Abstract
Motor unit firing rates and voluntary activation of muscle decline during sustained isometric contractions. After exercise, the responses to motor cortical and corticospinal stimulation are reduced. These changes may reflect motoneuronal inhibition mediated by group III and IV muscle afferents. To determine whether the post-contraction depression of the responses to corticospinal or motor cortical stimulation could be maintained by continued firing of ischaemically sensitive group III and IV muscle afferents, we examined responses in muscles that were held ischaemic after exercise.
Following a sustained maximal voluntary contraction (MVC) of the elbow flexors lasting 2 min, the response to stimulation of the corticospinal tract was reduced but the usual recovery (over ∼2 min) was not delayed when the muscles were maintained ischaemic for 2 min after the contraction.
Following a sustained MVC, the time course of the reduction in the response to motor cortical stimulation (a gradual decrease over ∼2 min, maintained for > 10 min) was also not altered if the muscle was held ischaemic.
Mean arterial blood pressure rose to 155 ± 12 mmHg during the 2 min MVC, declined to 125 ± 9 mmHg immediately after it, but remained at this level without returning to pre-exercise levels (102 ± 10 mmHg) until circulation to the arm was restored. This confirms that the sustained MVC activated a reflex dependent on group III and IV muscle afferents.
This study shows that ischaemically sensitive group III and IV muscle afferents do not mediate depression of responses to motor cortical or corticospinal stimulation after fatiguing exercise. It also suggests that firing of such afferents does not directly inhibit motoneurones or motor cortical output cells.
Muscles are richly innervated by group III and IV muscle afferents. These afferents respond to a range of chemical and mechanical changes within the muscle. In particular, potassium, lactic acid, bradykinin and arachidonic acid activate many group III and IV muscle afferents (e.g. Mense, 1977; Kniffki et al. 1978; Rotto & Kaufman, 1988) with minimal effect on group I muscle afferents (e.g. Mense, 1977; Prochazka & Somjen, 1986). During a contraction, the activity of many group IV afferents may take some seconds to develop but it is enhanced by muscle ischaemia (e.g. Kaufman et al. 1983, 1984; Mense & Stahnke, 1983; Adreani & Kaufman, 1998).
It is widely believed that the central actions of group III and IV muscle afferents involve direct inhibition of the activity of human motoneurones during voluntary contractions, although the mechanism through which this may be achieved is poorly understood (for review see Garland & Kaufman, 1995). If present, such an effect would limit the firing frequencies of motor units during fatiguing contractions and would reduce the effectiveness of voluntary ‘drive’ to motoneurones in fatigue. There is evidence that these changes occur during sustained maximal voluntary contractions (MVCs) in human subjects.
First, during sustained maximal isometric contractions the discharge frequency of motor units declines (e.g. Marsden et al. 1983; Bigland-Ritchie et al. 1983; Gandevia et al. 1990). This decline occurs together with a slowing of contractile speed of the whole muscle (e.g. Bigland-Ritchie & Woods, 1984). Importantly, if muscle ischaemia is maintained after the effort with a sphygmomanometer cuff, the firing rates of motoneurones do not recover (e.g. Bigland-Ritchie et al. 1986; Woods et al. 1987). The decline in motoneuronal output is absent when muscle afferent feedback is prevented from reaching the spinal cord (Gandevia et al. 1990; Macefield et al. 1993). In addition, the tendon jerk and H-reflex are diminished in fatigue (Garland & McComas, 1990; Balestra et al. 1992; Duchateau & Hainaut, 1993). Second, voluntary activation of muscles during an isometric contraction declines despite being driven by ‘maximal’ effort. This has been measured from force increments produced during the effort by motor nerve stimulation (e.g. Woods et al. 1987; McKenzie & Gandevia, 1987; McKenzie et al. 1992; Gandevia et al. 1996). A plausible explanation for all these findings is that the input from ischaemically sensitive group III and IV afferents reduces motoneuronal output in fatigue.
There are observations which suggest that human muscle fatigue is accompanied by a depression in the effectiveness of descending outputs to motoneurones. Responses to transcranial stimulation of the motor cortex are depressed in relaxed muscles for many minutes after fatiguing exercise (e.g. Brasil-Neto et al. 1993; Zanette et al. 1995; McKay et al. 1995; Liepert et al. 1996; Gandevia et al. 1999), while recent evidence shows that the responses to stimulation of the corticospinal tract at the cervicomedullary junction are depressed for about 2 min after a maximal contraction (Gandevia et al. 1999).
We have previously demonstrated that EMG responses to transcranial magnetic stimulation elicited during brief maximal contractions after fatiguing exercise are not affected by maintained firing of group III and IV afferents although the force increments elicited by the stimulus are (Gandevia et al. 1996; Taylor et al. 1996). We concluded that group III and IV afferents acted ‘upstream’ of the motor cortex to prevent 100 % voluntary activation but did not affect motor cortical or motoneuronal responses during MVC. However, stimulation of the cortex during voluntary contraction is not an adequate test of the actions of afferents at a segmental level during fatigue. Neither the level of descending drive to the motoneurones nor the corticofugal volleys evoked by cortical stimulation can be controlled. Thus, increased descending drive while insufficient to activate the muscle fully could mask a reflex inhibition of the motoneurones.
