Abstract
Osmolarity-dependent ionic currents from follicle-enclosed Xenopus oocytes (follicles) were studied using electrophysiological techniques. Whole follicle currents were monitored using a two-electrode voltage clamp and single-channel activity was measured using the patch-clamp technique.
In follicles held at -60 mV two chloride currents were activated in external hyposmotic solutions. One was the habitual volume-regulated current elicited by external hyposmolarity (ICl,swell), and the second was a slow and smooth current (Sin) generated by ACh or ATP application.
In follicles, the permeability ratios for different anions with respect to Cl− were similar for both ICl,swell and Sin, with a sequence of: SCN− > I− > Br−≥ NO3−≥ Cl− > gluconate ≥ cyclamate > acetate > SO42−.
Extracellular ATP blocked the outward component of Sin. Also, extracellular pH modulated the inactivation kinetics of Sin elicited by ACh; e.g. inactivation at +80 mV was ∼100% slower at pH 8.0 compared with that at pH 6.0.
Lanthanides inhibited ICl,swell and Sin. La3+ completely inhibited ICl,swell with a half-maximal inhibitory concentration (IC50) of 17 ± 1.9 μm, while Sin was blocked up to 55% with an apparent IC50 of 36 ± 2.6 μm.
Patch-clamp recordings in follicular cells showed that hyposmotic challenge opened inward single-channel currents. The single channel conductance (4.7 ± 0.4 pS) had a linear current-voltage relationship with a reversal membrane potential close to −20 mV. This single-channel activity was increased by application of ACh or ATP.
The ICl,swell generation was not affected by pirenzepine or metoctramine, and did not affect the purinergic activation of the chloride current named Fin. Thus, ICl,swell was not generated via neurotransmitters released during cellular swelling.
All together, equal discrimination for different anions, similar modulatory effects by extracellular pH, the blocking effects by ATP and La3+, and the same single-channel activity, strongly suggest that ICl,swell and Sin currents depend on the opening of the same type or a closely related class of volume-regulated chloride channels.
Follicle-enclosed Xenopus oocytes (follicles) generate ionic current responses when they are exposed to hyposmotic external media. These osmolarity-dependent current responses are carried principally by potassium and chloride ions (Arellano & Miledi, 1993, 1995). The ionic currents carried by Cl− (ICl(osm); Arellano & Miledi, 1995; Arellano et al. 1996) seem to be very similar to the volume-regulated chloride currents, named ICl,swell, that have been characterised in many cell types and which may contribute to the regulatory volume decrease, a process activated in most cells in response to an hyposmotic challenge (Strange et al. 1996; Nilius et al. 1997; Okada, 1997; for consistency, this follicle osmolarity-dependent Cl− current will be denoted here as ICl,swell). In follicles, the volume-regulated currents are principally generated in the membrane of the follicular cells. It is possible to monitor these currents with electrodes inserted into the oocyte, due to the electrical coupling via gap junction channels that exist between the oocyte and its follicular cells (van den Hoef et al. 1984; Woodward & Miledi, 1987; Arellano & Miledi, 1993). ICl,swell is not the only chloride current regulated by reduction of external osmolarity in follicles. Perhaps as important is a potentiation of currents that from their time course were named slow and smooth inward currents (Sin); these are elicited by several neurotransmitters, hormones, and intracellular increase of cAMP (Arellano & Miledi 1993, 1994). Sin originate also in the membrane of the follicular cells and are carried mainly by chloride ions. Several of the Sin characteristics show that they are distinct from Ca2+-dependent chloride currents that arise in the membrane of the oocyte itself (Kusano et al. 1982), and which are also activated by several neurotransmitters and other substances (Miledi et al. 1989). Moreover, Sin are different from another type of follicular chloride current activated by ACh or ATP, which has been named Fin from its fast time course (Arellano & Miledi, 1993; Arellano et al. 1998, 1999). The Sin elicited by hormones such as follicle stimulating hormone (FSH), or neurotransmitters such as noradrenaline and dopamine, are mimicked and potentiated by an intracellular cAMP increase, suggesting that those substances activate Sin via cAMP acting as an intermediary. Also, other studies suggest osmolarity-dependent current modulation through a cAMP increase in different cellular systems (e.g. Meng & Weinman, 1996; Carpenter & Peers, 1997). On the other hand the mechanisms of Sin activated by ACh or ATP remain poorly understood (Arellano & Miledi, 1994; Arellano et al. 1998).
In general, not much information is available on the signal transduction pathways involved in controlling the gating of the volume-regulated channels; but it seems clear that G protein-dependent mechanisms and phosphorylation pathways are involved (Doroshenko et al. 1991; Voets et al. 1998; Nilius et al. 1999). The molecular mechanisms of the gating and modulation of the volume-regulated channels are complex and our understanding of these phenomena is incipient. It is very probable that those mechanisms involve several cellular processes that might interact in order to maintain or control the cell volume, which is clearly a fundamental process for cellular functioning (Okada, 1999).
Due to the similarities between both follicle osmolarity-dependent currents it is tempting to suggest that they may be due to activation of the same type of chloride channel, even though they might be activated (or modulated) via distinct mechanistic pathways (Arellano et al. 1996). In this work we investigated whether the follicular-based ICl,swell and Sin (elicited by ACh or ATP) involve activation of a single type of channel.
In addition, various ionic channels and membrane receptors have been described in the ovarian follicle of many species; for several of them their function can be directly related to follicular development (e.g. gonadotropin hormone receptors). For several other molecules located in the ovarian follicle their functions are still not clear, such is the case for muscarinic and purinergic receptors and several of the ionic channels that they activate or inhibit (Arellano et al. 1996, 1999). Although the stimulation of muscarinic receptors and ionic channels have been implicated as modulators of important physiological processes, such as maturation (Dascal et al. 1984; Skoblina & Huhtaniemi, 1997), the cellular mechanisms involved are poorly understood. The presence of muscarinic and purinergic receptors in follicles is not particular to Xenopus frogs, and it has been shown that other species contain similar molecules (Eusebi et al. 1984; Kamada et al. 1994; Fritz et al. 1999). Moreover, several studies have shown possible sources for neurotransmitters within the ovarian follicle of other species (Sporrong et al. 1985; Dees et al. 1995; Mayerhofer et al. 1998). Thus, knowledge regarding the effects provoked by their stimulation might help to elucidate their role in ovarian follicle physiology.
METHODS
Cell preparation
Xenopus laevis frogs were obtained from Xenopus I (Ann Arbor, MI, USA) and Xenopus Express (Homosassa, FL, USA). Ovary lobules (4-8) were surgically removed under sterile conditions from frogs that were anaesthetised using 0.1 % 3-aminobenzoic acid ethyl ester and rendered hypothermic. After surgery, frogs were sutured, and allowed to recover from anaesthesia and hypothermia. Frogs were maintained for 15–20 days in individual tanks until healing was complete, and were then returned to a larger group housing. No further oocytes were taken for at least 2 months. After the final taking of oocytes the anaesthetised frogs were killed by decerebration and pithing. The institutional animal use committees approved this procedure. The lobules were placed in sterile unsupplemented modified Barth's medium containing (mM): 88 NaCl, 0.2 KCl, 2.4 NaHCO3, 0.33 Ca(NO3)2, 0.41 CaCl2, 0.82 MgSO4, 0.88 KH2PO4, 2.7 Na2HPO4, with gentamicin 70 μg ml−1 and adjusted to pH 7.4.
For studies on follicle-enclosed oocytes, the follicles (stage VI; Dumont, 1972) were dissected as epithelium-removed, where their inner epithelia, together with thecal blood vessels, were separated using sharp forceps. This procedure leaves the follicular cell basal membrane in place which thus provides protection and a natural environment for these cells (Arellano et al. 1998). Also, the follicle epithelium-removed dissection facilitates electrode insertion, improves the stability of electrophysiological recording and simplifies the interpretation of results by eliminating the possible participation of epithelium and other surrounding thecal tissues in the responses. Epithelium-removed follicles were incubated (18-20°C) in sterile modified Barth's medium supplemented with glucose (5 mM), and fetal bovine serum (0.1-0.2 %). In these conditions, follicular cell-oocyte electrical coupling and follicular responses can be maintained for more than 10 days.
