Abstract
Aquaporin (AQP) water channels provide a major pathway for osmotically driven water movement across epithelial and microvascular barriers in the lung. We used mice deficient in each of the three principal lung aquaporins, AQP1, AQP4 and AQP5, to test the hypothesis that aquaporins are important in neonatal lung fluid balance, adult lung fluid clearance and formation of lung oedema after acute lung injury.
Wet-to-dry weight ratios (W/D) in lungs from wild-type mice decreased from 7.9 to 5.7 over the first hour after spontaneous delivery. AQP deletion did not significantly affect W/D at 45 min after birth.
Alveolar fluid clearance was measured in living ventilated mice in which 0.5 ml saline containing radiolabelled albumin was instilled into the airspaces. Fluid clearance was 17.4% in 15 min and inhibited >90% by amiloride, but clearance was not affected by AQP deletion.
W/D was measured in established models of acute lung injury – acid aspiration and thiourea administration. Two hours after intratracheal administration of HCl, W/D increased from 3.7 to 7.5 but was not affected by AQP deletion. Three hours after intraperitoneal infusion of thiourea, W/D increased to 5.5 and marked pleural effusions appeared, but there were no differences in wild-type and AQP knockout mice.
Hyperoxic subacute lung injury was induced by 95% oxygen. Neither mean survival (143 h) nor W/D at 65 h (5.1) were significantly affected by AQP deletion.
Despite their role in osmotically driven lung water transport, aquaporins are not required for the physiological clearance of lung water in the neonatal or adult lung, or for the accumulation of extravascular lung water in the injured lung.
Water transport occurs during rapid fluid absorption in the perinatal lung, airspace hydration in the adult lung, and fluid accumulation/absorption in response to lung injury. In normal lung, water permeabilities are exceptionally high in alveolar epithelial and microvascular endothelial barriers (Folkesson et al. 1994; Carter et al. 1996, 1998) and in isolated type I alveolar epithelial cells (Dobbs et al. 1998). Water permeability is moderately high across the epithelial barrier in large and small airways (Folkesson et al. 1996; Farinas et al. 1997). Airspace-capillary water permeability increases near the time of birth (Carter et al. 1997).
It has been proposed that aquaporin-type water channels may be important in physiological and pathophysiological processes in the lung. Three aquaporins have been localized in lung: AQP1 in microvascular endothelia and some pneumocytes (Nielsen et al. 1993; Folkesson et al. 1994; Schnitzer & Oh, 1996; King et al. 1997; Effros et al. 1997), AQP4 at the basolateral membrane of airway epithelium (Frigeri et al. 1995; Nielsen et al. 1997), and AQP5 at the apical membrane of type I alveolar epithelial cells (Nielsen et al. 1997; Funaki et al. 1998). The expression of AQP1 and AQP4 are strongly upregulated near the time of birth, whereas AQP5 expression increases slowly during the first week of life (Umenishi et al. 1996; Yasui et al. 1997). Recently, decreased AQP1 and AQP5 expression were found after acute viral infection in lung (Towne et al. 2000), suggesting a role for aquaporins in lung fluid accumulation. Each of the lung aquaporins has been deleted in mice by targeted gene disruption. In lung, deletion of AQP1 produced a 10-fold decrease in osmotically driven water transport between the airspace and capillary compartments, and an even greater decrease in transcapillary water permeability (Bai et al. 1999). AQP1 deletion produced a small decrease in transcapillary water movement in response to hydrostatic pressure differences, but did not affect active near-isosmolar alveolar fluid reabsorption. In AQP1-deficient mice, AQP4 deletion further decreased airspace-capillary water permeability (Song et al. 2000), indicating that the small airways offer a small contribution to net lung water permeability. AQP5 deletion produced a 10-fold decrease in airspace-capillary water permeability, but had no effect on hydrostatically driven lung fluid accumulation or active alveolar fluid absorption (Ma et al. 2000). Water transport in mice lacking AQP1 and AQP5 was reduced nearly 30-fold (Ma et al. 2000). It was concluded from these results that aquaporins provide a major route for osmotically driven water transport across epithelial and endothelial barriers in lung.
The purpose of this study was to test the hypothesis that the three principal lung aquaporins play a role in several important physiological processes: fluid absorption in the neonatal lung, alveolar fluid clearance in the in vivo adult lung, and lung oedema in response to a lung injury. Studies of fluid accumulation in the neonatal lung required harvesting of lungs (from heterozygote breedings) at specified times after spontaneous delivery. For studies in the adult lung, experiments were done on wild-type and knockout (null) mice deficient in each of the lung aquaporins in which the investigator was blinded to genotype information until completion of the analysis. The principal finding was that despite their proven role in osmotically driven lung water transport, the three major lung aquaporins do not appear to have an important role in physiological lung fluid balance.
METHODS
Transgenic mice
Transgenic knockout mice deficient in AQP1, AQP4 and AQP5 proteins were generated by targeted gene disruption as described previously (Ma et al. 1997, 1998, 1999). Litter-matched mice aged 8–10 weeks with a CD1 genetic background were used. The mice had free access to water and food and were kept in a standard air-filtered ventilated animal house with 12 h light cycles. A total of 19 pregnant mice (and 113 pups therefrom), 64 wild-type mice, 35 AQP1 null mice, 35 AQP4 null mice, 35 AQP5 null mice and 6 AQP1-AQP5 double knockout mice were used. In all studies the investigator was blinded to mouse genotype until completion of the data analysis. All protocols were approved by the University of California, San Francisco Committee on Animal Research.
Lung fluid clearance in neonatal mice
Timed-pregnant mice were obtained from breeding of pairs of aquaporin heterozygous mice. From 1 day before the expected delivery, pregnant mice were observed every 15–30 min by one of the investigators. Only pups from observed spontaneous deliveries (50-60 % of deliveries) were used. The mice usually delivered in the early morning hours and generally the duration of spontaneous delivery was ∼90 min, with 4–10 min between pups. The exact time of delivery of each pup was recorded. After the mother cleaned the pup, it was placed in a marked aluminum dish on soft cotton. At a specified time the pup was weighed and killed by decapitation. The chest was opened via a mid-line incision, the lungs were carefully dissected from large airways, heart and mediastinal structures, and excess fluid was absorbed with soft tissue paper. A segment of tail was removed for genotype analysis. The lungs were immediately weighed in pre-weighed aluminum dishes (wet weight), and again after 72 h at 70°C in a drying oven (dry weight) for computation of the wet-to-dry weight ratio (W/D).
