Abstract
Detection of the specific Salmonella serovar Gallinarum, which is divided into the biovars Pullorum and Gallinarum, is compulsory under the national hygienic and sanitary control regulations of France for breeding flocks whose offspring are exported. Our aim was to examine the suitability of bacteriologic and serologic methods routinely used in France to screen serum samples and organs for S. Gallinarum. Since bacteriologic reference techniques are designed to isolate the commonly occurring non-typhoid serovars, such as S. Typhimurium, S. Enteritidis, and others that cause outbreaks of foodborne illness, they may not be particularly suitable for detecting S. Pullorum and S. Gallinarum. This hypothesis was confirmed by the inoculation of 10-wk-old chickens and 1-d-old chicks with various strains of S. Pullorum and S. Gallinarum. The most reliable enrichment media were selenite cystine and Rappaport-Vassiliadis broths. Moreover, on the usual plating media, colonies were small, grew more slowly than the common serovars (in 48 h instead of 24 h), and had an unusual appearance. Since the rapid slide agglutination (RSA) test is based only on antigens from standard and variant strains of S. Pullorum, it may not readily detect S. Gallinarum. In our study, it detected infection in all 10-wk-old chickens inoculated with S. Pullorum strains but did not detect any antibodies against S. Gallinarum. Therefore, S. Gallinarum antigens must be added to the S. Pullorum antigens used in the RSA test in order to detect antibodies produced by birds infected with either biovar.
Introduction
The Salmonella serovar Gallinarum may be divided into biovars Gallinarum and Pullorum, which are respectively responsible for fowl typhoid and the Pullorum disease of breeding flocks. Fowl typhoid is a disease of mature fowl that results in either acute enteritis with greenish diarrhea or a chronic disease of the genital tract that reduces egg production. Pullorum disease causes a high mortality rate (50% to 100%) among embryos and then chicks, as well as weakness and white diarrhea. Subacute, acute, or chronic clinical signs may follow one another.
Even if these diseases were virtually eradicated in France, a search for the S. Gallinarum serovar and its biovar Pullorum is compulsory under the national hygienic and sanitary control regulations for breeding flocks whose offspring are exported. Authorities must be aware of the risk of a new infection by these strains, which are responsible for considerable economic loss for poultry production in South and Central America, the Middle East, Africa, the Pacific Rim, and some parts of Southern Europe (1,2,3,4). Outbreaks in an integrated broiler operation were also reported in 1990 in the United States (5,6): a grandparent male-line breeding flock contaminated 19 parent flocks and 261 roaster flocks in 5 southern states. The same problem occurred in Germany in 1992 and in Denmark during the same period (7), probably because of a truck that transported hens at the end of the laying period.
The sampling and the bacteriologic analyses used currently are mainly indicated to find the ubiquitous Salmonella serovars that induce food poisoning. But both S. Gallinarum and S. Pullorum are highly adapted to the species (including chickens, turkeys, pheasants, and quail) and have little, if any, public health significance (1). The aim of this study was to determine whether S. Gallinarum and S. Pullorum can be found by routine bacteriologic methods in 10-wk-old chickens and 1-d-old chicks inoculated intramuscularly (IM) or orally, respectively, with 108 colony-forming units (CFU) of various strains.
A rapid slide agglutination (RSA) test, based on S. Pullorum antigens from standard (O: 1, 9, 121, and 123) and variant (O: 1, 9, 121, and 122) strains, is used in France in the surveillance program for S. Pullorum and S. Gallinarum. This traditional easy and cheap test, developed by Runnells et al (8), assisted in the control or elimination of fowl typhoid and Pullorum disease in France and elsewhere when used as a flock test (9). But it can produce variable and erratic results (2,3), such as non-specific reactions, and a lack of sensitivity is also suspected. Therefore, we tested its specificity and sensitivity in experimentally immunized 10-wk-old chickens.
