Abstract
Nicotinic acetylcholine receptors (nAChRs) that bind α-bungarotoxin (αBgt) were studied on isolated rat superior cervical ganglion (SCG) neurons using whole-cell patch clamp recording techniques.
Rapid application of ACh onto the soma of voltage clamped neurons evoked a slowly desensitizing current that was reversibly blocked by αBgt (50 nm). The toxin-sensitive current constituted on average about half of the peak whole-cell response evoked by ACh.
Nanomolar concentrations of methyllycaconitine blocked the αBgt-sensitive component of the ACh-evoked current as did intracellular dialysis with an anti-α7 monoclonal antibody. The results indicate that the slowly reversible toxin-sensitive response elicited by ACh arises from activation of an unusual class of α7-containing receptor (α7-nAChR) similar to that reported previously for rat intracardiac ganglion neurons.
A second class of functional α7-nAChR was identified on some SCG neurons by using rapid application of choline to elicit responses. In these cases a biphasic response was obtained, which included a rapidly desensitizing component that was blocked by αBgt in a pseudo-irreversible manner. The pharmacology and kinetics of the responses resembled those previously attributed to α7-nAChRs in a number of other neuronal cell types.
Experiments measuring the dissociation rate of 125I-labelled αBgt from SCG neurons revealed two classes of toxin-binding site. The times for toxin dissociation were consistent with those required to reverse blockade of the two kinds of αBgt-sensitive response.
These results indicate that rat SCG neurons express two types of functional α7-nAChR, differing in pharmacology, desensitization and reversibility of αBgt blockade.
Nicotinic acetylcholine receptors (nAChRs) that bind α-bungarotoxin (αBgt) and contain the α7 gene product comprise one of the most abundant type of nicotinic receptor in both the central and peripheral nervous systems (Couturier et al. 1990; Schoepfer et al. 1990; Anand et al. 1993; Conroy & Berg, 1998). The α7-nAChRs have a high relative permeability to calcium (Bertrand et al. 1993; Seguela et al. 1993), and therefore can influence a variety of calcium-dependent events in neurons. These have been shown to include release of neurotransmitter from presynaptic sites (McGehee et al. 1995; Gray et al. 1996), second messenger cascades (Vijayaraghavan et al. 1995), neurite extension (Pugh & Berg, 1994; Fu et al. 1998), neuronal survival (Messi et al. 1997) and apoptosis (Berger et al. 1998). The receptors can also participate in postsynaptic signalling (Zhang et al. 1996; Frazier et al. 1998; Chang & Berg, 1999; Hefft et al. 1999).
In most instances native α7-nAChRs have been found to generate nicotinic responses that rapidly desensitize and are blocked almost irreversibly by αBgt. This is true in rat hippocampal neurons (Zorumski et al. 1992; Alkondon & Albuquerque, 1993), chick ciliary ganglion neurons (Zhang et al. 1994) and the rat phaeochromocytoma cell line PC12 (Blumenthal et al. 1997). It has been shown recently, however, that α7-nAChRs expressed by rat intracardiac ganglion neurons have quite different properties: the receptors desensitize slowly and are blocked by αBgt in a rapidly reversible manner (Cuevas & Berg, 1998). It is not known how widespread such receptors might be, but they offer the potential of having sustained effects on calcium-dependent processes.
A mammalian preparation that has been widely used for analysis of neuronal nAChRs is the rat superior cervical ganglion (SCG). Neurons of the rat SCG express a variety of nAChR genes, including α7, and display nicotine-induced responses. None of the reported responses, however, are rapidly desensitizing or sensitive to αBgt, despite the fact that the neurons have αBgt-binding sites (Brown & Fumagalli, 1977; Trouslard et al. 1993; Mandelzys et al. 1995; Kristufek et al. 1999). These observations raised the prospect of dysfunctional α7-nAChRs or α7-nAChRs that have unusual activation properties.
The present experiments were undertaken to determine whether functional α7-nAChRs could be demonstrated on rat SCG neurons and whether they shared properties either with the rapidly desensitizing α7-nAChRs of rat hippocampal neurons and PC12 cells or with the slowly desensitizing receptors of rat intracardiac ganglion neurons. The results show that both types of α7-nAChR can be found on SCG neurons, sometimes co-expressed on the same cell. Moreover, two classes of αBgt-binding site can be distinguished on the neurons, and toxin retention by the sites correlates with the duration of toxin blockade for the two types of α7-nAChR response.
METHODS
Rat SCG neurons
Membrane currents were studied in acutely dissociated sympathetic neurons from neonatal rat superior cervical ganglia. The procedure for isolating the neurons was similar to that previously described by Mathie et al. (1990). Neonatal rats (10-14 days old) were killed by inhalation of a rising concentration of carbon dioxide. The superior cervical ganglia were rapidly excised and placed in high glucose medium (Dulbecco's modified Eagle's medium; DMEM) containing 1 mg ml−1 Type 1 collagenase (Worthington) and 1 mg ml−1 Type 1 trypsin (Sigma). Ganglia were incubated at 37°C for 1 h, triturated with a fine-bore Pasteur pipette and centrifuged at low speed for 5 min. The supernatant fraction was removed, and the cell pellet was resuspended in high glucose DMEM containing 10 % (v/v) fetal calf serum, 100 U ml−1 penicillin and 0.1 mg ml−1 streptomycin. Cells were then plated onto 18 mm coverslips coated with polylysine and fibronectin and incubated at 37°C for ≥ 1 h under a 95 % air-5 % CO2 atmosphere.
Electrical recordings
Electrophysiological recordings were conducted as previously described (Cuevas & Berg, 1998). In most experiments recordings were obtained using the perforated-patch variation of the patch clamp method in voltage clamp mode (Horn & Marty, 1988). The pipette solution in this case contained 75 mM K2SO4, 55 mM KCl, 5 mM MgSO4, 360 μg ml−1 amphotericin B, 0.6 % DMSO and 10 mM Hepes; pH 7.2 with N-methyl-D-glucamine. When antibodies were to be dialysed into the cell via the patch pipette, recordings were obtained using conventional whole-cell patch clamp techniques (Hamill et al. 1981). For conventional (dialysing) whole-cell experiments, the intracellular solution contained (mM): 140 KCl, 10 glucose, 2 EGTA and 10 Hepes; pH 7.2 with KOH. In some conventional whole-cell experiments cells were dialysed (≥ 10 min) with patch pipette solution containing rat anti-α7 monoclonal antibody (mAb) 319 (2 μg ml−1), which was raised against and is specific for the putative cytoplasmic loop sequence of α7 (Schoepfer et al. 1990). In control experiments, rat anti-α3 mAb 313 (Whiting et al. 1991) was substituted for mAb 319.