Alveolar fluid clearance in ventilated lungs of adult mice
Mice were anaesthetized with pentobarbital (50 mg kg−1i.p.). The trachea was cannulated through a tracheotomy and lungs were mechanically ventilated using a mouse ventilator (Harvard Apparatus, Holliston, MA, USA) (inspired O2 fraction 1.0, tidal volume 9–10 ml kg−1, respiratory rate 90 breaths min−1, peak airway pressure 8–12 cmH2O, positive end-expiratory pressure 2–3 cmH2O). Body temperature was kept constant at 37–38°C with an infrared lamp. After 20 min, the lungs were infused via the trachea with 10–13 ml (kg body weight)−1 Ringer lactate solution (340 mosmol kg−1 (to match mouse serum osmolality), 5 % bovine albumin) containing 0.1 μCi 131I-labelled albumin. In some experiments, isoproterenol (10−3 M) or amiloride (10−3 M) was added to the instillate solutions. Fluid samples were withdrawn 15 min after airspace infusion. Blood samples were obtained by cardiac puncture and lungs were removed through a sternotomy. Alveolar fluid samples were assayed for total protein concentration. Alveolar fluid clearance was computed from radioactivities and protein concentrations as described (Bai et al. 1999; Fukuda et al. 2000). Extravascular lung water was calculated from wet and dry weights of blood and lung homogenates as described by Jayr et al. (1994).
Acid aspiration lung injury
An established acid aspiration model was used (Folkesson et al. 1995, 1997). Adult mice were anaesthetized using inhaled isoflurane (Rezaiguia-Delclaux et al. 1998). In the supine position, a mid-line incision was made in the skin of the neck, and the trachea was exposed. A 25-gauge feeding catheter was passed via the mouth into the trachea just above the carina. HCl (0.063 M) in 1/3 normal saline (pH 1.2, 4 ml kg−1) was rapidly instilled during a spontaneous inspiration. Control mice were instilled with 1/3 normal saline without added HCl. The mouse was moved around and rotated to ensure that the instillate was distributed throughout the lungs. The skin was closed with 3–0 silk suture and the mouse was returned to the cage with free access to food and water. There was little evidence of increased respiratory effort. No mouse died or was killed because of overt respiratory distress. After 2 h, mice were killed with an overdose of pentobarbital (150 mg kg−1i.p.), a mid-line abdominal incision was made, and the descending aorta was incised for blood collection. The chest was opened by a median sternotomy, and the lungs were isolated and placed in a pre-weighed container. After addition of 1 ml distilled water, lungs were homogenized for determination of W/D and haemoglobin concentration (see details below).
Hyperoxic lung injury
Mice were kept in a large sealed Lucite chamber with constant inflow of 100 % oxygen to maintain a > 95 % oxygen content (measured by oxygen sensor probe, Teledyne Analytical Instruments); the continuous flow flushed away expired carbon dioxide. The mouse had free access to food and water with 12 h light-dark cycles. In one set of studies, mouse survival was recorded. Mice were observed every 6 h or less, and dead mice were removed from the chamber. In a separate set of studies, mice were removed from the chamber at 65 h, and blood samples and lungs were harvested as described above. Mice were killed by an overdose of pentobarbital (150 mg kg−1i.p.) at the end of the study.
Thiourea-induced lung injury
An established thiourea lung injury model was used (Mais & Bosin, 1984). Mice were infused intraperitoneally with thiourea (Sigma) dissolved in saline (5 mg ml−1) at a dose of 10 mg kg−1. Control mice were infused with saline which did not contain thiourea. Mice were returned to a clean cage and given free access to food and water. After 3 h, mice were killed with an overdose of pentobarbital (150 mg kg−1i.p.) and the abdominal cavity was opened for blood collection from the aorta. The pleural cavities were carefully opened for aspiration of pleural fluid into pre-weighed vials. Lungs were isolated and homogenized as described above.
Measurement of lung wet-to-dry ratios
In lung injury models, blood samples (1-1.2 ml) were assayed for haemoglobin concentration and wet/dry weight ratios. Lungs were weighed, homogenized (after addition of 1 ml distilled water), and the homogenate was weighed. A portion of the homogenate (∼0.6 ml) was centrifuged (16000g, 8 min) for assay of haemoglobin concentration in the supernatant. The remainder of the lung homogenate was dessicated in an oven (70°C for 24 h) for determination of dry weight. Lung wet-to-dry weight ratio (W/D) was computed from lung wet and dry weights, lung and blood haemoglobin concentrations, and blood wet and dry weights by standard procedures (Jayr et al. 1994).
RESULTS
Lung fluid clearance in the neonatal mice
As a quantitative measure of fluid clearance in neonatal lungs, wet-to-dry weight ratios (W/D) were measured in lungs removed from pups killed at specified times after spontaneous delivery. The time course of W/D for a series of wild-type mice was first established. As summarized in Fig. 1A, W/D values decreased sharply over the first hour after delivery, and continued to decline during the first 12 h. Further analysis showed no systematic difference in W/D ratios in pups from different mothers, or in pups delivered at the beginning versus the end of labour (not shown). There was no observed mortality between the time of birth and the time of killing. We therefore chose to make subsequent comparisons in pups from breeding of AQP heterozygous mice 45 min after birth. It was reasoned that inhibition of fluid clearance would be apparent as an increase in W/D at 45 min; in addition, uncertainties in recording the exact time of delivery are minimal at 45 min, but could be substantial at earlier times.
Figure 1. Lung fluid clearance in neonatal mice.
