Abstract
Kir5.1 is an inwardly rectifying K+ channel (Kir) subunit, whose physiological function is unknown. Human embryonic kidney HEK293T cells co-transfected with rat Kir5.1 and Kir4.1 cDNA expressed a functional K+ channel, whose properties were significantly different from those of the homomeric Kir4.1 channel. Formation of a Kir4.1/Kir5.1 assembly in HEK293T was confirmed biochemically.
We found that heteromeric Kir4.1/Kir5.1 channel activity was affected by internal pH levels between 6.0 and 8.0, when the homomeric Kir4.1 channel activity was relatively stable. Changing external pH in this range had no effect on either Kir channel.
Western blot analysis using specific antibodies revealed that Kir4.1 and Kir5.1 proteins were expressed in kidney and brain, but co-immunoprecipitated only from kidney.
These results indicate that the co-assembly of Kir5.1 with Kir4.1 occurs in vivo, at least in kidney. The heteromeric Kir4.1/Kir5.1 channel may therefore sense intracellular pH in renal epithelium and be involved in the regulation of acid-base homeostasis.
The inwardly rectifying potassium (Kir) channel family is now known to possess more than 20 members, which can be classified into four major subfamilies (Jan & Jan, 1997; Isomoto et al. 1997). These play pivotal roles in determining the resting membrane potential (Kir2.0), in G protein- or intracellular metabolism-dependent regulation of cell excitability (Kir3.0 and Kir6.0, respectively), and in transporting K+ ions in epithelial tissues and glial cells (Kir1.1 and Kir4.0). Kir5.1 does not belong to any of these subfamilies and its physiological roles are unknown (Bond et al. 1994), though with Kir4.1 it may form a functional K+ channel in Xenopus oocytes (Pessia et al. 1996).
To clarify the physiological role of Kir5.1, we addressed two questions: (1) what is the functional difference between homomeric Kir4.1 channels and heteromeric Kir4.1/Kir5.1 channels? and (2) does the heteromeric assembly of Kir5.1 and Kir4.1 occur in vivo? We found that in addition to differences in the conductance and kinetic properties between the two channels, the heteromeric Kir4.1/Kir5.1 channel was much more sensitive to internal pH than the homomeric Kir4.1 channel. Using specific antibodies for Kir4.1 and Kir5.1 subunits, we also found that rat kidney and brain contained both Kir subunit proteins, but they were co-immunoprecipitated only from kidney. Therefore the heteromeric Kir4.1/Kir5.1 channel exists in vivo, at least in kidney, and may be involved in intracellular pH-dependent alteration of renal handling of ion transport.
METHODS
Transient expression of Kir4.1 and Kir5.1 in HEK293T cells
Kir5.1 cDNA was cloned from a rat kidney cDNA library as described previously (Takumi et al. 1995). The coding regions of rat Kir1.1, Kir4.1 and Kir5.1 cDNAs were subcloned into an expression vector, pCDNA3 (Invitrogen, San Diego, CA, USA). HEK293T cells were then transfected with the plasmid vectors using LipofectAMINE (Life Technologies, Gaithersburg, MD, USA). To express the Kir4.1/Kir5.1 heteromer, Kir4.1 and Kir5.1 cDNAs were co-transfected, with the Kir5.1 cDNA:Kir4.1 cDNA ratio being 5:1. Electrophysiological measurements were conducted 48–96 h after transfection.
Electrophysiological recordings
The currents flowing through the channels expressed in HEK293T cells were measured using the patch-clamp method in the whole-cell, cell-attached patch and inside-out patch configurations. Currents were measured using a patch-clamp amplifier (EPC-7, List Electronics, Darmstadt, Germany) and recorded on videocassette tapes with PCM converter system (RP-880, NF Electronic Circuit Design, Yokohama, Japan). The data were reproduced, low-pass filtered at 1 kHz (-3 dB) through an eight-pole Bessel filter, sampled at 5 kHz, and analysed off-line on a computer. All experiments were performed at room temperature (22-24°C). The pipette and bath solutions contained (mM): 90 KCl, 5 EGTA, and 50 Hepes potassium salt (pH 7.4). The bath solution for whole-cell recording contained (mM): 120 NaCl, 20 KCl, 5 EGTA, 2 MgCl2, and 5 Hepes potassium salt (pH 7.4). To prepare external or internal solutions with different pH values, Mes (< pH 7), Hepes (pH 7-pH 8) and Tris buffers (> pH 8) were used. The pH of the solutions was adjusted to the desired value by adding NaOH for external solutions or KOH for internal solutions.