Materials and methods
Salmonella strains
Twelve strains were tested: 11 S. Pullorum (SP) strains and 1 S. Gallinarum (SG) strain. Six SP strains were provided by the Veterinary Laboratories Agency (Weybridge, England): 3 strains (1168/isolated in 2000, 3116/99, and 8534/99) had been found in chickens and 3 others (5233/98, 5299/99, and 6007/99) had been isolated from pheasants. One other SP strain (711/95) had contaminated a familial flock of hens in France, and a further SP strain had been found in hens in Pakistan. The other 4 strains were provided by the National Veterinary Services Laboratories (Ames, Iowa, USA) and used as reference strains: SP standard (SPS 17368/97), SP variant (SPV 1635/97), and SP intermediate (SPI 1637/97) strains, as well as an SG strain (SG 7995/92).
Biochemical characteristics (Table I)
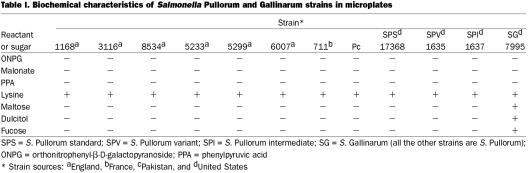
Table I.
The biochemical characteristics of all strains were tested. First, 160 μL of physiological water was placed in each well of 2 flat-bottom microplates (Nunc, Roskilde, Denmark). Then 1 μL of Kligler-Hajna (KH) medium was added to each well of 1 microplate, and a drop of orthonitrophenyl-β-D-galactopyranoside (ONPG) (Sigma, Steinheim, Germany) was added to each well containing colonies. The microplate was incubated at 37°C and read after half an hour and again after 2 h. When a Salmonella strain produces β-galactosidase, the suspension becomes yellow and the reaction is considered positive; when the strain does not have this enzyme, the suspension remains uncolored. Most Salmonella strains are ONPG negative. With the 2nd microplate, 80 μL of malonate (Difco, Detroit, Michigan, USA), 160 μL of phenylpyruvic acid reactant (PPA) (Difco), or 220 μL of lysine decarboxylase (Difco) was added to the wells, and the microplate was incubated for 24 h at 37°C. A strain using malonate gives a blue coloration (basic medium) and is considered malonate positive, whereas when the medium remains green (neutral), the strain is considered malonate negative. Salmonella strains are malonate negative. After the incubation, 1 or 2 drops of PPA reactant was added to reveal PPA produced by the considered strain. When green coloration appears, the strain is considered PPA positive, whereas when the medium remains uncolored, the strain is considered PPA negative. Salmonella strains are PPA negative, as they do not produce PPA by using phenylalanine. A strain positive for lysine decarboxylase produces cadaverine, and the broth becomes purple (basic medium), whereas a negative strain colors the broth yellow (acid medium), owing to the use of glucose. Salmonella strains are lysine positive except for S. Paratyphi. Strains were also tested for the use of maltose (Sigma), dulcitol (Fluka-Sigma-Aldrich Chimie, St-Quentin-Fallavier, France), and L(-)fucose (Sigma): for each analysis, 160 μL of a 1% dilution of the sugar in purple broth base (Difco) was added to each well. The biovars are differentiated by their use of sugars as maltose: Gallinarum uses dulcitol and fucose, but Pullorum does not.
Inoculation
The Salmonella strains were stocked at −70°C in a brain–heart infusion (AES, Combourg, France) containing 20% glycerol (Acros Organics, a Fisher Scientific Worldwide company, Springfield, New Jersey, USA). TSA-YE agar (AES) was used to enhance colonies after 24 h of incubation at 37°C. The purity of the strain was verified, and 1 colony was transferred to TSB-YE broth (AES), which was warmed at room temperature and then incubated for 20 h at 37°C. The broth was diluted to concentrations of 10−1 to 10−7 with salt tryptone, and 50 μL from the 10−5, 10−6, and 10−7 dilutions were distributed with a spiral plater (AES) onto 2 TSA-YE agar plates per dilution. Because the TSB-YE broth contained 5 × 108 CFU/mL, the chickens were inoculated IM with 1 mL of a 1:5 dilution in physiological water, whereas the chicks received 200 μL of pure TSB-YE broth orally.