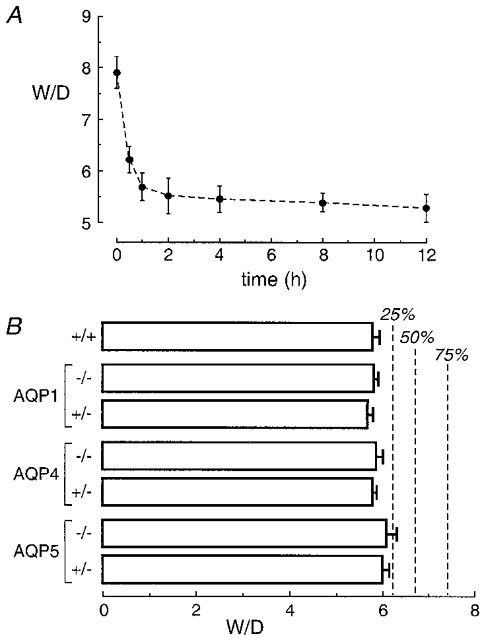
A, wet-to-dry weight ratios (W/D) of lungs removed from pups at indicated times after spontaneous delivery in wild-type mice. Each time point is the mean and s.e.m. for data from 6 pups. B, W/D of lungs 45 min after spontaneous delivery in pups from breeding of AQP heterozygous mice. Values are means and s.e.m. for data from 5–10 wild-type and AQP null pups and 6–16 AQP heterozygous pups. Dashed lines indicate predicted W/D values for the indicated percentages of inhibition of fluid clearance, assuming an exponential decrease in W/D. Differences in W/D not significant (ANOVA, P= 0.82 between each group).
Mean total body weight for pups was not affected by AQP deletion: 1.48 ± 0.04 g (wild-type), 1.52 ± 0.04 g (AQP1 knockout), 1.50 ± 0.03 g (AQP4) and 1.48 ± 0.09 g (AQP5) (P= 0.90 between groups, ANOVA). The pups were not distinguishable in terms of appearance or activity level. Figure 1B shows that W/D values did not differ significantly in wild-type versus AQP heterozygous and knockout pups. For comparison, based on exponential regression of the data in Fig. 1A, predicted W/D values are provided for 25, 50 and 75 % inhibition of fluid clearance (vertical dashed lines). These results show that AQP deletion does not affect physiological alveolar fluid clearance in neonatal mice.
Lung fluid clearance in ventilated adult mice
Previous measurements of alveolar fluid clearance in mice were done in isolated lungs ex vivo or in in situ perfused lungs in dead mice. In the present experiments, alveolar fluid clearance was measured in living ventilated mice. Lungs were instilled with 10–13 mg kg−1 of an isosmolar solution, a volume that was tolerated well by ventilated mice. After 15 min, a small airspace fluid sample was removed for determination of alveolar fluid clearance and the lungs were harvested for determination of extravascular lung water content. As summarized in Fig. 2A, alveolar fluid clearance was 11.6 % in control wild-type mice, inhibited by 90 % by the apical Na+ channel blocker amiloride, and increased by 51 % by the β-agonist isoproterenol. These findings are in general agreement with data from the isolated and in situ perfused lung models in mice (Garat et al. 1998; Bai et al. 1999; Ma et al. 2000).
Figure 2. Alveolar fluid clearance in adult ventilated mice.
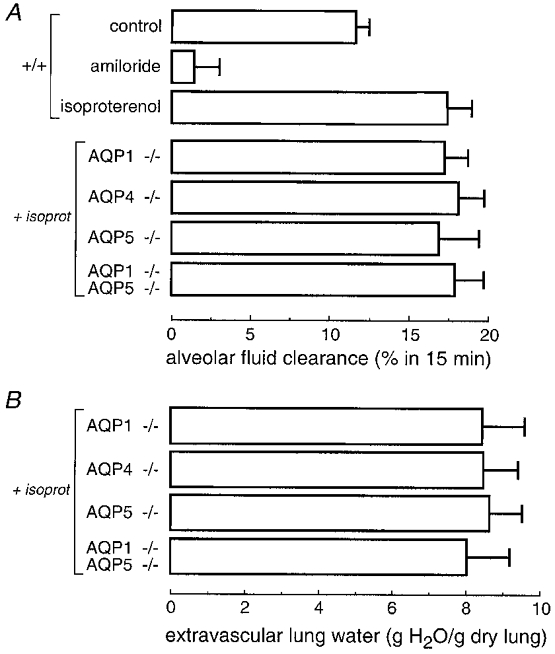
A, alveolar fluid clearance in mice of indicated genotype measured 15 min after instillation of an isosmolar solution containing a membrane-impermeant volume marker (see Methods). Where indicated, instillate solutions contained isoproterenol (10−3 M) or amiloride (10−3 M). Differences among genotypes in the isoproterenol (isoprot) group were not significant (P > 0.1 ANOVA). B, extravascular lung water measured at 15 min after fluid instillation. Differences not significant (P > 0.1 ANOVA).
Alveolar fluid clearance was compared in wild-type and AQP knockout mice with isoproterenol stimulation in order to maximize fluid clearance and to avoid uncertainties in endogenous catacholamine levels. Figure 2A shows no significant effect of deletion of AQP1, AQP4 and AQP5 on alveolar fluid clearance. Deletion of AQP1 and AQP5 were found to individually inhibit airspace-capillary osmotic water permeability approximately 10-fold (Bai et al. 1999; Ma et al. 2000). In addition, alveolar fluid clearance was not affected by deletion of AQP1 and AQP5 together, a manouevre which decreased airspace-capillary water permeability 30-fold. Fluid from the alveolar compartment is cleared by active transport across the alveolar epithelium into the interstitium, followed by transport into the microvasculature and drainage into lymphatics. To estimate lung interstitial water content in the mice studied here, extravascular lung water was determined as described in Methods. Figure 2B shows that total extravascular lung was not significantly affected by AQP deletion. Together these results provide evidence against a role of lung aquaporins in alveolar fluid clearance.
Lung fluid accumulation in an acid aspiration model of lung injury
Acid aspiration is a clinically relevant model of acute lung epithelial injury. Intratracheal instillation of fluid (acid-containing or control) in anaesthetized mice often produced transient apnoea followed by wheezing; however, ∼90 % of the mice recovered from anaesthesia and were able to eat and drink with little or no apparent distress. Mice were killed 2 h after acid/saline instillation. Lungs from most acid-treated mice had a mildly bloody appearance, which was never seen in saline-treated mice. Figure 3 shows significantly increased W/D values in acid-treated mice. AQP deletion did not affect lung water accumulation. Blood haemoglobin concentrations measured at the time of death were not significantly different (12.8 ± 1.6 g dl−1 (wild-type), 13.2 ± 1.3 g dl−1 (AQP1 knockout), 11.9 ± 1.7 g dl−1 (AQP4), 12.6 ± 0.7 g dl−1 (AQP5)), suggesting that altered hydration resulting from renal abnormalities (e.g. nephrogenic diabetes insipidus in AQP1 null mice; Ma et al. 1998) was not a factor in lung fluid accumulation.