Antibodies
Polyclonal anti-Kir4.1 and anti-Kir5.1 antibodies were raised in rabbits against the synthetic peptides EKEGSALSVRISNV and LAKMATARKRAQTIRFSYF which correspond to amino acids 366–379 of Kir4.1 and 169–187 of Kir5.1, respectively. These antibodies were purified with antigenic peptide-coupled Sulfonlink resin (Pierce, Rockford, IL, USA) and specifically detected, using Western blotting analysis, Kir4.1 or Kir5.1 heterologously expressed in HEK293T cells. Both immunoreactivities were prevented by their respective antigenic peptides. Rabbit polyclonal anti-green fluorescent protein (GFP) and mouse monoclonal anti-FLAG M2 antibodies were purchased from Clontech Laboratories (Palo Alto, CA, USA) and Eastman Kodak (New Haven, CT, USA), respectively.
Immunoblot analysis and immunoprecipitation analysis
An adult male Spraque-Dawley rat was anaesthetized with ether and killed by decapitation, according to the regulations of the Animal Care Committee of Osaka University Medical School. Membrane preparations of Kir4.1- and/or Kir5.1-transfected HEK293T cells and of several rat tissues were obtained. The membrane proteins were solubilized in a lysis buffer containing 150 mM NaCl, 50 mM Tris-HCl (pH 7.4), 1 μg ml−1 aprotinin, 100 μg ml−1 PMSF, 0.02 % sodium azide, 0.1 % SDS, 0.5 % sodium deoxycholate, and 1 % Triton X-100. About 40 μg of the membrane proteins were separated using SDS-PAGE (10 %) and transferred to PVDF (polyvinylidene di-fluoride) membranes. The membranes were incubated for 12 h at 4°C with a blocking buffer containing 80 mM NaCl, 50 mM Tris-HCl (pH 7.5), 5 % (w/v) skimmed milk and 0.2 % Triton X-100. They were then incubated in blocking buffer containing antibodies at a concentration of 0.5 μg ml−1 for 12 h at 4°C. After incubation with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG antibodies (EnVision Plus; Dako, Carpinteria, CA, USA) diluted to 1:500 (v/v) in blocking buffer, membranes were washed once with a washing buffer (2 % Lubrol-PX in PBS) and twice with PBS. Immunoreactive bands were detected with a SuperSignal chemiluminescence immunostaining kit (Pierce, Rockford, IL, USA).
For immunoprecititation analysis, protein A-Sepharose beads were pretreated with 1 % (w/v) BSA in the lysis buffer for 2 h at 4°C. The protein A-Sepharose beads were then incubated with antibodies (1 μg antibodies per 20 μl protein A-Sepharose) in the lysis buffer for 4 h at 4°C. The solubilized membrane fractions of each tissue, prepared as described above, were incubated with the antibody-pretreated protein A-Sepharose beads for 16 h at 4°C. Pellets of protein A-Sepharose beads were then washed five times with the lysis buffer. Immunoprecipitated proteins were resolved using SDS-PAGE and analysed as described above.
RESULTS
Formation of heteromeric Kir4.1/Kir5.1 channels in HEK293T cells
HEK293T cells were transfected with Kir4.1 alone, Kir5.1 alone, or both Kir5.1 and Kir4.1. Figure 1Aa shows whole-cell currents recorded from these cells. The current flowing through the Kir4.1 channel exhibited a rapid activation upon hyperpolarization which decreased time dependently at -120 mV. The Ba2+-sensitive component showed intermediate inward rectification (Fig. 1Ab). In the cells transfected only with Kir5.1, no significant Ba2+-sensitive component was detected (n= 10). The currents measured in the cells co-transfected with Kir4.1/Kir5.1 exhibited a Ba2+-sensitive inwardly rectifying K+ current, which increased gradually during hyperpolarizing voltage steps. The Kir4.1/Kir5.1 channel showed stronger inward rectification than Kir4.1 (Fig. 1Ab).
