Abstract
The immunophilin FKBP12 associates with intracellular Ca2+ channels and this interaction can be disrupted by the immunosuppressant FK506. We have investigated the effect of FK506 on Ca2+ release and Ca2+ uptake in permeabilized cell types.
Changes in medium free [Ca2+] were detected by the fluorescent Ca2+ indicator fluo-3 in digitonin-permeabilized SH-SY5Y human neuroblastoma cells, DT40 and R23-11 (i.e. triple inositol 1,4,5-trisphosphate (IP3) receptor knockout cells) chicken B lymphocytes and differentiated and undifferentiated BC3H1 skeletal muscle cells. 45Ca2+ fluxes were studied in saponin-permeabilized A7r5 rat smooth muscle cells.
Addition of FK506 to permeabilized SH-SY5Y cells led to a sustained elevation of the medium [Ca2+] corresponding to ∼30% of the Ca2+ ionophore A23187-induced [Ca2+] rise. This rise in [Ca2+] was not dependent on mitochondrial activity.
This FK506-induced [Ca2+] rise was related to the inhibition of the sarcoplasmic/endoplasmic reticulum Ca2+-Mg2+-ATPase (SERCA) Ca2+ pump. Oxalate-facilitated 45Ca2+ uptake in SH-SY5Y microsomes was inhibited by FK506 with an IC50 of 19 μm.
The inhibition of the SERCA Ca2+ pump was not specific since several macrocyclic lactone compounds (ivermectin > FK506, ascomycin and rapamycin) were able to inhibit Ca2+ uptake activity.
FK506 (10 μm) did not affect IP3-induced Ca2+ release in permeabilized SH-SY5Y and A7r5 cells, but enhanced caffeine-induced Ca2+ release via the ryanodine receptor (RyR) in differentiated BC3H1 cells.
In conclusion, FK506 inhibited active Ca2+ uptake by the SERCA Ca2+ pump; in addition, FK506 enhanced intracellular Ca2+ release through the RyR, but it had no direct effect on IP3-induced Ca2+ release.
FK506 is a macrocyclic lactone immunosuppressant, which is used for the prevention of allograft rejection in organ transplantation (Spencer et al. 1997). An important cellular target of FK506 is the ubiquitous FK506-binding protein, FKBP12, a 12 kDa member of the immunophilin family (Marks, 1996). The FK506-FKBP12 complex binds to calcineurin, a Ca2+-calmodulin-dependent protein phosphatase and suppresses the interleukin2-dependent proliferation of T cells (Schreiber, 1991).
FKBP12 does not only play an important role in T cells, but it is present in many cell types and it is very well conserved from plants and yeast to humans (Kay, 1996). FKBP12 can participate in other cellular processes, including neurotransmitter release, neural nitric oxide production, nerve growth, signal transduction via the type 1 receptor for transforming growth factor-β and intracellular Ca2+ release via the ryanodine receptor (RyR) and the inositol 1,4,5-trisphosphate receptor (IP3R) (Snyder et al. 1998).
The RyR and IP3R are tetrameric intracellular Ca2+ release channels, which mediate mobilization of stored Ca2+ from the sarcoplasmic reticulum (SR) and the endoplasmic reticulum (ER) into the cytoplasm (Berridge, 1993). The RyR plays an important role in excitation-contraction coupling in skeletal muscle cells (RyR1) and in heart muscle cells (RyR2). The IP3R is a ubiquitous Ca2+ release channel activated by IP3 produced from phosphatidylinositol 4,5-bisphosphate by phospholipase C in response to several extracellular stimuli (Berridge, 1993).
The interaction of FKBP12 with the RyR is well characterized. FKBP12 binds stoichiometrically to the RyR1, i.e. four FKBP12 molecules bind per tetrameric channel (Jayaraman et al. 1992). Moreover, the RyR can be affinity purified from terminal cisternae based on its tight association with FKBP12 (Xin et al. 1995). FK506 disrupts the interaction of FKBP12 with the RyR1, thereby increasing the open probability and mean open time of the channel (Ahern et al. 1994). Furthermore, associated FKBP12 stabilizes the RyR channel (Brillantes et al. 1994) and induces a rectification of the Ca2+-release channel, which favours unidirectional Ca2+ flux from the lumen of the ER to the cytosol (Chen et al. 1994). FKBP12 also stabilizes the interaction between different RyRs, thereby enhancing coupled gating of a cluster of channels (Marx et al. 1998). Recent studies revealed that this interaction is conserved, as FKBP12 forms an integral part of the RyR1 and modulates the RyR1 in each of the five classes of vertebrates (Qi et al. 1998). These biochemical and functional data suggest that FKBP12 can function as a modulator of the Ca2+ flux properties of the RyR1 and that interaction with FKBP12 can influence Ca2+ signalling by the RyR.
Recently, Cameron et al. (1995b) demonstrated that FKBP12 could also interact directly with the type 1 IP3 receptor (IP3R1), thereby modulating the Ca2+ flux through this channel. Dissociation of FKBP12 from cerebellar microsomes using FK506 potentiated Ca2+ release via IP3R1. In contrast to this observation, intracellular Ca2+ oscillations mediated by the IP3R in intact tracheal epithelial cells were strongly inhibited by FK506 (Kanoh et al. 1999). This effect of FK506 was accompanied by a decrease of the Ca2+ content of the ER Ca2+ stores. Furthermore, FKBP12 could target calcineurin to the IP3R1. The associated calcineurin could rapidly modulate the phosphorylation state of the IP3R1 and might induce a fine-tuning of the Ca2+ flux properties of this channel (Cameron et al. 1995a). Besides these direct effects, FK506 attenuated IP3R expression over more extended periods by inhibiting the calcineurin pathway (Genazzani et al. 1999).
Although the IP3R is the most prevalent intracellular Ca2+ release mechanism, little is known to what degree IP3-induced Ca2+ release is affected by FK506 in a cellular context. In order to clarify the potential role of immunophilins in cellular Ca2+ handling, we have investigated the functional effects of FK506 on intracellular Ca2+ release in permeabilized cells.
The SH-SY5Y neuroblastoma cell line was chosen since it expresses predominantly IP3R1 (Wojcikiewicz, 1995). Cell lines that express high levels of RyR (differentiated BC3H1 muscle cells; De Smedt et al. 1991) or triple IP3R knockout B cells (R23-11) (Sugawara et al. 1997) were used for comparison purposes.
Our results indicate that FK506 inhibited the intracellular SERCA Ca2+ pumps. In addition, FK506 did not affect IP3-induced Ca2+ release, but enhanced Ca2+ flux through the RyR. The effects of FK506 on intracellular Ca2+ signalling may be relevant for the potential side effects of this drug which is widely applied in allograft transplantation surgery.
METHODS
Materials
FK506 was kindly provided by Dr A. Friedrich, Fujisawa GmbH, München, Germany. Rapamycin, cyclosporin A, midecamycin, ivermectin and A23187 were purchased from Sigma (St Louis, MO, USA). Ascomycin and polyclonal rabbit anti-FKBP12 antibody were purchased from Affinity Bioreagents, Inc. (Golden, CO, USA). Fluo-3 was obtained from Molecular Probes (Junction City, OR, USA) and 45Ca2+ was obtained from Amersham Pharmacia Biotech AB (Uppsala, Sweden). IP3 was from Boehringer (Mannheim, Germany).