Figure 3. Increased lung water 2 h after acid aspiration.
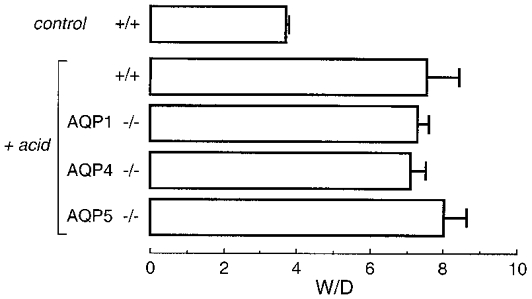
Anaesthetized mice underwent tracheotomy and instillation of 4 ml kg−1 of either 1/3 normal saline (control mice) or pH 1.2 HCl (see Methods). Lungs were harvested after 2 h for determination of W/D. Data are means and s.e.m. for 5–7 mice per genotype. Differences among genotypes in acid-treated mice were not significant (P > 0.05 ANOVA); the difference between saline- and acid-treated mice was significant (P < 0.01).
Lung fluid accumulation and survival in a model of hyperoxic lung injury
Hyperoxic lung injury was produced by placing mice in a normobaric chamber containing a 95 % oxygen atmosphere. Survival was taken as the endpoint in the first set of studies. Generally the mice showed normal activity during the first day, decreased activity on the second day, and then lethargy and mild difficulty breathing. The first mouse died at 84 h and the last at 174 h. Examination of lungs at autopsy showed a homogeneous haemorrhagic appearance. Figure 4A shows that mean survival time was 143 h in wild-type mice, and was not significantly affected by AQP deletion.
Figure 4. Survival and increased lung water after hyperoxic lung injury.
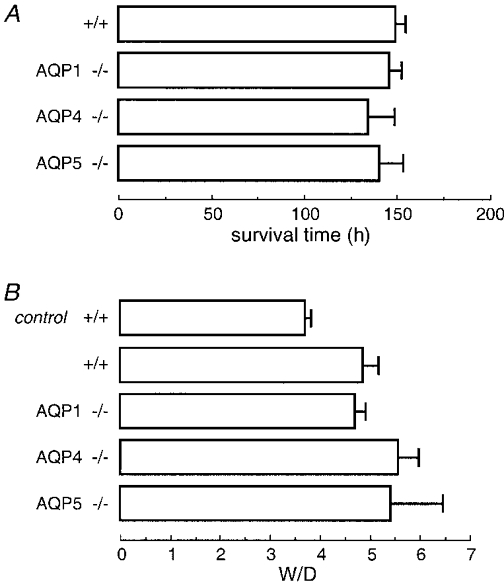
Mice were maintained on normal food and water in a 95 % oxygen atmosphere. A, mean survival time in mice of indicated genotype (6 mice per genotype). Differences were not significant (P > 0.05 ANOVA). B, in a separate set of mice, lungs were harvested after 65 h of hyperoxia for determination of W/D. W/D values are means and s.e.m. for 5–8 mice per genotype. Differences in W/D in lung from hyperoxia-exposed mice were not significant (P > 0.05 ANOVA).
In the second set of studies, mice were killed after 65 h of hyperoxia for determination of lung fluid accumulation. Figure 4B shows increased W/D values in mice exposed to hyperoxia, without significant effect of AQP deletion. Blood haemoglobin concentrations were similar in each group: (13.6 ± 0.6 g dl−1 (wild-type), 14.3 ± 1.1 g dl−1 (AQP1), 14.7 ± 1.4 g dl−1 (AQP4) and 16.2 ± 1.2 g dl−1 (AQP5)). The mildly greater haemoglobin concentrations in this study might be related to mild dehydration and/or increased erythropoesis. Aquaporins thus do not play a role in the pathophysiology of hyperoxic lung injury.
Lung fluid accumulation and pleural effusion in thiourea-induced lung injury
Intraperitoneal infusion of thiourea produces acute lung endothelial cell injury. Mouse activity and appearance were normal after thiourea infusion. After 3 h mice were killed. Their lungs appeared to be moderately damaged without haemorrhage, and there was a marked accumulation of non-bloody clear pleural fluid. Figure 5A shows that thiourea produced accumulation of lung water with W/D increasing to 5.3 in wild-type mice. However, there was no effect of AQP deletion on lung water accumulation. Blood haemoglobin concentrations were: 12.9 ± 0.4 g dl−1 (wild-type), 15.2 ± 1.0 g dl−1 (AQP1), 12.2 ± 0.6 g dl−1 (AQP4) and 13.4 ± 0.2 g dl−1 (AQP5). Figure 5B shows marked accumulation of pleural fluid in thiourea-treated mice, but with similar pleural fluid accumulation in wild-type and AQP knockout mice.
Figure 5. Increased lung water and development of pleural effusions in thiourea-induced lung injury.
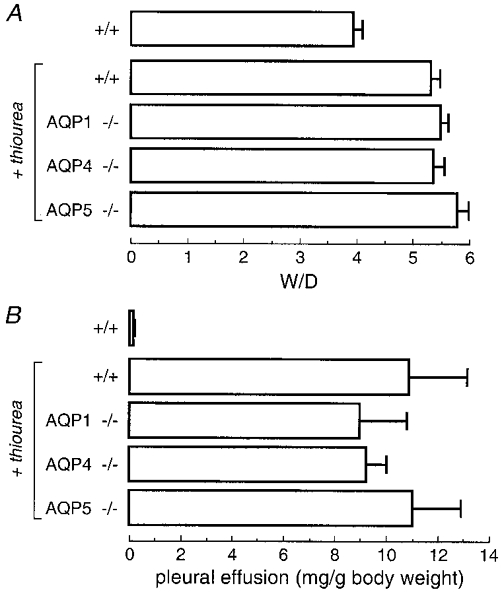
Lung injury was induced by intraperitoneal injection of 10 mg kg−1 thiourea. A, lungs were harvested 3 h after thiourea injection for determination of W/D. W/D values are means and s.e.m. for 5–10 mice per genotype. Differences between thiourea-treated mice were not significant (P= 0.2 ANOVA); the difference between control and thiourea-treated mice was significant (P < 0.02). Control mice were injected identically but without thiourea. B, amount of pleural fluid (mean and s.e.m.) in the same mice 3 h after thiourea injection. Differences in thiourea-treated mice were not significant (P= 0.85 ANOVA).