The external solution for whole-cell recordings was physiological saline solution (PSS) containing (mM): 140 NaCl, 3 KCl, 2.5 CaCl2, 1.2 MgCl2, 7.7 glucose and 10 Hepes; pH 7.2 with NaOH. Neurons were continuously perfused with PSS before and after agonist application and for washout of drugs. All patch clamp recordings were conducted at room temperature. A rapid application system was used as previously reported (Zhang et al. 1994) to administer agonists and antagonists. In some experiments neurons were pre-incubated at 37°C in PSS containing 100 nM αBgt for 1 h prior to recording. When acetylcholine (ACh) was used as agonist, 100 nM atropine was included to block activation of muscarinic ACh receptors. The rate of solution exchange, as determined by recording the liquid junction potential change from an open patch pipette, was < 3 ms.
Whole-cell membrane currents were amplified and filtered (5 kHz) with an Axopatch 200A (Axon Instruments Inc., Foster City, CA, USA) and digitized with a Digidata 1200B (Axon Instruments Inc.). Data were acquired using Clampex 6 at a sampling rate of 20 kHz and analysed using Clampfit 6 (Axon Instruments Inc.). Current decay records were fitted with Clampex 6 using the Simplex fitting algorithm, and data from dose-response relationships were fitted using SigmaPlot software (SPSS Science, Inc., Chicago, IL, USA) which employs the Marquardt-Levenberg algorithm. Both of these programs use the minimum χ2 method to optimize the fit.
RT-PCR
RT-PCR was used to detect nAChR gene expression in tissues exactly as previously described for rat intracardiac ganglion and pituitary samples (Cuevas & Berg, 1998). The same oligonucleotide primers were used and the same conditions were followed for amplification and analysis.
Binding experiments
Binding studies with 125I-αBgt were carried out on surface nAChRs expressed on SCG and chick ciliary ganglion neurons in dissociated cell culture. Ciliary ganglion neurons were isolated from 8-day-old chick embryos as previously described (Margiotta & Gurantz, 1989) and SCG neurons from neonatal pups as indicated above. SCG and ciliary ganglion neuronal cell cultures were prepared by plating neurons onto polystyrene culture dishes coated with polylysine and fibronectin followed by incubation at 37°C under a 95 % air-5 % CO2 atmosphere for 36–72 h. Cultured neurons were incubated with 10 nM 125I-αBgt in PSS either for 10 min at room temperature or for 1 h at 37°C. The radiolabelled toxin was then replaced with 100 nM αBgt in PSS, and at the indicated times the cells were rinsed 3 times within 2 min in PSS, scraped into 1 n NaOH and bound radioactivity quantified with a gamma counter.
Reagents and statistical analysis
All chemicals were analytical grade. ACh, choline chloride and atropine sulfate were purchased from Sigma Chemical Co. (St Louis, MO, USA). Methyllycaconitine (MLA) was purchased from RBI (Natick, MA, USA). αBgt was purchased from Biotoxins (St Cloud, FL, USA) and radioiodinated using chloramine T to a specific activity of 0.3 × 1018-0.7 × 1018 c.p.m. mol−1. Anti-α7 mAb 319 and anti-α3 mAb 313 were generously supplied by Dr Jon Lindstrom (University of Pennsylvania, PA, USA).
All animal care and handling was in strict accordance with the policies of the University of California, San Diego, Committee on Animal Subjects and the American Association for Accreditation of Laboratory Animal Care. The University holds a currently approved National Institutes of Health Assurance and a United States Department of Agriculture License.
Data are presented as means ± s.d. unless otherwise stated, and were compared using Student's paired or unpaired t tests as appropriate.
RESULTS
Blockade of ACh-evoked currents in SCG neurons by αBgt
αBgt in the nanomolar range is thought to be an effective antagonist of α7-nAChRs. To test for functional α7-nAChRs on rat SCG neurons, we examined the whole-cell ACh-evoked responses of freshly dissociated cells before and after exposure to αBgt. Patch clamp recordings showed substantial currents evoked by rapid focal application of ACh (500 μM, 1.5 s) onto the soma of voltage clamped (-60 mV) neurons electrically accessed using the perforated-patch method (Fig. 1A, control). Bath application of αBgt (50 nM, 5 min) attenuated the peak ACh-evoked current (Fig. 1A, αBgt) in nearly three-quarters of the cells examined (11 of 15 cells). In cells exhibiting a toxin-sensitive response, the mean amplitude of the ACh-evoked current before and after exposure to αBgt was 3.2 ± 0.5 and 1.9 ± 0.3 nA (n = 11), respectively. The difference was statistically significant (P < 0.01). Toxin blockade was maximal at 50 nM: testing the same neurons with first 20 nM and then 200 nM αBgt produced no additional inhibition of the ACh-evoked currents (38 ± 5 vs. 37 ± 5 % inhibition; 3 cells).
Figure 1. ACh responses that are reversibly blocked by αBgt and slowly desensitize in mammalian SCG neurons.

A, whole-cell currents evoked by rapid focal application of 500 μM ACh onto the soma of an isolated rat SCG neuron (-60 mV) in the absence (Control) and presence (αBgt) of 50 nM αBgt. B, net αBgt-sensitive ACh-evoked current, determined by subtracting the current induced by ACh in the presence of 50 nM αBgt from that recorded in the absence of the toxin; traces taken from A. The continuous line represents the best fit to the data and is the sum of two exponential functions with τ1= 162 ms and τ2= 1.3 s, in this case. C, family of currents evoked by 500 μM ACh recorded from a single SCG neuron at -60 mV in the absence (Control), presence (αBgt) and following washout (Wash) of 50 nM αBgt. D, ACh-evoked whole-cell current amplitudes recorded before, during and after removal of 50 nM αBgt (horizontal bar). Values have been normalized to the maximal response from the same cell, and plotted as a function of time.
The time course of the toxin-sensitive component of the response was assessed by subtracting ACh-evoked current recorded in the presence of αBgt from that recorded in the absence of toxin. The resulting profile suggested a relatively slow rate of desensitization (Fig. 1B). The decay phase was best fitted by the sum of two exponential functions with τ1 of 153 ± 20 ms and τ2 of 1.4 ± 0.2 s (n = 5). These values are in the range of those seen for the αBgt-sensitive response of rat intracardiac ganglion neurons (Cuevas & Berg, 1998) and are much slower than the rapidly decaying responses seen in rat hippocampal neurons (Zorumski et al. 1992; Alkondon & Albuquerque, 1993) and PC12 cells (Blumenthal et al. 1997).