Cell culture and preparation of cell homogenates and microsomes
The SH-SY5Y human neuroblastoma cell line (9403034) was obtained from the European Collection of Animal Cell Cultures (Wiltshire, UK). The BC3H1 mouse muscle cell line (CRL-1443) and the A7r5 rat aorta smooth muscle cell line (CRL-1444) were obtained from the American Type Culture Collection (Rockville, MD, USA). The DT40 and R23-11 chicken B lymphocyte cell lines were a generous gift from Dr T. Kurosaki (Tokyo, Japan). Undifferentiated and differentiated BC3H1 (De Smedt et al. 1991), A7r5 (Missiaen et al. 1992a) and SH-SY5Y cell monolayers (Wojcikiewicz, 1995) as well as DT40 and R23-11 cell suspensions (Miyakawa et al. 1999) were cultured as described. Cell harvest and microsomal preparations were performed according to earlier published procedures (Parys et al. 1995). SH-SY5Y homogenates for immunoblot analysis were prepared by a brief sonication step (4 times 10 s with a probe sonicator MSE Ltd, Westminster, UK) in 50 mM Tris-HCl, 300 mM sucrose and 0.5 % Triton X-100 at pH 7.4. Mouse heart homogenates (Ji et al. 1999) and cardiac SR (Kirchberger & Antonetz, 1982) were prepared as described. All preparations were frozen in liquid nitrogen and stored at -80°C until use. Protein concentrations were measured by the method of Lowry et al. (1951) with bovine serum albumin as standard after precipitation of the proteins with 10 % (w/v) trichloroacetic acid.
Ca2+ release studies in permeabilized cells
Cell pellets were resuspended in intracellular medium (120 mM KCl, 30 mM Hepes pH 7.4, 1 mM MgCl2) in the presence of 1 mM ATP, 25 mM creatine phosphate, 50 i.u. creatine kinase and 2.5-5 μM fluo-3 and transferred to a 37°C thermostatically controlled 4 ml fluorescence quartz cuvette. Cell density was 15 × 106 cells ml−1 for SH-SY5Y, 50 × 106 cells ml−1 for DT40 and R23-11 and 3.5 × 106 cells ml−1 for BC3H1. Mild treatment of the cells with digitonin (50 μM) disrupted the plasma membrane. Free [Ca2+] was estimated from the fluorescence signal upon binding to fluo-3. The fluorescence (excitation: 503 nm, emission: 530 nm) was measured with a Perkin Elmer LS50 fluorimeter and recorded as a function of time by the Fluorescence Luminescence Data Manager (version 2.50, Perkin Elmer) software. A23187 (8 μM) was added at the end of each experiment to control store loading and to measure the total releasable Ca2+. The fluorescence signal (F) was calibrated by first adding 0.5 mM Ca2+ (Fmax) and then 5 mM EGTA (Fmin).
The free [Ca2+] was calculated by the equation:
(Grynkiewicz et al. 1985), using a dissociation constant of 864 nM (Kd), as determined in cytosol-like medium at 37°C (Missiaen et al. 1991).
45Ca2+ uptake experiments
45Ca2+ uptake was determined using a rapid filtration technique and was basically performed as previously described (Ji et al. 1999). Cardiac homogenates (100 μg protein ml−1) or SH-SY5Y microsomes (200 μg protein ml−1) were incubated at 37°C in Ca2+ uptake medium (1.5 ml) containing 40 mM imidazole-HCl pH 7.0, 100 mM KCl, 5 mM MgCl2, 5 mM NaN3, 5 mM potassium oxalate, 0.5 mM EGTA, 1 μM ruthenium red and a free [Ca2+] of 3.16 μM as determined by CaBuf software (Dr G. Droogmans, K.U.Leuven, Belgium). 45Ca2+ (10 μCi ml−1) uptake was started by the addition of 5 mM ATP and the initial uptake rate was determined by sampling after 30, 60 and 120 s for cardiac homogenates and after 6, 12 and 18 min for SH-SY5Y microsomes and performing a linear regression analysis. The non-specific 45Ca2+ uptake was determined before the addition of ATP and subtracted from the sample values. Microsomes were pre-incubated for 5 min with drug or vehicle prior to the addition of ATP, which initiates 45Ca2+ uptake. Longer pre-incubation times (up to 30 min) did not affect the inhibitory effect, indicating that an equilibrium distribution was obtained.
Ca2+-Mg2+-ATPase activity of cardiac SR vesicles
The Ca2+-Mg2+-ATPase activity was measured at 37°C in 1 ml of medium containing 20 mM Mops pH 6.8, 100 mM KCl, 1 mM EGTA, 5 mM NaN3, 5 mM ATP, 5.77 mM MgCl2, 100 μM ouabain, 40 i.u. pyruvate kinase, 36 i.u. lactate dehydrogenase, 1.5 mM phosphoenolpyruvate, 0.63 mM disodium NADH and 10 μM A23187. The decrease in absorbance at 340 nm was recorded with a Beckman DU-1 spectrophotometer. After thermal equilibration of the cuvette, 25 μg of cardiac SR vesicles were added. A final free [Ca2+] of 10 μM was obtained by adding 0.97 mM Ca2+. To determine the rate of the Ca2+-Mg2+-ATPase activity, the difference between the ATPase activities in the presence and absence of Ca2+ was measured. FK506 or ethanol (vehicle) was added to the thermostatically controlled cuvette and incubated for 5 min before recording the ATPase activity.
45Ca2+ fluxes on saponin-permeabilized A7r5 cells
45Ca2+ fluxes on monolayers of saponin-permeabilized A7r5 cells at 25°C were measured exactly as described previously (Missiaen et al. 1992a).
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis
Cell homogenates and microsomes were separated on 13 % SDS-PAGE followed by semidry electrophoretic transfer to a polyvinylidene difluoride membrane (Immobilon-P, Millipore Corp., Bedford, USA). Transfer was performed for 2 h in transfer buffer, containing 25 mM Tris, 192 mM glycine, 0.0375 % SDS and 20 % methanol. After blocking the membrane with PBS-T (phosphate-buffered saline with 1 % Tween-20) containing 5 % non-fat dried milk powder, the membrane was incubated overnight with the rabbit anti-FKBP12 antibody (diluted 1/1000) at 4°C. The membrane was further incubated with an alkaline phosphatase-conjugated anti-rabbit IgG antibody for 1 h at room temperature. The immunoreactive bands were visualized by conversion of the Vistra ECF substrate (Amersham Pharmacia Biotech AB) into a fluorescent probe and analysed and quantified as described previously (Vanlingen et al. 1997).
Statistics
Values are given as means ±s.d., unless otherwise stated. Statistical comparisons between two groups were performed by Student's t test. P < 0.05 was considered significantly different.
RESULTS
FK506-induced Ca2+ responses in SH-SY5Y cells
Permeabilization of the plasma membrane of SH-SY5Y cells in the uptake medium with digitonin caused an immediate decrease of the free [Ca2+] in the cuvette, due to Ca2+ uptake in the ER and in the mitochondria. The steady-state [Ca2+] after Ca2+ accumulation was typically 106 ± 39 nM (n= 20). Addition of 100 μM FK506 resulted in a sustained elevation of [Ca2+] to about 500 nM (Fig. 1A). After reaching the new Ca2+ level, 100 nM IP3 still released Ca2+ from the Ca2+ stores (Fig. 1A). At the end of the experiment, the Ca2+ ionophore A23187 (8 μM) was given to measure the medium [Ca2+] after maximal discharge of the intracellular stores, which was set as 100 % in further calculations. The FK506-induced [Ca2+] rise did not occur when the vehicle (0.1 % ethanol) was given alone and FK506 had no effect on the fluo-3 fluorescence in the absence of cells (data not shown). This indicates that the rise in [Ca2+] was neither due to an effect of the solvent nor to autofluorescence properties of FK506.