DISCUSSION
The principal conclusion of this study was that despite their demonstrated role in epithelial and endothelial osmotic water permeabilities, the major lung aquaporins do not appear to play an important role in active alveolar fluid clearance in the neonatal and adult lung, or in the accumulation of pulmonary oedema in various types of acute lung injury. This finding is in contrast to the proven role of aquaporins in kidney (Deen et al. 1994; Ma et al. 1998; Schnermann et al. 1998; Chou et al. 1999; Pallone et al. 2000), salivary gland (Ma et al. 1999), brain (Manley et al. 2000) and other organ systems. A role for aquaporins in lung physiology had been assumed based on the specific aquaporin expression pattern, the high water permeabilities at sites of aquaporin expression, the altered aquaporin expression after insults such as acute viral infection, and as discussed below, the correlation between developmental aquaporin expression and increased airspace-capillary water permeability. The negative results reported in these studies suggest that interventions to inhibit or increase water permeability in the lung would have little clinical utility.
In the prenatal lung, the distal airway epithelium actively secretes Cl− into the lumen which creates an osmotic gradient to drive water transport into the airspace compartment. The airspace fluid is isosmolar with plasma, but is relatively high in K+ and Cl−, and low in HCO3− and protein (Bland, 1991). Near the time of birth net fluid secretion decreases and the amount of luminal liquid decreases. During labour and the postnatal period the pulmonary epithelium becomes a predominantly Na+ absorbing membrane (Bland, 1990). Fluid absorption in neonatal lung has been divided into a rapid phase, within 60 min after birth, followed by a slower phase. We found that 50 % of the luminal fluid was absorbed within ∼25 min, with a fluid absorption rate substantially greater than that in adult mice. Finely et al. (1998) reported that alveolar fluid clearance in guinea-pig neonatal lung rapidly decreased from the time of birth and reached adult levels at postnatal day 5. The high alveolar clearance was mediated by increased plasma catecholamine concentrations after spontaneous delivery. Just before birth, there is upregulation of Na+,K+-ATPase (Folkesson et al. 1998). The ENaC Na+ channel plays an important role based on inhibition of fluid clearance in the postnatal lung by amiloride (O'Brodovich et al. 1990; Finely et al. 1998), and the early death of α-ENaC knockout mice (Hummler et al. 1996).
Aquaporin expression in lung is minimal before birth, but there is a sharp increase in AQP4 expression near the time of birth and a less dramatic increase in AQP1 expression (Umenishi et al. 1996; Yasui et al. 1997); AQP5 expression increases slowly over the first week of life. In rabbits, the increased lung aquaporin expression correlated with increased airspace-capillary osmotic water permeability (Carter et al. 1997). It was also found that glucocorticoid administration increased AQP1 expression in neonatal rats (King et al. 1996). However, despite these lines of indirect evidence suggesting a role for aquaporins in neonatal lung fluid clearance, we found no effect of AQP1, AQP4 or AQP5 deletion on lung water content in the first hour after birth, nor was pup survival over the first day of life impaired by aquaporin deletion. Thus, water transport in neonatal lung is not a rate-limiting step in the active transport of fluid out of the airspaces across the lung epithelium or for removal of water from the lung interstitium across the endothelium.
We reported previously using perfused and in situ lung models that aquaporin deletion does not impair alveolar fluid clearance in the adult lung (Bai et al. 1999; Ma et al. 2000). Fluid absorption across the alveolar epithelium involves active Na+ transport, which is energized by the basolateral membrane Na+,K+-ATPase and requires apical membrane Na+ channels (ENaC and non-ENaC proteins) and possibly Cl− transporters (Matalon & O'Brodovich, 1999). Water is osmotically driven from the airspace to interstitial compartments, where it is subsequently removed by transport into pulmonary microvessels and drainage into lymphatics (Berthiaume et al. 1989). Alveolar fluid clearance in the mouse has been shown to be very rapid, strongly inhibited by amiloride, and increased by β-agonists (Garat et al. 1998). In this study we used a ventilated adult mouse model to study in vivo alveolar fluid clearance. The ventilated mouse model preserves blood flow and normal pulmonary pressures. Alveolar fluid clearance in living mice was rapid, amiloride inhibitable, and isoproterenol stimulated. However, aquaporin deletion did not affect alveolar fluid clearance, even in AQP1-AQP5 double knockout mice where osmotically driven water permeability was decreased ∼30-fold compared with wild-type mice. Together our results indicate that in vivo alveolar fluid clearance in neonatal and adult mice does not require aquaporins.
Three established models of acute/subacute lung injury were used to test the hypothesis that the aquaporins play a role in lung fluid accumulation following injury. Acid aspiration is a common clinical cause of increased-permeability pulmonary oedema. The aspiration of acidic stomach fluid produces an inflammatory pneumonitis (Cassiere & Niederman, 1998; Matthay et al. 1996). Acid aspiration results in injury to the alveolar epithelium and marked inflammation with neutrophil recruitment and release of numerous mediators of inflammation (e.g. tumour necrosis factor and interleukin-1) and oxygen radicals (Weiser et al. 1997). In our study the aspiration of stomach contents was mimicked in using a hypo-osmotic, low pH solution as established previously (Folkesson et al. 1995, 1997). Lung water accumulation was assayed 2 h after acid instillation, a time selected based on previous work at which there was a substantial increase in W/D yet minimal animal distress. Control studies showed that instillation of non-acidic fluid did not result in increased lung water, whereas acid instillation caused a remarkable increased in W/D. However, aquaporin deletion did not affect the amount of lung water accumulation.