The blockade of αBgt-sensitive current was reversible. Rinsing αBgt-treated SCG neurons with PSS alone to remove toxin allowed substantial recovery of the response (Fig. 1C). Comparison of the peak amplitude of the response as a function of time after toxin removal indicated that recovery was almost complete by this criterion within 5 min (Fig. 1D). Recovery over the same time frame was seen when the cells were incubated with αBgt for as long as 1 h at 37°C before rinsing (data not shown). The relatively rapid reversibility is similar to that seen for αBgt-sensitive responses in intracardiac ganglion neurons but not for the nearly irreversible blockade of responses previously reported in hippocampal neurons and PC12 cells.
Effects of MLA and anti-α7 mAbs on the αBgt-sensitive component
Two other methods for selectively blocking α7-nAChR responses are application of nanomolar concentrations of MLA extracellularly (Alkondon & Albuquerque, 1993; Wilkie et al. 1996) or infusion of anti-α7 mAbs intracellularly via the patch pipette (Cuevas & Berg, 1998). MLA at picomolar concentrations produced a significant depression of the ACh-evoked response and the change in response profile mimicked that caused by αBgt (Fig. 2A). Data from a number of neurons were combined to generate an extended dose-response curve. Both high and low affinity components are suggested by the broad concentration range over which MLA exerted a depressive effect on the ACh-induced current (Fig. 2B). At concentrations below 5 nM, MLA appeared to interact with a site that inhibited about half of the total peak current in neurons subsequently shown to have an αBgt-sensitive response (three-quarters of the neurons). Under these same conditions, MLA had no effect on the αBgt-resistant ACh-evoked current as seen by testing αBgt-treated neurons (data not shown). At 100 nM and higher concentrations, MLA inhibited the αBgt-resistant ACh response (Fig. 2B). The MLA sensitivity of the first component is that expected for α7-nAChRs (Alkondon & Albuquerque, 1993; Wilkie et al. 1996).
Figure 2. MLA blocks a component of the ACh-evoked response in SCG neurons at concentrations specific for α7-nAChRs.

A, family of currents evoked from a single neuron (-60 mV) by focal application of 500 μM ACh in the presence of MLA at the indicated concentrations. B, peak ACh-evoked current amplitude plotted as a function of MLA concentration. Points represent means ±s.e.m. for 21 neurons. The continuous line represents a best fit to the data and is the sum of two single-site adsorption isotherms with IC50 values of 120 pM and 200 nM, respectively. Individual components of the fit are shown as dashed lines; the high and low affinity MLA components had relative amplitudes of about 60 and 40 %, respectively. The response in the absence of MLA is shown as C.
One other class of neuronal nicotinic receptor in rat has been shown to be capable of binding αBgt and MLA. This class is the α9-containing nAChR which has a very restricted pattern of expression (Elgoyhen et al. 1994). PCR analysis was previously used to show that rat intracardiac ganglia, which have slowly desensitizing αBgt-sensitive responses like those seen here in SCG neurons, express the α7 but not the α9 gene (Cuevas & Berg, 1998). Similar experiments carried out here showed that the α7 transcript was expressed in the SCG as expected but that there was no detectable α9 transcript (Fig. 3). As a positive control for the procedure, both transcripts were shown to be present in rat pituitary. The results further support the contention that α7-nAChRs are responsible for the slowly desensitizing αBgt- and MLA-sensitive responses in SCG neurons.
Figure 3. RT-PCR analysis demonstrates α7 but not α9 mRNA in the rat SCG.

RT-PCR products generated with the indicated tissue samples and oligonucleotide primers were analysed as shown. Primers/template RNA: lanes 1 (on left) and 6, 100 bp standards; lane 2, α7/SCG; lane 3, α9/SCG; lane 4, α7/pituitary; lane 5, α9/pituitary; lane 7, α7/water (Control); lane 8, α9/water. The expected sizes for the α7 (476 bp) and α9 (573 bp) PCR products are indicated by arrows. The results show α7 but not α9 transcripts in the SCG; pituitary samples served as positive controls for the method of detecting both kinds of transcript.
Intracellular dialysis with the anti-α7 mAb 319 has previously been found to block responses attributed to α7-nAChRs in rat ganglionic neurons (Cuevas & Berg, 1998). Prolonged intracellular dialysis of rat SCG neurons electrically accessed using conventional whole-cell patch clamp techniques did not produce significant run-down of either the whole-cell ACh-evoked current or the αBgt-resistant portion of it (Fig. 4A). Intracellular dialysis with 2 μg ml−1 mAb 319, in contrast, significantly reduced the whole-cell response within 30 min while having no effect on the αBgt-resistant component (Fig. 4B). Compiling the results from a number of neurons demonstrated that the mAb 319 effect was specific for the αBgt-sensitive portion of the response and that it blocked over half of it within 30 min (Fig. 4C and D). Moreover, the blockade was specific for the anti-α7 mAb because very little run-down was observed when mAb 319 was omitted from the pipette or was replaced with an equivalent concentration of the anti-α3 mAb 313 (Fig. 4D). Taken together, the results show that α7-nAChRs are responsible for the αBgt-sensitive portion of the slowly desensitizing ACh-evoked responses in rat SCG neurons.
Figure 4. Intracellular dialysis with anti-α7 mAbs confirms that the slowly decaying, αBgt-sensitive responses found in SCG neurons are generated by α7-nAChRs.

A, family of currents evoked from a single neuron dialysed with normal pipette solution. Currents were evoked by focal application of 500 μM ACh in the absence (top) and presence (bottom) of 50 nM αBgt after 10 and 30 min dialysis. B, similar currents evoked from a neuron dialysed with the anti-α7 mAb 319 (2 μg ml−1 in the pipette). C, peak currents evoked by 500 μM ACh (Control) and ACh plus 50 nM αBgt from neurons dialysed with normal pipette solution (no mAb) or pipette solution containing mAb 319. Currents were recorded following 10 and 30 min of dialysis. D, fractional decrease in peak αBgt-sensitive current (IαBgt) amplitude over time (10 vs. 30 min) for neurons dialysed with normal pipette solution (no mAb) and neurons dialysed with either the anti-α3 mAb 313 (negative control; 2 μg ml−1) or the anti-α7 mAb 319. The decrease was determined by dividing the net αBgt-sensitive current recorded following 30 min of dialysis by that recorded following 10 min of dialysis.