Figure 1. Formation and electrophysiological properties of heteromeric Kir4.1/Kir5.1 channels expressed in HEK293T cells.
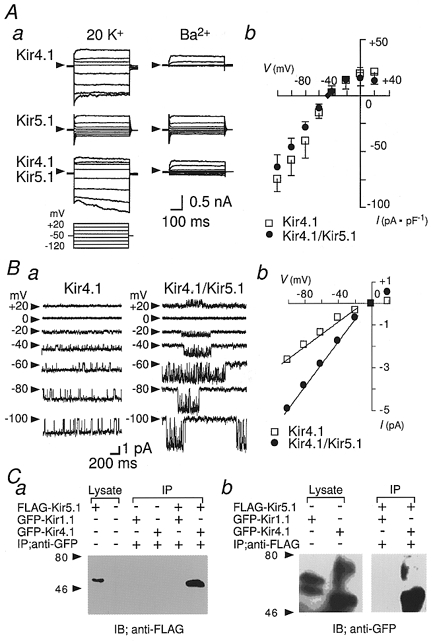
A, whole-cell currents from HEK293T cells transfected with Kir4.1, Kir5.1, or Kir4.1/Kir5.1 cDNA. Aa, voltage steps (500 ms in duration) from a holding potential of -50 mV were applied to potentials between -120 and +20 mV. Ba2+ (1 mM) was then added. Ab, the current-voltage relationship of the Ba2+-sensitive component recorded at the end of command pulses. □, Kir4.1; •, Kir4.1/Kir5.1; ♦, holding current. Data are means ±s.d. (n= 4). B, cell-attached single channel recordings of Kir4.1 and Kir4.//Kir5.1 channels. Ba, single channel currents recorded at the indicated membrane potentials. Arrowheads indicate the closed channel current level. Bb, the single channel current-voltage relationships. □, Kir4.1; •, Kir4.1/Kir5.1. C, FLAG-tagged Kir5.1 (FLAG-Kir5.1) and GFP-tagged Kir1.1 (GFP-Kir1.1) or Kir4.1 (GFP-Kir4.1) were co-transfected in HEK293T cells. The cell lysates from these cells were immunoprecipitated (IP) with anti-GFP antibody (anti-GFP) (a), or anti-FLAG antibody (anti-FLAG) (b). Immunoprecipitated FLAG-Kir5.1 and GFP-Kir4.1 proteins were the same size as those in the cell lysates, as indicated. Values to the left of the blots indicate the molecular mass in kDa.
Single channel properties of Kir4.1 and Kir4.1/Kir5.1 channels were compared in the cell-attached configuration (Fig. 1B). With 145 mM K+ in the pipette solution, the single channel conductance of homomeric Kir4.1 channels was 27.2 ± 2.0 pS (mean ±s.d., n= 4), while that of heteromeric Kir4.1/Kir5.1 channels was 49.6 ± 3.2 pS (n= 4) (Fig. 1B b). The Kir4.1 channel opened almost continuously with short closures (Fig. 1Ba). At -60 mV, the open time histogram could be fitted by a single exponential with a time constant (τ) of ∼100 ms, and the closed time histogram was the sum of two exponentials with τ values of ∼4 and ∼20 ms. The bursts of openings lasted > 15 s and were sometimes interrupted by long closures (gaps) of 100–200 ms. In contrast, in the Kir4.1/Kir5.1 channel the bursts of openings were clearly separated (Fig. 1Ba). The open time histogram of Kir4.1/Kir5.1 channel currents at -60 mV could be fitted by a single exponential with a τ value of ∼20 ms. The closed time histogram was the sum of three exponentials with τ values of ∼6 ms, ∼15 ms and ∼3 s. The duration of the bursts between gaps was 3–5 s.