For follicular cell isolation and culture, and patch-clamp recording (see below), the follicles (stage VI) were dissected as ‘unzipped’ follicles (Arellano et al. 1996), where the external layers were removed together with the follicular cell basal membrane. With this dissection, the follicular cells were exposed but remained attached to the vitelline envelope, allowing them to be dislodged for cell culture (Miledi & Woodward, 1989), or the unzipped follicle to be used directly for patch-clamping of follicular cells (Arellano et al. 1996). For cell cultures, unzipped follicles were enzymatically treated for 5 min in Hanks’ balanced salt solution (Gibco BRL) containing 0.05 % trypsin and 0.5 mM EDTA, then gently washed in medium containing 10 % fetal bovine serum. The treated follicles were then sucked repeatedly with a polished Pasteur pipette, and the dislodged follicular cells were recovered on glass coverslips prepared with collagen as the substrate. Follicular cells were maintained in L-15 culture medium (supplemented with 0.2 % fetal bovine serum) at 18°C (R. Reyes, S. Alshihabi, R. O. Arellano & R. Miledi, unpublished results) until their use in patch-clamp experiments.
For patch-clamp experiments on the denuded oocyte membrane, follicular cells from unzipped follicles were removed by rolling the follicles on a poly-L-lysine treated coverslip (Miledi & Woodward, 1989). After ∼6 h the defolliculated oocytes were incubated in normal Ringer solution with 50 mM sucrose for 20 min, and the vitelline envelope was removed with fine forceps to patch clamp the oocyte membrane.
Electrophysiological measurements and analysis
Whole currents in epithelium-removed follicles were monitored using a two-microelectrode voltage clamp (Miledi, 1982). Unless otherwise stated, follicles were voltage clamped at -60 mV, and superfused with normal Ringer (NR) solution containing (mM): 115 NaCl, 2 KCl, 1.8 CaCl2, 5 Hepes, pH 7.0, or hyposmotic Ringer solution (HR90), where the NaCl concentration in NR was decreased from 115 to 90 mM. HR90 was used to potentiate osmolarity-dependent Sin activated via stimulation of muscarinic or purinergic receptors by ACh or ATP, respectively. Different hyposmotic Ringer solutions, with either 80 or 50 mM NaCl (HR80 or HR50), were used to activate ICl,swell. Unless otherwise stated, all follicles were injected with ethylene glycol-bis(β-amino-ethylether) N,N,N′,N′-tetraacetic acid (EGTA) (∼0.1 nmol per oocyte) and tetraethylammonium (TEA+) chloride (∼0.25 nmol per oocyte) by pneumatic pressure ejection, via a third micropipette inserted into the oocyte filled with a solution (100 mM EGTA plus 250 mM TEA+) made up in 5 mM Hepes, pH adjusted to 7.0 with KOH. The EGTA-TEA+ follicle loading effectively eliminated Ca2+-dependent Cl− currents and blocked K+ currents elicited by muscarinic and purinergic stimulation in follicles. TEA+ also blocked osmolarity-dependent K+ currents generated in some follicles by hyposmotic medium (Arellano & Miledi, 1993). The injection apparatus was also used for extracellular delivery of ATP (1 μM in NR). For these studies ATP was ejected from a micropipette positioned ∼50 μm from the follicle, this method allowed us to generate repeatedly focal Fin currents in an area of the follicle and concurrently to apply a general stimulation through the bath superfusion (Arellano et al. 1998). In these cases the superfusion rate was lowered from 10 to 2 ml min−1 in order to allow current activation by the ejected ATP.
Ionic substitutions were made in either NR or the hyposmotic solutions, replacing all the NaCl by any of the following salts: NaSCN, NaI, NaBr, NaNO3, sodium acetate, sodium cyclamate, Na2SO4, or sodium gluconate. Each follicle was tested first in NR and then in one other anion solution. Changes in junction potentials generated by ion substitution (and osmolarity) using the different external solutions were measured directly. This was completed before follicle recording. The microelectrode tip potential was set to zero in the bath filled with NR, which was grounded through a salt bridge with a chlorided silver wire. Then the bath solution was replaced with each testing solution and the stable value for the potential generated was confirmed by returning to NR. The final reversal potentials (Erev) were corrected for the respective junction potentials. Permeability ratios for the different anions (X) relative to Cl− (PX/PCl) were estimated using the formula:
| (1) |
where [Cl−]o and [Cl−]t are the external Cl− concentrations in control and test anion-substituted solutions, respectively, ΔErev is the difference in reversal potential value between those two solutions, and [X−]o is the external concentration of the substituting anion, while F and R are the Faraday and gas constants and T is the absolute temperature.
Blockage effects by ATP and La3+ were evaluated using a one-site blockade model which assumes that the effect of the compounds is fast (Woodhull, 1973; Garcia & Miledi, 1996). The model estimates the half-blocking concentration (IC50(0)) of the compounds at a membrane potential of 0 mV, and the fraction of the membrane potential sensed by the binding site for the blocking agent, applying the following equation:
| (2) |
where ICl is the amplitude of the chloride current studied, either the ICl,swell or Sin in the absence of the blocker B (ATP or La3+), and ICl(B) is the current in the presence of the blocker. [B] is the concentration of the blocker, Vm the membrane potential, δ the electrical distance of the binding site measured from the extracellular side, z the valence of the blocker, while F and R are the Faraday and gas constants and T is the absolute temperature.
Membrane currents in either isolated follicular cells in culture, in freshly dissected (within the first 6 h) unzipped follicles, or denuded (i.e. defolliculated) oocytes, were recorded using the cell-attached configuration of the patch-clamp technique. Unzipped follicles and denuded oocytes were not loaded with EGTA-TEA+. Polished patch pipettes were made from borosilicate glass capillaries and had a resistance of 5–8 MΩ when filled with NR. Currents were monitored using a patch-clamp amplifier APC-8 (Medical Systems, Japan) and stored on-line in a DAT recorder, for their later digitisation and analysis using the pCLAMP6 and Axoscope software (Axon Instruments, Foster City, CA, USA).
Substances
ACh, ATP, EGTA, collagenase type I, fetal bovine serum, collagen, 3-aminobenzoic acid ethyl ester, and all salts were from Sigma Chemical Co. (St Louis, MO, USA). Pirenzepine dihydrochloride and metoctramine tetrahydrochloride were purchased from RBI (Natick, MD, USA). TEA chloride was from Baker Co. (Phillisburg, NJ, USA). Trypsin, gentamicin, and L-15 culture medium were purchased from Life Technologies (Gibco BRL, Rockville, MD, USA).
RESULTS
Characteristics and anion permeability of ICl,swell and Sin follicle currents
The follicles loaded with EGTA and TEA+ had a resting potential in NR of -29.3 ± 6.7 mV (all results quoted are means ±s.e.m. unless otherwise stated), and an input resistance of 0.37 ± 0.03 MΩ (165 follicles, 14 frogs). In general, the ICl,swell generated by HR80 reached a peak amplitude in 4–6 min (Fig. 1A), and inactivated slowly when follicles were maintained in this solution. However ICl,swell deactivated rapidly, and in most follicles completely, upon returning to NR solution. Defolliculation (manual or enzymatic) completely eliminated the ICl,swell elicited by superfusion with hyposmotic solutions (HR80 or HR50), while EGTA injection (together with TEA+) failed to abolish it, thus confirming that this current is follicular cell based and Ca2+ independent (Arellano & Miledi, 1993, 1995). The ICl,swell had a reversal potential (Erev) of -23.5 ± 0.7 mV (78 follicles, 14 frogs), close to the equilibrium potential for Cl− ions in Xenopus follicles (Kusano et al. 1982) and very similar to that for other Cl− carried currents in follicles (Arellano et al. 1998).
Figure 1. Osmolarity-dependent membrane currents elicited in Xenopus follicles.