A second class of functional α7-nAChR on SCG neurons
A second class of functional α7-nAChR was detected on some rat SCG neurons by using choline as an agonist. Choline appears to be a preferential agonist of nAChRs containing the α7 subunit and is much less able to activate α3-containing receptors (Papke et al. 1996; Alkondon et al. 1998). These latter receptors are usually responsible for much, if not all, of the αBgt-resistant portion of ganglionic ACh responses (Zhang et al. 1994; Mandelzys et al. 1995; but see Yu & Role, 1998). Rapid focal application of 10 mM choline (300 ms) onto the soma of a voltage clamped SCG neuron (-60 mV), electrically accessed using the perforated-patch method, elicited a biphasic response (Fig. 5A). The large, rapidly desensitizing portion was blocked by 50 nM αBgt while the smaller, slowly desensitizing portion was unaffected by the toxin. No recovery of the rapidly desensitizing component was obtained when the neurons were rinsed (> 10 min) to remove toxin. In control experiments in which αBgt was omitted, the peak amplitude of both the rapidly desensitizing and slowly desensitizing components remained stable over a period of ≥ 30 min. The rapidly desensitizing component of the choline-evoked response was best fitted by a single exponential function with a τ of 23.0 ± 2.1 ms (n = 10), which is comparable to the approximately 25 ms decay rate observed for α7-nAChR-mediated currents in rat brain neurons (Alkondon et al. 1997). A dose-response curve for the peak response was generated by combining the data from a number of neurons challenged with a range of choline concentrations (Fig. 5B). An EC50 of about 6 mM for choline activation of the rapidly desensitizing peak response was obtained when a single-site adsorption isotherm was used to fit the data. This value is comparable to that reported previously (1.6 mM) for rat brain α7-nAChRs (Alkondon et al. 1997), but should only be considered as an estimate here because the choline concentrations tested did not achieve a maximal response. Higher choline concentrations would have made substantive changes to the composition of the extracellular solution, directly affecting current amplitude.
Figure 5. Some SCG neurons display a second αBgt-sensitive response that rapidly desensitizes and can be elicited by choline.

A, currents evoked from a single neuron by application of 10 mM choline in the absence (Control), presence (50 nM αBgt) and following 10 min washout (Wash) of αBgt. B, dose-response curve for peak choline-evoked αBgt-sensitive current. Toxin-sensitive current was determined by subtracting the choline-induced current in the presence of αBgt (50 nM) from that elicited in the absence of toxin. Points are means ±s.e.m. for 23 neurons. The continuous line represents the best fit to the data using a single-site adsorption isotherm and indicates an EC50 of about 6 mM for choline.
The properties of the rapidly desensitizing component evoked by choline were those expected for the αBgt-sensitive response previously attributed to α7-nAChRs in rat hippocampal neurons and PC12 cells. This suggested that SCG neurons might express two functional classes of α7-nAChR: one class that rapidly desensitized and bound αBgt almost irreversibly, and a second class that slowly desensitized and bound the toxin in a rapidly reversible manner. The former might have been largely obscured by the toxin-resistant component when ACh was used as agonist, and the latter might not be activated by choline. To examine this possibility further, we compared ACh- and choline-evoked currents in the same cells before and after αBgt blockade. Whereas ACh elicited responses with similar desensitization kinetics over a range of current amplitudes among cells (Fig. 6A, left traces), choline evoked currents showing considerable variation in this parameter in the same cells (Fig. 6A, right traces). In approximately one-fifth of the neurons (5 out of 27), choline evoked a rapidly desensitizing current with an amplitude significantly greater than that of the slowly desensitizing component (Fig. 6Aa, Type I), though neither was large in these cases. About half of the neurons (13 out of 27) displayed no rapidly desensitizing current in response to choline (Fig. 6Ab, Type II), and a third (9 out of 27) exhibited both a rapidly desensitizing current and a slowly desensitizing one of about equivalent amplitude (Fig. 6Ac, Type III).
Figure 6. Individual SCG neurons can express two types of αBgt-sensitive response mediated by distinct populations of α7-nAChRs.
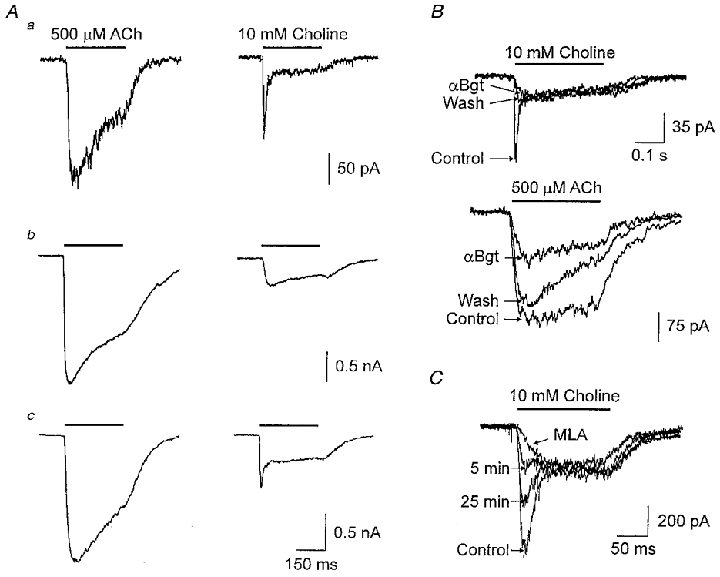
A, currents evoked from three individual neurons (a, b, c) by application of ACh (500 μM; left traces) or choline (10 mM; right traces). The peak ACh-evoked currents have been scaled to permit easy visual comparison of the relative amplitudes of choline-evoked current in each case. B, ACh- and choline-evoked currents recorded from an individual neuron in the absence (Control), presence (αBgt) and following 10 min washout (Wash) of 50 nM αBgt. C, choline-evoked currents recorded from a neuron before (Control), during (MLA), and following washout of 5 nM MLA for the indicated times (5 and 25 min).
Application of αBgt to the Type I neurons blocked the rapidly desensitizing current elicited by choline and the blockade was not reversible (Fig. 6B, upper panel). In contrast, αBgt blocked a portion of the slowly desensitizing ACh response in the same cells but in this case most of the block was readily reversible within minutes (Fig. 6B, lower panel). Since the toxin block of the choline-induced rapidly desensitizing component persisted while the toxin block of the ACh-induced slowly desensitizing component in the same cells was largely reversed, the two kinds of responses almost certainly would have had to have been produced by activation of different classes of α7-nAChR.