In Fig. 1C, the formation of a heteromeric complex of Kir4.1 and Kir5.1 in HEK293T cells was confirmed biochemically. GFP-tagged Kir1.1 or Kir4.1 (GFP-Kir1.1, GFP-Kir4.1) was co-transfected with FLAG-tagged Kir5.1 (FLAG-Kir5.1). The immunoprecipitants obtained with anti-GFP antibody contained FLAG-Kir5.1 only in the cells co-transfected with GFP-Kir4.1 and FLAG-Kir5.1 but not in those with GFP-Kir1.1 and FLAG-Kir5.1 (Fig. 1C a). On the other hand, the immunoprecipitants obtained with the anti-FLAG antibody contained GFP-Kir4.1 but did not contain GFP-Kir1.1 (Fig. 1C b).
The effects of intracellular and extracellular pH on Kir4.1 and Kir4.1/Kir5.1 channels
Kir currents were measured from inside-out macro-patches of cells transfected with Kir4.1 alone (Fig. 2Aa) or both Kir4.1 and Kir5.1 (Fig. 2Ab). The internal solutions with different pH (pHi) values were perfused continuously. Each solution was perfused for more than 3 min to obtain a stable effect on the channel activity. The activity of homomeric Kir4.1 channels was relatively stable at pHi levels between 6.0 and 8.0 (Fig. 2Aa and c); it increased at pHi 8.0 and decreased at pHi 6.0, and disappeared at pHi < 5.5. On the other hand, the currents flowing through heteromeric Kir4.1/Kir5.1 channels were much more sensitive to pHi (Fig. 2Ab and c). When the internal solution was acidified from pHi 7.3 to 7.0, 6.5 and 6.0, channel activity was reduced to 20, 5 and 0 % of the value at pHi 7.3, respectively. When the internal solution was alkalinized from pHi 7.3 to 7.7 and 8.0, the channel activity increased to 120 and 200 %, respectively. The effects of pHi between 5 and 8 on the channel activities were reversible for both homomeric Kir4.1 channels and heteromeric Kir4.1/Kir5.1 channels (not shown).
Figure 2. Modulation of currents flowing through Kir4.1 homomeric and Kir4.1/Kir5.1 heteromeric channels by intracellular pH.
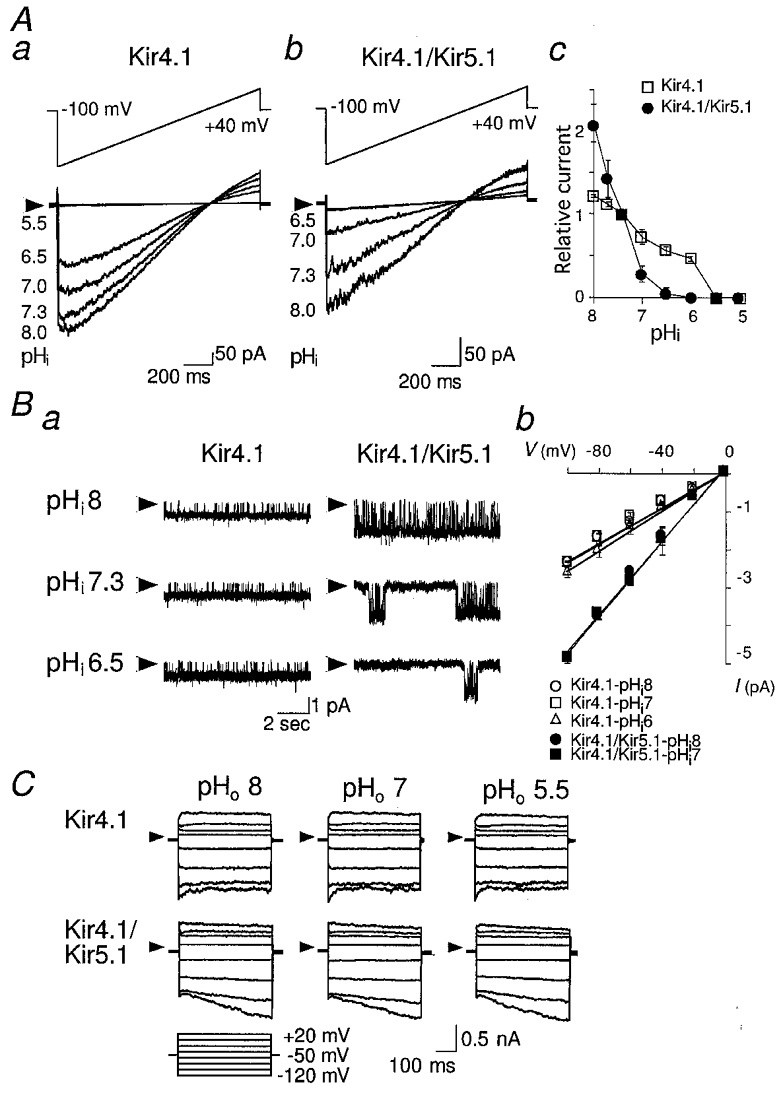
A, currents recorded from inside-out macro-patches from HEK293T cells transfected with Kir4.1 (a) or Kir4.1/Kir5.1 (b) cDNA. Voltage ramps from -100 mV to +40 mV were applied from a holding potential of 0 mV. The internal solutions of different pH (pHi) were applied as indicated. Ac, the current amplitude at -100 mV at different pHi levels is plotted relative to the value at pHi 7.3. □, Kir4.1; •, Kir4.1/Kir5.1. Data are means ±s.d. (n= 3). B, single channel currents of Kir4.1 and Kir4.1/Kir5.1 channels at various pHi levels. Ba, single channel recordings from inside-out patches. The traces were recorded at a holding potential of -60 mV with internal solutions of different pH, as indicated. In Kir4.1-transfected cells, inter-burst durations did not change significantly at different pHi levels. Bb, the