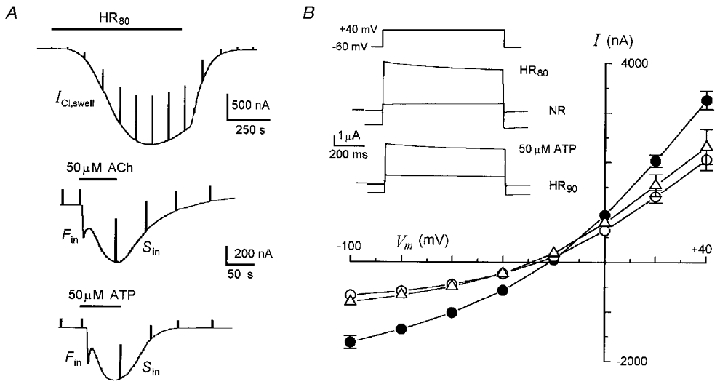
A, ICl,swell elicited by HR80 in a follicle superfused originally in NR, and Fin and Sin elicited by ACh and ATP in follicles bathed in HR90. All different epithelium-removed follicles were held at -60 mV. In this and subsequent records test solutions and drugs were applied during times indicated by bars at top, and steps to -40 mV were applied periodically to monitor membrane conductance. B, current-voltage relationships for ICl,swell (•), and Sin elicited in HR90 by 50 μM ACh (○) and 50 μM ATP (▵); steady-state currents at different potentials were measured by application of a voltage step series in control conditions and at the peak of the currents. Superimposed records are examples of currents during the voltage steps to +40 mV during the indicated conditions. Data (here and throughout are quoted as means ±s.e.m.) from follicles of 7–14 frogs.
Follicle osmo-dependent responses to ACh or ATP had the same characteristics described previously (Arellano & Miledi, 1993; Arellano et al. 1998). Briefly, in NR both agonists evoked principally a fast inward current (Fin) followed by a slow and smooth current (Sin). The Sin current was potentiated several (5-8) times when the same concentrations of ACh or ATP were applied in an external hyposmotic medium, such as HR90 (Fig. 1A and B). Sin elicited by ACh or ATP were dependent on the presence of the follicular cells and their coupling with the oocyte, and were also Ca2+ independent (Arellano & Miledi, 1993). In HR90Sin had an Erev of -25.8 ± 0.6 mV (26 follicles, 11 frogs) and of -29 ± 1.4 mV (21 follicles, 7 frogs) when elicited by ACh or ATP, respectively (Fig. 1B).
Usually, follicles in HR90 (a solution with a similar osmolarity to Barth's incubation medium) had a lower input resistance (0.14 ± 0.01 MΩ; n= 80) than that of the same follicles in NR (0.2-0.4 MΩ, cf. Arellano & Miledi 1995). Apparently, the lower resistance is mainly due to the presence of a steady inward current in this hyposmotic solution (Arellano et al. 1996). This current did not seem to determine the presence or amplitude reached by ICl,swell or Sin current, because these were not directly correlated with the amplitude of the steady current. Due to the fact that the later current was blocked by La3+ (1 mM, see below) and it seemed to be carried mainly by Cl− ions (not shown), we suggest that this current corresponds with a population of ICl,swell channels that remains active in Barth's or other hyposmotic solutions.
The amplitudes of both ICl,swell and Sin were variable among follicles from different frogs, as has been shown in previous studies. Here, ICl,swell generated by HR80 had amplitudes that ranged from a few nanoamperes to 3.9 μA, and Sin elicited in HR90 activated either by ACh or ATP were also in the range of a few nanoamperes to 1.2 μA.
Given the similarity in the general characteristics of ICl,swell and Sin, we proceeded to compare their channel permeability to the following anions: Cl−, Br−, I−, NO3−, SCN−, SO42−, acetate (acet), cyclamate (cycl), and gluconate (gluc). The permeability ratios for the different anions with respect to chloride (PX/PCl) were calculated applying eqn (1). For this purpose, current-voltage relationships were obtained for both osmolarity-dependent responses by giving a series of voltage pulses (duration of 650 ms) applied in the range of -100 to +40 mV (20 mV steps) from a holding potential of -60 mV.
For ICl,swell the control voltage pulses were applied during superfusion of the testing solution, where all NaCl in NR was substituted by the corresponding testing salt (NaX; e.g. NaI, sodium cyclamate). Then voltage pulses were reapplied during the peak ICl,swell elicited by reducing the osmolarity of the testing solution from 115 mM NaX to either 80 or 50 mM NaX. For every voltage step, the control pulse currents were subtracted from those obtained during ICl,swell, and these values were plotted as in Fig. 2A.
Figure 2. Permeability ratios for ICl,swell and Sin.
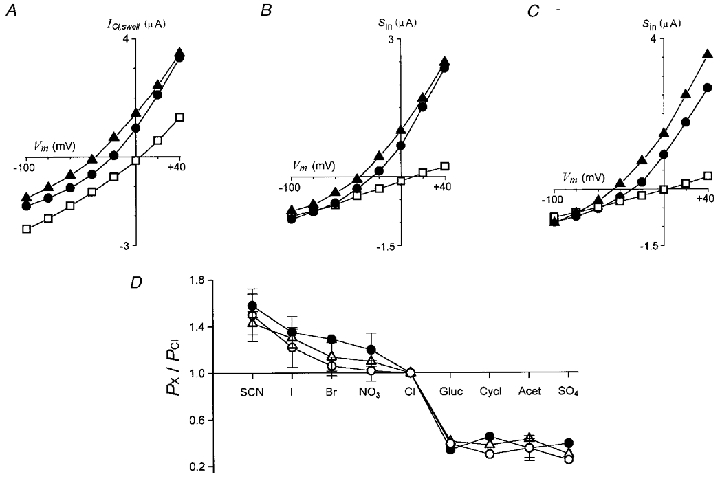
Current-voltage relationships for A, ICl,swell generated by HR80 or HR50, and Sin elicited in HR90 by application of 50 μM ACh, B, or 50 μM ATP, C, in Ringer solution containing NaCl (•), or in solutions in which chloride was substituted by SCN (▴) or cyclamate (□). D, permeability ratios for different anions of ICl,swell (•) and Sin elicited by ACh (▵), or ATP (○). Data are from 4–10 follicles (3-8 frogs) in each condition.
For Sin, follicles maintained in Barth's medium were initially superfused in HR90 solution, thus most ICl,swell was inactivated. The follicles in HR90 were superfused with the control testing solution where the NaCl was substituted by 90 mM NaX, and a series of voltage pulses was applied in order to obtain the control membrane current. Then 50 μM ACh or 50 μM ATP was applied in the same testing solution and voltage steps were reapplied at the Sin peak amplitude. At this time (∼1 min) the Fin also elicited by both neurotransmitters was usually completely inactivated. Therefore, the Fin did not contribute significantly to the peak current of Sin. Again, for every voltage step, control membrane currents in HR90 with chloride substituted by the testing anion were subtracted from those obtained during Sin elicited by ACh or ATP and plotted as in Fig. 2B and C, respectively.
The values for Erev for ICl,swell and Sin, the latter elicited by either ACh or ATP, were estimated from the current-voltage (I–V) relations obtained in each test solution. The ΔErev was then used to estimate the permeability ratios PX/PCl that were plotted in Fig. 2D. All three osmolarity-dependent currents presented the same sequence of permeability for the different anions: SCN− > I− > Br−≥ NO3−≥ Cl− > gluconate ≥ cyclamate > acetate > SO42−.
These results suggested that ICl,swell and Sin currents elicited either by ACh or ATP, were driven through a similar type of anionic channel. To explore this further, we performed pharmacological studies on the osmolarity-dependent currents, and patch-clamp recording on the follicular cell and oocyte membranes to obtain direct information on the characteristics of the channels involved.
pH modulation and inhibition by ATP and lanthanides on ICl,swell and Sin
There are two pharmacological effects that have been consistently shown on the ICl,swell from several cellular types including Xenopus follicles (Ackerman et al. 1994; Jackson & Strange, 1995b; Voets et al. 1996), which are: (1) a blocking effect by extracellular ATP, and (2) modulation of the time course of ICl,swell inactivation at positive potentials by extracellular pH. Thus, these two aspects were studied here with respect to their possible effects on the generation of Sin elicited by neurotransmitters.
The Sin currents were elicited by ACh or ATP at different maximal concentrations from 10 μM to 1 mM, and voltage steps to different potentials were applied at the peak amplitude of the Sin generated (Fig. 3A and B). The inward peak current amplitudes were similar (at -30 to -120 mV) independent of the agonist and the maximal concentration used, but when 1 mM ATP was applied, the outward currents were potently inhibited (13 follicles, 3 frogs). This blocking effect was voltage dependent, being stronger at positive potentials (Fig. 3A), suggesting that the binding site for ATP is located inside the Sin channel pore. When this effect was analysed using a one-site blockade model (eqn (2)), the parameter δ that indicates the fraction of the membrane electrical field sensed by the binding site for ATP was estimated as 0.26 ± 0.03. And the IC50 at a membrane potential of 0 mV was 0.57 ± 0.12 μM (see Fig. 5C). The inhibitory effect by 1 mM ATP was specific for this agonist because a similar concentration of ACh did not produce blockage (6 follicles, 2 frogs). This effect of ATP on the Sin is similar to that reported for ICl,swell in other cells (Strange et al. 1996).