The two kinds of α7-nAChR identified here differ not only in their kinetic properties and in their relative proportions among SCG neurons, but also in their pharmacology. While the slowly desensitizing α7-nAChR response can clearly be elicited by ACh, this was less obvious for the rapidly desensitizing α7-nAChR response due to the overshadowing effect of the usually large αBgt-resistant component. Nonetheless, in two neurons the αBgt-resistant component was sufficiently small to allow detection of a rapidly desensitizing ACh response that could be blocked by αBgt (data not shown). A clear pharmacological difference in the two kinds of α7-nAChR response is that only the rapidly desensitizing response can be significantly activated by choline. This follows from the observation that little, if any, of the slowly decaying choline response can be blocked by αBgt, indicating that it is different from that portion of the slowly decaying ACh response which is reversibly blocked by the toxin. Measurement of the choline-induced current 150 ms after initiation of the response yielded values before and after αBgt exposure, respectively, of 136 ± 32 vs. 123 ± 41 pA for 3 mM choline (n = 4), 209 ± 74 vs. 175 ± 60 pA for 10 mM choline (n = 7) and 304 ± 89 vs. 243 ± 70 pA (n = 5) for 30 mM choline. None of these nominal differences is statistically significant.
Though the two kinds of α7-nAChR response differ in their response to agonists and in their desensitization kinetics, they are similar with respect to MLA blockade. Comparison of the responses of neurons before and after incubation with 5 nM MLA for 5 min showed that the compound completely blocked the rapidly decaying response elicited by 10 mM choline (Fig. 6C) as it did the slowly decaying αBgt-sensitive response elicited by 500 μM ACh (Fig. 2). A small portion of the slowly decaying choline response may also have been affected by MLA (Fig. 6C), either because choline may cause marginal activation of slowly decaying α7-nAChRs or because MLA at this concentration has some effect on α3-containing nAChRs. Moreover, a partial reversal of the MLA block could be demonstrated with continued wash (Fig. 6C) as previously seen for MLA blockade of α7-nAChR responses in rat PC12 cells (Rangwala et al. 1997). It was not possible to use intracellular dialysis to test the effects of anti-α7 mAbs on the rapidly decaying response because it underwent significant rundown with time of recording when conventional patch clamp methods were used to dialyse the interior of the cells.
Distinguishing between α7-nAChR subclasses with 125I-αBgt binding
The fact that αBgt blockade could be reversed for one of the two toxin-sensitive responses suggested that SCG neurons should have a class of reversible αBgt-binding sites on the cell surface. Previous binding studies with 125I-αBgt on membrane fragments and detergent-solubilized receptors, however, had identified only toxin sites associated with α7-nAChRs that showed nearly irreversible binding over the time periods used here for electrophysiological recording (for review see Sargent, 1993). We obtained similar results when we examined 125I-αBgt binding to and dissociation from either SCG membrane fragments or solubilized receptors (data not shown). This motivated us to measure the reversibility of 125I-αBgt binding to intact SCG neurons in culture.
SCG neurons in dissociated cell culture were incubated with 10 nM 125I-αBgt for 1 h at 37°C to label toxin-binding sites. The radiolabelled toxin was then replaced with 100 nM αBgt to prevent rebinding of labelled toxin after dissociation. The cells were then incubated for various lengths of time, rapidly rinsed and the retained radioactivity quantified. Monitoring of the time course of 125I-αBgt dissociation from SCG neurons under these conditions revealed a biphasic curve (Fig. 7). About three-quarters of the toxin had completely dissociated within 5–10 min while the remainder appeared much more stable and followed over time the dissociation seen for 125I-αBgt bound to chick ciliary ganglion neurons in culture (Fig. 7). Both classes of SCG sites were specific in that binding of 125I-αBgt was reproducible, significant, and blocked by unlabelled αBgt. The rapidly dissociating toxin component in the case of SCG neurons was too fast to permit an accurate determination of the off-rate, given the required rinse time in the assay, but it was clearly consistent with the rapid reversal of αBgt blockade seen for the slowly desensitizing response attributed above to α7-nAChRs. The second class of 125I-αBgt-binding site on SCG neurons displayed the slow off-rate more commonly expected for α7-nAChRs and was consistent with the pseudo-irreversible blockade seen for the rapidly decaying αBgt-sensitive response of such receptors.
Figure 7. Reversal of 125I-αBgt binding to surface receptors on rat SCG and chick ciliary ganglion neurons in culture.

Cells were incubated at 37 °C for 1 h in 125I-αBgt (10 nM in PSS). At t= 0, 125I-αBgt was removed and 100-fold excess of αBgt (in PSS) added. Points represent means ±s.e.m. of 3–4 experiments (triplicate cultures per experiment) for ciliary ganglion (○) and SCG (•) neurons. The curves are the best fit to the data using a single exponential function for ciliary ganglion neurons and two exponential functions for SCG neurons. The SCG rapidly reversing component (τ1 < 5 min) was absent from the ciliary ganglion population.
DISCUSSION
The principal finding reported here is that rat SCG neurons express two classes of functional α7-nAChR. One class rapidly desensitizes, recognizes choline as an agonist and is blocked almost irreversibly by αBgt. The other class desensitizes much more slowly, does not respond well, if at all, to choline and is blocked by αBgt in a readily reversible manner. The two classes vary in their relative abundance among SCG neurons with most cells having the slowly desensitizing species and fewer having the rapidly desensitizing species. These properties may help to explain previous failures to detect functional α7-nAChRs on mammalian sympathetic neurons. The small and variable response of the rapidly desensitizing species could have been obscured by responses from other kinds of nAChRs, and the slowly desensitizing species would not have been recognized as αBgt sensitive if much of the toxin quickly dissociated prior to testing.
