Abstract
A perforated patch recording method was used to determine how plating cells on laminin (20 μg ml−1; >2 h) alters cholinergic regulation of L-type Ca2+ current (ICa,L) in atrial myocytes.
Acetylcholine (ACh; 1 μm)-induced inhibition of basal ICa,L was not different between cells on glass and laminin. However, stimulation of ICa,L elicited by ACh withdrawal was significantly smaller in cells on laminin (10 ± 2 %) than on glass (48 ± 5 %) (P = 0.001).
Stimulation of ICa,L induced by either spermine-NO (200 μm), milrinone (10 μm), IBMX (100 μm) or forskolin (1 μm) was significantly smaller in cells plated on laminin than on glass. However, stimulation of ICa,L by 100 μm 8-CPT-cAMP or intracellular dialysis with 50 μM cAMP was not different between cells plated on laminin or glass.
Basal, forskolin- and IBMX-stimulated cAMP content was significantly smaller in cells plated on laminin than on glass.
Stimulation of ICa,L by ACh withdrawal was significantly smaller in cells plated on an αβ1-integrin antibody (10 ± 4 %) than on glass (38 ± 6 %; P = 0.001). In cells on laminin, prior exposure to 100 μg ml−1 YIGSR, a laminin receptor-binding peptide, restored ACh-induced stimulation of ICa,L (58 ± 14 %)laminin alone (7 ± 2 %; P = 0.05).
Addition of 20 μm cytochalasin D or 1 μM latrunculin A, agents that prevent actin polymerization, to cells on laminin restored ACh-induced stimulation of ICa,L.
We conclude that laminin binding to β1 integrins acts in association with the actin-based cytoskeleton to attenuate adenylate cyclase activity. As a result, laminin inhibits NO-mediated stimulation of ICa,L elicited by ACh withdrawal. Laminin-integrin signalling may be relevant to changes in autonomic regulation that occur during cardiac development and/or disease.
Attachment of a variety of cells to constituents of the extracellular matrix (ECM) can promote dramatic changes in cell differentiation, gene expression, migration, shape and function (Hynes, 1992; Schwartz et al. 1995; Clark & Brugge, 1995; Dedhar, 1999). The information stored within the ECM that is necessary to provoke these changes is communicated to the intracellular environment through integrins. Integrins are a family of heterodimeric transmembrane proteins consisting of alpha (α) and beta (β) chains which combine to give the integrin complex a variety of ECM-binding specificities. At least 16 α subunits and 8 β subunits have been described so far. In the adult cardiac myocyte, integrins are of the β1 subtype, with several different α chains. Through their association with structural and regulatory elements of the cytoskeleton, integrins can influence the assembly and distribution of actin microfilaments (Hilenski et al. 1989; Borg et al. 1990; Schwartz et al. 1995; Miyamoto et al. 1995). Freshly isolated adult cardiac myocytes readily attach to laminin, a cardiac ECM component, via β1 integrin receptors. In addition to cell adhesion, integrins transmit signals from the ECM to elicit changes in intracellular signalling pathways in a variety of cell types (Schwartz et al. 1995; Clark & Brugge, 1995). However, little is known about the influence of the ECM- integrin-cytoskeletal complex on intracellular signalling pathways in cardiac myocytes.
In cat atrial myocytes exposure to acetylcholine (ACh) inhibits basal L-type Ca2+ current (ICa,L) and withdrawal of ACh elicits a pronounced stimulation of ICa,L above control levels (Wang & Lipsius, 1995; Wang et al. 1998). ACh withdrawal also elicits rebound stimulation of cAMP-stimulated ICa,L in guinea-pig ventricular Purkinje fibres (Ehara & Mitsuiye, 1984), cAMP-stimulated chloride current in guinea-pig ventricular myocytes (Ono & Noma, 1994; Zakharov & Harvey, 1997), cAMP concentrations in embryonic chick heart cells (Linden, 1987) and intracellular Ca2+ release and contraction in rabbit atrial muscle (Endoh & Blinks, 1984). Rebound stimulation of cAMP and Ca2+ influx via ICa,L may function to rapidly restore and enhance atrial pacemaker (Wang & Lipsius, 1996) and contractile activities (Endoh & Blinks, 1984; Wang & Lipsius, 1995) following cholinergic suppression, and prime the heart for reciprocal β-adrenergic stimulation. The purpose of the present study is to determine whether the ECM protein laminin can modulate intracellular signalling mechanisms responsible for cholinergic regulation of ICa,L. The present results demonstrate that in atrial myocytes engagement of β1 integrins by laminin acts in association with the actin-based cytoskeleton to inhibit adenylate cyclase, thereby inhibiting the stimulation of ICa,L elicited by ACh withdrawal. These findings may be relevant to the changes in autonomic regulation that occur during cardiac development and/or disease. Portions of this work have been presented in abstract form (Wang et al. 1999).
