the ranked values for pi after − pi before in the cattle that received in-feed chlortetracycline only. This hypothesis was tested with the Wilcoxon sum-rank test because of the small sample sizes and because the prevalence of resistance has been reported to have distributions other than normal (8,9). The test was conducted for each antibiotic — that is, 19 times.
Results
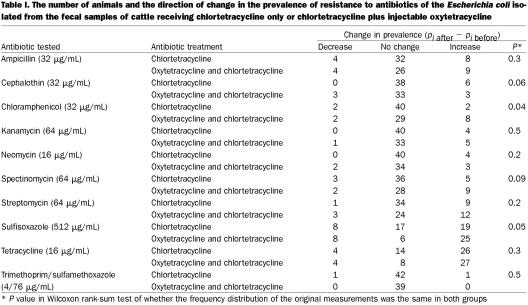
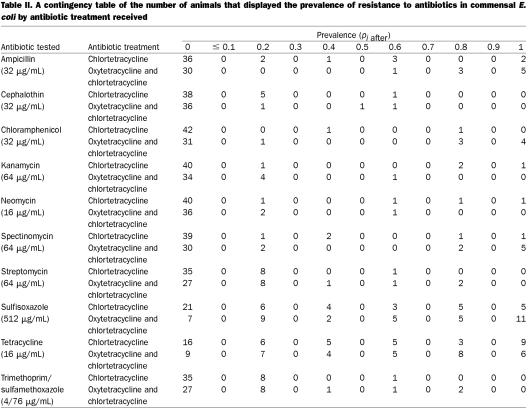
Of the 139 animals in the study population, complete data (samples collected both before and after the addition of chlortetracycline to the diet) were available for 83. This data set contained matched samples from 39 animals that received both injectable oxytetracycline and in-feed chlortetracycline and 44 animals that received only in-feed chlortetracycline. The ratio of animals was not 1:2 because untreated animals could become treated. For amikacin, ceftiofur, ciprofloxacin, nalidixic acid, polymyxin B, and tobramycin, there was no difference in the change in the prevalence of resistance (P = 0.5). For apramycin, ceftriaxone, and gentamicin, only 1 animal each showed any change in the prevalence of resistance: 1 animal that received both oxytetracycline and chlortetracycline showed a 0.2 increase in the prevalence of resistance to apramycin (P = 0.15), 1 animal that received only in-feed chlortetracycline showed a 0.8 increase in the prevalence of resistance to ceftriaxone (P = 0.2), and 1 animal that received only in-feed chlortetracycline showed a 0.2 increase in the prevalence of resistance to gentamicin (P = 0.2). For Table I, the data for the remaining antibiotics were arranged in 3 categories: (1) a decrease in prevalence, meaning the prevalence of resistance was lower at the February 9 sampling [(pi after − pi before) < 0], (2) no change in the prevalence of resistance [(pi after − pi before) = 0], and (3) an increase in prevalence, meaning that the prevalence of resistance was higher in the February 9 sampling [(pi after − pi before) > 0]; Table I presents the number of animals in each category and the P value for the Wilcoxon rank-sum test that used the original value of (pi after − pi before). A contingency table showing the number of animals and the associated prevalence of resistance among the E. coli isolated from the samples collected February 9 is reported in Table II. For amikacin, ceftiofur, ciprofloxacin, nalidixic acid, polymyxin B, and tobramycin, the prevalence at that time was zero. For apramycin, 1 animal receiving injectable oxytetracycline and in-feed chlortetracycline had a prevalence of resistance of 0.2. For both ceftriaxone and gentamicin, 1 animal receiving in-feed chlortetracycline had a prevalence other than zero: 0.8 for ceftriaxone and 0.2 for gentamicin.
Table I.
Table II.
Discussion
Generic E. coli are considered commensal bacteria in cattle (i.e., they are normally nonpathogenic). The use of antibiotics in food-producing animals is the subject of debate owing to concern about the impact on the prevalence of resistance among bacterial populations and the corresponding impact on human health (10). The use of antimicrobial agents for growth promotion and prophylaxis has been criticized by several groups (2). However, little is known about the effect of therapeutic uses of injectable antibiotics on enteric bacteria that may be associated with food-safety hazards.
In our study, the use of injectable oxytetracycline in cattle receiving in-feed chlortetracycline was associated with an increase in the prevalence of resistance to chloramphenicol and sulfisoxazole (Table I). This is against a background of selection pressure imposed by the exposure of all animals to chlortetracycline in the feed. It would appear that resistance to chloramphenicol and sulfisoxazole is selected by the use of injectable oxytetracycline. We did not detect any additional effect of the injectable oxytetracycline on the prevalence of resistance to other antibiotics. We had expected that any such effect would result in an increase in tetracycline resistance; however, this effect was not found. One explanation may be that the in-feed chlortetracycline increased the level of resistance to tetracycline to “saturation” and, therefore, injectable oxytetracycline could have no detectable additional impact on the prevalence of resistance. Dunlop and colleagues (11) failed to find an association between farm-level rates of individual-animal treatment and the farm-level prevalence of resistance in swine when the antibiotic was included in the ration; however, they did report an association between individual-animal treatment rates for gentamicin and the farm-level prevalence of resistance to gentamicin, an antibiotic not included in the ration. They suggested that the resistance developing from the in-feed medication may have overshadowed any effect of individual-animal treatment (9). A similar mechanism may have been at work in our population. Alternatively, the failure to observe an effect of oxytetracycline on tetracycline resistance may have been due to the short-lived nature of tetracycline resistance, as reported by Stabler and associates (3), and the length of time between the samplings in our study. This explanation would be unlikely for chloramphenicol resistance, which is persistent (12,13) and, therefore, was more likely to be detected. Because the animals were together in pens and came in contact during handling, it is also possible that changes in resistance due to injectable oxytetracycline were transferred in the months between samplings to the animals who received only chlortetracycline, and therefore no difference was detectable.