Growth capacity
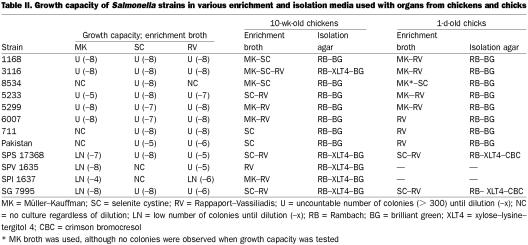
Müller-Kauffman (MK) broth (AES), selenite cystine (SC) broth (AES), and Rappaport–Vassialidis (RV) broth (Oxoid, Basingstoke, England) were tested for Salmonella strain enrichment. The TSB-YE broth containing colonies was diluted from 10−1 to 10−8 with salt tryptone, and the 10−4 to 10−8 dilutions were distributed into 2 or 3 tubes of the media as follows: MK broth, 1:10 dilution; selenite broth, 1:10 dilution; and RV broth, 1:100 dilution. The media were incubated at 42°C, 37°C, and 42°C, respectively, for 18 to 24 h. After that, 50 μL of each medium was distributed with a spiral plater onto TSA-YE agar. Growth capacity is presented for each strain in Table II. The enrichment media were chosen when the TSA-YE agars corresponding to the highest dilutions of Salmonella contained more than 300 colonies and were considered uncountable. MK and RV broth were preferred over SC broth, as SC broth was outdated after 1 d.
Table II.
Experimental design
Experiment 1 — Twelve groups of specific-pathogen-free (SPF) 10-wk-old chickens were inoculated IM with 108 CFU of Salmonella contained in 1 mL of TSB-YE broth diluted 1:5 with physiological water. This route was chosen since at this age the competitive flora may inhibit Salmonella. Consequently, the immune system may not be stimulated. A control group of 12 chickens received 1 mL of physiological water. Blood, feces, and environmental swabs were sampled weekly for 4 wk. Organs (liver, spleen, cecal tonsils, and ovaries or testicles) were analyzed when the birds were slaughtered, at 13 wk of age.
Experiment 2 — Ten groups of 1-d-old SPF chicks were orally inoculated with 108 CFU of Salmonella contained in 200 μL of TSB-YE. Another group of 10 chicks received 200 μL of sterile TSB-YE. Survivors were sacrificed at 8 d of age and organs (liver, spleen, and cecal tonsils) collected for bacteriologic analysis.
Serologic analysis
Serum was tested with an RSA test produced by Analysis and Development Laboratory 22 of the Agence Française de Securité Sanitaire des Aliments (AFSSA)-Ploufragan (Ploufragan, France). The test is based on somatic antigens of 2 strains of S. Pullorum: antigens O: 1, 9, 121, and 123 of a standard strain and antigens O: 1, 9, 121, and 122 of a variant strain. Serum was also analyzed with an enzyme-linked immunosorbent assay (ELISA) previously developed by the AFSSA laboratory on the basis of lipopolysaccharide (LPS) antigens of S. Typhimurium (O: 1, 4, 5, and 12) and S. Enteritidis (O: 1, 9, and 12) (10). In addition, 4 specific ELISAs were developed with S. Pullorum standard, variant, and intermediate strains and with S. Gallinarum to test the homologous serum samples. Strains were cultivated overnight at 37°C in 50 mL of buffered peptone water (BPW) (AES) and centrifuged for 30 min at 12 000 × g.