METHODS
Details of the isolation and recording methods have been published previously (Wu et al. 1991). Adult cats of either sex were anaesthetized with sodium pentobarbital (70 mg kg−1i.p.). After bilateral thoracotomy, hearts were rapidly excised and mounted on a Langendorff perfusion apparatus for cell isolation. Experiments were performed on either right or left atrial cells, with no discernable differences in responses. Cells studied were isolated on the same morning of each experiment.
Within approximately 1 h of isolation, cells from the same hearts were plated on uncoated glass coverslips or glass coverslips coated with one of the following substrates: laminin (20 μg ml−1) (Sigma), poly-L-lysine (20 μg ml−1) (Sigma), goat anti-human αβ1 integrin IgG (20 μg ml−1) (antibody generously provided by Dr T. K. Borg, University of South Carolina Medical School, Charleston, SC, USA) or non-immune goat IgG (20 μg ml−1) (Chemicon). Cells were plated on substrates as follows: a drop of substrate-containing solution was placed on individual glass coverslip. Atrial cells in solution were carefully pipetted into the drop and allowed to settle onto the coverslip for about 30 min. This method provides a more physiological three-dimensional exposure of cells to substrate rather than on one cell surface. Once the cells were settled, the dish containing the cells on substrate-coverslips was completely bathed in Tyrode solution. Cells were plated on each of these substrates for at least 2 h before recordings were performed. Attachment of cardiac myocytes to laminin requires about 30-60 min (Borg & Terracio, 1988). Cells were exposed to 100 μg ml−1 YIGSR, a laminin receptor-binding peptide, for 30 min prior to being plated on laminin. Control experiments showed no differences in the responses to ACh among cells plated on glass from 1 to 6 h. Coverslips containing cells were transferred to a small tissue bath on the stage of an inverted microscope and superfused with a modified Tyrode solution containing (mM): NaCl, 137; KCl, 5.4; MgCl2, 1.0; CaCl2, 2.0; Hepes, 5; glucose, 11; and titrated with NaOH to a pH of 7.4. Solution was perfused through a small (0.3 ml) chamber by gravity at ∼5 ml min−1. The system requires ∼20 s to completely exchange the bath contents. All experiments were performed at 35 ± 1°C. In most experiments, ionic currents were recorded using a nystatin-perforated patch (Horn & Marty, 1988) whole-cell recording method (Hamill et al. 1981), as previously described (Wang & Lipsius, 1995). This method minimizes dialysis of intracellular contents thereby maintaining physiological buffering of intracellular Ca2+ and second messenger signalling pathways, and also avoids run down of ICa,L. The internal pipette solution contained (mM): caesium (Cs+) glutamate, 100; CsCl, 40; MgCl2, 1.0; Na2-ATP, 4; EGTA, 0.5; Hepes, 5; titrated with CsOH to a pH of 7.2. ICa,L was isolated by replacing K+ with Cs+ in the internal pipette solution and adding 5 mM CsCl to the external solutions to block K+ currents. In one set of experiments, a ruptured patch recording method was used to dialyse the cell interior with cAMP. In these experiments the internal pipette solution contained (mM): caesium (Cs+) glutamate, 100; CsCl, 40; MgCl2, 1.0; Na2-ATP, 4; EGTA, 6; CaCl2, 4; Hepes, 5; titrated with CsOH to a pH of 7.2, without nystatin. The pipette tip was filled with cAMP-free solution and the pipette was back-filled with cAMP-containing solution. This method allows time to record basal ICa,L before cAMP diffuses into the cell interior and stimulates ICa,L. A single glass pipette recorded ionic currents (discontinuous mode) using an Axoclamp2A amplifier (Axon Instruments, Inc.). The discontinuous (switch) clamp precludes the need to compensate for series resistance. When filled with internal solution the pipette tip resistance was ∼3 MΩ. Once a gigaseal was formed access resistance stabilized at ∼15-20 MΩ. The sampling rate of the switch clamp was ∼10-12 kHz and a second oscilloscope was used to monitor the duty cycle to ensure that the voltage transient settled between cycles. Computer software (pCLAMP7.0; Axon Instruments, Inc.) was used to deliver voltage protocols, acquire and analyse data.