single channel current-voltage relationship at different pHi levels. Open symbols, Kir4.1; filled symbols, Kir4.1/Kir5.1. Data are means ±s.d. (n= 3). C, effects of external pH (pHo). Representative whole-cell currents of Kir4.1 and Kir4.1/Kir5.1 channels. The experimental conditions were as in Fig. 1Aa, except for the pHo of the bath solution, which is indicated above the traces. Currents flowing through both Kir4.1 and Kir4.1/Kir5.1 channels were unaffected by changing pHo between 5.5 and 8.0. Arrowheads in A, B and C indicate zero current level.
Figure 2B shows single channel recordings of homomeric Kir4.1 and heteromeric Kir4.1/Kir5.1 channels at various pHi levels. The unitary channel conductance and open- closed kinetics of Kir4.1 channels were not altered significantly. The long gaps between bursts seemed to increase with acidic pHi, but the number of gaps was too small to be measured accurately. At pHi 5.5, channel openings disappeared completely. In contrast, the open probability of heteromeric Kir4.1/Kir5.1 channels was dramatically altered, without significant alteration of the unitary conductance. At pHi 7.3, the duration of the bursts was 3–5 s. At pHi 8.0, the gaps became shorter and the bursts continued for more than 20 s. At pHi 6.5, the gaps became longer and the duration of bursts became shorter than 1 s. The open-closed kinetics within bursts was not affected. At pHi 6.0, channel activity disappeared completely.
Extracellular pH had no effect upon homomeric Kir4.1 and heteromeric Kir4.1/Kir5.1 channel currents (Fig. 2C). Therefore protons may act on the Kir channels specifically from the internal side.
Heteromeric assembly of Kir4.1 and Kir5.1 subunits occurs in vivo
Next, using Western blot analysis, we addressed the question of whether the heteromeric Kir4.1/Kir5.1 channel exists in vivo (Fig. 3A). Significant amounts of Kir4.1 were detected in brain, eye and kidney, but not in heart, as shown previously (Isomoto et al. 1997). Lysates from HEK293T cells expressing Kir4.1 contained several bands detected by anti-Kir4.1 antibody. Because the lysate from mock-transfected HEK293T cells did not show any bands, this may indicate that the channel is subject to post-translational modification. The smallest band, ∼35 kDa, was the size predicted from the amino acid sequence for Kir4.1, and was detected in kidney. The Kir4.1 bands from brain and eye were slightly larger (∼40 kDa). On the other hand, only a single band of protein was detected with anti-Kir5.1 antibody in the cells transfected with Kir5.1. It was detected in brain and kidney, but not in eye or heart. Using Northern blot analysis, Kir5.1 mRNA was also detected in brain and kidney, but not in eye or heart (data not shown).