The present studies were designed to look for an inhibition of the motoneurones of elbow flexor muscles by group III and IV muscle afferents excited by sustained voluntary contractions. The responses of motoneurones were tested during relaxation so that changes in descending input were minimized while occlusion of the blood supply to and from the muscle prevented recovery from fatigue. The metabolic status of the muscle does not improve during post-contraction ischaemia. Force output from the muscle does not recover, neither does voluntary activation nor motor unit firing rate. Firing of ischaemically sensitive muscle afferents continues even with the muscle at rest (Kaufman et al. 1984; see also McCloskey & Mitchell, 1972). Thus, a reflex inhibition of motoneurones related to the fatigued state of the muscle should be prolonged by ischaemia, and should not recover until blood flow resumes.
There were two test inputs. The first was derived from an electrical stimulus at the cervicomedullary junction, which is likely to evoke a single excitatory corticospinal volley (Ugawa et al. 1991; Gandevia et al. 1999). This input is not subject to presynaptic inhibition (Nielsen & Petersen, 1994). The second input was generated by transcranial magnetic stimulation over the motor cortex. The effectiveness of these inputs was assessed after a sustained MVC with the muscle relaxed but held ischaemic, and the results compared with those when there was no post-contraction ischaemia (Gandevia et al. 1999). Because prior activity in muscle fibres alters the size of their action potentials (e.g. Cupido et al. 1996; Taylor et al. 1999), supramaximal stimuli to the brachial plexus were also delivered to assess the relative size of the test responses. Preliminary findings have been reported previously (Petersen et al. 1998).
METHODS
Subjects
Seven healthy volunteers (3 females; age, 27–45 years) were studied. Written informed consent was obtained from the subjects, and the studies were approved by the local ethics committee and conducted according to the Declaration of Helsinki. The subject sat comfortably at a table with the shoulders flexed and the elbows flexed to 90 deg (Fig. 1). During the experiment each subject performed a single sustained maximal voluntary contraction (MVC) of the right elbow flexor muscles. This maximal isometric elbow flexion lasted 2 min and subjects received continuous visual feedback and verbal encouragement throughout. No strong contractions were undertaken by the subjects in the 1–2 h preceding the studies. As many of the procedures were similar to those recently described (Gandevia et al. 1999), only a brief description is given below.
Figure 1. Experimental protocol.
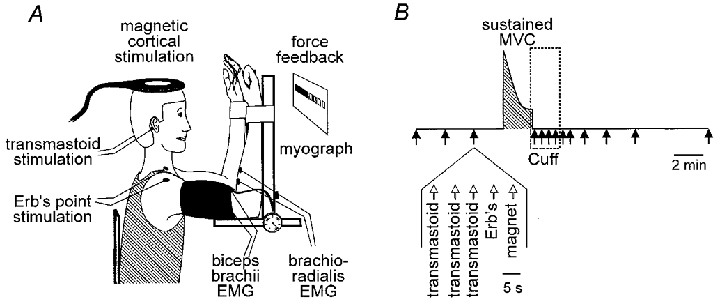
A, the experimental set-up, showing the position of stimulating and recording electrodes. B, the hatched region denotes the period of the maximal voluntary contraction (MVC) and the box denotes the period of ischaemia produced by inflation of a sphygmomanometer cuff just before the end of the contraction (Cuff). The timing of each ‘set’ of stimuli is shown by the arrows. The various stimuli within each set are also shown.
Recordings and stimulation
Surface electromyographic (EMG) activity was recorded with electrodes overlying biceps brachii and brachioradialis (Ag-AgCl discs, 10 mm diameter) firmly stuck to the skin (bandpass, 1.6 Hz to 1 kHz). The isometric force produced by MVC of the right elbow flexors was measured with a linear strain gauge (Xtran).
EMG responses to stimulation at three sites were measured: the motor cortex, corticospinal tract and motor axons. Transcranial magnetic stimuli were delivered via a circular coil (13.5 cm outer diameter) positioned over the vertex (Magstim 200). The output was usually set at 80–90 % of maximal stimulator output. The direction of current flow favoured activation of the left motor cortex.
Corticospinal stimulation was achieved with electrical shocks between two 9 mm Ag-AgCl electrodes fixed over the left (cathode) and right (anode) mastoid processes (100 μs duration, up to 750 V; Digitimer, D180). Such stimuli activate axons in the descending motor tracts at the level of the cervicomedullary junction with a large part of the evoked muscle response occurring through activation of corticospinal axons (Gandevia et al. 1999; see also Ugawa et al. 1991). The intensity of stimulation at the transmastoid level was set while responses were monitored at high gain to assess whether the onset latency of responses in the relaxed muscles was reduced markedly (by ∼2 ms) with an increase in stimulus intensity. Such a shift in latency indicated that stimulation was inadvertently occurring distal, rather than proximal, to the motoneurone cell bodies. With the subject instructed to relax, the stimulus intensity was adjusted to produce a compound potential (termed the cervicomedullary motor-evoked potential, CMEP) in the right brachioradialis of 20–30 % of the maximal M-wave. The usual stimulus intensity was ∼500 V. Once this level had been selected, the stimulus intensity was increased to check that a shortening in latency occurred and thus confirm that the test stimulus was below the intensity required to activate peripheral motor axons. At the test intensity, the size of the CMEP increased during weak voluntary contraction.