Figure 1. FK506-induced [Ca2+] rise in permeabilized SH-SY5Y cells and FKBP12 expression in SH-SY5Y homogenates and microsomes.

A, a representative recording of the free [Ca2+] measured by fluo-3 fluorescence. Calibration was performed as described in Methods. After permeabilization of the plasma membrane with digitonin (50 μM), cells accumulated Ca2+ in their Ca2+ stores. After stabilization of the signal, FK506 (100 μM) and IP3 (0.1 μM) were successively added. At the end of each experiment, A23187 (8 μM) was added to estimate the total releasable amount of Ca2+. B, dose-response curve for the FK506-induced [Ca2+] rise, normalized to the A23187-induced [Ca2+] rise, which was set as 100 %. Each data point is the mean ±s.d. of at least three independent experiments. Sigmoidal fitting was performed by using Microcal Origin (version 6.0) software. C, Western blot analysis (anti-FKBP12, diluted 1/1000) of SH-SY5Y cell homogenates (lane 1, 75 μg), SH-SY5Y microsomes (lane 2, 75 μg) and human recombinant FKBP12 (lane 3, 50 ng) after separation on 13 % SDS-PAGE and electroblotting to Immobilon-P.
The FK506-induced [Ca2+] rise, calculated as a percentage of the A23187-induced [Ca2+] rise, was dose dependent (Fig. 1B). The concentration dependence was fitted by a sigmoidal curve and characterized by an apparent EC50 of 49 ± 6 μM FK506. The maximum [Ca2+] rise induced by FK506 was reached at about 150 μM FK506. This concentration led to a sustained [Ca2+] elevation corresponding to 31 ± 6 % of the A23187-induced [Ca2+] rise. No detectable [Ca2+] increase was induced by FK506 at a concentration lower than 10 μM. A more pronounced [Ca2+] increase could not be achieved, since it is technically impossible to use concentrations higher than 200 μM FK506, due to its low solubility in an aqueous environment and due to potential solvent (ethanol) effects on intracellular Ca2+ signals. It should also be pointed out that the effective dose of lipophilic compounds such as FK506 is determined by the partition of the drug between aqueous and lipid compartments (Heirwegh et al. 1992). The EC50 and maximum level of the FK506-induced [Ca2+] rise will therefore be dependent on the amount of membrane material used in the assays. For this reason, it will be difficult to make a quantitative comparison between different cell types or membrane preparations.
FKBP12, the best characterized cellular target for FK506, could be immunologically detected not only in a total homogenate of SH-SY5Y cells but also in microsomes of these cells, suggesting a molecular link between FKBP12 and an ER component (Fig. 1C). The microsomal fraction contained 20 ± 4 % (n= 4) of the total FKBP12 expressed in SH-SY5Y cells. These results confirmed the previous observations that FKBP12 is a ubiquitously expressed protein and is abundant in cells of neural origin (Steiner et al. 1992).
IP3R-dependent Ca2+ mobilization pathway
FK506 may dissociate FKBP12 from IP3R1 and increase its sensitivity to IP3 (Cameron et al. 1995b). We therefore investigated whether the [Ca2+] rise induced by FK506 was mediated by the IP3R. We compared the effect of FK506 on permeabilized DT40 cells that express the three IP3R isoforms and on R23-11 cells, which are genetically manipulated DT40 cells deficient for all three IP3R isoforms (Sugawara et al. 1997; Miyakawa et al. 1999). Addition of 50 μM FK506 after permeabilization of both cell strains led to a similar sustained [Ca2+] elevation amounting to 28 ± 6 % (n= 5) and 27 ± 7 % (n= 5) of the A23187-induced [Ca2+] rise for the DT40 and R23-11 cell strains, respectively (Fig. 2). As illustrated in Fig. 2C, IP3 could not induce Ca2+ release in permeabilized R23-11 cells. This indicates that other pathways different from the IP3R mainly mediated the FK506-induced [Ca2+] rise. The very similar [Ca2+] elevation observed for R23-11 cells and the parent DT40 cells seems to exclude a major contribution of Ca2+-induced Ca2+ release mechanisms via intracellular Ca2+ release channels, particularly since these cells also did not show caffeine-induced Ca2+ release (data not shown). On the other hand, thapsigargin could empty the R23-11 Ca2+ stores and provoked store-operated Ca2+ entry (Sugawara et al. 1997). This may indicate that a pathway for rapid store depletion is still operative in these triple IP3R knockout cells.
Figure 2. FK506-induced [Ca2+] rise in permeabilized DT40 and R23-11 cells.

FK506 (50 μM)-induced [Ca2+] rise in permeabilized DT40 (A) and R23-11 (B) cells. Typical traces out of 5 experiments are shown. C shows the absence of IP3-induced Ca2+ release in R23-11 cells. The experimental procedure was identical to that described in Fig. 1A.
Identity of the FK506-sensitive Ca2+ store
There are different intracellular Ca2+ stores, which could be modulated by FK506. We first investigated the effect of FK506 on mitochondrial Ca2+ accumulation in permeabilized SH-SY5Y cells. First, preferential Ca2+ uptake in the mitochondria was obtained by application of thapsigargin (10 μM), which blocked any Ca2+ uptake in the ER and addition of spermine (1 mM), which activated the mitochondrial Ca2+ uniporter (Lenzen et al. 1992). The mitochondrial set point for Ca2+ is about 1 μM, which correlates with the low affinity (Kd∼1-5 μM) of the mitochondrial Ca2+ uniporter (Duchen, 1999). According to the characteristics of the mitochondrial Ca2+ uniporter, optimal Ca2+ uptake in the mitochondria occurred in the presence of 1 mM spermine. In these experimental conditions with thapsigargin blocking the high-affinity ER Ca2+ pump, the equilibrium [Ca2+] never dropped below 1 μM. From Fig. 3A, it is evident that the [Ca2+] rise induced by FK506 (50 μM) did not originate from the mitochondria. Second, FK506 was still able to induce a sustained [Ca2+] elevation, when Ca2+ was accumulated in the absence of thapsigargin and when the mitochondrial Ca2+ accumulation was inhibited by mitochondrial inhibitors, such as azide (10 mM). In Fig. 3B, intact cells were pre-treated during 10 min with azide (10 mM NaN3) in the extracellular medium. After permeabilization of the plasma membrane and reaching the steady-state [Ca2+], azide (10 mM) was added during the experiment to confirm that the mitochondria did not contain any Ca2+. The ‘hump’ in the free [Ca2+] after application of azide could be due to a small amount of contaminating Ca2+ in the azide solution and was also observed in earlier reports (Missiaen et al. 1992b). FK506 (50 μM) still induced a [Ca2+] rise. Similar results were obtained with ruthenium red (4 μM) and carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone (FCCP, 20 μM; data not shown).
Figure 3. Source of the FK506-induced [Ca2+] rise.

A, accumulation of Ca2+ in the mitochondrial compartment of permeabilized SH-SY5Y cells in the presence of 10 μM thapsigargin and 1 mM spermine. Addition of FK506 (50 μM), IP3 (0.1 μM) and A23187 (8 μM) are indicated. B, accumulation of Ca2+ in non-mitochondrial Ca2+ stores of permeabilized SH-SY5Y cells, pre-treated with 10 mM NaN3 10 min before digitonin addition. As a control on its inhibitory effect, NaN3 (10 mM) was again administered in the course of the experiment, 2 min before FK506 (50 μM). A23187 (8 μM) was given at the end of the experiment. Typical for 3 experiments.
Taken together, these results suggest that the ER, but not the mitochondria, was the most likely source for the FK506-induced [Ca2+] rise.