Hyperoxia is an important experimental model of subacute lung injury that has clinical relevance to the adult respiratory distress syndrome, where 100 % oxygen is sometimes required to prevent hypoxaemia. Sustained hyperoxia can produce endothelial and epithelial cell damage in the lung, with increased permeability, neutrophil recruitment, and release of oxygen free radicals, cytokines and other mediators of inflammation (Folz et al. 1999). Numerous hyperoxia-induced lung injury animal models have been described. In rats, hyperoxia produced an increase in lung wet-to-dry ratio from 5.1 to 6.3 after 60 h (Clerch & Massaro, 1993). In one study in mice, exposure to > 95 % oxygen for 5 days did not induce an increase in lung wet-to-dry weight ratio (Arkovitz et al. 1997); however, a substantial increase in lung water was reported in other studies (Folz et al. 1999). In our experiments, the oxygen concentration was maintained above 95 % at all times. Although there was considerable variability in the degree of hyperoxia-induced lung injury from mouse to mouse, on average there was no effect of aquaporin deletion on survival or lung fluid accumulation.
A third commonly used model of lung injury is that induced by α-naphthylthiourea (ANTU), which produces marked pulmonary oedema and pleural effusions resulting from increased permeability changes in the lung microvasculature (Latta, 1947; Pine et al. 1976). Morphological studies by light and electron microscopy indicated that the capillary endothelial cell is the primary cellular target of ANTU toxicity (Cunningham & Hurley, 1972; Meyrick et al. 1972). Two hours after thiourea injection in mice, lung wet-to-dry weight ratio increased, was maximal at 3–6 h, and then decreased slowly with generally full recovery by 24 h (Mais & Bosin, 1984). In our study, intraperitoneal thiourea infusion produced marked accumulation of lung water and formation of pleural effusions. However, aquaporin deletion neither affected the amount of lung water accumulation nor the size of the pleural effusions.
Together these studies provide evidence against an important role of lung aquaporins in the clearance of oedema fluid from the airspace in the neonatal and adult lung, and in the response of the adult lung to different forms of lung injury. Although aquaporins are responsible for high osmotic water permeabilities in lung, neither aquaporins nor high lung water permeabilities appear to be important in regulating lung fluid balance at the time of birth or after clinically relevant forms of experimental lung injury. The major difference between the lung and tissues in which aquaporins have been shown to be important, such as kidney and salivary gland, is that the absolute rates of fluid movement across epithelia and endothelia are substantially lower in the lung. Active fluid clearance involves water movement driven by osmotic gradients. Slow rates of fluid movement probably do not require high water permeability because of the increased time available for osmotic equilibration across epithelial or endothelial barriers. Because paracellular water permeability is probably substantially greater than transcellular water permeability in lung microvessels (Song et al. 2000), as is the case in renal microvessels (Pallone et al. 2000), it is unlikely that a microvascular endothelial water channel would be important in physiologically relevant types of water transport that do not involve osmotic gradients of small solutes. In addition, various non-osmotic types of fluid movement occur in lung such as hydrostatic filtration and lymphatic drainage. As reviewed recently (Verkman et al. 2000) hydrostatically driven lung oedema generally involves transient breakdown of the alveolar epithelial barrier. Although we believe that it is unlikely, our studies do not rule out the possibility that aquaporins might be important in other forms of lung injury not tested in these studies, or that aquaporins might be important in human but not rodent lung physiology. We reported recently that AQP1 does not facilitate carbon dioxide transport in the in vivo lung (Yang et al. 2000); however, there may be other as yet unidentified novel functions of aquaporins in lung. Finally, although we conclude that aquaporins are probably not important in the physiology and pathophysiology of fluid movement in peripheral lung, they may be important in the larger airways in airspace humidification, airway surface liquid fluid properties, and in the pathophysiology of diseases such as cystic fibrosis.
Acknowledgments
We thank Ms Liman Qian for breeding and genotype analysis of transgenic mice. This study was supported by NIH grants HL59198, HL51854, DK35124, HL60288 and DK43840, and grant R613 from the National Cystic Fibrosis Foundation.
References
- Arkovitz M, Garcia V, Szabo C, McConnell K, Bove K, Wispe J. Decreased pulmonary compliance is an early indicator of pulmonary oxygen injury. Journal of Surgical Research. 1997;67:193–198. doi: 10.1006/jsre.1996.4980. [DOI] [PubMed] [Google Scholar]
- Bai C, Fukuda N, Song YL, Ma T, Matthay MA, Verkman AS. Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice. Journal of Clinical Investigation. 1999;103:555–561. doi: 10.1172/JCI4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiaume Y, Albertine KH, Grady M, Fick G, Matthay MA. Protein clearance from the air spaces and lungs of unanesthetized sheep over 144 h. Journal of Applied Physiology. 1989;67:1887–1897. doi: 10.1152/jappl.1989.67.5.1887. [DOI] [PubMed] [Google Scholar]
- Bland RD. Lung epithelial ion transport and fluid movement during the perinatal period. American Journal of Physiology. 1990;259:L30–37. doi: 10.1152/ajplung.1990.259.2.L30. [DOI] [PubMed] [Google Scholar]
- Bland RD. Fetal lung liquid and its removal near birth. In: Crystal RG, West JB, Banes PJ, Cherniack NS, editors. The Lung Scientific Foundations. New York: Raven Press; 1991. pp. 1677–1685. [Google Scholar]