Supramaximal stimulation of the right brachial plexus (at Erb's point) was used to assess changes in the maximal compound muscle action potential (M-wave). This allowed changes in response to stimulation above the motoneurone to be adjusted according to changes in the maximal M-wave (see Taylor et al. 1999; Gandevia et al. 1999), although the normalization was not necessary for the key qualitative conclusions in this study. Single electrical stimuli were delivered with a cathode in the supraclavicular fossa and an anode on the acromion (100 μs duration; Digitimer, DS7, modified to deliver up to 1 A). The intensity of stimulation was set at least 50 % above the level needed to produce a maximal compound muscle action potential (M-wave) in both elbow flexor muscles (see Gandevia et al. 1999).
Effects of maintained muscle ischaemia on corticospinal and motor cortical responses
Each subject performed a sustained MVC lasting 2 min and the muscle was maintained ischaemic for 2 min after the contraction ended. Before and shortly after the MVC a ‘set’ of stimuli comprising stimulation at each of the three sites was delivered. The set of five stimuli consisted of three corticospinal shocks, then one stimulus to the brachial plexus bilaterally and finally one to the motor cortex. The interval between stimuli in a set was 5 s. The subject was warned to relax before every stimulus. Before the MVC, the set of stimuli was repeated three times at intervals of 1–2 min. The subject stopped the sustained MVC on instruction and sets of stimuli were delivered 5, 35, 65, 95, 125, 150, 210, 300, 420 and 720 s after the contraction ceased. Care was taken to avoid any unnecessary or unpredictable stimulation which may have altered spinal excitability.
Muscle ischaemia
To maintain the arm ischaemic, 5 s prior to completion of the sustained MVC a sphygmomanometer cuff around the upper arm was rapidly inflated to 300 mmHg by switching a solenoid-operated valve connecting the cuff to a cylinder of compressed air.
Although we have previously used this method to maintain muscle ischaemia (e.g. Gandevia et al. 1996), as indicated in Results, concern arose as to whether the muscle ischaemia was sufficient to produce significant reflex changes within the CNS. Hence, on a separate day, the study with maintained muscle ischaemia was repeated, in the same subjects, but without stimuli, while arterial blood pressure was measured non-invasively at the digital arteries of the middle finger on the left (non-contracting) side (Finapres, Ohmeda, Englewood, CO, USA).
Data recording and analyses
Analyses of the size of EMG responses and statistical testing were similar to those reported previously (Gandevia et al. 1999). To compare the changes in responses to corticospinal and motor cortical stimulation associated with maintained ischaemia to those under conditions without ischaemia, we used control data from Gandevia et al. (1999). These data were obtained in the same subjects tested about 1 week apart from the current studies.
Every response generated in each study was sampled at 5 kHz and stored on disk (CED 1401, Cambridge Electronic Design) for later analysis using customized software. Because of activity-dependent and other changes in the size of the maximal M-wave, we expressed the size of supraspinally evoked responses relative to the maximal M-wave recorded in the same set of stimuli. The size of the responses was formally assessed using the area of the compound muscle action potential. However, measurements of the peak-to-peak amplitude provided the same qualitative conclusion (see Fig. 2). Area was measured above and below baseline between two cursors set at the onset and end of the potentials. The cursor positions were the same for each type of response throughout a study.
Figure 2. EMG responses recorded from brachioradialis in a single subject before and after a 2 min MVC with maintained ischaemia of the forearm muscles.
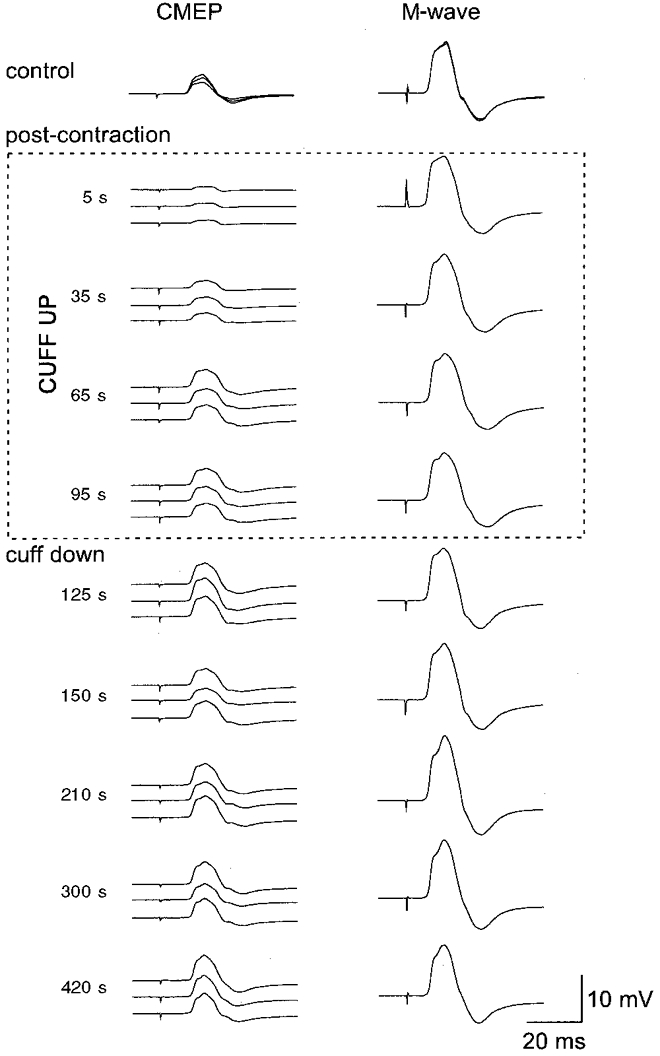
Responses to transmastoid stimulation are shown on the left (CMEP; cervicomedullary tract motor-evoked potential) and responses to supramaximal peripheral nerve stimulation on the right (M-wave). All responses were recorded in the relaxed muscle. At the top, control responses recorded before the MVC are shown superimposed. The next four sets of traces were recorded from 5 to 20 s, 35 to 50 s, 65 s to 80 s and 95 s to 110 s after the MVC but while the muscle was held ischaemic. The response to transmastoid stimulation is depressed following the MVC but recovers despite the ischaemia. The M-wave is slightly increased in size. The remaining sets of responses were recorded after blood flow to the forearm was allowed to resume.