ICa,L was activated by clamp steps from a holding potential of -40 to 0 mV for 200 ms every 5 s. This voltage protocol avoids activation of fast Na+ and T-type Ca2+ currents. Peak ICa,L was measured with respect to steady-state current and was not compensated for leak currents. ICa,L density was calculated by normalizing total peak current to total membrane capacitance. Drugs or chemicals used include: milrinone, spermine-NO, 3-isobutyl-1-methylxanthine (IBMX), adenosine 3′,5′-cyclic monophosphate (cAMP), forskolin, cytochalasin D, YIGSR (Sigma Chemical Co.), adenosine 3′,5′-cyclic monophosphate, 8-(4-chlorophenylthio) (8-PTC-cAMP) (Calbiochem) and latrunculin A (Molecular Probes).
Intracellular concentrations of cAMP were measured as follows: atrial myocytes were allowed to attach (2 h) onto un-coated, 100 mm glass Petri dishes, or 100 mm Petri dishes coated with laminin or poly-L-lysine. Then cells were either maintained in plating medium to measure basal cAMP or stimulated with medium containing 1 μm forskolin for 5 min or 100 μm IBMX for 4 min. The medium was then aspirated, and ice-cold trichloroacetic acid (TCA, 6 %, 2 ml) was added. The TCA and cells were scraped from the dishes and the suspension was incubated on ice (15 min). Following centrifugation (2000 g, 15 min), the precipitates were solubilized in 0.3 n NaOH for determination of total protein by the Lowry method. The supernatant fractions were washed five times with 2 volumes of water-saturated diethyl ether to remove the TCA. After removing any remaining ether under a stream of N2, the aqueous extracts were lyophilized to dryness, and the dried residues were re-suspended in assay buffer. cAMP was measured using a commercial radioimmunoassay kit (RPA509, Amersham Pharmacia Biotech, Piscataway, NJ, USA). The aqueous cell extracts were appropriately diluted (5- to 20-fold) so that the cAMP concentration of the unknowns fell within the linear range of the assay system. Samples from four individual experiments were run in duplicate and the data were expressed as femptomoles per microgram of total protein (fmol (μg total protein)−1).
For the measurements of ICa,L, data are expressed as means ±s.e.m. Data obtained from two groups of cells (glass vs. laminin) from the same hearts were analysed using Student's unpaired t test with significance at P = 0.05. Data obtained from among three groups of cells were analysed for significance using a one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls test at P = 0.05. For the cAMP measurements, data are expressed as means ±s.e.m. for four replicate experiments performed on cells derived from four different hearts. One-way blocked ANOVA followed by the Student-Newman-Keuls test were used for the statistical comparison of multiple groups (i.e. cAMP levels in basal and forskolin-treated cardiomyocytes plated on glass, laminin, or poly-l-lysine). Statistical comparison of two groups (i.e. IBMX-treated myocytes plated on glass or laminin) was accomplished by paired t tests. Data were analysed using SigmaStat Statistical Software Package, version 1.0 (Jandel Scientific, San Rafael, CA, USA).
The animal procedures used in this study were in accordance with the guidelines of the Animal Care and Use Committee of Loyola University Medical Center.