Several lines of evidence indicate that both kinds of αBgt-sensitive response described here are generated by α7-nAChRs. The rapidly desensitizing species has the kinetics and pharmacology previously ascribed to α7-nAChRs in rat hippocampal neurons and PC12 cells (Zorumski et al. 1992; Alkondon & Albuquerque, 1993; Blumenthal et al. 1997). The slowly desensitizing species has the properties of α7-nAChRs previously described in rat intracardiac ganglion neurons (Cuevas & Berg, 1998), and can be blocked by intracellular dialysis with an anti-α7 mAb. The neurons are known to express the α7 gene (Mandelzys et al. 1995), and the present results show they do not express the α9 gene – the only other identified neuronal nAChR gene product in rat capable of binding αBgt. Moreover, the neurons have two classes of αBgt-binding site that are distinguishable by their rates of toxin dissociation, rates that are consistent with the times required to reverse toxin blockade of the responses. The relative amounts of the two classes of binding site are consistent with the relative amplitudes and occurrence of the two types of αBgt-sensitive response, but the results do not permit a close comparison because saturation binding was not confirmed for the two binding components and because the off-rate of the rapidly dissociating component was too fast for accurate quantification. It should be noted, however, that the results cannot exclude the possibility that one or both of the toxin-sensitive responses may be generated by an as yet unidentified gene product, but in this case the gene product must be expressed in SCG neurons, bind αBgt and MLA with high affinity and be inhibited by anti-α7 mAbs but not by anti-α3 mAbs.
The molecular basis for the two kinds of α7-nAChR response is unknown. One possibility is that two distinct receptor species are involved with different subunit compositions. The rapidly desensitizing species may be homomeric for the α7 gene product (Couturier et al. 1990; Schoepfer et al. 1990; Chen & Patrick, 1997) while the slowly desensitizing species may be heteromeric, containing other gene products in addition to α7. Heterologous expression studies in Xenopus oocytes indicate that the rat α7 gene product can co-assemble with one or more of the muscle nAChR gene products to produce functional receptors (Helekar et al. 1994), and the chick α7 gene product can co-assemble with a mutated β3 gene product under these conditions (Palma et al. 1999). In chick, the α7 gene product can also be found co-assembled with α8 in a subpopulation of native receptors (Schoepfer et al. 1990). However, none of these other genes – the muscle nAChR genes, β3 or α8 – are thought to be expressed in autonomic neurons.
Heteromeric α7-containing nAChRs have also been proposed recently to explain several ACh-evoked currents in chick sympathetic neurons in culture (Yu & Role, 1998). An αBgt-sensitive, slowly decaying response was seen, much like the αBgt-sensitive, slowly decaying response described here in SCG neurons, but it was not inhibited by low concentrations of MLA (Yu & Role, 1998). Instead, MLA inhibited a rapidly desensitizing, αBgt-resistant ACh-evoked response from the neurons. Neither the MLA-sensitive nor the αBgt-sensitive responses in chick sympathetic neurons were nearly as quick to desensitize as the rapidly decaying αBgt-sensitive response elicited by ACh and choline observed here in rat SCG neurons or previously in rat hippocampal neurons (Zorumski et al. 1992; Alkondon & Albuquerque et al. 1993), chick ciliary ganglion neurons (Zhang et al. 1994) or rat PC12 cells (Blumenthal et al. 1997). Both the MLA- and αBgt-sensitive responses in chick sympathetic neurons were attributed to heteromeric α7-containing nAChRs based on results from deletion experiments using antisense oligonucleotides (Yu & Role, 1998).
A different explanation for the two kinds of α7-nAChR response seen in rat SCG neurons is that post-translational modifications may affect receptor function. This option remains viable because as yet there is no clear biochemical evidence to support the hypothesis that native α7-nAChRs are heteromeric (with the exception of α7/α8 receptors in chick). For example, immunoprecipitation of native α7-nAChRs, followed by Western blot analysis, failed to detect other known nAChR gene products specifically associated with α7 (except for α8; Schoepfer et al. 1990; Vernallis et al. 1993; Chen & Patrick, 1997). Efforts to purify α7-nAChRs have led to reports that several components co-fractionate with the receptors (e.g. Gotti et al. 1992, and references therein), but it remains unknown whether the components represent non-α7 subunits, α7 degradation products or non-specifically associated components. It will be interesting in this context to determine the relationship between the two kinds of αBgt-binding site on SCG neurons when the receptors are solubilized; rapidly dissociating αBgt sites have not been reported for solubilized receptors to our knowledge, raising the possibility that the sites are either lost or have different binding properties under these conditions.
The fact that the α7-nAChR responses were obtained with freshly dissociated SCG neurons provides assurance that the neurons normally express such receptors in vivo. A previous report described ACh responses in SCG neurons that could be reversibly blocked by αBgt (Dun & Karczmar, 1980), but the high concentration of αBgt used in those experiments raised the possibility that the blockade might have been produced by contaminating toxins acting on other kinds of nAChRs (Chiappinelli et al. 1981). Some PC12 cell strains have a rapidly desensitizing αBgt-sensitive response (Blumenthal et al. 1997), but one report describes an αBgt-sensitive response in cells that appears to be relatively slow to desensitize (Rangwala et al. 1997). Though high concentrations of αBgt and MLA were used in that characterization, blockade could also be seen with low concentrations of MLA (W. Green, personal communication). Possibly the kind of α7-nAChR response produced by PC12 cells varies with the individual PC12 strain as is the case for the composite ACh response (Blumenthal et al. 1997).
What might be the functions of α7-nAChRs on SCG neurons? Previous studies have identified αBgt-binding sites at extrasynaptic locations on the neurons (Fumagalli & DeRenzis, 1984). Such sites presumably represent the rapidly desensitizing α7-nAChRs because the rinse procedures used to prepare samples for autoradiography should have removed most toxin bound to readily reversible sites. Nothing is known yet about the surface distribution of the slowly desensitizing α7-nAChRs. Even if both classes are confined to extrasynaptic sites, they may play important roles in synaptic signalling. On chick ciliary neurons α7-nAChRs are confined to somatic spines and are excluded from postsynaptic densities (Shoop et al. 1999) but nonetheless contribute importantly to synaptic currents (Zhang et al. 1996) and help ensure reliable, synchronized transmission through the ganglion during development (Chang & Berg, 1999). At later times ciliary ganglion α7-nAChRs are thought to be more important for influencing calcium-dependent events, given their high relative permeability to calcium, but dispensable for acute transmission at such times (Chiappinelli, 1983; Chang & Berg, 1999).
The rapidly desensitizing α7-nAChRs generate relatively small and variable currents in SCG neurons but they may still contribute importantly to fast synaptic signalling: depolarizing pulses as small as 50 pA in current clamp mode can elicit action potentials in SCG neurons. Alternatively, the rapidly desensitizing α7-nAChRs, particularly in extrasynaptic locations, may be designed to monitor extracellular choline released by hydrolysis of ACh (Papke et al. 1996; Alkondon et al. 1997), though it is not known whether extracellular choline concentrations in the SCG reach those required to activate the receptors. The slowly desensitizing α7-nAChRs generate substantial currents, accounting for about half of the whole-cell ACh-evoked current seen in three-quarters of the SCG neurons tested. Such receptors should have the capacity to contribute prominently to synaptic signalling.