- Carter EP, Matthay MA, Farinas J, Verkman AS. Transalveolar osmotic and diffusional water permeability in intact mouse lung measured by a novel surface fluorescence method. Journal of General Physiology. 1996;108:133–142. doi: 10.1085/jgp.108.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter EP, Olveczky BP, Matthay MA, Verkman AS. High microvascular endothelial water permeability in mouse lung measured by a pleural surface fluorescence method. Biophysical Journal. 1998;74:2121–2128. doi: 10.1016/S0006-3495(98)77919-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter EP, Umenishi F, Matthay MA, Verkman AS. Developmental changes in alveolar water permeability in perinatal rabbit lung. Journal of Clinical Investigation. 1997;100:1071–1078. doi: 10.1172/JCI119617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassiere HA, Niederman MS. Aspiration pneumonia, lipid pneumonia, and lung abscess. In: Baum GL, Crapo JD, Celli BR, Karlinsky JB, editors. Textbook of Pulmonary Disease. 6. Vol. 1. Philadelphia, New York: Lippincott-Raven publishers; 1998. pp. 645–655. [Google Scholar]
- Chou CL, Knepper MA, Van Hoek AN, Brown D, Yang B, Ma T, Verkman AS. Reduced water permeability and altered ultrastructure in thin descending limb of Henle in aquaporin-1 null mice. Journal of Clinical Investigation. 1999;103:491–496. doi: 10.1172/JCI5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerch LB, Massaro D. Tolerance of rats to hyperoxia-lung antioxidant and enzyme gene expression. Journal of Clinical Investigation. 1993;91:499–508. doi: 10.1172/JCI116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AL, Hurley JV. Alpha-naphylthiourea-induced pulmonary edema in the rat: A topographical and electron microscopy study. Journal of Pathology. 1972;106:25–35. doi: 10.1002/path.1711060103. [DOI] [PubMed] [Google Scholar]
- Deen PM, Verdijk MA, Knoers NV, Wieringa B, Monnens LA, Van Os CH, Van Oost BA. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994;264:92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- Dobbs LG, Gonzalez R, Matthay MA, Carter EP, Allen L, Verkman AS. Highly water-permeable type I alveolar epithelial cells confer high water permeability between the airspace and vasculature in rat lung. Proceedings of the National Academy of Sciences of the USA. 1998;95:2991–2996. doi: 10.1073/pnas.95.6.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RM, Darin C, Jacobs ER, Rogers RA, Krenz G, Schneeberger EE. Water transport and distribution of aquaporin-1 in the pulmonary airspaces. Journal of Applied Physiology. 1997;83:1002–1016. doi: 10.1152/jappl.1997.83.3.1002. [DOI] [PubMed] [Google Scholar]
- Farinas J, Kneen M, Moore M, Verkman AS. Plasma membrane water permeability in cultured cells and epithelia measured by light microscopy with spatial filtering. Journal of General Physiology. 1997;110:283–296. doi: 10.1085/jgp.110.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finely N, Norlin A, Baines DL, Folkesson HG. Alveolar epithelial fluid clearance is mediated by endogenous catacholamines at birth in guinea pigs. Journal of Clinical Investigation. 1998;101:972–981. doi: 10.1172/JCI1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkesson HG, Matthay MA. Inhibition of CD18 or CD11b attenuates acute lung injury after acid instillation in rabbits. Journal of Applied Physiology. 1997;82:1743–1750. doi: 10.1152/jappl.1997.82.6.1743. [DOI] [PubMed] [Google Scholar]
- Folkesson H, Matthay MA, Frigeri A, Verkman AS. High transepithelial water permeability in microperfused distal airways: evidence for channel-mediated water transport. Journal of Clinical Investigation. 1996;97:664–671. doi: 10.1172/JCI118463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkesson HG, Matthay MA, Haebert CA, Broaddus VC. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. Journal of Clinical Investigation. 1995;96:107–116. doi: 10.1172/JCI118009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkesson HG, Matthay MA, Hasegawa H, Kheradmand F, Verkman AS. Transcellular water transport in lung alveolar epithelium through mercury-sensitive water channels. Proceedings of the National Academy of Sciences of the USA. 1994;91:4970–4974. doi: 10.1073/pnas.91.11.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkesson HG, Norlin A, Baines D. Salt and water transport across the alveolar epithelium in the developing lung: Correlations between function and recent molecular biology and advances. International Journal of Molecular Medicine. 1998;2:515–531. doi: 10.3892/ijmm.2.5.515. [DOI] [PubMed] [Google Scholar]
- Folz RJ, Abshamaa AM, Suliman HG. Extracellular superoxide dismutase in the airway of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. Journal of Clinical Investigation. 1999;103:1055–1066. doi: 10.1172/JCI3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigeri A, Gropper M, Turck CW, Verkman AS. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proceedings of the National Academy of Sciences of the USA. 1995;92:4328–4331. doi: 10.1073/pnas.92.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda N, Folkesson HG, Matthay MA. Relationship of interstitial fluid volume to alveolar fluid clearance in mice: ventilated versus in situ studies. Journal of Applied Physiology. 2000 doi: 10.1152/jappl.2000.89.2.672. in the Press. [DOI] [PubMed] [Google Scholar]
- Funaki H, Yamamoto T, Koyama Y, Kondo D, Yaoita E, Kawasaki K, Kobayashi H, Sawaguchi S, Abe H, Kihara I. Localization and expression of AQP5 in cornea, serous salivary glands and pulmonary epithelial cells. American Journal of Physiology. 1998;275:C1151–1157. doi: 10.1152/ajpcell.1998.275.4.C1151. [DOI] [PubMed] [Google Scholar]
- Garat C, Carter EP, Matthay MA. New in situ mouse model to quantify alveolar epithelial fluid clearance. Journal of Applied Physiology. 1998;84:1763–1767. doi: 10.1152/jappl.1998.84.5.1763. [DOI] [PubMed] [Google Scholar]
- Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal liquid clearance in αENaC-deficient mice. Nature Genetics. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- Jayr C, Garat C, Meignan M, Pittet JF, Zelter M, Matthay MA. Alveolar liquid and protein clearance in anesthetised ventilated rats. Journal of Applied Physiology. 1994;76:2636–2642. doi: 10.1152/jappl.1994.76.6.2636. [DOI] [PubMed] [Google Scholar]
- King LS, Nielsen S, Agre P. Aquaporin-1 water channel protein in lung: ontogeny, steroid-induced expression, and distribution in rat. Journal of Clinical Investigation. 1996;97:2183–2191. doi: 10.1172/JCI118659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Nielsen S, Agre P. Aquaporins in complex tissues. I. Developmental patterns in respiratory and glandular tissues of rat. American Journal of Physiology. 1997;273:C1541–1548. doi: 10.1152/ajpcell.1997.273.5.C1541. [DOI] [PubMed] [Google Scholar]