RESULTS
We first measured the basal amplitude of peak ICa,L in cells plated on either glass or laminin. The amplitude of basal ICa,L density was significantly smaller in cells on laminin (2.8 ± 0.2 pA pF−1) than in cells on glass (3.6 ± 0.3 pA pF−1) (P = 0.05; n = 116). Figure 1A-D shows the effects of a 2 min exposure to 1 μm ACh in atrial myocytes plated on glass (A and B) or laminin (C and D). In an atrial cell on glass, exposure to ACh elicited a typical inhibition of basal ICa,L (-21 %), and withdrawal of ACh elicited a transient stimulation of peak ICa,L above control levels (+69 %). ICa,L returned toward baseline within 4-5 min. Similar results have been reported previously (Wang & Lipsius, 1995). In an atrial cell plated on laminin, exposure to ACh elicited a typical inhibition of basal ICa,L (-17 %). However, withdrawal of ACh failed to stimulate ICa,L above control. In a total of 19 cells studied, ACh-induced inhibition of ICa,L in cells plated on glass (-12 ± 2 %) and on laminin (-12 ± 2 %) were not different, whereas stimulation of ICa,L elicited by ACh withdrawal was significantly smaller in cells on laminin (10 ± 2 %) than on glass (48 ± 5 %) (P = 0.001). Laminin preferentially inhibited the signalling mechanisms responsible for stimulation of ICa,L induced by ACh withdrawal. This is consistent with our previous findings that ACh-induced inhibition and rebound stimulation of ICa,L result from different signalling mechanisms (Wang & Lipsius, 1995). To determine whether the effects of laminin were simply due to non-specific cell attachment, cells were plated on either glass or 20 μg ml−1 poly-l-lysine. In cells plated on glass vs. poly-l-lysine, ACh-induced inhibition (-16 ± 4 vs. -15 ± 5 %) and rebound stimulation of ICa,L (54 ± 7 vs. 32 ± 10 %), respectively, were not significantly different (n = 3).
Figure 1. Effects of ACh on basal ICa,L in atrial cells plated on either glass (A and B) or laminin (C and D).

Top row: selected original ICa,L traces. Bottom row: consecutive measurements of peak ICa,L in individual experiments. Cells on glass (A and B) and laminin (C and D) were exposed to 1 μm ACh for 2 min followed by withdrawal of ACh. On glass, exposure to ACh inhibited peak ICa,L (b) followed, upon ACh withdrawal, by a prominent stimulation of ICa,L (c), which returned to baseline (d). On laminin, exposure to ACh inhibited peak ICa,L (b) but withdrawal of ACh failed to stimulate ICa,L (c). a-d indicate the times when the individual ICa,L traces were recorded during each experiment.
We next sought to determine how laminin alters the NO-signalling mechanisms responsible for rebound stimulation of ICa,L (Wang et al. 1998). NO stimulates basal ICa,L via activation of cGMP-induced inhibition of phosphodiesterase type 3 (PDE3) activity (Kirstein et al. 1995; Wang et al. 1998). As shown in Fig. 2A-C, in an atrial cell on glass (A), 200 μm spermine-NO, an NO donor (Maragos et al. 1991), elicited a prominent increase in basal ICa,L. In an atrial cell plated on laminin (B), the stimulatory effect of spermine-NO on basal ICa,L was markedly attenuated. Spermine alone has no effect on ICa,L (Wang et al. 1998). As summarized in the bar graph (Fig. 2C) and Table 1, spermine-NO elicited a significantly smaller increase in basal ICa,L in cells on laminin (57 ± 21 %) than in cells on glass (163 ± 37 %) (P = 0.05).
Figure 2. Effects of 200 μm spermine-NO on basal ICa,L recorded from atrial cells plated on either glass (A) or laminin (B).