It seems reasonable to suppose that the two kinds of α7-nAChR on SCG neurons have different roles due to their different time courses of action. The variation in relative abundance of the two kinds of α7-nAChR among SCG neurons may reflect the different physiological assignments of neurons within the ganglion. Previous studies have identified multiple neuronal subtypes in rat SCG on the basis of their morphological and neurochemical properties and have demonstrated that such characteristics correlate with the target tissues innervated by the neurons (Andrews et al. 1996). Preliminary results suggest that the slowly desensitizing α7-nAChRs on rat SCG and intracardiac ganglion neurons may have high relative permeabilities to calcium (J. Cuevas, unpublished results), as do rapidly desensitizing α7-nAChRs (Bertrand et al. 1993; Seguela et al. 1993). If so, both classes of receptor could have profound effects on a variety of neuronal events and signalling capabilities in the ganglion.
Acknowledgments
We thank Dr Jon Lindstrom (University of Pennsylvania, PA, USA) for generously supplying mAbs, and Eleanor Hewitt and Nicholas Haliotis for expert help with tissue dissections and culture preparation. Grant support was provided by NIH grants NS12601 and NS35469 and Tobacco-Related Disease Research Program grant RT65-0050 to D.K.B., and by American Heart Association grant 9930259N to J.C. A.L.R. is a HFSP Fellow.
References
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. Journal of Pharmacology and Experimental Therapeutics. 1993;265:1455–1473. [PubMed] [Google Scholar]
- Alkondon M, Pereira EFR, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of α7 nicotinic acetylcholine receptors in the rat brain neurons. European Journal of Neuroscience. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EFR, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. Journal of Neuroscience. 1998;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R, Peng X, Lindstrom J. Homomeric and native α7 acetylcholine receptors exhibit remarkably similar but non-identical pharmacological properties, suggesting that the native receptor is a heteromeric protein complex. FEBS Letters. 1993;327:241–246. doi: 10.1016/0014-5793(93)80177-v. [DOI] [PubMed] [Google Scholar]
- Andrews TJ, Thrasivoulou C, Nesbit W, Cowen T. Target-specific differences in the dendritic morphology and neuropeptide content of neurons in the rat SCG during development and aging. Journal of Comparative Neurology. 1996;368:33–44. doi: 10.1002/(SICI)1096-9861(19960422)368:1<33::AID-CNE3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Berger F, Gage FH, Vijayaraghavan S. Nicotinic receptor-induced apoptotic cell death of hippocampal progenitor cells. Journal of Neuroscience. 1998;18:6871–6881. doi: 10.1523/JNEUROSCI.18-17-06871.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Galzi JL, Devillers-Thiery A, Bertrand S, Changeux JP. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal α7 nicotinic receptor. Proceedings of the National Academy of Sciences of the USA. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal EM, Conroy WG, Romano SJ, Kassner PD, Berg DK. Detection of functional nicotinic receptors blocked by α-bungarotoxin on PC12 cells and dependence of their expression on post-translational events. Journal of Neuroscience. 1997;17:6094–6104. doi: 10.1523/JNEUROSCI.17-16-06094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Fumagalli L. Dissociation of α-bungarotoxin binding and receptor block in the rat superior cervical ganglion. Brain Research. 1977;129:165–168. [Google Scholar]
- Chang K, Berg DK. Dependence of circuit function on nicotinic acetylcholine receptors containing α7 subunits. Journal of Neuroscience. 1999;19:3701–3710. doi: 10.1523/JNEUROSCI.19-10-03701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Patrick JW. The α-bungarotoxin-binding nicotinic acetylcholine receptor from rat brain contains only the α7 subunit. Journal of Biological Chemistry. 1997;272:24024–24029. doi: 10.1074/jbc.272.38.24024. [DOI] [PubMed] [Google Scholar]
- Chiappinelli VA. Kappa-bungarotoxin: a probe for the neuronal nicotinic receptor in the avian ciliary ganglion. Brain Research. 1983;277:9–21. doi: 10.1016/0006-8993(83)90902-2. [DOI] [PubMed] [Google Scholar]
- Chiappinelli VA, Cohen JB, Zigmond RE. The effects of α- and β-neurotoxins from the venoms of various snakes on transmission in autonomic ganglia. Brain Research. 1981;211:107–126. doi: 10.1016/0006-8993(81)90070-6. [DOI] [PubMed] [Google Scholar]
- Conroy WG, Berg DK. Nicotinic receptor subtypes in the developing chick brain: appearance of a species containing the α4, β2 and α5 gene products. Molecular Pharmacology. 1998;53:392–401. doi: 10.1124/mol.53.3.392. [DOI] [PubMed] [Google Scholar]
- Couturier S, Bertrand D, Matter J-M, Hernandez M-C, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- Cuevas J, Berg DK. Mammalian nicotinic receptors with α7 subunits that slowly desensitize and rapidly recover from α-bungarotoxin blockade. Journal of Neuroscience. 1998;18:10335–10344. doi: 10.1523/JNEUROSCI.18-24-10335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun NJ, Karczmar AG. Blockade of ACh potentials by α-bungarotoxin in rat superior cervical ganglion cells. Brain Research. 1980;196:536–540. doi: 10.1016/0006-8993(80)90421-7. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. α9: An acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV. Synaptic potentials mediated via α-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. Journal of Neuroscience. 1998;18:8228–8235. doi: 10.1523/JNEUROSCI.18-20-08228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W-M, Liou H-C, Chen Y-H. Nerve terminal currents induced by autoreception of acetylcholine release. Journal of Neuroscience. 1998;18:9954–9961. doi: 10.1523/JNEUROSCI.18-23-09954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, DeRenzis G. Extrasynaptic localization of α-bungarotoxin receptors in the rat superior cervical ganglion. Neurochemistry International. 1984;6:355–364. doi: 10.1016/0197-0186(84)90078-0. [DOI] [PubMed] [Google Scholar]