It could be argued that the reported findings may have occurred as a result of multiple hypothesis testing and therefore could be considered a likely type I error, given the number of antibiotics tested. The need to adjust for multiple comparisons has been criticized in the epidemiologic literature, although it remains a common practice (14,15).
In line with the NCCLS guidelines, the isolates were categorized as sensitive or resistant on the basis of breakpoints, but this system does not allow for the detection of changes in sensitivity above or below the breakpoints. There may have been large changes in the distribution of MICs above and below the breakpoints that were undetected because of the classification system chosen. Also, we measured phenotypic expression of resistance rather than the presence of genes that confer resistance, which may be present but not expressed. From a public health point of view, the latter is also important and should be monitored when resources permit.
In conclusion, injectable oxytetracycline may be associated with increases in the prevalence of resistance to sulfisoxazole and chloramphenicol among E. coli in lot-fed cattle beyond those due to in-feed chlortetracycline, and these changes appear to be detectable a long time after the use of the injectable oxytetracycline.
Footnotes
Acknowledgments
We acknowledge the financial assistance of the Ontario Cattlemen's Association and the technical assistance of staff at the Beef Improvement Ontario Bull Test Station. We thank Jennifer Becker and Steven Moore, co-op students from the University of Guelph, for their assistance in isolating, identifying and determining the antimicrobial resistance of the E. coli.
Address correspondence and reprint requests to Dr. Annette M. O'Connor, Veterinary Diagnostic and Production Animal Medicine, College of Veterinary Medicine, Iowa State University, Ames, Iowa 50010, USA, tel: 515-294-5012, fax: 515-294-1072, e-mail: oconnor@iastate.edu
Received May 29, 2001. Accepted February 26, 2002.
References
- 1.van Den Bogaard AE, Stobberingh EE. Epidemiology of resistance to antibiotics. Links between animals and humans. Int J Antimicrob Agents 2000;14:327–335. [DOI] [PubMed]
- 2.World Health Organization. The Medical Impact of the Use of Antimicrobials in Food Animals. Report of a WHO Meeting, Berlin, Germany, 13–17 1997. Geneva: WHO, 1997. http://www.who.int/emc/diseases/zoo/zoo97_4.html.
- 3.Stabler SL, Fagerberg DJ, Quarles CL. Effects of oral and injectable tetracyclines on bacterial drug resistance in feedlot cattle. Am J Vet Res 1982;43:1763–1766. [PubMed]
- 4.Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiological Research. Scarborough, Ontario: Van Nostrand Reinhold, 1982.
- 5.National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing; Eighth Informational Supplement. NCCLS document M100-S8. Wayne, Pennsylvania, 1998.
- 6.National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard. NCCLS document M31-A. Wayne, Pennsylvania, 1999.
- 7.Food and Drug Administration, National Antimicrobial Resistance Monitoring Program — Enteric Bacteria, Veterinary Isolates. Final Report. Washington, DC: USDA/FDA/CDC, 1997.
- 8.Snedecor GW, Cochran WG. Statistical Methods. Ames, Iowa: Iowa State University Press, 1989.
- 9.Dunlop RH, McEwen SA, Meek AH, Friendship RM, Black WD, Clarke RC. Sampling considerations for herd-level measurement of faecal Escherichia coli antimicrobial resistance in finisher pigs. Epidemiol Infect 1999;122:485–496. [DOI] [PMC free article] [PubMed]
- 10.Dunne EF, Fey PD, Kludt P, et al. Emergence of domestically acquired ceftriaxone-resistant Salmonella infections associated with AmpC beta-lactamase. JAMA 2000;284:3151–3156. [DOI] [PubMed]
- 11.Dunlop RH, McEwen SA, Meek AH, Clarke RC, Black WD, Friendship RM. Associations among antimicrobial drug treatments and antimicrobial resistance of fecal Escherichia coli of swine on 34 farrow-to-finish farms in Ontario, Canada. Prev Vet Med 1998;34:283–305. [DOI] [PubMed]
- 12.Campos J, Roman F, Georgiou M, et al. Long-term persistence of ciprofloxacin-resistant Haemophilus influenzae in patients with cystic fibrosis. J Infect Dis 1996;174:1345–1347. [DOI] [PubMed]
- 13.Jorgensen ST, Grinsted J, Bennett P, Richmond MH. Persistence and spread of chloramphenicol resistance-mediating plasmid in antigenic types of Escherichia coli pathogenic for piglets. Plasmid 1980;4:123–129. [DOI] [PubMed]
- 14.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–46. [PubMed]
- 15.Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia: Lippincott, Williams and Wilkins, 1998.