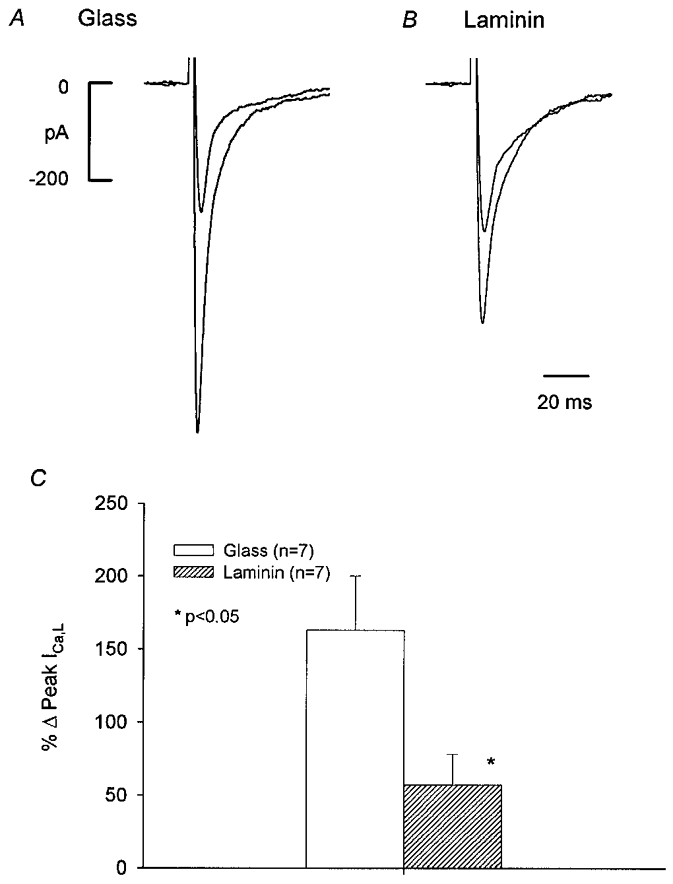
Spermine-NO stimulated ICa,L significantly less in cells on laminin than on glass. C, bar graph summarizing the effects of spermine-NO on ICa,L in the two groups.
Table 1.
Effects of NO–adenylate cyclase–cAMP signalling on basal ICa,L amplitude in cells plated on glass or laminin
| Glass | (n) | Laminin | (n) | |
|---|---|---|---|---|
| Spermine–NO (200 μm) | 163 ± 37 | (7) | 57 ± 21* | (7) |
| Milrinone (10 μm) | 66 ± 15 | (5) | 28 ± 7* | (5) |
| 8-PTC-cAMP (100 μm) | 35 ± 7 | (4) | 40 ± 6 | (3) |
| IBMX (100 μm) | 619 ± 104 | (6) | 263 ± 52* | (6) |
| Forskolin (1 μm) | 230 ± 35 | (10) | 127 ± 25* | (9) |
Values indicate percentage changes compared with controls; n, total number of cells studied.
P = 0·05.
Since NO stimulates basal ICa,L via inhibition of PDE3, we next examined whether laminin alters stimulation of ICa,L elicited by direct inhibition of PDE3. As summarized in Table 1, 10 μm milrinone, a specific inhibitor of PDE3 (Harrison et al. 1986), elicited a significantly smaller increase in basal ICa,L in cells on laminin (28 ± 7 %) than on glass (66 ± 15 %) (P = 0.05). The fact that the stimulatory effects of both NO and milrinone are attenuated by laminin suggests that laminin is not acting by specifically interfering with ACh-induced production of NO or the ability of NO to access and inhibit PDE3. Alternatively, the foregoing results suggest the possibility that laminin may alter the ability of PDE3 to regulate cAMP or the ability of cAMP to stimulate ICa,L. These two possibilities were examined by determining the direct effects of cAMP on ICa,L using two different approaches. As summarized in Table 1, 100 μm 8-CPT-cAMP, a membrane-permeant analogue of cAMP, stimulated ICa,L to a similar extent in cells plated on glass (35 ± 7 %) or laminin (40 ± 6 %). These findings eliminate the possibility that laminin alters the sensitivity of L-type Ca2+ channels to cAMP. It is also important to note that because this concentration of 8-CPT-cAMP does not maximally stimulate ICa,L (compare response to IBMX; Table 1) we can conclude that laminin does not alter endogenous phosphatase-related mechanisms that regulate cAMP-mediated phosphorylation of L-type Ca2+ channels. In the second approach, dialysing the cell interior with 50 μm cAMP elicited a similar stimulation of ICa,L in cells plated on glass (33 ± 8 %; n = 3) and laminin (37 ± 7 %; n = 3). These findings support those obtained with 8-CPT-cAMP and further indicate that laminin does not alter PDE-related mechanisms that could attenuate cAMP-mediated effects.