Unless indicated otherwise, results are given as the mean ±s.e.m. When grouped results are shown (Figs 3 and 4), we have superimposed mean values previously reported from the same subjects when identical MVCs were performed without a post-contraction ischaemia (Gandevia et al. 1999).
Figure 3. Grouped data for the responses to transmastoid stimulation before and after a 2 min MVC with maintained ischaemia of the forearm muscles.
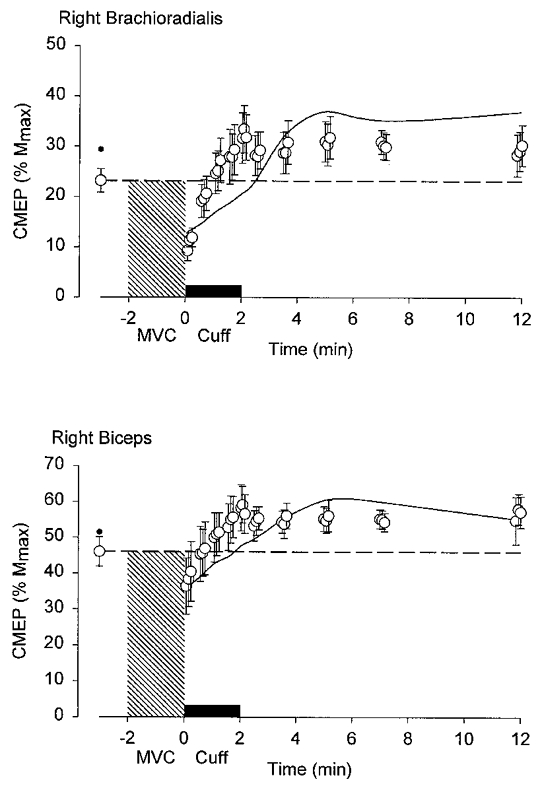
Upper and lower panels show the normalized responses (percentage maximal M-wave, Mmax) from brachioradialis and biceps brachii. The hatched region denotes the period of contraction and the filled bar above the x-axis denotes the period of ischaemia produced by inflation of a sphygmomanometer cuff just before the end of the contraction. Means ±s.e.m. (n= 7). Time zero corresponds to the end of the MVC. The response to transmastoid stimulation is depressed following the MVC but it recovers despite ischaemia of the arm. For comparison, data from a previous study in the same subjects in whom there was no maintained ischaemia are given as a small filled circle (before the MVC) and as a continuous line between original data points (Gandevia et al. 1999).
Figure 4. Grouped data for the responses to transcranial magnetic stimulation of the cortex before and after a 2 min MVC with maintained ischaemia of the forearm muscles.
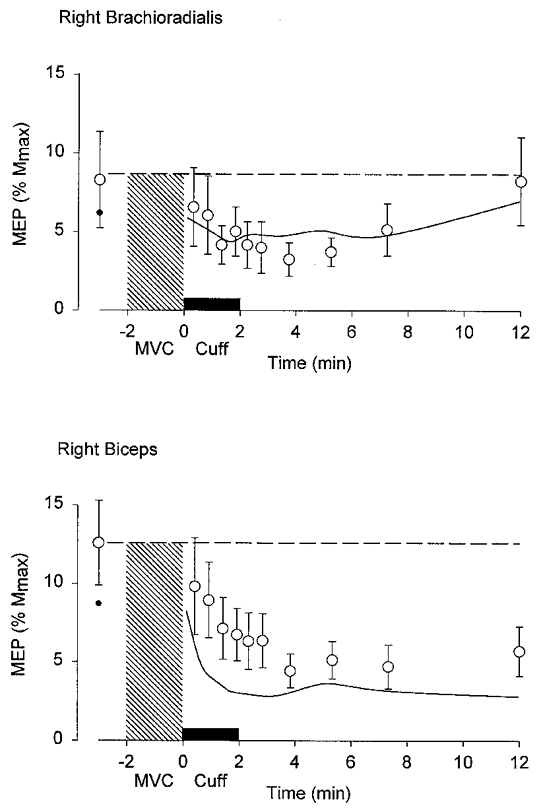
Upper and lower panels show the normalized responses from brachioradialis and biceps brachii. The hatched region denotes the period of contraction and the filled bar above the x-axis denotes the period of ischaemia produced by inflation of a sphygmomanometer cuff just before the end of the contraction. Means ±s.e.m. (n= 7). Time zero corresponds to the end of the MVC. The response to transcranial stimulation of the cortex declines in the 2 min after the MVC. For comparison, data from a previous study in the same subjects in whom there was no maintained ischaemia are given as a small filled circle (before the MVC) and as a continuous line between original data points (Gandevia et al. 1999). Note that the control MEPs recorded from biceps were smaller in the previous study than in the current study.