Bacteriologic analysis
Experiment 1 — As recommended by the routine reference method used in France, 50 g of feces was diluted 1:5 with BPW, whereas 100 mL of BPW was added to each environmental swab. The samples were incubated for 16 to 24 h at 37°C. With the reference method, MK and another enrichment media, such as SC and RV broth, must be used. We used MK, SC, and RV broth after 1:10, 1:10, and 1:100 dilution, respectively. MK and RV broth were incubated for 24 h at 42°C, whereas SC broth was incubated at 37°C for 24 h. For the reference method, 4 isolation agars may be used: Rambach, xylose–lysine–tergitol 4 (XLT4), SMID, or Hektoen agar. We plated 10 μL of suspension on Rambach (Merck, Darmstadt, Germany), XLT4 (Biokar, Beauvais, France), and brilliant green (BG) (AES) agar. BG agar is less selective than SMID and Hektoen agar. The agar plates were incubated for 24 to 48 h at 37°C. Liver, spleen, and genital tract were diluted 1:2 in BPW and then cut into small pieces. Then 10 μL was distributed on crimson bromocresol (CBC) (AES) for direct isolation and incubated for 24 to 48 h at 37°C. Half-diluted BPW was diluted again to reach a final dilution of 1:10, and bags were incubated for 16 to 20 h at 37°C. One to 3 of MK, SC, and RV were used as enrichment media, depending on the growth capacity of each strain; 10 μL of the medium was distributed onto Rambach and BG agar for all the strains and onto XLT4 agar for the US strains and the 3116 strain. Dilution, temperature, and time of incubation were the same as described for the feces and environmental swabs. Characteristic colonies were biochemically tested for glucose, lactose, hydrogen sulfide, and gas on KH medium incubated for 24 h at 37°C. The colonies were small (≤ 2 mm); they were light pink or salmon-colored on Rambach agar and translucent on BG and XLT4 agar.
Experiment 2 — Liver, spleen, and cecum samples from the chicks were diluted with 5 mL of BPW and incubated for 16 to 20 h at 37°C. Enrichment was done with 1 or 2 media (MK, SC, or RV). Most of the colonies were isolated on Rambach and BG agar, but for S. Pullorum variant and S. Gallinarum strains 3 media were used: Rambach, XLT4, and CBC. For the chicks that died following inoculation with S. Pullorum standard and S. Gallinarum strains, liver and spleen samples were directly plated on CBC, whereas cecum samples were directly plated on Rambach and XLT4 agar.
Results
Clinical signs
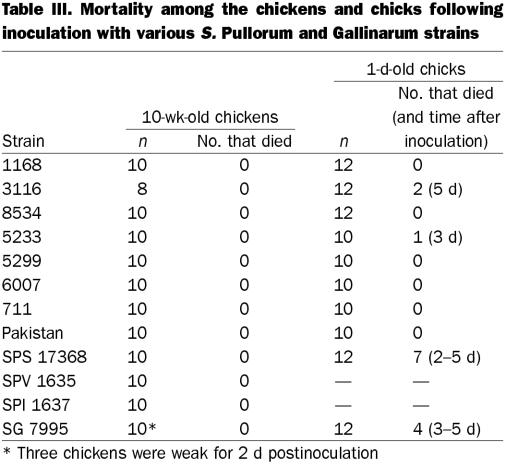
Transitory weakness occurred for 1 or 2 d in 3 of the 10-wk-old chickens inoculated with S. Gallinarum. All the chickens were alive at the end of the experiment, whereas a few chicks died no later than 5 d postinoculation (Table III). The yolk sac was still present and the spleen was normal in most of these chicks, but a few had a mottled liver and white, clay-like cecal contents.
Table III.
Serologic results
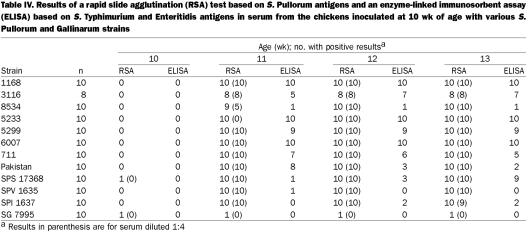
With the RSA, 2 chickens were found to be positive before inoculation when the serum was tested full-strength but not when it was tested at a 1:4 dilution. The RSA was positive in almost all the serum samples (full-strength or diluted) from birds inoculated with the S. Pullorum strains. All of the birds inoculated with the S. Gallinarum strain were negative in tests with a 1:4 dilution (Table IV).
Table IV.