- Latta H. Pulmonary edema and pleural effusion produced by acute alpha-naphthylthiourea poisoning in rats and dogs. Johns Hopkins Medical Journal. 1947;80:181–197. [PubMed] [Google Scholar]
- Ma T, Fukuda N, Song YL, Matthay MA, Verkman AS. Lung fluid transport in aquaporin-5 knockout mice. Journal of Clinical Investigation. 2000;105:93–100. doi: 10.1172/JCI8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Song YL, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. Journal of Biological Chemistry. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Generation and phenotype of a transgenic knock-out mouse lacking the mercurial-insensitive water channel aquaporin-4. Journal of Clinical Investigation. 1997;100:957–962. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. Journal of Biological Chemistry. 1998;273:4296–4299. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- Mais DE, Bosin TR. A role for serotonin in α-naphthylthiourea-induced pulmonary edema. Toxicology and Applied Pharmacology. 1984;74:185–194. doi: 10.1016/0041-008x(84)90142-x. [DOI] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Filiz F, Bollen A, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema following acute water intoxication and ischemic stroke. Nature Medicine. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Matalon S, O'Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties and physiological significance. Annual Review of Physiology. 1999;61:627–661. doi: 10.1146/annurev.physiol.61.1.627. [DOI] [PubMed] [Google Scholar]
- Matthay MA. Acid aspiration induced lung injury. American Journal of Respiratory and Critical Care Medicine. 1996;154:277–278. doi: 10.1164/ajrccm.154.2.8756794. [DOI] [PubMed] [Google Scholar]
- Matthay MA, Folkesson HG, Verkman AS. Salt and water transport across alveolar and distal airway epithelium in adult lung. American Journal of Physiology. 1996;270:L487–503. doi: 10.1152/ajplung.1996.270.4.L487. [DOI] [PubMed] [Google Scholar]
- Meyrick B, Miller J, Reid L. Pulmonary edema induced by ANTU or by high or low oxygen concentrations in rats-an electron microscopic study. British Journal of Experimental Pathology. 1972;53:347–358. [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, King LS, Christensen BM, Agre P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. American Journal of Physiology. 1997;273:C1549–1561. doi: 10.1152/ajpcell.1997.273.5.C1549. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Smith BL, Christensen EI, Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proceedings of the National Academy of Sciences of the USA. 1993;90:7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brodovich H, Hannam V, Seear M, Mullen JB. Amiloride impairs lung water clearance in newborn guinea pigs. Journal of Applied Physiology. 1990;68:1758–1762. doi: 10.1152/jappl.1990.68.4.1758. [DOI] [PubMed] [Google Scholar]
- Pallone TL, Edwards A, Ma T, Silldorff E, Verkman AS. Requirement of aquaporin-1 for NaCl driven water transport across descending vasa recta. Journal of Clinical Investigation. 2000;105:215–222. doi: 10.1172/JCI8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine MB, Beach PM, Cottrel TS, Scala M, Turino GH. The relationship between right duct lymph flow and extravascular lung water in dogs given ANTU. Journal of Clinical Investigation. 1976;58:482–492. doi: 10.1172/JCI108492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaiguia-Delclaux S, Jayr C, Luo DF, Saïdi NE, Meignan M, Duvaldestin P. Halothane and isoflurane decrease alveolar epithelial fluid clearance in rats. Anesthesiology. 1998;88:751–760. doi: 10.1097/00000542-199803000-00027. [DOI] [PubMed] [Google Scholar]
- Richter CP. The physiology and cytology of pulmonary edema and pleural effusion produced in rats by ANTU. Journal of Thoracic Surgery. 1952;23:66–90. [PubMed] [Google Scholar]
- Schnermann J, Chou J, Ma T, Knepper MA, Verkman AS. Defective proximal tubule reabsorption in transgenic aquaporin-1 null mice. Proceedings of the National Academy of Sciences of the USA. 1998;95:9660–9664. doi: 10.1073/pnas.95.16.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P. Aquaporin-1 in plasma membrane and caveolae provide mercury-sensitive water channels across lung endothelium. American Journal of Physiology. 1996;270:H416–422. doi: 10.1152/ajpheart.1996.270.1.H416. [DOI] [PubMed] [Google Scholar]
- Song YL, Ma T, Matthay MA, Verkman AS. Role of aquaporin-4 in airspace-to-capillary water permeability in intact mouse lung measured by a novel gravimetric method. Journal of General Physiology. 2000;115:17–27. doi: 10.1085/jgp.115.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towne JE, Harrod KS, Krane CM, Menon AG. Decreased expression of aquaporin (AQP)1 and AQP5 in mouse lung after acute viral infection. American Journal of Respiratory Cell and Molecular Biology. 2000;22:34–44. doi: 10.1165/ajrcmb.22.1.3818. [DOI] [PubMed] [Google Scholar]
- Umenishi F, Carter EP, Yang B, Oliver B, Matthay MA, Verkman AS. Sharp increase in rat lung water channel expression in the perinatal period. American Journal of Respiratory Cell and Molecular Biology. 1996;15:673–679. doi: 10.1165/ajrcmb.15.5.8918374. [DOI] [PubMed] [Google Scholar]
- Verkman AS, Matthay MA, Song Y. Aquaporin water channels and lung physiology. American Journal of Physiology Lung Cellular and Molecular Physiology. 2000;278:L867–879. doi: 10.1152/ajplung.2000.278.5.L867. [DOI] [PubMed] [Google Scholar]
- Weiser MR, Pechet TT, Williams JP, Ma M, Frenette PS, Moore FD, Kobzik L, Hines RO, Wager DD, Carroll MC, Hechtman HB. Experimental murine acid aspiration injury is mediated by neutrophils and the alternative complement pathway. Journal of Applied Physiology. 1997;83:1090–1095. doi: 10.1152/jappl.1997.83.4.1090. [DOI] [PubMed] [Google Scholar]
- Yang B, Fukuda N, Van Hoek AN, Matthay MA, Ma T, Verkman AS. Carbon dioxide permeability of aquaporin-1 measured in erythrocytes and lung of aquaporin-1 null mice and in reconstituted proteoliposomes. Journal of Biological Chemistry. 2000;275:2686–2692. doi: 10.1074/jbc.275.4.2686. [DOI] [PubMed] [Google Scholar]
- Yasui M, Serlachius E, Lofgren M, Belusa R, Nielsen S, Aperia A. Perinatal changes in expression of aquaporin-4 and other water and ion transporters in rat lung. The Journal of Physiology. 1997;505:3–11. doi: 10.1111/j.1469-7793.1997.003bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