RESULTS
Effects of muscle ischaemia following muscle contractions
After a sustained MVC of the elbow flexors, when the muscles are relaxed, there is a depression of the responses to motor cortical stimulation and to corticospinal stimulation in the muscles involved in the contraction (Gandevia et al. 1999). Depression of the responses to corticospinal stimulation at the cervicomedullary level (CMEPs) occurs as early as 2 s after the MVC and responses recover over about 2 min, while depression of the responses to motor cortical stimulation (MEPs) develops much more slowly and recovery does not occur for over 10 min. These data form the control for the results of the present study. To determine whether the post-contraction depression and recovery of the response to corticospinal stimulation in the contracting muscles were due to declining inhibition from group III and IV afferents, we assessed whether the depression would continue as long as the muscle was held ischaemic after the voluntary contraction. This did not occur. Typical data from a single subject are shown in Fig. 2. Responses to corticospinal stimulation declined immediately after the sustained contraction, but even when a cuff on the upper arm kept the elbow flexors ischaemic (and the subject was asked to relax), the response to corticospinal stimulation recovered to control levels within 2 min after the contraction. Pooled data from the seven subjects are given in Fig. 3. Responses in biceps and brachioradialis behaved in a similar way, although greater emphasis is attached to data for brachioradialis because it lies distal to the cuff, whereas biceps is compressed under the cuff. While it is likely that the cuff affects the biceps EMG electrodes, the normalization of the responses to the maximal M-wave should minimize this problem.
After a sustained MVC, responses to transcranial magnetic stimulation of the cortex showed a prolonged depression (see above; Fig. 4), but when blood flow to the exercising muscles was prevented by inflation of a cuff on the upper arm, the behaviour of the responses to motor cortical stimulation was similar to that observed when muscle perfusion was unimpeded. Presumably, group III and IV afferents activated by ischaemia do not depress overall responsiveness at cortical and motoneuronal levels, at least in relaxed subjects.
The failure of maintained muscle ischaemia to prolong any inhibition of motoneurones after MVC was surprising. Therefore, we sought direct evidence that our maintenance of muscle ischaemia after a 2 min MVC produced some sustained reflex effect. Fatiguing voluntary contractions are well known to produce reflex increases in blood pressure through the firing of group III and IV muscle afferents (for review see Kaufman & Forster, 1996). Hence, we measured arterial blood pressure in our subjects. As shown in Fig. 5, mean arterial pressure increased during the contraction by about 50 mmHg. It reached a plateau 30–60 s after the contraction began. Blood pressure declined immediately the contraction ceased but remained well above the control level (by about 25 mmHg) as long as the cuff was inflated.
Figure 5. Changes in mean arterial pressure before, during and after a 2 min MVC when a cuff was applied at the end of the contraction.

Data for the group of seven subjects. Time zero corresponds to the end of the MVC. Mean arterial pressure (means ±s.e.m.) increases during the contraction but does not return to control levels if a cuff is inflated just prior to the end of the voluntary contraction. When the cuff is deflated and muscle circulation is restored, blood pressure returns rapidly to the level prior to the contraction. Each point represents data obtained in each subject averaged for 15 s intervals.
DISCUSSION
The main result of the study is that while maintenance of muscle ischaemia was an effective technique to examine the reflex effects of small-diameter muscle afferents, post-contraction ischaemia failed to prolong the depression of responses to corticospinal stimulation. Responses to motor cortical stimulation were also unaffected by post-contraction ischaemia. The demonstration that arterial blood pressure was maintained at a raised level throughout the period of muscle ischaemia confirmed that small-diameter muscle afferents were activated sufficiently to produce reflex cardiovascular effects. However, this continued firing of group III and IV muscle afferents when the subject was at rest did not inhibit the motoneurone pool. This is a surprising result given that post-contraction muscle ischaemia does maintain the slowing of motoneurone firing rates associated with fatigue (Bigland-Ritchie et al. 1986), and voluntary activation also remains low (Woods et al. 1987; Gandevia et al. 1996).