Effect on SERCA Ca2+ pumps
To probe the effect of FK506 on Ca2+ pump activity, cells were permeabilized with digitonin in the presence of 20 mM oxalate. Oxalate immobilizes luminal Ca2+ and thus enables the measurement of unidirectional Ca2+ uptake properties of permeabilized SH-SY5Y cells. After reaching the steady-state [Ca2+], a 10 nmol pulse of CaCl2 was added to the medium (Fig. 4A). This caused an immediate increase in fluorescence, followed by an exponential decrease in resting [Ca2+] due to the Ca2+ pump activity. After four Ca2+ pulses, either ethanol (0.2 %), FK506 (10-100 μM) or thapsigargin (1 μM) was added. Three minutes after this addition the cells were again challenged with four pulses of 10 nmol CaCl2. These four pulses were averaged and normalized. The mean rate of Ca2+ uptake (n= 4) was compared before and after addition of FK506.
Figure 4. Effect of FK506 on the uptake of exogenously added Ca2+ in permeabilized SH-SY5Y cells under oxalate-facilitated uptake conditions.

A, the effect of FK506 (50 μM) on Ca2+ uptake was measured by adding 4 pulses of exogenous Ca2+ (10 nmol) before and after FK506 application. The fluorescence signal is indicated in arbitrary units (A.U.), since it was difficult to estimate the [Ca2+] accurately in the presence of 20 mM oxalate. B, the 4 pulses before and after FK506 were normalized, averaged and superimposed. The mean decay (n= 4) of the [Ca2+] in control conditions and after addition of FK506 (50 μM) is plotted. This Ca2+ decay can be fitted by a monoexponential function (y= e−t/τ), which resulted in a significant increase in the time constant τ of ≈35 % (P < 0.01 by Student's paired t test). Typical for 3 experiments. The inset shows the effect of the vehicle (0.1 % ethanol) on the uptake of exogenously added Ca2+ (average of 4 pulses of 10 nmol Ca2+). C, the effect of thapsigargin (4 nM or 1 μM) on the uptake of exogenously added Ca2+ (average of 4 pulses of 10 nmol Ca2+).
The normalized uptake of the exogenously added Ca2+ (10 nmol) was fitted by a monoexponential function (y= e−t/τ). Up to 10 μM FK506 did not significantly inhibit the uptake rate of exogenously added Ca2+, but 50 μM FK506 had a significant inhibitory effect on the uptake rate (τ−1) of the exogenously added Ca2+ (Fig. 4B) and caused an increase in τ of 35 ± 5 % (mean ±s.e.m., n= 3). At 100 μM, FK506 caused an increase in τ of 46 ± 7 % (mean ±s.e.m., n= 3). A similar inhibition of the SERCA pump occurred in R23-11 cells (data not shown). Furthermore, these changes in transient kinetics by FK506 were clearly absent in the presence of the vehicle alone (see inset Fig. 4B), which indicates that FK506, but not the steady increase of the ER Ca2+ content, was responsible for the reduced Ca2+ uptake rate. Moreover, 4 nM thapsigargin caused a similar increase in τ of ∼36 % as observed for 50 μM FK506 (Fig. 4C). This concentration of thapsigargin elevated the free [Ca2+] to a value corresponding to ∼16 % of the A23187-induced [Ca2+] rise, which is also very similar to the [Ca2+] rise induced by 50 μM FK506 (Fig. 1B). In the presence of 1 μM thapsigargin, accumulation of the exogenously added Ca2+ into the ER Ca2+ stores was completely inhibited (Fig. 4C).
For a quantitative assessment of the inhibitory effect of FK506 on the initial Ca2+ uptake rate mediated by the SERCA pump, we also performed 45Ca2+ uptake experiments in SH-SY5Y microsomes in the presence of oxalate and mitochondrial inhibitors using a rapid filtration technique (Fig. 5A). The Ca2+ uptake rate at a free [Ca2+] of 3.16 μM in control conditions was 1.3 ± 0.2 nmol mg−1 min−1 (n= 17), determined by linear regression. The thapsigargin-insensitive Ca2+ uptake activity represented 10 ± 6 % (n= 9) of the Ca2+ uptake in control conditions. Figure 5A also shows the time-dependent uptake in the presence of 100 μM FK506 (0.22 nmol mg−1 min−1) or 10 μM thapsigargin (0.08 nmol mg−1 min−1). No 45Ca2+ uptake was detected in the absence of ATP. FK506 inhibited the rate of the thapsigargin-sensitive part of the Ca2+ uptake in SH-SY5Y microsomes with an IC50 of about 19 μM (Fig. 5B).
Figure 5. Effect of FK506 on oxalate-facilitated 45Ca2+ uptake in SH-SY5Y microsomes.

A, the ATP-driven 45Ca2+ uptake at a free [Ca2+] of 3.16 μM was measured in control conditions (in the presence of 0.2 % ethanol; •), in the presence of 100 μM FK506 (▪), 10 μM thapsigargin (▴) or without ATP (▾). The vehicle, FK506 or thapsigargin was added 5 min prior to ATP, which initiates 45Ca2+ uptake. A typical experiment out of 4 experiments is shown. B, dose-response curve for the effect of FK506 on the thapsigargin-sensitive part of the initial rate of Ca2+ uptake (% of control). The initial Ca2+ uptake rate was obtained by linear regression through 4 time points each performed in duplicate. Each result is the mean of at least three independent experiments. s.e.m. is indicated, unless smaller than the symbol. Sigmoidal fitting was performed by using Microcal Origin (version 6.0) software.
A similar inhibitory effect of FK506 on Ca2+ uptake activity was found for cardiac homogenates, which contain the related SERCA2a Ca2+ pump. The dose-response curve in these cells was characterized by an IC50 of about 39 μM (data not shown).
To verify whether the inhibition of the Ca2+ pump activity was accompanied by a lower activity of the Ca2+-Mg2+-ATPase, we measured the effect of FK506 on this Ca2+- Mg2+-ATPase activity in cardiac microsomes. We observed a partial inhibition (∼30 %) of the Ca2+-Mg2+-ATPase activity by 100 μM FK506. The Ca2+-Mg2+-ATPase activity in SH-SY5Y microsomes was below the detection limit of the assay (data not shown).
In order to elucidate whether the inhibition of Ca2+ uptake by FK506 represented a specific effect, we tested several related compounds with an immunosuppressive action (rapamycin, cyclosporin A and ascomycin) or with a macrocyclic lactone structure (rapamycin, ascomycin, ivermectin and midecamycin) (Fig. 6). Ivermectin (30 μM) was the most potent inhibitor of ATP-dependent Ca2+ uptake. At the same concentration, FK506, rapamycin and ascomycin inhibited the SERCA pump to a similar extent, while cyclosporin A and midecamycin were not effective.
Figure 6. Effect of functional and structural analogues of FK506 on 45Ca2+ uptake in SH-SY5Y microsomes.

The initial Ca2+ uptake rate was obtained by linear regression as explained in Methods. The Ca2+ uptake rate in control conditions (0.1 % ethanol) was set as 100 %. All drugs were tested at a concentration of 30 μM. Each result is the mean of at least three independent experiments, each performed in duplicate. s.e.m. is indicated.
This inhibition of Ca2+ uptake activity by FK506 depended on the microsomal concentration, since 30 μM FK506 reduced the Ca2+ uptake rate to 45 ± 2 % of control (mean ±s.e.m., n= 6) when 200 μg ml−1 SH-SY5Y microsomes were used. However, the same concentration of FK506 reduced the Ca2+ uptake rate to only 68 ± 5 % of control (mean ±s.e.m., n= 3) when 400 μg ml−1 SH-SY5Y microsomes were used.