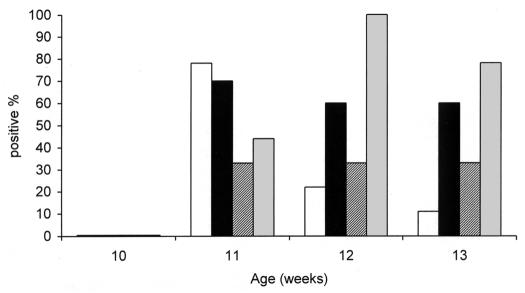
The ELISA based on antigens from S. Typhimurium and S. Enteritidis was positive for all or some of the chickens inoculated with the S. Pullorum strains but none of those inoculated with the S. Gallinarum biovar (Table IV). At least 30% of the birds were seropositive with the ELISA specific for the strain with which they were inoculated, and all the birds inoculated with the S. Gallinarum strain were seropositive with the SG-specific ELISA 2 wk after inoculation (Figure 1).
Figure 1. Percentage of chickens seropositive in the enzyme-linked immunosorbent assays specific for Salmonella Pullorum standard (white bars), variant (black bars), and intermediate (striped bars) strains, or S. Gallinarum (gray bars).
Bacteriologic results
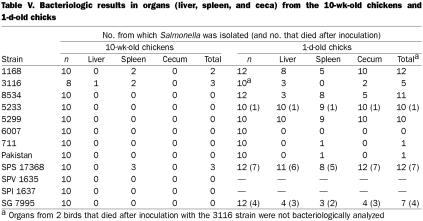
Experiment 1 — All the control birds remained free of Salmonella until the end of the experiment. Although various techniques were used, depending on the growth capacity of each strain, Salmonella was isolated only from the liver or spleen (Table V). Feces and environmental swabs from the room of the inoculated chickens were never found to be contaminated.
Table V.
Experiment 2 — All organs were uncontaminated in the control chicks at 1 wk of age. The organs of all the chicks that died after oral inoculation were contaminated by Salmonella; the cecum was the most contaminated organ. The survivors were less contaminated than the birds that died after inoculation, and Salmonella was also mainly isolated from the cecum of the survivors. Only 3 of the 8 chicks inoculated with S. Gallinarum and only 5 of the 10 chicks inoculated with the 3116 strain were bacteriologically positive (Table V). The strains originating from pheasants (5233, 5299, and 6007) were isolated from all 1-d-old inoculated chicks.
Discussion
No contamination was detected in the feces or the environmental swabs collected in the room where the 10-wk-old inoculated chickens were housed. As discussed by Berchieri, Iba, and Barrow (9), unlike other Salmonella serotypes, Pullorum and Gallinarum are not excreted extensively in the feces. In fact, later, Berchieri and associates (11) showed that excretion depended on the susceptibility of the fowl line to Salmonella infection. Whereas transmission of infection between birds in susceptible lines may be primarily horizontal, through ingestion of feces and mucus containing S. Gallinarum and through cannibalism, vertical transmission is much more likely to occur in lines that are genetically resistant to fowl typhoid. Vertical transmission may result directly from contamination of the egg in the genital tract or indirectly from chick-to-chick contact in the hatchery (12). These findings led to 2 conclusions: most of the time, limited environmental contamination may decrease the risk of horizontal infection; however, if, as in France, surveillance is based on bacteriologic analysis of feces and environmental swabs, it will not be easy to isolate S. Pullorum or S. Gallinarum in the absence of clinical signs.
Among the chicks, the mortality rate was high, and contamination of the organs was revealed on non-selective media. In contrast, among the 10-wk-old adults, clinical signs were not clearly observed, there were no deaths, and the organs were poorly contaminated. Salmonella Pullorum and S. Gallinarum strains were rarely isolated, their growth capacity depending on the enrichment media. Considering the uncountable number of colonies on the isolation agars following the use of enrichment media, either SC or RV medium may be recommended. However, the SC medium must be discarded after 1 d and is not routinely used to isolate other serovars; RV medium may, therefore, be the most reliable broth. Semi-solid RV is used for ubiquitous motile Salmonella, whose flagella allow migration through this medium. Obviously, this medium is not reliable for a non-motile serovar, such as S. Gallinarum. Moreover, the colonies were small on the isolation media, they grew slowly (most were detected after 48 h of incubation), and their appearance was unusual: uncolored or salmon-colored on Rambach agar, translucent without a dark center on XLT4 agar. These problems may cause delayed isolation from breeding flocks, with resultant spread through offspring.