Figure 3. Formation of heteromeric Kir4.1/Kir5.1 channels in vivo.
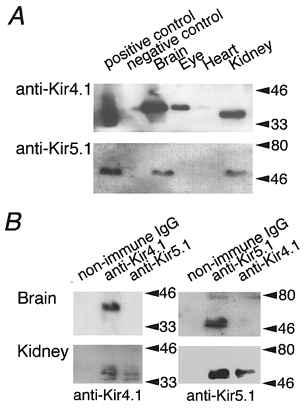
A, Western blot analysis of Kir4.1 and Kir5.1 in various rat organs using antibodies specific to Kir4.1 and Kir5.1. The positive controls were the lysates from HEK293T cells transfected with Kir4.1 and Kir5.1 cDNA. The negative controls were lysates from mock-transfected HEK293T cells. B, immunoprecipitation analysis of the heteromeric assembly of Kir5.1 and Kir4.1. Lysates of the membrane fraction of brain and kidney were incubated with non-immune control IgG, or anti-Kir4.1 or anti-Kir5.1 antibodies. The immunoprecipitants obtained were blotted with anti-Kir4.1 (left panels) or with anti-Kir5.1 (right panels) antibodies.
The question of whether the heteromeric Kir4.1/Kir5.1 channel might exist in brain or kidney was examined by immunoprecipitation analysis (Fig. 3B). The anti-Kir5.1 immunoprecipitant from kidney contained Kir4.1 immunoreactivity but that from the brain did not; similarly the anti-Kir4.1 immunoprecipitant from kidney contained Kir5.1 immunoreactivity but that from brain did not. These results indicate that heteromeric Kir4.1/Kir5.1 channels exist in kidney.
DISCUSSION
This study shows that a heteromeric assembly of Kir5.1 and Kir4.1 occurs in vivo and suggests a physiological function for the heteromeric Kir channel in the kidney where Kir4.1 is localized at the basolateral membrane of the distal convoluted tubules (Ito et al. 1996). Kir channels in the basolateral membrane of renal epithelium are thought to assist Na+-K+-ATPase activity by recycling K+ ions across the membrane (Koeppen & Stanton, 1992). In this way the transport of Na+ ions would be affected by an intracellular pH (pHi) dependence of the Kir4.1/Kir5.1 channel. For example, respiratory acidosis in the epithelium of the distal convoluted tubules would suppress Kir4.1/Kir5.1 channel activity and thus reduce Na+ reabsorption. The consequent increase in luminal Na+ ions in the collecting ducts would enhance H+ excretion, because in the collecting ducts Na+ ions are reabsorbed in exchange for H+ ions (Gennari & Maadox, 1992). The reverse would occur at each stage under respiratory alkalosis. The pHi-dependent regulation of Kir4.1/Kir5.1 channels, and to a lesser extent Kir4.1 channels, may therefore be involved in renal compensatory mechanisms for disorders of acid-base balance.
The heteromeric assembly of Kir4.1 and Kir5.1 channel subunits resulted in a channel with a larger conductance, different kinetics and increased sensitivity to pHi compared with channels composed of homologous Kir4.1 subunits. The heteromeric Kir4.1/Kir5.1 channel showed openings in bursts. The closed periods between bursts were influenced by intracellular pH, as has also been shown for the pHi-dependent Kir1.1 channel (Fakler et al. 1996). In Kir1.1, residue K80 was found to be responsible for pHi modulation. H72 and K67 are found at the respective corresponding regions in Kir5.1 and Kir4.1 (Yang & Jiang, 1999). Further studies are required to determine the importance of these residues in the regulation of the heteromeric Kir4.1/Kir5.1 channel by pHi.