Effect of FK506 on IP3-induced Ca2+ release
Although the major effect of FK506 on Ca2+ signalling was due to an inhibition of the SERCA pumps, we cannot exclude that FK506 may in addition alter Ca2+ flux through the IP3R (Cameron et al. 1995b). We therefore investigated whether low FK506 concentrations that did not affect the SERCA pumps could still modulate the intracellular Ca2+ channels. We measured the effect of FK506 on IP3-induced Ca2+ release in permeabilized SH-SY5Y cells. After permeabilization of the cells and a 5 min pre-incubation with FK506 (or 0.1 % ethanol) IP3 (0.1 μM) was added to the intracellular medium. IP3 caused an immediate, transient increase in the free cytosolic [Ca2+]. We have determined in a large number of observations that the IP3-induced peak [Ca2+] in control conditions (19 ± 3 % of the A23187-releasable Ca2+, n= 11) did not differ from the IP3-induced peak [Ca2+] released in the presence of 10 μM FK506 (19 ± 4 % of the A23187-releasable Ca2+, n= 19) (Fig. 7A). Similar results were obtained in permeabilized undifferentiated BC3H1 cells, which also exhibit predominantly IP3-induced Ca2+ release (De Smedt et al. 1991; data not shown).
Figure 7. Effect of FK506 on IP3-induced Ca2+ release in permeabilized SH-SY5Y or permeabilized A7r5 cells.
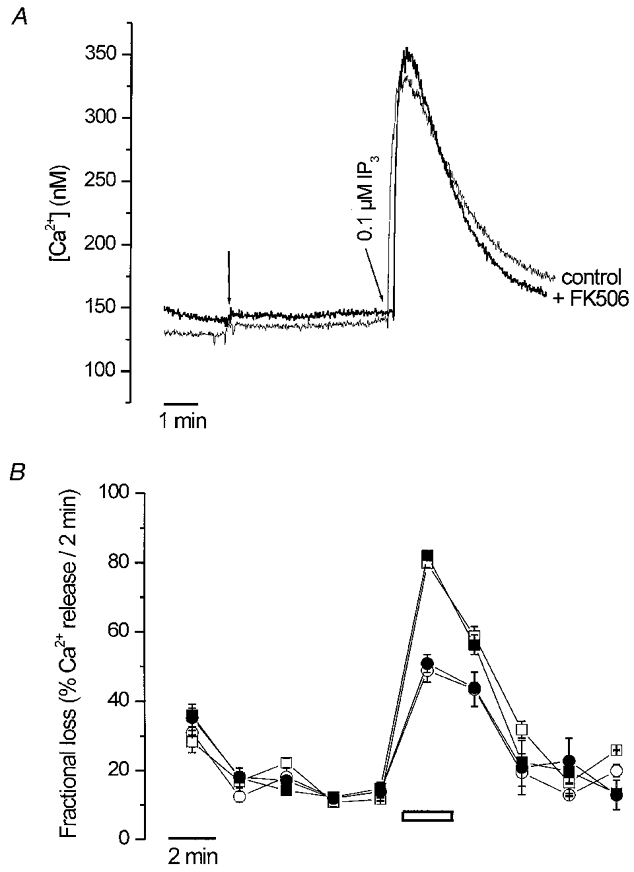
A, SH-SY5Y permeabilization and Ca2+ uptake were as described in Fig. 1A. After reaching a stable steady-state level for Ca2+, either FK506 (10 μM) or ethanol (0.1 %) were added (first arrow), 5 min before administration of IP3 (0.1 μM; second arrow). Two separate experiments out of more than 10 are superimposed. B, permeabilization and loading of A7r5 monolayers was as described in Methods. The non-mitochondrial Ca2+ stores, loaded to steady state with 45Ca2+, released Ca2+ passively when incubated in efflux medium in the continuous presence of 0.1 % ethanol (○ and □) or 10 μM FK506 (• and ▪) for 10 min, at which time 1 μM IP3 (○ and •) or 10 μM A23187 (□ and ▪) were added for 2 min, as indicated by the horizontal bar. Ca2+ release was plotted as the fractional loss, i.e. the amount of Ca2+ leaving the stores in 2 min divided by the total store Ca2+ content at that time. The result is the mean of three experiments.
Permeabilized monolayers of A7r5 cells are a well-characterized model system which allows a very accurate analysis of IP3-induced Ca2+ release in unidirectional flux conditions (Missiaen et al. 1992a). As shown in Fig. 7B, the increase in fractional loss in the presence of 1 μM IP3 (i.e. the amount of Ca2+ released by IP3 in 2 min divided by the total store Ca2+ content at that time) did not differ significantly between control cells and 10 μM FK506- treated A7r5 cells.
Taken together, these data indicate that FK506 did not affect IP3-induced Ca2+ release in permeabilized SH-SY5Y cells, undifferentiated BC3H1 cells or A7r5 cells.
Effect of FK506 on caffeine-induced Ca2+ release
To validate our assay, we controlled whether FK506 could indeed potentiate caffeine-induced Ca2+ flux through the RyR, as described in several reports (Timerman et al. 1993; Brillantes et al. 1994). Neither SH-SY5Y cells nor A7r5 cells demonstrate caffeine-induced Ca2+ release (data not shown). We therefore investigated the effect of FK506 in differentiated BC3H1 cells, which predominantly express the skeletal muscle isoform type 1 RyR as a major Ca2+ release channel (De Smedt et al. 1991). In Fig. 8, caffeine-induced Ca2+ release in permeabilized differentiated BC3H1 cells is shown in control conditions and in the presence of 10 μM FK506. FK506 potentiated the caffeine-induced Ca2+ release by a factor of 1.8 ± 0.2 (n= 3). This increase in Ca2+ release was not due to an inhibition of the SERCA pump, since under these conditions 10 μM FK506 had no significant effect on the Ca2+ uptake of exogenously added Ca2+ in the presence of oxalate (data not shown). Furthermore, the caffeine-induced Ca2+ release, both in the presence and in the absence of FK506, could be largely blocked by 100 μM ryanodine (see Fig. 8, inset) and 20 μM ruthenium red (data not shown), both inhibitors of the RyR.
Figure 8. Effect of FK506 on caffeine-induced Ca2+ release in permeabilized differentiated BC3H1 cells.

Permeabilization and Ca2+ loading of BC3H1 cells was similar to that described for SH-SY5Y cells in Fig. 1A. After reaching a stable steady-state level for Ca2+, either FK506 (10 μM) or ethanol (0.1 %) was added (first arrow), 3 min before administration of caffeine (7.5 mM; second arrow). The results were not normalized to the total releasable Ca2+, since it is known that caffeine can interact with fluorescent Ca2+ dyes (Muschol et al. 1999). This interaction distorts the accurate estimation of the total releasable Ca2+. Two separate experiments are superimposed. The figure is representative of three experiments. The inset shows the effect of 10 μM FK506 or vehicle (first arrow) on caffeine-induced Ca2+ release in the presence of 100 μM ryanodine.
DISCUSSION
Effects of FK506 on Ca2+ uptake mechanisms (SERCA) in permeabilized cells
FK506 induced a sustained rise in the free [Ca2+] in permeabilized SH-SY5Y cells. A similar [Ca2+] elevation was also observed in permeabilized R23-11 cells, which do not possess any functional IP3R. This indicates that FK506 could induce a [Ca2+] rise independently of the IP3R. This FK506-induced [Ca2+] rise was also independent of the RyR, since neither R23-11 nor SH-SY5Y cells expressed a functional RyR. Furthermore, the effect of FK506 on the sustained elevation of [Ca2+] was not dependent on Ca2+ sequestration in the mitochondria, but required Ca2+ uptake in the endoplasmic reticulum.