Consequently, further serologic screening of breeding flocks must be recommended, although sensitivity may be an issue. During the outbreak in the 90s in the United States, a particularly disturbing outcome of the investigation was the isolation of S. Pullorum from cultured tissues taken from parent flocks that had earlier tested negative in RSA screening (6). This suggests that either the flock became infected after testing or the testing failed to detect existing infection (13). In fact, detection depended on the particular strain. Gast (13) observed that the agglutination tests prescribed by the National Poultry Improvement Plan identified chickens experimentally infected with recent field isolates of S. Pullorum; however, infections with variant or intermediate strains were less often detected serologically than were infections with the standard strain. Consequently, the RSA based on antigens from both standard (O: 1, 9, 121, and 123) and variant (O: 1, 9, 121, and 122) strains of S. Pullorum was used in the serologic testing program (12). Similarly, we observed a lack of sensitivity. The RSA detected all antibodies against S. Pullorum but did not detect antibodies against S. Gallinarum. Theoretically, S. Pullorum and S. Gallinarum are members of the same serotype and have the same antigens (O: 1, 9, and 12). Although ribotyping (14) and plasmid profiles (15) differentiate S. Gallinarum strains, most of the strains are very similar in chromosomal markers (16). Thus, a new RSA must be developed and tested. At the least, antigens from S. Gallinarum must be added to the pool of antigens. Since we tested only 1 S. Gallinarum strain, as soon as the new RSA, based on antigens from S. Pullorum variant and standard strains as well as Gallinarum antigens, is developed, it should be tested on other strains.
The lack of specificity of the RSA was resolved by analysis of serum diluted 1:4. The non-specific reactions had previously been observed and may be attributed to cross-reactions with Escherichia coli antigen, probably due to the somatic antigen O:122 (17,18).
On the other hand, the reference ELISA, based on S. Typhimurium and S. Enteritidis antigens, partially detected S. Pullorum antibodies. This may be explained by the fact that S. Pullorum and S. Gallinarum have somatic antigens in common with S. Enteritidis (O: 1, 9, and 12). Barrow, Berchieri, and Al-Haddad (19) showed that either soluble protein or LPS could be used to develop an ELISA that would detect experimental S. Pullorum and S. Gallinarum infections. As our reference ELISA, based on S. Typhimurium and S. Enteritidis LPS, did not detect antibodies against S. Gallinarum, we decided to develop ELISAs specific to S. Pullorum or S. Gallinarum. Although the RSA did not detect S. Gallinarum antibodies, the specific ELISA based on S. Gallinarum antigens did, in 100% of the serum samples from infected birds; all the birds were producing anti-S. Gallinarum antibodies as early as 2 wk after inoculation.
Finally, we observed that strains isolated from pheasants were not strictly host-adapted, as they infected all of the 1-d-old chicks.
In conclusion, Pullorum disease and fowl typhoid have a high economic cost to fowl production and, although the risk of infection is low, the surveillance program for S. Pullorum and S. Gallinarum strains must be reliable. From our preliminary results, the addition of S. Gallinarum antigens is recommended, but, because we tested only 1 S. Gallinarum strain, additional strains must be tested.
Footnotes
Acknowledgments
We thank Yannick Morin and his staff for taking care of the animals, and Carole Guillemoto and Isabelle Pierre for their technical support.
Address correspondence and reprint requests to Dr Karine Proux, tel: 00-33-96-01-62-77, fax: 00-33-96-01-62-63, e-mail: k.proux@ploufragan.afssa.fr
Received December 12, 2001. Accepted April 2, 2002.
References
- 1.Pomeroy BS, Nagaraja KV. Fowl typhoid. In: Calnek BW, Barnes HJ, Beard CW, Reid WM, Yoder HW, eds. Diseases of Poultry. 9th ed. Ames, Iowa: Iowa State University Press, 1991:87–99.