We next examined whether laminin alters basal adenylate cyclase activity by inhibiting PDE activities with IBMX, a non-specific PDE inhibitor. With PDE activity inhibited and phosphatase activity unchanged, any differences in basal ICa,L amplitude between cells on laminin or glass can be attributed primarily to alterations in endogenous adenylate cyclase activity. As shown in Table 1, 100 μm IBMX elicited a significantly smaller stimulation of ICa,L in cells plated on laminin (263 ± 52 %) than on glass (619 ± 104 %), suggesting that basal adenylate cyclase activity is attenuated by laminin. To test this interpretation further we considered the effects of forskolin, a direct stimulator of adenylate cyclase. Forskolin (1 μm) elicited a significantly smaller increase in basal ICa,L in cells on laminin (127 ± 25 %) than on glass (230 ± 35 %) (Table 1).
As shown in the graph in Fig. 3, direct measurements of cAMP concentration revealed that cells, obtained from the same hearts, plated on laminin (8.5 ± 1.3 fmol (μg protein)−1) exhibited a significantly lower basal cAMP concentration than cells plated on glass (11.1 ± 1.0 fmol (μg protein)−1) (-23 %; P = 0.05; n = 4). Exposure to 1 μm forskolin stimulated cAMP significantly less in cells on laminin (28.9 ± 8.8 fmol (μg protein)−1) than in cells on glass (43.4 ± 11.8 fmol (μg protein)−1) (-33 %; P = 0.05; n = 4). Exposure to 100 μm IBMX also stimulated cAMP levels significantly less in cells on laminin (20.0 ± 1.8 fmol (μg protein)−1) than in cells on glass (28.2 ± 2.2 fmol (μg protein)−1) (-29 %; P = 0.05; n = 4). In cells plated on poly-l-lysine, basal cAMP (11.3 ± 0.6 fmol (μg protein)−1) and forskolin-stimulated cAMP (39.8 ± 11.4 fmol (μg protein)−1) levels were not different from basal and forskolin-stimulated cAMP in cells plated on glass but were significantly larger than basal and forskolin-stimulated cAMP in cells on laminin, respectively (P = 0.05). When both the ICa,L and cAMP measurements are considered together, these findings indicate that laminin attenuates adenylate cyclase activity and thereby attenuates the stimulatory response to NO signalling.
Figure 3. Measurements of cAMP concentration.
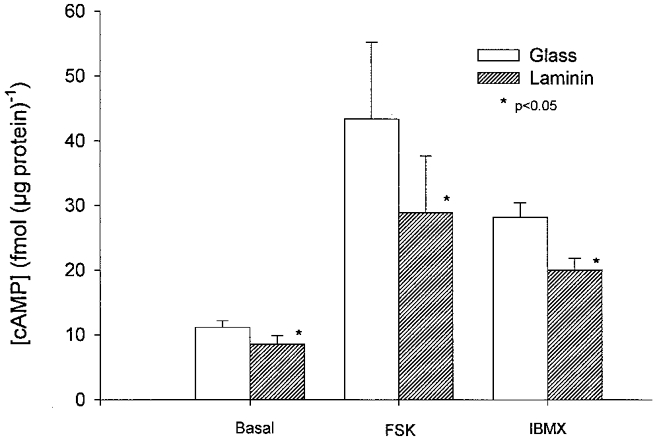
cAMP concentration in cells plated on glass (open bars) or laminin (hatched bars) under basal conditions, stimulated by exposure to 1 μm forskolin (FSK) or by exposure to 100 μm IBMX. Cells plated on laminin exhibited significantly smaller basal, forskolin- and IBMX-stimulated cAMP levels than cells plated on glass.