Electrical stimulation between the mastoids activates descending tracts. It is likely that the descending volley is carried largely by corticospinal axons to evoke a monosynaptic excitatory response from the motoneurone pool (Ugawa et al. 1991; Gandevia et al. 1999). The lack of presynaptic inhibition at this synapse (e.g. Nielsen & Petersen, 1994) makes transmastoid stimulation a particularly direct way to test the excitability of the motoneurone pool in awake human subjects. Immediately following a maximal contraction, the response to transmastoid stimulation is depressed by about 50 % and recovers to control levels over 2 min. However, this depression does not appear to be directly related to fatigue. It is similar after a 5 s or a 2 min MVC (Gandevia et al. 1999) and thus is not correlated with the duration of contraction or loss of maximal voluntary force. In the present study, maintained post-contraction ischaemia failed to affect the behaviour of the response to transmastoid stimulation. This strongly implies that small-diameter muscle afferents do not directly inhibit α-motoneurones after a fatiguing voluntary contraction.
Depression of H-reflexes and of the short-latency response to muscle stretch have been demonstrated in human subjects after fatiguing contractions (Garland & McComas, 1990; Balestra et al. 1992; Duchateau & Hainaut, 1993). Although these changes might suggest motoneuronal inhibition, they could also be due to changes in the afferent volley. Presynaptic inhibition of Ia afferents would decrease both the H-reflex and the short-latency stretch response. Such a decrease in the presynaptic Ia volley by group III and IV muscle afferents has recently been demonstrated in anaesthetized rats (Pettorossi et al. 1999). Moreover, an increase in the thresholds of axons to electrical stimulation occurs through repetitive firing even at physiologically realistic rates (e.g. Bergmans, 1970; Vagg et al. 1998). This effect alone would alter the afferent volley of the H-reflex and reduce its efficacy (Burke & Gandevia, 1999).
Direct measurement of the effects of chemical stimulation of group III and IV muscle afferents on lumbar α-motoneurones in cats showed varied responses in different motoneurones and motoneurone pools. Although motoneurones innervating the extensor muscles tended to hyperpolarize, which implies inhibition, many motoneurones innervating the flexor muscles showed depolarization (Kniffki et al. 1979, 1981a,b). This is consistent with our finding that motoneurones to the flexors of the arm in humans show no overall inhibition associated with maintained muscle ischaemia. Indeed, responses to corticospinal stimulation recovered more quickly under ischaemic conditions than in the absence of ischaemia (see Fig. 3).
The lack of inhibition of motoneurones by group III and IV muscle afferents at rest after a fatiguing contraction does not necessarily imply that motoneurones do not become less excitable during a fatiguing contraction. The slowing of motor unit firing rates with fatigue is good evidence that the motoneurones are less easily excited, are receiving less net excitatory input, or both. Furthermore, the lack of recovery of motor unit firing rates when the muscle is held ischaemic in the post-contraction period means that feedback from the muscle must be important. One possible explanation is that reflex inhibition of motoneurones by group III and IV muscle afferents could be gated by descending inputs and thus only be apparent during a voluntary contraction. Alternatively, other effects such as an increase in recurrent inhibition or an increased length of afterhyperpolarization would not be apparent in the response to a single test pulse but would act during a contraction. Subject to the caveat about axonal excitability given above, there is indirect evidence that recurrent inhibition is increased during fatigue induced by a MVC (Kukulka et al. 1986) and reduced during a fatiguing submaximal contraction (Löscher et al. 1996). Similarly, any effects of muscle afferents that fire in response to contraction might only be evident during a voluntary contraction. However, there is a major difficulty in the measurement of segmental effects on motoneurone behaviour during a fatiguing voluntary contraction (see Gandevia, 1998). It is likely that descending drive to the motoneurones also changes with fatigue and the way in which it changes cannot be measured directly and cannot be controlled. Thus any change in motoneurone behaviour that is measured during a voluntary contraction will reflect combined descending and segmental influences.
In the present study, we also examined the effect of post-contraction muscle ischaemia on the responses to magnetic stimulation of the motor cortex. In the relaxed muscle after a fatiguing contraction, the MEP follows a completely different pattern of recovery from the response to transmastoid stimulation (Brasil-Neto et al. 1993; McKay et al. 1995; Zanette et al. 1995; Liepert et al. 1996; Samii et al. 1997; Gandevia et al. 1999). The cortically evoked MEP is initially increased in size and then becomes depressed for many minutes. These changes must reflect increased and then decreased excitability of neurones in the motor cortex and are unlikely to be due to the activation of small-diameter muscle afferents during fatigue. The early excitation occurs after non-fatiguing as well as fatiguing voluntary contractions (Samii et al. 1996). The depression is fatigue related with an onset within the first 30 s of relaxation and can last for up to 30 min (Zanette et al. 1995; Samii et al. 1997). Thus, neither change in the MEP after exercise follows the time course of the activation of group III and IV afferents. After a 2 min sustained MVC, the MEP remained depressed for more than 12 min (Gandevia et al. 1999). When the muscle was held ischaemic at the end of a fatiguing contraction, the time course of MEP changes was not altered. Taken together with the lack of effect on the responsiveness of the motoneurone pool, this suggests that maintained post-contraction ischaemia has no effect on output neurones in the motor cortex as demonstrated with transcranial magnetic stimulation.