Figure 3. Effects of extracellular ATP and pH on Sin.
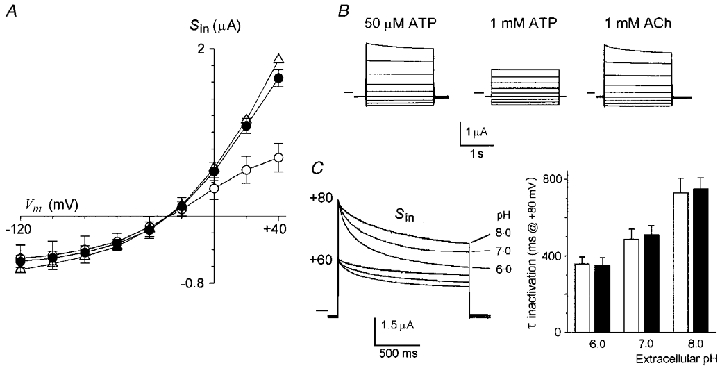
A, current-voltage relations of Sin elicited by 50 μM ATP (•), 1 mM ATP (○), or 1 mM ACh (▵). Data are from 5–13 follicles (2-3 frogs) in each condition held at -60 mV. B, superimposed current traces representative of the Sin elicited by the agonists at membrane potentials from +40 to -120 mV. Horizontal lines indicate zero current. C, superimposed current traces obtained at the peak of Sin elicited by 50 μM ACh at the membrane potentials stepped to +80 and +60 mV from a holding potential of -60 mV. At each potential the set of current traces corresponds with Sin generated in extracellular medium buffered to pH 8, 7, or 6. The inactivation kinetics at +80 mV were fitted to simple exponentials and the mean of the time constants (τ) in each value of extracellular pH were plotted in the bar graph. The open bars are the mean τ inactivation for the ICl,swell generated by HR80 (3 follicles, 2 frogs), while the filled bars are those for the Sin elicited by 50 μM ACh in HR90 (7 follicles, 2 frogs) in follicles from the same frogs.
Figure 5. Voltage dependence of La3+ block on ICl,swell and ATP on Sin.
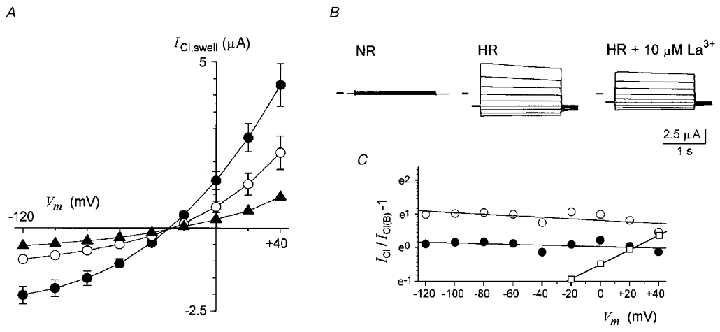
A, current-voltage relations of ICl,swell elicited by HR50 in presence of 0 μM (•), 10 μM (○) or 25 μM La3+ (▴). Data are the means of 6 follicles (2-3 frogs) in each condition held at -60 mV. B, superimposed traces are examples of ICl,swell in each condition. C, comparison of the voltage dependency of La3+ block on ICl,swell (•, 10 μM; ○, 25 μM) and of 1 mM ATP on Sin (□, data from Fig. 3A); chloride currents in each potential in the absence (ICl) and the presence of the blocker (ICl(B)) were analysed using eqn (2) that was linearized. Parameters from the lines fitted to the data points were used to estimate the IC50 at 0 mV and δ values.
The other characteristic of ICl,swell is that the time course of its inactivation process at positive potentials is accelerated by a decrease of extracellular pH. We have confirmed the effect of pH on the inactivation kinetics of ICl,swell and studied this for the Sin elicited by ACh. Although similar experiments were made for ATP, this agonist at lower pH had additional effects that seemed to involve modifications in the interaction of the agonist with the purinergic receptor, and further experiments will be necessary to completely evaluate them. Thus, the Sin was elicited by 50 μM ACh in HR80, and at the peak current amplitude voltage steps to +60 and +80 mV were applied, first in HR80 at pH 7.0. After a wash period of 15 min, 50 μM ACh was again superfused in solution adjusted to pH 8.0 or 6.0, and the depolarizing voltage steps were applied at the peak Sin. The Sin inactivation kinetics was accelerated by reducing the external solution pH from 8.0 to 6.0 at both membrane potentials (Fig. 3C), as was shown by the time constants of the inactivation time course estimated by fitting with single exponentials. The time constants of inactivation at +80 mV for the Sin currents elicited by ACh (7 follicles, 2 frogs) were 347 ± 18 ms for pH 6.0, 514 ± 39 ms for pH 7.0 and 760 ± 46 ms for pH 8.0. The strength of this effect is very similar to that observed on the inactivation rates of ICl,swell in follicles (cf. Voets et al. 1996), and experiments eliciting ICl,swell in follicles (n= 3 at each pH) from the same frogs used here, confirmed this result (Fig. 3C).
Thus these results indicated that Sin showed a similar sensitivity to extracellular pH and ATP to that demonstrated for ICl,swell, not only from Xenopus follicles but also that from other cell types. This strengthened the suggestion that both currents were carried through a similar channel.
One important characteristic of ICl,swell in the Xenopus follicle is its sensitivity to lanthanides, specifically La3+ (Ackerman et al. 1994; Arellano & Miledi, 1995) and Gd3+. This effect on volume-activated chloride channels has been reported also for other cells (Robson & Hunter, 1994). Two aspects were studied here. First it was of interest to know if Sin currents elicited by agonists were similarly blocked by lanthanides (Fig. 4), and second we investigated the voltage dependency of the blocking effect on ICl,swell in order to obtain some information about the inhibition mechanisms involved (Fig. 5).
Figure 4. Follicle osmo-dependent currents inhibition by La3+ and Gd3+.
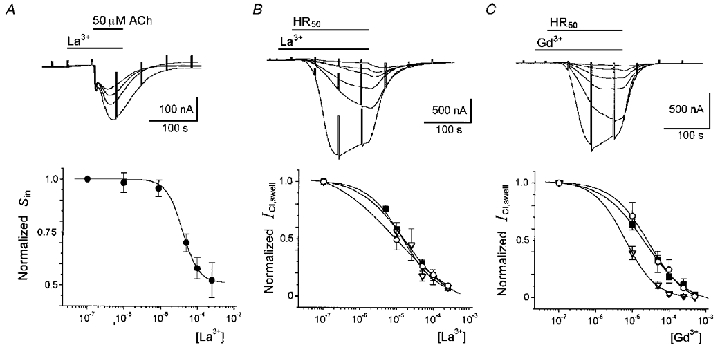
The Sin currents were elicited in HR90 by 50 μM ACh, in the absence or presence of different concentrations of La3+ that were first applied alone for ∼1 min. In its maximal effect, La3+ (around 500 μM) blocked more than 55 % of Sin, with an apparent IC50 of 30 ± 2.6 μM (Fig. 4A). The effect of La3+ was completely reversible after washing in HR90 for 10 min, and was specific on Sin because the ACh-elicited Fin was not affected by the polycation. This strongly suggested that lanthanides had similar effects on ICl,swell and Sin currents, and further supported the notion that both currents are carried via the same type of channel.