On the other hand, we clearly demonstrated that FK506 caused an inhibition of the SERCA Ca2+ pump activity. This inhibition of the Ca2+ uptake activity by FK506 was observed in permeabilized SH-SY5Y cells, SH-SY5Y microsomes and cardiac microsomes.
In order to maintain the steady-state cytosolic [Ca2+], the intracellular Ca2+ stores have to continuously resequester the Ca2+ that passively leaks out of these stores. Interrupting this cycle of passive Ca2+ leak and active Ca2+ uptake by inhibiting the Ca2+ pumps with FK506 or thapsigargin leaves the Ca2+ efflux temporarily uncompensated, leading to a partial discharge of the stored Ca2+ from the ER. As a consequence, there will be a net Ca2+ efflux out of the Ca2+ stores until the new and higher steady-state cytosolic [Ca2+] is reached. This was observed as a sustained elevation of the medium [Ca2+]. Similarly, Thastrup et al. (1990) demonstrated that thapsigargin caused a sustained [Ca2+] rise by specifically inhibiting Ca2+ uptake through the SERCA pump. The pathway for Ca2+ efflux out of the intracellular Ca2+ stores is as yet unidentified, but is generally described as ‘passive leak’. It is important to note that experiments in permeabilized A7r5 monolayers, where the ‘passive leak’ can be measured in the absence of Ca2+ uptake activity, showed no increase of this ‘passive leak’ pathway by FK506 (authors’ unpublished results).
We have to point out that the concentration of FK506 required for inhibition of the Ca2+ uptake activity was rather high (>10 μM) compared with the effects of FK506 on other cellular processes. Concentrations in the nanomolar range are required for the inhibition of calcineurin and subsequent suppression of the interleukin2-dependent T cell activation (Schreiber, 1991) and stoichiometrical concentrations (<5 μM) are required for the dissociation of FKBP12 from the RyR (Timerman et al. 1993). Nevertheless, several reports demonstrated the effects of higher concentrations (10-50 μM) of FK506 on cellular Ca2+-dependent mechanisms, including the effects on voltage-operated Ca2+ channels in the porcine coronary artery (Yasutsune et al. 1999), elementary Ca2+ events in cardiac myocytes (Xiao et al. 1997), cardiac intracellular [Ca2+] and contraction (Valdivia, 1998) and voltage-operated Ca2+ channel-dependent long-term potentiation in the hippocampus (Onuma et al. 1998).
Furthermore, FK506 (up to 20 μM) increased the cytosolic [Ca2+] of intestinal smooth muscle cells (Bielefeldt et al. 1997). The Ca2+ originated from the ryanodine-sensitive stores, since depletion of these stores with caffeine abolished the effect. These data indicate that FK506 had at least two effects resulting in a cytosolic [Ca2+] increase. Besides the effect of low concentrations of FK506 (<5 μM) on the RyR, there might be an additional effect on the SERCA Ca2+ pump activity, since the [Ca2+] increase did not saturate at the stoichiometrical concentrations of FK506 (<5 μM) needed for dissociation of the RyR from FKBP12. Instead, higher concentrations of FK506 (20 μM) still enhanced the FK506-induced cytosolic [Ca2+] increase.
Our observations are also compatible with the attenuating effect of FK506 on ATP-induced Ca2+ oscillations in tracheal epithelium (Kanoh et al. 1999). If FK506 would potentiate Ca2+ flux through the IP3R (Cameron et al. 1995b), an enhancement of the Ca2+ oscillations should have been observed. A reduced Ca2+ uptake activity by the ER in contrast would suppress the ATP-induced Ca2+ oscillations.
A recent report showed that compounds with a macrocyclic lactone ring structure (e.g. ivermectin) could reduce SERCA-mediated Ca2+ uptake in terminal cisternae of skeletal muscle in an FKBP12-independent way (Ahern et al. 1999). Taken together, the relative high concentrations needed and the observation that other macrocyclic compounds also inhibit Ca2+ uptake, suggest that the observed inhibition of the SERCA pump by FK506 is non-specific and FKBP12 independent.
Effects of FK506 on intracellular Ca2+ release channels (IP3R and RyR) in permeabilized cells
Evidence has been presented for a potentially important role of the immunophilin FKBP12 in the regulation of intracellular Ca2+ release through interaction with both the RyR and the IP3R (Marks, 1996).
To study functional effects of FKBP12 on intracellular Ca2+-release channels, we investigated the effects of FK506 on Ca2+ release in permeabilized SH-SY5Y cells. This cell type expresses almost exclusively the neuronal IP3R1 isoform and is therefore used as a model system for IP3-induced Ca2+ release via this IP3R isoform (Wojcikiewicz, 1995). We demonstrated that FKBP12 is also expressed in this cell type and that a significant fraction of the FKBP12 protein is retained in microsomal membranes.
FK506 had no effect on the IP3-induced Ca2+ release in permeabilized SH-SY5Y, A7r5 cells and undifferentiated BC3H1 cells which expressed the IP3R as the predominant intracellular Ca2+ release channel. On the other hand, FK506 clearly stimulated caffeine-induced Ca2+ mobilization in differentiated BC3H1 cells which expressed the RyR. This observation is in agreement with previous data indicating that dissociation of FKBP12 from the RyR by FK506 enhanced Ca2+ flux through the RyR (Brillantes et al. 1994). In addition, a recent report showed that compounds with a macrocyclic lactone ring structure could activate RyRs directly independently of FKBP12 (Ahern et al. 1999).
Cameron et al. (1995b) showed a reduced accumulation of Ca2+ into IP3-sensitive stores when cerebellar microsomes were treated with FK506. Dissociation of FKBP12 from the IP3R was found to change the phosphorylation state of this receptor and increase its sensitivity towards IP3 (Cameron et al. 1995a). We did not find a potentiating effect of FK506 on IP3-induced Ca2+ release in the investigated cell types whereas such an effect was clearly evident for caffeine-induced Ca2+ release in RyR-expressing cells. It should be pointed out, however, that the proposed FKBP12 interaction site on IP3R1 is located in close proximity to a number of other potential regulatory sites, such as the SII-splice site, a Ca2+-binding site, a calmodulin-binding site, a PKA-phosphorylation site and an ATP-binding site (Patel et al. 1999). It can therefore not be excluded that more subtle effects, potentially dependent on other modulatory compounds of the IP3R, were not present in our assay. It is, however, clear that the major effects of FK506 on intracellular Ca2+ signalling could not be attributed to a direct or indirect effect on the IP3R.
Pharmacological and physiological implications
FK506 is an immunosuppressant widely used in primary and rescue therapy for various organ transplantations (e.g. liver, kidney, heart, lung, pancreas and intestine; Spencer et al. 1997). However, treatment of patients with FK506 is often associated with side effects, e.g. renal, hepatic and metabolic toxicity (Spencer et al. 1997).
The concentrations needed for inhibition of calcineurin activity (Schreiber, 1991) and subsequent suppression of allograft rejection in organ transplants are in the nanomolar range (1-25 nM) (Venkataramanan et al. 1987). The concentrations required for affecting Ca2+ uptake activity are at least 1000-fold higher than the concentrations needed for immunosuppression. Nevertheless, due to the lipophilic structure of FK506 (Tanaka et al. 1987) and its repetitive application during therapy in allograft patients (Spencer et al. 1997), it may accumulate in cellular membranes. It was reported that FK506 has the capacity to accumulate in T lymphocytes and concentrate up to 900-fold the extracellularly added concentration (Dumont et al. 1994). This intracellular accumulated FK506 can lead to a reduction in Ca2+ accumulation in the ER of cells and subsequently to dysfunction.