- 2.Snoeyenbos GH. Pullorum disease. In: Calnek BW, Barnes HJ, Beard CW, Reid WM, Yoder HW, eds. Diseases of Poultry. 9th ed. Ames, Iowa: Iowa State University Press, 1991:73–86.
- 3.Silva EN. The Salmonella Gallinarum problem in Central and South America. Proc Intern Symp Salmonella 1985:150–156.
- 4.Sato Y, Sato G, Tuchili L, et al. Status of Salmonella Gallinarum–Pullorum infections in poultry in Zambia. Avian Dis 1997;41: 490–495. [PubMed]
- 5.Salem M, Odor EM, Pope C. Pullorum disease in Delaware roasters. Avian Dis 1992;36:1076–1080. [PubMed]
- 6.Johnson DC, David M, Goldsmith S. Epizootiological investigation of an outbreak of Pullorum disease in an integrated broiler operation. Avian Dis 1992;36:770–775. [PubMed]
- 7.Christensen JP, Skov MN, Hinz KH, Bisgaard M. Salmonellaenterica serovar Gallinarum biovar Gallinarum in layers: epidemiological investigations of a recent outbreak in Denmark. Avian Pathol 1994;23:489–501. [DOI] [PubMed]
- 8.Runnells RA, Coon CJ, Farley H, Thorp F. An application of the rapid method agglutination test to the diagnosis of bacillary white diarrhoea infection. J Am Vet Med Assoc 1927;70:660–667.
- 9.Berchieri A Jr, Iba AM, Barrow PA. Examination by ELISA of sera obtained from chicken breeder and layer flocks showing evidence of fowl typhoid or Pullorum disease. Avian Pathol 1995;24:411–420. [DOI] [PubMed]
- 10.Proux K, Jouy E, Houdayer C, et al. Reliable ELISAs showing differences between resistant and susceptible lines in hens orally inoculated with Salmonella Enteritidis. Vet Res 2002;33:23–33. [DOI] [PubMed]
- 11.Berchieri A Jr, de Oliveira GH, Soeiro Pinheiro LA, Barrow PA. Experimental Salmonella Gallinarum infection in light laying hen lines. Braz J Microbiol 2000;31:50–52.
- 12.Shivaprasad HL. Fowl typhoid and Pullorum disease. Rev Sci Tech Off Intern Epiz 2000;19:405–424. [DOI] [PubMed]
- 13.Gast RK. Detecting infections of chickens with recent Salmonella Pullorum isolates using standard serologic methods. Poultry Sci 1997;76:17–23. [DOI] [PubMed]
- 14.Christensen JP, Olsen JE, Bisgaard M. Ribotypes of Salmonella enterica serovar Gallinarum biovars Gallinarum and Pullorum. Avian Pathol 1993;22:725–738. [DOI] [PubMed]
- 15.Christensen JP, Olsen JE, Hansen HC, Bisgaard M. Characterization of Salmonella enterica serovar Gallinarum biovars Gallinarum and Pullorum by plasmid profiling and biochemical analysis. Avian Pathol 1992;21:461–470. [DOI] [PubMed]
- 16.Olsen JE, Skov MN, Christensen JP, Bisgaard M. Genomic lineage of Salmonella enterica serotype Gallinarum. J Med Microbiol 1996;45:413–418. [DOI] [PubMed]
- 17.Burton WH, Garrard EH. Non-Pullorum agglutination reactions — IV: Reactions with Pullorum antigen from fowl inoculated with coliform types. Can J Comp Med 1948;12:20. [PMC free article] [PubMed]
- 18.Papageorgiou C, Valette L, Beranger G, Joubert L. Spécificité, fidélité et sensibilité des antigènes pulloriques de dépistage. Bull Acad Vet 1968;90:107–117. [PubMed]
- 19.Barrow PA, Berchieri A Jr, Al-Haddad O. Serological response of chickens to infection with Salmonella Gallinarum–S. Pullorum detected by enzyme-linked immunosorbent assay. Avian Dis 1992;36:227–236. [PubMed]