The mechanism of lanthanide inhibition of ICl,swell is unknown. Among other possibilities, it might be due to a direct blocking effect in the pore of the channel. Therefore, we obtained dose-response relations for the effects of La3+ and Gd3+ on ICl,swell in follicles held at different potentials. We also obtained current-voltage relations in the presence and the absence of La3+, which were analysed using a one-site blockade model (Fig. 5). Similar to that made for the analysis of Sin inhibition, follicles were first superfused with NR solution containing a known concentration of either La3+ or Gd3+, after ∼1 min in this solution, the follicles were then exposed to HR50 containing the same testing concentration of the lanthanide. The ICl,swell generated in the presence of each concentration of the polycation was normalized with respect to the current generated in its absence. The dose-response relations of inhibition were constructed in follicles held at -100, -60 and -40 mV (Fig. 4B and C). At a holding potential of -60 mV the IC50 for La3+ and Gd3+ were 17.1 ± 1.9 μM (23 follicles, 6 frogs) and 20.9 ± 1.4 μM (11 follicles, 4 frogs), respectively. Similar IC50 values were obtained in follicles held at -40 mV; 9.1 ± 0.8 μM for La3+ (2 follicles, 1 frog) and 24.8 ± 2.3 μM for Gd3+ (2 follicles, 2 frogs). And at -100 mV where IC50 was 14.2 ± 5.9 μM for La3+ (17 follicles, 8 frogs) and 6.7 ± 3.6 μM for Gd3+ (6 follicles, 3 frogs), indicating that in general the inhibition effect is not strongly voltage dependent (Fig. 4B and C, bottom).
Similar results were obtained when the La3+ blocking effect on ICl,swell was evaluated obtaining the I–V relations in the absence and the presence of the polycation. For this, ICl,swell were elicited by HR50 superfusion and I–V curves were obtained by stepping the potential from +40 to -120 mV at the peak current; this procedure was repeated in the presence of 10 or 25 μM La3+ (6 follicles, 2 frogs). From the I–V curves obtained (Fig. 5A) it is clear that the potency of the inhibitory effect of La3+ on ICl,swell behaved linearly at the entire voltage range tested, a result that is different from that observed for the inhibition produced by ATP on Sin (Figs 3A and 5C). When the I–V curves were analysed using a one-site blockade model, the parameter δ was estimated to be very close to 0, and was 0.03 ± 0.013 for 25 μM La3+ and 0.016 ± 0.01 for 10 μM. These results suggested that the site for La3+ and Gd3+ actions did not sense the transmembrane voltage, and that the ICl,swell inhibition was not due to a blocking effect within the pore of the channel.
Recording of osmo-dependent ion channel currents in the follicular cells
More direct evidence for the similarity between ICl,swell and Sin channels was obtained by monitoring the membrane channel activity in patches of follicular cells either isolated and maintained in culture, or recorded from the surface of unzipped follicles. Patches from both preparations behaved similarly. Patch-clamp recordings, in the cell-attached configuration, were obtained from follicular cells maintained in NR. After a high resistance seal was formed, with pipettes filled with NR, superfusion of the cells with HR90 activated inward membrane currents (Fig. 6). Single channel activity was resolved in most of the patches made (Fig. 6A and B), and the unitary conductance was 4.7 ± 0.4 pS (Fig. 6B). Unzipped follicles had an average membrane potential of -55 ± 8 mV (13 follicles, 3 frogs) in NR, as measured with an electrode inserted into the oocyte. Assuming a follicular cell resting membrane potential of -60 mV in NR, the activated channels had an Erev of approximately -20 mV, as expected for osmolarity dependent and other currents carried by chloride ions in follicles (16 unzipped follicles, 3 frogs, and 32 isolated follicular cells, 4 frogs) (e.g. Kusano et al. 1982). Accordingly, potassium channels (Arellano et al. 1996) activated in some patches (not shown) had an Erev of -95 ± 6 mV, which is also that expected for these currents.
Figure 6. Osmo-dependent single channel activity generated in follicular cell membrane.
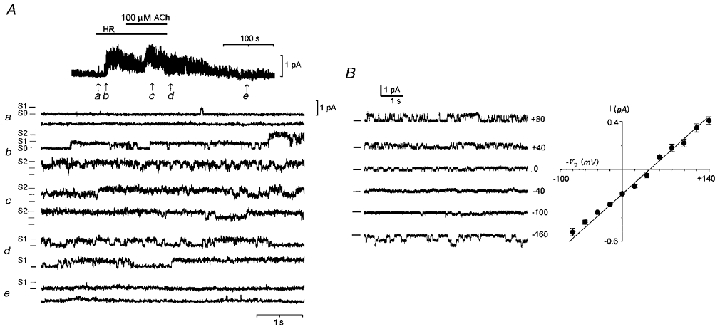
A, cell-attached patch-clamp current recorded at a pipette potential (Vp) of +50 mV from a follicular cell (unzipped follicle) maintained in NR (a). In b subsequent superfusion with HR80 solution opened inward membrane currents, that increased in activity when the hyposmotic solution was applied together with 100 μM ACh (c–d). Activity returned to basal levels when superfused back with NR (e). S0, S1 and S2 signal the opening level of at least 2 channels in this patch. Similar records were obtained in another 8 unzipped follicles and in 5 isolated follicular cells maintained in culture (7 frogs). B, current-voltage relationship for single-channel currents opened by HR80 in an unzipped follicle. A similar relation was obtained for single currents in presence of ACh or ATP. Records are examples of currents at different Vp; at least three channels were present in this particular case. Data represent the means of single current in five different patches performed in cells from three frogs. All records were digitised at 1 kHz and filtered at 100 Hz.
The single inward current activity was potentiated when HR90 was superfused with 50 μM ACh (Fig. 6Ac and d) or ATP. The increase in activity was evidenced by an increase in the open time of the channels (Fig. 6Ac), and also in several patches by the number of units activated. The channels frequently remained open for long periods (several seconds), and occasionally several channels gated in concert, producing larger openings or closings (not shown). The unitary conductance did not change in amplitude when the agonist was superfused (see Fig. 6Ac and d). Osmolarity-dependent current activity was almost completely reversible after some seconds of superfusion with NR (see Fig. 6Ae). It seems that the channels are present at high density since very few (2 from > 40) patches of follicular cells had a single channel, and they regularly contained three or more (> 10) channels.
In several patches from unzipped follicles some channels were active even in NR, or they opened infrequently in this condition (Fig. 6Aa). In follicular cells maintained in culture this phenomenon seemed to be increased and many membrane patches displayed a large basal activity, apparently due to the opening of various channels with characteristics similar to those activated by HR. However, their activity and/or their number were always potentiated upon application of hyposmotic solutions. Thus, it is clear that the increase in single-channel current activity is a consequence of hyposmotic conditions. Because the single-channel conductance was the same in the absence or presence of the agonists, the potentiation of Sin is probably due to an increase in the number and open-time of channels activated. In patches performed on the manually defolliculated oocyte membrane (10 oocytes, 3 frogs) no current activity was generated by HR80, alone or together with ACh or ATP, even after periods of 15 min of continuous superfusion with hyposmotic solution.
Therefore, these results show that HR solution alone or together with ACh or ATP activated inward currents with similar unitary characteristics in the follicular cell membrane but not in the oocyte per se. Since single-channel currents were detected in practically all the follicular cell patches studied, it seems very likely that these contribute significantly to the generation of total ICl,swell and Sin.
The Sin and neurotransmitter release induced by cell swelling
Cell swelling induces the release of various substances from the intracellular medium; several of them participate in the regulatory volume decrease that tends to restore the cellular volume. One of the molecules that has been shown to be commonly released in this condition is cellular ATP (Taylor et al. 1998; Hazama et al. 1999). Since our results seemed to indicate that ICl,swell and Sin were carried via a channel type with similar characteristics, it is possible that the release of substances produced by cellular swelling might be related to the generation of ICl,swell or Sin. At least two mechanistic relations can be directly proposed: (1) stimulation by the agonists produces follicular cell swelling and the concomitant ICl,swell (Sin) activation, and (2) cell swelling elicited by hyposmotic solution produces the release of ACh and/or ATP with the consequent generation of Sin (ICl,swell). The first suggestion was not directly examined here; however, some previous indirect evidence (Arellano et al. 1996) seems to indicate that agonists did not produce or potentiate swelling of the cells (see discussion). The second possibility was assessed with the following experiments. For the case of ACh release and receptor activation we have compared the development of ICl,swell (activated by HR80) in the presence of potent and selective antagonists of muscarinic receptors. It is known that follicles are endowed with at least two different muscarinic receptors very similar to M3 and M2 subtypes, which are strongly blocked by substances such as pirenzepine and metoctramine (Kusano et al. 1982; Arellano et al. 1999). However, the amplitude and rate of development of ICl,swell was not affected by addition of these muscarinic antagonists (10 μM) in the superfusion medium (7 follicles, 3 frogs; Fig. 7A). In follicles from the same frogs the typical muscarinic response in the oocyte is the production of an oscillatory current (ICl(Ca); activation of this current was made in follicles not injected with EGTA-TEA+) and the Fin. Both were potently inhibited by the antagonists (6 follicles, 3 frogs; Fig. 7B). This result suggested that if release of ACh exists during cell swelling produced by the hyposmotic challenge, the possible activation of muscarinic receptors in this condition did not importantly affect the generation of ICl,swell.