For instance, a hypertrophic cardiomyopathy was associated with application of FK506 in paediatric patients (Atkison et al. 1995). Another report about paediatric liver transplantation demonstrated heart failure during application of FK506, which was resolved when treatment with FK506 was discontinued (Whitington et al. 1994). It is interesting to note that animal models, with dilated cardiomyopathy, had a reduced Ca2+ uptake and binding activity of the SR (Edes et al. 1991) and humans with idiopathic dilated cardiomyopathy had a decreased SR Ca2+-uptake rate (Limas et al. 1987) and Ca2+-Mg2+-ATPase activity.
In conclusion, our findings demonstrate that the immunosuppressant FK506 reduced Ca2+ uptake in the endoplasmic Ca2+ stores via an FKBP12-independent inhibition of the SERCA pump. Furthermore, FK506 did not affect Ca2+ flux through the IP3R in permeabilized SH-SY5Y and A7r5 cells. On the other hand, FK506 potentiated caffeine-induced Ca2+ release through the RyR in permeabilized differentiated BC3H1 cells. Our findings may explain some side effects of FK506 during treatment of allograft transplant patients.
Acknowledgments
We thank A. Florizoone, M. Crabbé, H. Van Weijenbergh, L. Bauwens, Y. Parijs, I. Willems and L. Heremans for their skilful technical assistance. We thank Dr N. Blanckaert, G. Michiels and G. Wuyts for the use of the Perkin Elmer LS50 fluorimeter. We thank Dr A. Friedrich (Fujisawa GmbH, München, Germany) for kindly providing us with FK506. DT40 and R23-11 cells were a generous gift from Dr T. Kurosaki (Tokyo, Japan). We thank Dr G. Droogmans for the CaBuf software and Dr F. Wuytack for stimulating discussions. G.B. has a pre-doctoral fellowship of the ‘Vlaams Instituut voor de Bevordering van het Wetenschappelijk-Technologisch Onderzoek in de Industrie (I.W.T.)‘. P.D.S. is a Research Assistant and J.B.P. is a Research Associate of the Foundation for Scientific Research – Flanders (F.W.O.). A.F.W. has a postdoctoral fellowship of the ‘Onderzoekfonds’ (K.U.Leuven). This work was supported by grants 3.0207.99 of the F.W.O., by grant P4/23 of the Program on Interuniversity Poles of Attraction and by grant 99/08 of the Concerted Actions of the K.U.Leuven.
References
- Ahern GP, Junankar PR, Dulhunty AF. Single channel activity of the ryanodine receptor calcium release channel is modulated by FK-506. FEBS Letters. 1994;352:369–374. doi: 10.1016/0014-5793(94)01001-3. [DOI] [PubMed] [Google Scholar]
- Ahern GP, Junankar PR, Pace SM, Curtis S, Mould JA, Dulhunty AF. Effects of ivermectin and midecamycin on ryanodine receptors and the Ca2+-ATPase in sarcoplasmic reticulum of rabbit and rat skeletal muscle. The Journal of Physiology. 1999;514:313–326. doi: 10.1111/j.1469-7793.1999.313ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkison P, Joubert G, Barron A, Grant D, Paradis K, Seidman E, Wall W, Rosenberg H, Howard J, Williams S, Stiller C. Hypertrophic cardiomyopathy associated with tacrolimus in paediatric transplant patients. Lancet. 1995;345:894–896. doi: 10.1016/s0140-6736(95)90011-x. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Sharma RV, Whiteis C, Yedidag E, Abboud FM. Tacrolimus (FK506) modulates calcium release and contractility of intestinal smooth muscle. Cell Calcium. 1997;22:507–514. doi: 10.1016/s0143-4160(97)90078-6. [DOI] [PubMed] [Google Scholar]
- Brillantes AB, Ondrias K, Scott A, Kobrinsky E, Ondriasová E, Moschella MC, Jayaraman T, Landers M, Ehrlich BE, Marks AR. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Cameron AM, Steiner JP, Roskams AJ, Ali SM, Ronnett GV, Snyder SH. Calcineurin associated with the inositol 1,4,5-trisphosphate receptor-FKBP12 complex modulates Ca2+ flux. Cell. 1995a;83:463–472. doi: 10.1016/0092-8674(95)90124-8. [DOI] [PubMed] [Google Scholar]
- Cameron AM, Steiner JP, Sabatini DM, Kaplin AI, Walensky LD, Snyder SH. Immunophilin FK506 binding protein associated with inositol 1,4,5-trisphosphate receptor modulates calcium flux. Proceedings of the National Academy of Sciences of the USA. 1995b;92:1784–1788. doi: 10.1073/pnas.92.5.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Zhang L, MacLennan DH. Asymmetrical blockade of the Ca2+ release channel (ryanodine receptor) by 12-kDa FK506 binding protein. Proceedings of the National Academy of Sciences of the USA. 1994;91:11953–11957. doi: 10.1073/pnas.91.25.11953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt H, Parys JB, Himpens B, Missiaen L, Borghgraef R. Changes in the mechanism of Ca2+ mobilization during the differentiation of BC3H1 muscle cells. Biochemical Journal. 1991;273:219–223. doi: 10.1042/bj2730219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. The Journal of Physiology. 1999;516:1–14. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont FJ, Kastner C, Iacovone FJ, Fischer PA. Quantitative and temporal analysis of the cellular interaction of FK-506 and rapamycin in T-lymphocytes. Journal of Pharmacology and Experimental Therapeutics. 1994;268:32–41. [PubMed] [Google Scholar]
- Edes I, Talosi L, Kranias EG. Sarcoplasmic reticulum function in normal heart and in cardiac disease. Heart Failure. 1991;6:221–237. [Google Scholar]
- Genazzani AA, Carafoli E, Guerini D. Calcineurin controls inositol 1,4,5-trisphosphate type 1 receptor expression in neurons. Proceedings of the National Academy of Sciences of the USA. 1999;96:5797–5801. doi: 10.1073/pnas.96.10.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Heirwegh KP, De Smedt H, Vermeir M. Analysis of membrane-bound acceptors. A correction function for non-specific accumulation of poorly water-soluble hydrophobic or amphipathic ligands based on the ligand partition concept. Biochemical Pharmacology. 1992;43:701–704. doi: 10.1016/0006-2952(92)90233-9. [DOI] [PubMed] [Google Scholar]
- Jayaraman T, Brillantes AM, Timerman AP, Fleischer S, Erdjument Bromage H, Tempst P, Marks AR. FK506 binding protein associated with the calcium release channel (ryanodine receptor) Journal of Biological Chemistry. 1992;267:9474–9477. [PubMed] [Google Scholar]
- Ji Y, Loukianov E, Periasamy M. Analysis of sarcoplasmic reticulum Ca2+ transport and Ca2+ ATPase enzymatic properties using mouse cardiac tissue homogenates. Analytical Biochemistry. 1999;269:236–244. doi: 10.1006/abio.1999.4059. [DOI] [PubMed] [Google Scholar]
- Kanoh S, Kondo M, Tamaoki J, Shirakawa H, Aoshiba K, Miyazaki S, Kobayashi H, Nagata N, Nagai A. Effect of FK506 on ATP-induced intracellular calcium oscillations in cow tracheal epithelium. American Journal of Physiology. 1999;276:L891–899. doi: 10.1152/ajplung.1999.276.6.L891. [DOI] [PubMed] [Google Scholar]
- Kay JE. Structure-function relationships in the FK506-binding protein (FKBP) family of peptidylprolyl cis-trans isomerases. Biochemical Journal. 1996;314:361–385. [PMC free article] [PubMed] [Google Scholar]