- Gotti C, Hanke W, Schlue W-R, Briscini L, Morretti M, Clementi F. A functional α-bungarotoxin receptor is present in chick cerebellum: purification and characterization. Neuroscience. 1992;50:117–127. doi: 10.1016/0306-4522(92)90386-g. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hefft S, Hulo S, Bertrand D, Muller D. Synaptic transmission at nicotinic acetylcholine receptors in rat hippocampal organotypic cultures and slices. The Journal of Physiology. 1999;510:709–716. doi: 10.1111/j.1469-7793.1999.769ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helekar SA, Char D, Neff S, Patrick J. Prolyl isomerase requirement for the expression of functional homo-oligomeric ligand-gated ion channels. Neuron. 1994;12:179–189. doi: 10.1016/0896-6273(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. Journal of General Physiology. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristufek D, Stocker E, Boehm S, Huck S. Somatic and prejunctional nicotinic receptors in cultured rat sympathetic neurones show different agonist profiles. The Journal of Physiology. 1999;516:739–756. doi: 10.1111/j.1469-7793.1999.0739u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee D, Heath M, Gelber S, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1697. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- Mandelzys A, De Koninck P, Cooper E. Agonist and toxin sensitivities of ACh-evoked currents on neurons expressing multiple nicotinic ACh receptor subunits. Journal of Neurophysiology. 1995;74:1212–1221. doi: 10.1152/jn.1995.74.3.1212. [DOI] [PubMed] [Google Scholar]
- Margiotta JF, Gurantz D. Changes in the number, function, and regulation of nicotinic acetylcholine receptors during neural development. Developmental Biology. 1989;135:326–339. doi: 10.1016/0012-1606(89)90183-8. [DOI] [PubMed] [Google Scholar]
- Mathie A, Colquhoun D, Cull-Candy SG. Rectification of currents activated by nicotinic acetylcholine receptors in rat sympathetic ganglion neurones. The Journal of Physiology. 1990;427:625–655. doi: 10.1113/jphysiol.1990.sp018191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messi ML, Renganathan M, Grigorenko E, Delbono E. Activation of α7 nicotinic acetylcholine receptor promotes survival of spinal cord motoneurons. FEBS Letters. 1997;411:32–38. doi: 10.1016/s0014-5793(97)00600-5. [DOI] [PubMed] [Google Scholar]
- Palma E, Maggi L, Barabino B, Eusebi F, Ballivet M. Nicotinic acetylcholine receptors assembled from the α7 and β3 subunits. Journal of Biological Chemistry. 1999;274:18335–18340. doi: 10.1074/jbc.274.26.18335. [DOI] [PubMed] [Google Scholar]
- Papke RL, Bencherif M, Lippiello P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the α7 subtype. Neuroscience Letters. 1996;213:201–204. doi: 10.1016/0304-3940(96)12889-5. [DOI] [PubMed] [Google Scholar]
- Pugh PC, Berg DK. Neuronal acetylcholine receptors that bind α-bungarotoxin mediate neurite retraction in a calcium-dependent manner. Journal of Neuroscience. 1994;14:889–896. doi: 10.1523/JNEUROSCI.14-02-00889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala F, Drisdel RC, Rakhilin S, Ko E, Atluri P, Harkins AB, Fox AP, Salman SB, Green WN. Neuronal α-bungarotoxin receptors differ structurally from other nicotinic acetylcholine receptors. Journal of Neuroscience. 1997;17:8201–8212. doi: 10.1523/JNEUROSCI.17-21-08201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annual Review of Neuroscience. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Schoepfer R, Conroy WG, Whiting P, Gore M, Lindstrom J. Brain α-bungarotoxin binding protein cDNAs and mAbs reveal subtypes of this branch of the ligand-gated ion channel gene superfamily. Neuron. 1990;5:35–48. doi: 10.1016/0896-6273(90)90031-a. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain α7: a nicotinic cation channel highly permeable to calcium. Journal of Neuroscience. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoop RD, Martone ME, Yamada N, Ellisman MH, Berg DK. Neuronal acetylcholine receptors with α7 subunits are concentrated on somatic spines for synaptic signaling in embryonic chick ciliary ganglia. Journal of Neuroscience. 1999;19:692–704. doi: 10.1523/JNEUROSCI.19-02-00692.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouslard J, Marsh SJ, Brown DA. Calcium entry through nicotinic receptor channels and calcium channels in cultured rat superior cervical ganglion cells. The Journal of Physiology. 1993;468:53–71. doi: 10.1113/jphysiol.1993.sp019759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernallis AB, Conroy WG, Berg DK. Neurons assemble acetylcholine receptors with as many as three kinds of subunits while maintaining subunit segregation among receptor subtypes. Neuron. 1993;10:451–464. doi: 10.1016/0896-6273(93)90333-m. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Huang B, Blumenthal EM, Berg DK. Arachidonic acid as a possible negative feedback inhibitor of nicotinic acetylcholine receptors on neurons. Journal of Neuroscience. 1995;15:3679–3687. doi: 10.1523/JNEUROSCI.15-05-03679.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ, Schoepfer R, Conroy WG, Gore MJ, Keyser KT, Shimasaki S, Esch F, Lindstrom JM. Expression of nicotinic acetylcholine receptor subtypes in brain and retina. Molecular Brain Research. 1991;10:61–70. doi: 10.1016/0169-328x(91)90057-5. [DOI] [PubMed] [Google Scholar]
- Wilkie GI, Hutson P, Sullivan JP, Wonnacott S. Pharmacological characterization of a nicotinic autoreceptor in rat hippocampal synaptosomes. Neurochemical Research. 1996;21:1141–1148. doi: 10.1007/BF02532425. [DOI] [PubMed] [Google Scholar]
- Yu CR, Role LW. Functional contribution of the α7 subunit to multiple subtypes of nicotinic receptors in embryonic chick sympathetic neurones. The Journal of Physiology. 1998;509:651–665. doi: 10.1111/j.1469-7793.1998.651bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z-W, Coggan JS, Berg DK. Synaptic currents generated by neuronal acetylcholine receptors sensitive to α-bungarotoxin. Neuron. 1996;17:1231–1240. doi: 10.1016/s0896-6273(00)80253-6. [DOI] [PubMed] [Google Scholar]
- Zhang Z-W, Vijayaraghavan S, Berg DK. Neuronal acetylcholine receptors that bind α-bungarotoxin with high affinity function as ligand-gated ion channels. Neuron. 1994;12:167–177. doi: 10.1016/0896-6273(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Thio LL, Isenberg KE, Clifford DB. Nicotinic acetylcholine currents in cultured postnatal rat hippocampal neurons. Molecular Pharmacology. 1992;41:931–936. [PubMed] [Google Scholar]