Although responses to both corticospinal and motor cortical stimulation are depressed when the muscle is relaxed after fatiguing exercise, neither response is altered by maintained muscle ischaemia. Neither the motoneurones nor motor cortical cells are directly inhibited by the firing of ischaemically sensitive group III and IV afferents.
References
- Adreani CM, Kaufman MP. Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. Journal of Applied Physiology. 1998;84:1827–1833. doi: 10.1152/jappl.1998.84.6.1827. [DOI] [PubMed] [Google Scholar]
- Balestra C, Duchateau J, Hainaut K. Effects of fatigue on the stretch reflex in a human muscle. Electroencephalography and Clinical Neurophysiology. 1992;85:46–52. doi: 10.1016/0168-5597(92)90101-g. [DOI] [PubMed] [Google Scholar]
- Bergmans J. The Physiology of Single Human Nerve Fibres. Louvain: Vander; 1970. [Google Scholar]
- Bigland-Ritchie B, Dawson NJ, Johansson RS, Lippold OC. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. The Journal of Physiology. 1986;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Johansson RS, Lippold OCJ, Smith S, Woods JJ. Changes in motoneurone firing rates during sustained maximal voluntary contractions. The Journal of Physiology. 1983;340:335–346. doi: 10.1113/jphysiol.1983.sp014765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Woods JJ. Changes in muscle contraction properties and neural control during human muscular fatigue. Muscle and Nerve. 1984;7:691–699. doi: 10.1002/mus.880070902. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Pascual-Leone A, Valls-Solé J, Cammarota A, Cohen LG, Hallett M. Postexercise depression of motor evoked potentials: a measure of central nervous system fatigue. Experimental Brain Research. 1993;93:181–184. doi: 10.1007/BF00227794. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia SC. Properties of human peripheral nerves: implications for studies of human motor control. In: Binder MD, editor. Progress in Brain Research, Peripheral and Spinal Mechanisms in the Neural Control of Movement. Vol. 123. Amsterdam and New York: Elsevier; 1999. pp. 427–435. [DOI] [PubMed] [Google Scholar]
- Cupido CM, Galea V, McComas AJ. Potentiation and depression of the M wave in human biceps brachii. The Journal of Physiology. 1996;491:541–550. doi: 10.1113/jphysiol.1996.sp021238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Behaviour of short and long latency reflexes in fatigued human muscles. The Journal of Physiology. 1993;471:787–799. doi: 10.1113/jphysiol.1993.sp019928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC. Neural control in human muscle fatigue: changes in muscle afferents, motor neurones and motor cortical drive. Acta Physiologica Scandinavica. 1998;162:275–283. doi: 10.1046/j.1365-201X.1998.0299f.x. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. The Journal of Physiology. 1996;490:529–536. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Macefield G, Burke D, McKenzie DK. Voluntary activation of human motor axons in the absence of muscle afferent feedback: the control of the deafferented hand. Brain. 1990;113:1563–1581. doi: 10.1093/brain/113.5.1563. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Petersen N, Butler JE, Taylor JL. Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. The Journal of Physiology. 1999;521:749–759. doi: 10.1111/j.1469-7793.1999.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland SJ, Kaufman MP. Role of muscle afferents in the inhibition of motoneurons during fatigue. In: Gandevia SC, Enoka RM, McComas AJ, Stuart DG, Thomas CK, editors. Advances in Experimental Medicine and Biology, Fatigue: Neural and Muscular Mechanisms. Vol. 384. New York and London: Plenum Press; 1995. pp. 271–278. [DOI] [PubMed] [Google Scholar]
- Garland SJ, McComas AJ. Reflex inhibition of human soleus muscle during fatigue. The Journal of Physiology. 1990;429:17–27. doi: 10.1113/jphysiol.1990.sp018241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York and Oxford: Oxford University Press; 1996. pp. 381–447. [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. Journal of Applied Physiology. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. Journal of Applied Physiology. 1984;57:644–650. doi: 10.1152/jappl.1984.57.3.644. [DOI] [PubMed] [Google Scholar]
- Kniffki K-D, Mense S, Schmidt RF. Responses of group IV afferent units from skeletal muscle to stretch, contraction and chemical stimulation. Experimental Brain Research. 1978;31:511–522. doi: 10.1007/BF00239809. [DOI] [PubMed] [Google Scholar]
- Kniffki K-D, Schomburg ED, Steffens H. Synaptic responses of lumbar α-motoneurones to chemical algesic stimulation of skeletal muscle in spinal cats. Brain Research. 1979;160:549–552. doi: 10.1016/0006-8993(79)91085-0. [DOI] [PubMed] [Google Scholar]
- Kniffki K-D, Schomburg ED, Steffens H. Synaptic effects from chemically activated fine muscle afferents upon α-motoneurones in decerebrate and spinal cats. Brain Research. 1981a;206:361–370. doi: 10.1016/0006-8993(81)90537-0. [DOI] [PubMed] [Google Scholar]
- Kniffki K-D, Schomburg ED, Steffens H. Effects from fine muscle and cutaneous afferents on spinal locomotion in cats. The Journal of Physiology. 1981b;319:543–554. doi: 10.1113/jphysiol.1981.sp013925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukulka CG, Moore MA, Russell AG. Changes in human α-motoneuron excitability during sustained maximum isometric contractions. Neuroscience Letters. 1986;68:327–333. doi: 10.1016/0304-3940(86)90511-2. [DOI] [PubMed] [Google Scholar]