Figure 7. ICl,swell generation and its relation with a likely ACh or ATP release elicited by cell swelling.
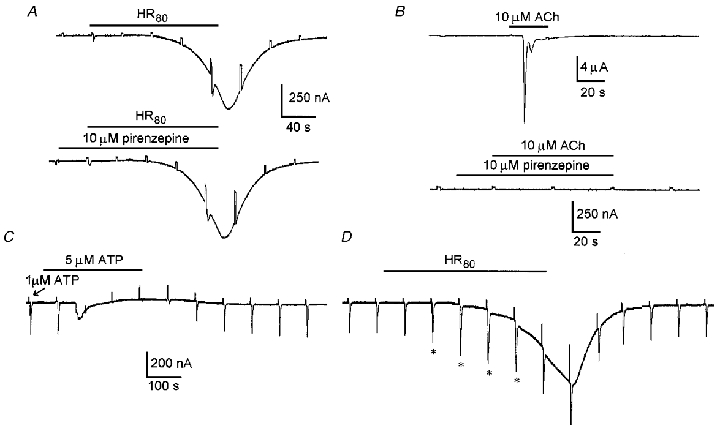
A, current traces correspond to the control ICl,swell activated by HR80 superfusion alone (upper trace) and in the presence of 10 μM pirenzepine (lower trace), a potent antagonist of muscarinic receptors in follicles. Similar results were obtained in 7 follicles more from 3 frogs. B, in follicles from the same frogs used in A, the muscarinic responses were completely inhibited by superfusion with the antagonist. The example traces showed that the typical oscillatory Ca2+-dependent chloride current elicited by 10 μM ACh was effectively eliminated by pirenzepine. C, Fin currents were elicited by short pulses of 1 μM ATP ejected periodically from a pipette positioned close to the follicle; each ejection pulse was applied after the voltage step of 20 mV (arrow). For the time indicated, 5 μM ATP in NR was superfused; while this solution is present in the bath and after its corresponding Fin was elicited, the Fin generated by the 1 μM ATP jets was potently inhibited. Similar results were obtained in 8 follicles from 3 different frogs. D, similar Fin currents generated by 1 μM ATP pulses were not decreased when ICl,swell was simultaneously generated; nevertheless, a transient potentiation of 30–60 % (*) was noted during the first minutes during ICl,swell activation in 4 experiments from 10 follicles tested (3 frogs).
Another possibility is the involvement of ATP release, but potent antagonists of follicle purinergic receptors are not known. However, it has been shown that Fin is strongly inactivated by several seconds in the presence of ACh or ATP (Arellano et al. 1998). If a swelling-elicited release of ATP occurs during HR superfusion one would anticipate that in this condition Fin might exhibit inactivation. In previous studies we have shown that Fin currents are not strongly affected during hyposmotic treatment (cf. Fig. 26 in Arellano et al. 1996), and the application of hyposmotic medium itself does not generate ionic currents with the characteristics of Fin (R. Miledi & R. O. Arellano, unpublished results). To confirm these observations, the follicles were stimulated focally by ejecting brief (0.2-0.5 s) jets of NR containing 1 μM ATP while the superfusion medium was set to 2–3 ml min−1. This stimulation allows Fin to be generated repetitively; if the ejection interval is adequate (> 40–60 s), Fin recovers well from each stimulation and the amplitude is maintained for several minutes. In these conditions, when ATP was superfused in similar concentrations (1-10 μM) to those released by cells exposed to hyposmotic medium (Hazama et al. 1999), the Fin were strongly inhibited during ATP application and remained so for several seconds (Fig. 7C; 8 follicles, 3 frogs). Contrary to this, during ICl,swell activation by HR superfusion (Fig. 7D), Fin elicited by the ATP jets were not abolished or decreased (10 follicles, 3 frogs). In fact, consistently during a short period of ICl,swell activation the Fin seemed to be potentiated (asterisks in Fig. 7D).
These results suggest that any release of the neurotransmitters, ACh or ATP, associated with follicular cells or oocyte swelling did not strongly affect ICl,swell generation. Further studies will be necessary to evaluate the possibility that stimulation by neurotransmitters provoke swelling of the follicular cells and consequently the activation of ICl,swell.
DISCUSSION
In the present study we show that follicle ICl,swell and Sin are carried via anionic channels with similar permeability, unitary conductance, and pharmacological characteristics, including sensitivity to extracellular pH, ATP and lanthanides. All this strongly suggests that both follicle currents flow through the same type of Cl− channels or a closely related class of volume-regulated channels. In addition, both follicle currents are similar to the ICl,swell from other cell types that are activated by external hyposmotic conditions, and which participate in the mechanisms of regulatory volume decrease by allowing a net efflux of Cl−, organic anions and osmolytes (e.g. Pasantes-Morales & Schousboe, 1988; Worrell et al. 1989; Lewis et al. 1993). The channel discrimination for different anions by ICl,swell (Sin) Xenopus channels is also similar to that reported previously (Arellano & Miledi, 1993; Ackerman et al. 1994; Voets et al. 1996), confirming that the selectivity sequence is lyotropic, as has been observed for many anionic channels. Once again, the differences in PX/PCl for different anions discriminate between ICl,swell and other chloride channels which are present in the follicle, namely the Ca2+-dependent Cl− channels present in the membrane of the oocyte itself, and the Fin channels located in the follicular cells (Arellano & Miledi, 1993; Arellano et al. 1998).
One conclusion of this work is that in Xenopus follicular cells ICl,swell channels are regulated by neurotransmitters via activation of muscarinic and purinergic receptors. Since the properties of Sin activated by follicle-stimulating hormone (FSH) and intra-oocyte injection of cAMP (Arellano & Miledi, 1993, 1994) are not different from those elicited by ACh or ATP, we suggest that these currents are also due to activation of ICl,swell channels. Indeed, preliminary results in follicular cells show that FSH generates single-channel activity, similar to that described here (R. O. Arellano & R. Miledi, unpublished results). The complete sequence of events involved in activating Sin still remains unknown. However, the possibility remains that stimulation by neurotransmitters (or hormones) provokes swelling of the follicular cells and hence the activation of Sin (ICl,swell). We have no direct evidence to support or reject this suggestion; nevertheless, some of our previous results seem to indicate that cellular swelling might not be the only mechanism involved in the activation of Sin by neurotransmitters. For example, assuming that ICl,swell generation maintains a close relationship with the time course of cell swelling, one would expect that potentiation of Sin might follow a similar time course for ICl,swell activation. However, this is not the case. Potentiation of Sin starts very early (seconds) after an hyposmotic medium is applied to the follicle, while ICl,swell is activated several seconds later and with a slower time course (cf. Fig. 26 in Arellano et al. 1996). Thus, Sin are near their maximum, 75–90 %, during the first minute in the hyposmotic medium, but the peak amplitude of ICl,swell is reached several minutes later, regularly 6–10 min. In conclusion, even though ICl,swell and Sin seemed to be carried via the same channel type, their generation seemed to occur independently, and the activation mechanisms seemed to differ, at least in some steps of the pathway used. An obvious possibility is that agonists modulate the Sin (ICl,swell) channels, producing facilitation upon a smaller change in osmolarity. Thus, activation of the channels might be through the same mechanism that activates ICl,swell, but the actions provoked by the agonists sensitise the channels to a smaller reduction in osmolarity (and cell-volume change); this mechanism might involve intracellular signalling pathways. In this context, it is well known that in other cells, G proteins and phosphorylation mechanisms might be involved in modulating ICl,swell. For example, it has been suggested that G proteins regulate the sensitivity of ICl,swell to cell swelling, and that this might be mediated through tyrosine phosphorylation (Voets et al. 1998; Nilius et al. 1999). It is possible that such modulation is not restricted to phosphorylation through a single type of protein kinase, and that in Xenopus follicular cells several signalling systems participate in modulating ICl,swell channel sensitivity. An example of this might be represented by the Sin currents activated via stimulation of receptors coupled to an increase in cAMP synthesis (Arellano & Miledi 1994; Arellano et al. 1996). Further studies are necessary to elucidate the mechanisms implicated in the actions of cAMP on ICl,swell modulation, as well as those activated by stimulation of muscarinic and purinergic receptors in follicular cells (Arellano et al. 1998) during Sin generation. Likewise, it will be important to study the effects of osmolarity-dependent current activation on volume control of the follicular cells and the whole follicle. The results presented in this study suggest that the activation of volume-regulated channels in follicular cells can be influenced by external signals, which might be part of a systemic mechanism for cell volume control via transmitters.