- Kirchberger MA, Antonetz T. Calmodulin-mediated regulation of calcium transport and (Ca2++ Mg2+)-activated ATPase activity in isolated cardiac sarcoplasmic reticulum. Journal of Biological Chemistry. 1982;257:5685–5691. [PubMed] [Google Scholar]
- Lenzen S, Münster W, Rustenbeck I. Dual effect of spermine on mitochondrial Ca2+ transport. Biochemical Journal. 1992;286:597–602. doi: 10.1042/bj2860597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limas CJ, Olivari MT, Goldenberg IF, Levine TB, Benditt DG, Simon A. Calcium uptake by cardiac sarcoplasmic reticulum in human dilated cardiomyopathy. Cardiovascular Research. 1987;21:601–605. doi: 10.1093/cvr/21.8.601. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Marks AR. Cellular functions of immunophilins. Physiological Reviews. 1996;76:631–649. doi: 10.1152/physrev.1996.76.3.631. [DOI] [PubMed] [Google Scholar]
- Marx SO, Ondrias K, Marks AR. Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors) Science. 1998;281:818–821. doi: 10.1126/science.281.5378.818. [DOI] [PubMed] [Google Scholar]
- Missiaen L, De Smedt H, Droogmans G, Casteels R. Ca2+ release induced by inositol 1,4,5-trisphosphate is a steady-state phenomenon controlled by luminal Ca2+ in permeabilized cells. Nature. 1992a;357:599–602. doi: 10.1038/357599a0. [DOI] [PubMed] [Google Scholar]
- Missiaen L, Taylor CW, Berridge MJ. Spontaneous calcium release from inositol trisphosphate-sensitive calcium stores. Nature. 1991;352:241–244. doi: 10.1038/352241a0. [DOI] [PubMed] [Google Scholar]
- Missiaen L, Taylor CW, Berridge MJ. Luminal Ca2+ promoting spontaneous Ca2+ release from inositol trisphosphate-sensitive stores in rat hepatocytes. The Journal of Physiology. 1992b;455:623–640. doi: 10.1113/jphysiol.1992.sp019319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO Journal. 1999;18:1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschol M, Dasgupta BR, Salzberg BM. Caffeine interaction with fluorescent calcium indicator dyes. Biophysical Journal. 1999;77:577–586. doi: 10.1016/S0006-3495(99)76914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onuma H, Lu YF, Tomizawa K, Moriwaki A, Tokuda M, Hatase O, Matsui H. A calcineurin inhibitor, FK506, blocks voltage-gated calcium channel-dependent LTP in the hippocampus. Neuroscience Research. 1998;30:313–319. doi: 10.1016/s0168-0102(98)00012-1. [DOI] [PubMed] [Google Scholar]
- Parys JB, De Smedt H, Missiaen L, Bootman MD, Sienaert I, Casteels R. Rat basophilic leukemia cells as model system for inositol 1,4,5-trisphosphate receptor IV, a receptor of the type II family: functional comparison and immunological detection. Cell Calcium. 1995;17:239–249. doi: 10.1016/0143-4160(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Patel S, Joseph SK, Thomas AP. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium. 1999;25:247–264. doi: 10.1054/ceca.1999.0021. [DOI] [PubMed] [Google Scholar]
- Qi Y, Ogunbunmi EM, Freund EA, Timerman AP, Fleischer S. FK-binding protein is associated with the ryanodine receptor of skeletal muscle in vertebrate animals. Journal of Biological Chemistry. 1998;273:34813–34819. doi: 10.1074/jbc.273.52.34813. [DOI] [PubMed] [Google Scholar]
- Schreiber SL. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Lai MM, Burnett PE. Immunophilins in the nervous system. Neuron. 1998;21:283–294. doi: 10.1016/s0896-6273(00)80538-3. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Goa KL, Gillis JC. Tacrolimus. An update of its pharmacology and clinical efficacy in the management of organ transplantation. Drugs. 1997;54:925–975. doi: 10.2165/00003495-199754060-00009. [DOI] [PubMed] [Google Scholar]
- Steiner JP, Dawson TM, Fotuhi M, Glatt CE, Snowman AM, Cohen N, Snyder SH. High brain densities of the immunophilin FKBP colocalized with calcineurin. Nature. 1992;358:584–587. doi: 10.1038/358584a0. [DOI] [PubMed] [Google Scholar]
- Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO Journal. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Kuroda A, Marusawa H, Hashimoto M, Hatanaka H, Kino T, Goto T, Okuhara M. Physicochemical properties of FK-506, a novel immunosuppressant isolated from Streptomyces tsukubaensis. Transplantation Proceedings. 1987;19:11–16. [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drøbak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promotor, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proceedings of the National Academy of Sciences of the USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timerman AP, Ogunbumni E, Freund E, Wiederrecht G, Marks AR, Fleischer S. The calcium release channel of sarcoplasmic reticulum is modulated by FK-506-binding protein. Dissociation and reconstitution of FKBP-12 to the calcium release channel of skeletal muscle sarcoplasmic reticulum. Journal of Biological Chemistry. 1993;268:22992–22999. [PubMed] [Google Scholar]
- Valdivia HH. Modulation of intracellular Ca2+ levels in the heart by sorcin and FKBP12, two accessory proteins of ryanodine receptors. Trends in Pharmacological Sciences. 1998;19:479–482. doi: 10.1016/s0165-6147(98)01269-3. [DOI] [PubMed] [Google Scholar]
- Vanlingen S, Parys JB, Missiaen L, De Smedt H, Wuytack F, Casteels R. Distribution of inositol 1,4,5-trisphosphate receptor isoforms, SERCA isoforms and Ca2+ binding proteins in RBL-2H3 rat basophilic leukemia cells. Cell Calcium. 1997;22:475–486. doi: 10.1016/s0143-4160(97)90075-0. [DOI] [PubMed] [Google Scholar]
- Venkataramanan R, Warty VS, Zemaitis MA, Sanghvi AT, Burckart GJ, Seltman H, Todo S, Makowka L, Starzl TE. Biopharmaceutical aspects of FK-506. Transplantation Proceedings. 1987;19:30–35. [PMC free article] [PubMed] [Google Scholar]
- Whitington PF, Alonso EM, Piper JB. Pediatric liver transplantation. Seminars in Liver Disease. 1994;14:303–317. doi: 10.1055/s-2007-1007320. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz RJ. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to downregulation and are expressed in markedly different proportions in different cell types. Journal of Biological Chemistry. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Valdivia HH, Bogdanov K, Valdivia C, Lakatta EG, Cheng H. The immunophilin FK506-binding protein modulates Ca2+ release channel closure in rat heart. The Journal of Physiology. 1997;500:343–354. doi: 10.1113/jphysiol.1997.sp022025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin HB, Timerman AP, Onoue H, Wiederrecht GJ, Fleischer S. Affinity purification of the ryanodine receptor/calcium release channel from fast twitch skeletal muscle based on its tight association with FKBP12. Biochemical and Biophysical Research Communications. 1995;214:263–270. doi: 10.1006/bbrc.1995.2283. [DOI] [PubMed] [Google Scholar]
- Yasutsune T, Kawakami N, Hirano K, Nishimura J, Yasui H, Kitamura K, Kanaide H. Vasorelaxation and inhibition of voltage-operated Ca2+ channels by FK506 in the porcine coronary artery. British Journal of Pharmacology. 1999;126:717–729. doi: 10.1038/sj.bjp.0702339. [DOI] [PMC free article] [PubMed] [Google Scholar]