Although both Kir 4.1 and Kir5.1 were expressed in brain and kidney, the heteromeric complex was detected only in kidney. While it is possible that in some restricted region of brain heteromeric Kir4.1/Kir5.1 channels exist, most Kir5.1 subunits seem to exist without assembling with Kir4.1. It has been reported that Kir5.1 could assemble with Kir4.2 (Pearson et al. 1999), and therefore Kir4.2/Kir5.1 channels might exist in brain. It is also possible that the channel becomes functional in situ by interacting with some associated proteins (Hibino et al. 1999). Further studies are needed to clarify the physiological function of Kir5.1 in different organs.
Acknowledgments
We thank Dr Ian Findlay (University of Tours, Tours, France) for his critical comments on this manuscript. This work was supported by grants from the Ministry of Education, Culture, Sports and Society of Japan, and from the Research for the Future Program of the Japanese Society for the Promotion of Science (96L00302).
References
- Bond CT, Pessia M, Xia XM, Lagrutta A, Kavanaugh MP, Adelman JP. Cloning and expression of a family of inward rectifier potassium channels. Receptors and Channels. 1994;2:183–191. [PubMed] [Google Scholar]
- Fakler B, Schultz JH, Yang J, Schulte U, Brandle U, Zenner HP, Jan LY, Ruppersberg JP. Identification of a titratable lysine residue that determines sensitivity of kidney potassium channels (ROMK) to intracellular pH. EMBO Journal. 1996;15:4093–4099. [PMC free article] [PubMed] [Google Scholar]
- Gennari FJ, Maddox DA. Renal Regulation of Acid-Base Homeostasis: Integrated Response. The Kidney. 2. New York: Raven Press; 1992. pp. 2695–2732. [Google Scholar]
- Hibino H, Inanobe A, Tanemoto M, Fujita A, Doi K, Kubo T, Hata Y, Takai Y, Kurachi Y. Anchoring proteins confer G protein sensitivity to an inward-rectifier K+ channel through the GK domain. EMBO Journal. 1999;19:78–83. doi: 10.1093/emboj/19.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Kurachi Y. Inwardly rectifying potassium channels: their molecular heterogeneity and function. Japanese The Journal of Physiology. 1997;47:11–39. doi: 10.2170/jjphysiol.47.11. [DOI] [PubMed] [Google Scholar]
- Ito M, Inanobe A, Horio Y, Hibino H, Isomoto S, Ito H, Mori K, Tonosaki A, Tomoike H, Kurachi Y. Immunolocalization of an inwardly rectifying K+ channel, KAB-2 (Kir4.1), in the basolateral membrane of renal distal tubular epithelia. FEBS Letters. 1996;388:11–15. doi: 10.1016/0014-5793(96)00502-9. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Cloned potassium channels from eukaryotes and prokaryotes. Annual Review of Neuroscience. 1997;20:91–123. doi: 10.1146/annurev.neuro.20.1.91. [DOI] [PubMed] [Google Scholar]
- Koeppen BM, Stanton BA. Sodium Chloride Transport: Distal Nephron. The Kidney. 2. New York: Raven Press; 1992. pp. 2003–2040. [Google Scholar]
- Pearson WL, Dourado M, Schreiber M, Salkoff L, Nichols CG. Expression of a functional Kir4 family inward rectifier K+ channel from a gene cloned from mouse liver. The Journal of Physiology. 1999;514:639–653. doi: 10.1111/j.1469-7793.1999.639ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessia M, Tucker SJ, Lee K, Bond CT, Adelman JP. Subunit positional effects revealed by novel heteromeric inwardly rectifying K+ channels. EMBO Journal. 1996;15:2980–2987. [PMC free article] [PubMed] [Google Scholar]
- Takumi T, Ishii M, Horio Y, Morishige K, Takahashi N, Yamada M, Yamashita T, Kiyama H, Sohmiya K, Nakanishi S, Kurachi Y. A novel ATP-dependent inward rectifier potassium channel expressed predominantly in glial cells. Journal of Biological Chemistry. 1995;270:16339–16346. doi: 10.1074/jbc.270.27.16339. [DOI] [PubMed] [Google Scholar]
- Yang Z, Jiang C. Opposite effects of pH on open-state probability and single channel conductance of Kir4.1 channels. The Journal of Physiology. 1999;520:921–927. doi: 10.1111/j.1469-7793.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