It will also be important to investigate whether ICl,swell in follicular cells might be regulated in a similar way as has been postulated in other cells. For example, caveolines have recently been postulated as modulators of the activity of volume-regulated channels (Trouet et al. 1999) in different cell lines. This protein is the main protein component of caveolae, which are invaginations that maintain microdomains within the plasma membrane (Anderson, 1998). Caveolaes have been proposed as structures associated with the activation mechanism of the volume-regulated channels (Okada, 1997, 1999), providing a scaffolding on which these channels can be assembled in a pre-activated form. In the plasma membrane microdomain formed by these invaginations are located a variety of membrane proteins which are involved in cell signalling, such as membrane receptors, G proteins, and other signal transduction molecules (Anderson, 1998; Okamoto et al. 1998). These molecules might be involved in the regulation of ionic channels.
Here, we confirm that ICl,swell is strictly dependent on the presence of follicular cells and their coupling with the oocyte (Arellano & Miledi, 1993, 1995). Osmolarity-dependent activated channels are not recorded from the membrane of denuded oocytes that were defolliculated manually; therefore, these currents seem to originate totally in the membrane of the follicular cells.
Single channels activated by hyposmotic solutions in follicular cells have a conductance of about 5 pS and their I–V relation is linear in the range of ±100 mV. This single-channel conductance seemed to be smaller that the one recorded in other cells (∼10 pS), and similarly its linear I–V behaviour does not seem to be in accord with the outward rectification of other ICl,swell channels. Further experiments will be necessary to confirm these characteristics. Nevertheless, it has been noted previously that rectification observed for the osmolarity-dependent currents of the whole follicles is weaker than that shown in several other cell types (Voets et al. 1996). A possibility is that the weak outward rectification of the ICl,swell in follicles may result from other factors such as changes in the number and open-time of the channels at positive potentials. A singular characteristic of the channels was their prolonged open-time, especially when ACh or ATP was applied. This characteristic is similar to that of volume-sensitive organic osmolyte and anion channels which switch abruptly from a closed to an open state, where their probability to stay in this conformation is near unity (Jackson & Strange, 1995a). Although the single-channel currents recorded here were not directly shown to be part of the whole ICl,swell or Sin, they were consistently seen to be activated by hyposmotic conditions. Moreover, their Erev values and time course were coincident with that of osmolarity-dependent currents, and we believe that their participation is fundamental to the generation of these currents in the whole follicle. Studies detailing the electrophysiological and pharmacological characteristics of these ionic channels in follicular cells will help to determine their participation in generating total ICl,swell and Sin.
Lanthanum and Gd3+ appear to block ICl,swell through a voltage-independent mechanism, strongly suggesting that these ions are not acting within the pore of the channel. Contrary to this, the ATP blocking effect was voltage dependent and sensitive to the direction of the net Cl− flux, similar to what has been shown in other cells (Jackson & Strange, 1995b), suggesting in this case that the binding site for ATP is located in the channel pore. The binding site for the polycations may still be on the channel protein, but located in a domain that does not sense the transmembrane voltage field, i.e. in residues that do not form the pore. A possible explanation is that the binding of the lanthanides to an external domain of the channel protein favours a conformation in the closed state. Alternatively, the inhibition effect might involve a binding site that indirectly interferes with the channel activation mechanism. However, some observations suggest that the effect of lanthanides is complex and probably involves more than one site of action. For example, there is some variability observed using different superfusion protocols. Application of La3+ directly on ICl,swell inhibits the current with an IC50 of ∼100 μM (Arellano & Miledi, 1995), while here a short (0.5-1 min) preincubation of the follicle with the cation alone gave an IC50 of 17 μM. Similarly, it has been reported that in some circumstances the La3+ blocking effect is irreversible (Ackerman et al. 1994). A mechanism of inhibition produced by lanthanides which involves actions on the activation mechanism versus one acting in the channel pore, might also explain the fact that lanthanum did not inhibit Sin as potently as ICl,swell. Gd3+ seemed to be relatively more potent on ICl,swell in follicles held at -100 mV than at -40 or -60 mV, and more experiments are needed to define a possible weak voltage dependency.
In summary, in this study we show results which strongly suggest that in Xenopus follicles the activation of ICl,swell and Sin involves the opening of a single type of ionic channel, alternatively, the two currents might involve different but closely related channels, which conform to a class of volume-regulated chloride channels. It appears that ICl,swell channels are opened, or sensitised to the activation mechanism (swelling), via stimulation by neurotransmitters and hormones that act on specific receptors in the follicular cell membrane. For example, the sensitisation mechanism may facilitate the activation of the channels producing a shift to a lower cellular volume threshold, as has been suggested (Voets et al. 1998). This mechanism of regulation of ICl,swell channels via signalling pathways may be important for volume control of the cells, and thus affect different aspects of ovarian physiology. In addition, we show that the endogenous osmolarity-dependent channels are mainly located in the membrane of the follicular cells, as has been previously suggested (Arellano & Miledi, 1993, 1995; Voets et al. 1996). Therefore, the oocyte expression system is clearly suitable for further experiments on the structural and functional details of volume-regulated chloride channels.
Acknowledgments
We are grateful to Dr Michael Jeziorski for help with the manuscript, and to Dr Edith Garay and Horacio Ramirez Leyva for technical assistance. This work was supported by grants from UNAM-DGAPA (IN200198 and 209596) and from CONACYT-México (3713-PN) to R. O. Arellano, and from CONACYT (G25775 N) to R. Miledi. A. Pérez-Samartín thanks Gobierno Vasco/Eusko Jaurlaritza – España for grant support PPMPI- numbers 4/97073 and 2/98033.