- Liepert J, Kotterba S, Tegenthoff M, Malin J-P. Central fatigue assessed by transcranial magnetic stimulation. Muscle and Nerve. 1996;19:1429–1434. doi: 10.1002/(SICI)1097-4598(199611)19:11<1429::AID-MUS7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Löscher WN, Cresswell AG, Thorstensson A. Recurrent inhibition of soleus alpha-motoneurons during a sustained submaximal plantar flexion. Electroencephalography and Clinical Neurophysiology. 1996;101:334–338. doi: 10.1016/0924-980x(96)95670-2. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. The Journal of Physiology. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Gandevia SC, Bigland-Ritchie B, Gorman RB, Burke D. The firing rates of human motoneurones voluntarily activated in the absence of muscle afferent feedback. The Journal of Physiology. 1993;471:429–443. doi: 10.1113/jphysiol.1993.sp019908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay WB, Tuel SM, Sherwood AM, Stokic DS, Dimitrijevic MR. Focal depression of cortical excitability induced by fatiguing muscle contraction: a transcranial magnetic stimulation study. Experimental Brain Research. 1995;105:276–280. doi: 10.1007/BF00240963. [DOI] [PubMed] [Google Scholar]
- McKenzie DK, Bigland-Ritchie B, Gorman RB, Gandevia SC. Central and peripheral fatigue of human diaphragm and limb muscles assessed by twitch interpolation. The Journal of Physiology. 1992;454:643–656. doi: 10.1113/jphysiol.1992.sp019284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie DK, Gandevia SC. Influence of muscle length on human inspiratory and limb muscle endurance. Respiration Physiology. 1987;67:171–182. doi: 10.1016/0034-5687(87)90039-9. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Meadows JC, Merton PA. Muscular wisdom’ that minimizes fatigue during prolonged effort in man: rates of motoneuron discharge and slowing of discharge during fatigue. Advances in Neurology. 1983;39:169–211. [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. The Journal of Physiology. 1977;267:75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S, Stahnke M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. The Journal of Physiology. 1983;342:383–397. doi: 10.1113/jphysiol.1983.sp014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N. Is presynaptic inhibition distributed to corticospinal fibres in man? The Journal of Physiology. 1994;477:47–58. doi: 10.1113/jphysiol.1994.sp020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Taylor JL, Butler JE, Gandevia SC. Depression of responses to transmastoid stimulation in human elbow flexor muscles after fatiguing voluntary contractions. The Journal of Physiology. 1998;511.P:65P. [Google Scholar]
- Pettorossi VE, Della Torre G, Bortolami R, Brunetti O. The role of capsaicin-sensitive muscle afferents in fatigue-induced modulation of the monosynaptic reflex in the rat. The Journal of Physiology. 1999;515:599–607. doi: 10.1111/j.1469-7793.1999.599ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Somjen GG. Insensitivity of cat muscle spindles to hyperkalaemia in the physiological range. The Journal of Physiology. 1986;372:26P. [Google Scholar]
- Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. Journal of Applied Physiology. 1988;64:2306–2313. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- Samii A, Wasserman EM, Hallett M. Post-exercise depression of motor evoked potentials as a function of exercise duration. Electroencephalography and Clinical Neurophysiology. 1997;105:352–356. doi: 10.1016/s0924-980x(97)00033-7. [DOI] [PubMed] [Google Scholar]
- Samii A, Wasserman EM, Ikoma K, Mercuri B, Hallett M. Characterization of postexercise facilitation of motor evoked potentials to transcranial magnetic stimulation. Neurology. 1996;46:1376–1382. doi: 10.1212/wnl.46.5.1376. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Allen GM, Gandevia SC. Changes in motor cortical excitability during human muscle fatigue. The Journal of Physiology. 1996;490:519–528. doi: 10.1113/jphysiol.1996.sp021163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Gandevia SC. Altered responses of human elbow flexors to peripheral nerve and cortical stimulation during a sustained maximal voluntary contraction. Experimental Brain Research. 1999;127:108–115. doi: 10.1007/s002210050779. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Annals of Neurology. 1991;29:418–427. doi: 10.1002/ana.410290413. [DOI] [PubMed] [Google Scholar]
- Vagg R, Mogyoros I, Kiernan MC, Burke D. Activity-dependent hyperpolarization of motor axons produced by natural activity. The Journal of Physiology. 1998;507:919–925. doi: 10.1111/j.1469-7793.1998.919bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JJ, Furbush F, Bigland-Ritchie B. Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. Journal of Neurophysiology. 1987;58:125–137. doi: 10.1152/jn.1987.58.1.125. [DOI] [PubMed] [Google Scholar]
- Zanette G, Bonato C, Polo A, Tinazzi M, Manganotti P, Fiaschi A. Long-lasting depression of motor-evoked potentials to transcranial magnetic stimulation following exercise. Experimental Brain Research. 1995;107:80–86. doi: 10.1007/BF00228019. [DOI] [PubMed] [Google Scholar]