References
- Ackerman MJ, Wickman KD, Clapham DE. Hypotonicity activates a native chloride current in Xenopus oocytes. Journal of General Physiology. 1994;103:153–179. doi: 10.1085/jgp.103.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RGW. The caveole membrane system. Annual Review of Biochemistry. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- Arellano RO, Garay E, Miledi R. Cl− currents activated via purinergic receptors in Xenopus follicles. American Journal of Physiology. 1998;274:C333–340. doi: 10.1152/ajpcell.1998.274.2.C333. [DOI] [PubMed] [Google Scholar]
- Arellano RO, Garay E, Miledi R. Muscarinic receptor heterogeneity in follicle-enclosed Xenopus oocytes. The Journal of Physiology. 1999;521:409–419. doi: 10.1111/j.1469-7793.1999.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano RO, Miledi R. Novel Cl− currents elicited by follicle stimulating hormone and acetylcholine in follicle-enclosed Xenopus oocytes. Journal of General Physiology. 1993;102:833–857. doi: 10.1085/jgp.102.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano RO, Miledi R. Osmo-dependent Cl− currents activated by cyclic AMP in follicle-enclosed Xenopus oocytes. Proceedings of the Royal Society. 1994;B258:229–235. doi: 10.1098/rspb.1994.0167. [DOI] [PubMed] [Google Scholar]
- Arellano RO, Miledi R. Functional role of follicular cells in the generation of osmolarity-dependent Cl− currents in Xenopus follicles. The Journal of Physiology. 1995;488:351–357. doi: 10.1113/jphysiol.1995.sp020971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano R, Woodward R, Miledi R. Ion Channels. Vol. 4. New York: Plenum Press; 1996. Ion channels and membrane receptors in follicle-enclosed Xenopus oocytes; pp. 203–259. [DOI] [PubMed] [Google Scholar]
- Carpenter E, Peers C. Swelling- and cAMP-activated Cl− currents in isolated rat carotid body type I cells. The Journal of Physiology. 1997;503:497–511. doi: 10.1111/j.1469-7793.1997.497bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N, Yekuel R, Oron Y. Acetylcholine promotes progesterone-induced maturation of Xenopus oocytes. Journal of Experimental Zoology. 1984;230:131–135. doi: 10.1002/jez.1402300117. [DOI] [PubMed] [Google Scholar]
- Dees WL, Hiney JK, Schultea TD, Mayerhofer A, Danilchik M, Dissen GA, Ojeda SR. The primate ovary contains a population of catecholaminergic neuron-like cells expressing nerve growth factor receptors. Endocrinology. 1995;136:5760–5768. doi: 10.1210/endo.136.12.7588334. [DOI] [PubMed] [Google Scholar]
- Doroshenko P, Penner C, Neher E. Novel chloride conductance in the membrane of bovine chromaffin cells activated by intracellular GTPγS. The Journal of Physiology. 1991;436:711–724. doi: 10.1113/jphysiol.1991.sp018575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont JN. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. Journal of Morphology. 1972;136:153–180. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Eusebi F, Pasetto N, Siracusa G. Acetylcholine receptors in human oocytes. The Journal of Physiology. 1984;346:321–330. doi: 10.1113/jphysiol.1984.sp015024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz S, Föhr KJ, Boddien S, Berg U, Brucker C, Mayerhofer A. Functional and molecular characterization of a muscarinic receptor type and evidence for expression of choline-acetyltransferase and vesicular acetylcholine transporter in human granulosa-luteal cells. Journal of Clinical Endocrinology and Metabolism. 1999;84:1744–1750. doi: 10.1210/jcem.84.5.5648. [DOI] [PubMed] [Google Scholar]
- Garcia J, Miledi R. Serotonergic modulation of muscle acetylcholine receptors of different subunit composition. Proceedings of the National Academy of Sciences of the USA. 1996;93:3990–3994. doi: 10.1073/pnas.93.9.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazama A, Shimizu T, Ando-Akatsuka Y, Hayashi S, Tanaka S, Maeno E, Okada Y. Swelling-induced, CFTR-independent ATP release from a human epithelial cell line. Journal of General Physiology. 1999;114:525–533. doi: 10.1085/jgp.114.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PS, Strange K. Single channel properties of a swelling-activated anion conductance: current activation occurs by abrupt switching of closed channels to an open state. Journal of General Physiology. 1995a;105:643–660. doi: 10.1085/jgp.105.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PS, Strange K. Characterization of the voltage-dependent properties of a volume-sensitive anion conductance. Journal of General Physiology. 1995b;105:661–677. doi: 10.1085/jgp.105.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada S, Blackmore PF, Oehninger S, Gordon K, Hogden GD. Existence of P2-purinoceptors on human and porcine granulosa cells. Journal of Clinical Endocrinology and Metabolism. 1994;78:650–656. doi: 10.1210/jcem.78.3.8126137. [DOI] [PubMed] [Google Scholar]
- Kusano K, Miledi R, Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. The Journal of Physiology. 1982;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RS, Ross PE, Cahalan MD. Chloride channels activated by osmotic stress in T lymphocytes. Journal of General Physiology. 1993;101:801–826. doi: 10.1085/jgp.101.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerhofer A, Smith GD, Danilchik M, Levine JE, Wolf DP, Dissen GA, Ojeda SR. Oocytes are a source of catecholamines in the primate ovary: evidence for a cell-cell regulatory loop. Proceedings of the National Academy of Sciences of the USA. 1998;95:10990–10995. doi: 10.1073/pnas.95.18.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X-J, Weinman SA. cAMP- and swelling-activated chloride conductance in rat hepatocytes. American Journal of Physiology. 1996;271:C112–120. doi: 10.1152/ajpcell.1996.271.1.C112. [DOI] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current evoked in Xenopus oocytes. Proceedings of the Royal Society B. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miledi R, Parker I, Sumikawa K. Fidia Research Foundation Neuroscience Award Lectures. New York: Raven Press; 1989. Transplanting receptors from brain into oocytes; pp. 57–90. [Google Scholar]
- Miledi R, Woodward RM. Effect of defolliculation on membrane responses of Xenopus oocytes. The Journal of Physiology. 1989;416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Progress in Biophysics and Molecular Biology. 1997;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- Nilius B, Voets T, Prenen J, Barth H, Aktories K, Kaibuchi K, Droogmans G, Eggermont J. Role of Rho and Rho kinase in the activation of volume-regulated anion channels in bovine endothelial cells. The Journal of Physiology. 1999;516:67–74. doi: 10.1111/j.1469-7793.1999.067aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. American Journal of Physiology. 1997;273:C755–789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Okada Y. A scaffolding for regulation of volume-sensitive Cl− channels. The Journal of Physiology. 1999;520:2. doi: 10.1111/j.1469-7793.1999.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing ‘preassembled signaling complexes’ at the plasma membrane. Journal of Biological Chemistry. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Schousboe A. Volume regulation in astrocytes: A role for taurine as an osmoeffector. Journal of Neuroscience Research. 1988;20:505–509. doi: 10.1002/jnr.490200415. [DOI] [PubMed] [Google Scholar]
- Robson L, Hunter M. Role of cell volume and protein kinase C in regulation of a Cl− conductance in single proximal tubule cells of Rana temporaria. The Journal of Physiology. 1994;480:1–7. doi: 10.1113/jphysiol.1994.sp020335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoblina MN, Huhtaniemi I. Involvement of chloride channels in progesterone production during meiotic maturation of follicle-enclosed oocytes of Rana temporaria and Xenopus laevis. Journal of Experimental Zoology. 1997;278:422–428. [PubMed] [Google Scholar]
- Sporrong B, Kannisto P, Owman C, Sjöberg NO, Walles B. Histochemistry and ultrastructure of adrenergic and acetylcholinesterase-containing nerves supplying follicles and endocrine cells in the guinea-pig ovary. Cell and Tissue Research. 1985;240:505–511. doi: 10.1007/BF00216338. [DOI] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume sensitive anion channels. American Journal of Physiology. 1996;270:C711–730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Taylor AL, Kudlow BA, Marrs KL, Gruenert DC, Guggino WB, Schwiebert EM. Bioluminescence detection of ATP release mechanisms in epithelia. American Journal of Physiology. 1998;275:C1391–1406. doi: 10.1152/ajpcell.1998.275.5.C1391. [DOI] [PubMed] [Google Scholar]
- Trouet D, Nilius B, Jacobs A, Remacle C, Droogmans G, Eggermont J. Caveolin-1 modulates the activity of the volume-regulated chloride channel. The Journal of Physiology. 1999;520:113–119. doi: 10.1111/j.1469-7793.1999.t01-1-00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Hoef MHF, Dictus WJAG, Hage WJ, Bluemink JG. The ultrastructural organization of gap junctions between follicle cells and the oocyte in Xenopus laevis. European Journal of Cell Biology. 1984;33:242–247. [PubMed] [Google Scholar]
- Voets T, Buyse G, Tytgat J, Droogmans G, Eggermont J, Nilius B. The chloride current induced by expression of the protein pICln in Xenopus oocytes differs from the endogenous volume-sensitive chloride current. The Journal of Physiology. 1996;495:441–447. doi: 10.1113/jphysiol.1996.sp021605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Manolopoulos V, Eggermont J, Ellory C, Droogmans G, Nilius B. Regulation of a swelling-activated chloride current in bovine endothelium by protein tyrosine phosphorylation and G proteins. The Journal of Physiology. 1998;506:341–352. doi: 10.1111/j.1469-7793.1998.341bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull AM. Ionic blockage of sodium channels in nerve. Journal of General Physiology. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward RM, Miledi R. Hormonal activation of membrane currents in follicle-enclosed Xenopus oocytes. Proceedings of the National Academy of Sciences of the USA. 1987;84:4135–4139. doi: 10.1073/pnas.84.12.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell RT, Butt AG, Cliff WH, Frizzell RA. A volume-sensitive chloride conductance in human colonic cell line T84. American Journal of Physiology. 1989;256:C1111–1119. doi: 10.1152/ajpcell.1989.256.6.C1111. [DOI] [PubMed] [Google Scholar]