Laminin mediates cell attachment via engagement and clustering of β1 integrin receptors. To determine whether laminin is modifying cholinergic signalling via β1 integrin receptors we tested the effects of ACh on atrial cells from the same hearts plated on either glass or glass coverslips coated with 20 μg ml−1 goat anti-human αβ1-integrin polyclonal IgG. As shown in Fig. 4A-E, in a cell plated on glass (A and B), ACh elicited a typical inhibition followed, upon ACh withdrawal, by stimulation of ICa,L. In a cell plated on the antibody (C and D), exposure to ACh elicited inhibition of ICa,L but withdrawal of ACh failed to elicit stimulation of ICa,L. The bar graph in panel E summarizes the effects of ACh in cells on glass (open bars) and antibody (hatched bars). ACh-induced inhibition of ICa,L was not different between glass (-13 ± 3 %) and antibody (-10 ± 2 %; P = 0.4). However, stimulation of ICa,L elicited by withdrawal of ACh was significantly smaller in cells on antibody (10 ± 4 %) than on glass (38 ± 6 %; P = 0.001). The response of cells on the αβ1-integrin antibody were essentially the same as cells plated on laminin. To confirm the specificity of the antibody, cells from the same hearts were plated on either glass or a non-immune goat IgG. Neither ACh-induced inhibition of ICa,L (glass, -16 ± 4 %vs. IgG, -25 ± 7 %) or rebound stimulation of ICa,L (glass, 32 ± 5 %vs. IgG, 47 ± 14 %), respectively, were significantly different between the two groups (n = 10). In fact, the non-immune antibody tended to enhance rather than inhibit the effects of ACh. These findings indicate that laminin is acting by binding to αβ1 integrin receptors to modify the response of adenylate cyclase.
Figure 4. Effects of 1 μm ACh on basal ICa,L recorded from atrial cells plated on either glass (A and B) or 20 μg ml−1 goat anti-human αβ1-integrin polyclonal IgG (C and D).

Top row: selected original ICa,L traces. Middle row: consecutive measurements of peak ICa,L. A and B, on glass, ACh elicited a typical inhibition followed by rebound stimulation of ICa,L. C andD, on antibody, ACh elicited a typical inhibition of ICa,L but failed to elicit rebound stimulation of ICa,L. E, bar graph summarizing ACh-induced inhibition and rebound stimulation of ICa,L obtained in cells plated on either glass (open bars) or antibody (hatched bars).
During cell attachment to laminin, myocytes bind via β1 integrins to a specific amino acid sequence (YIGSR) within the laminin polypeptide chain (Iwamoto et al. 1987). Therefore, occupancy of the laminin binding site by a synthetic YIGSR peptide should interfere with laminin-dependent adhesion and integrin clustering and thereby modify its inhibitory effects on adenylate cyclase. Figure 5A-D shows a typical experiment where ACh was tested in atrial cells from the same hearts that were either: (1) plated on glass, (2) plated on laminin or (3) exposed to 100 μg ml−1 YIGSR and then plated on laminin (Laminin + YIGSR). In a cell plated on glass (A) ACh exposure and withdrawal elicited typical inhibition and stimulation of ICa,L, respectively. In a cell on laminin (B), ACh elicited a typical inhibition followed by an attenuated stimulation of ICa,L. In a laminin + YIGSR cell (C), ACh inhibited ICa,L and the stimulation of ICa,L induced by ACh withdrawal was restored. As summarized in the graph (D), ACh-induced inhibition of ICa,L was similar in all three groups. However, ACh-induced stimulation of ICa,L was not different between cells plated on glass (52 ± 9 %) and those exposed to YIGSR and then plated on laminin (58 ± 14 %) whereas the response of cells plated on laminin alone (7 ± 2 %) was significantly smaller than cells on either glass or laminin + YIGSR (P = 0.05). These findings strongly support the idea that the effects of laminin are mediated via binding to specific integrin receptors used for cell attachment.
Figure 5. Effects of 1 μm ACh on basal ICa,L recorded from atrial cells plated on either glass (A), laminin (B) or exposed to 100 μg ml−1 YIGSR and then plated on laminin (C).
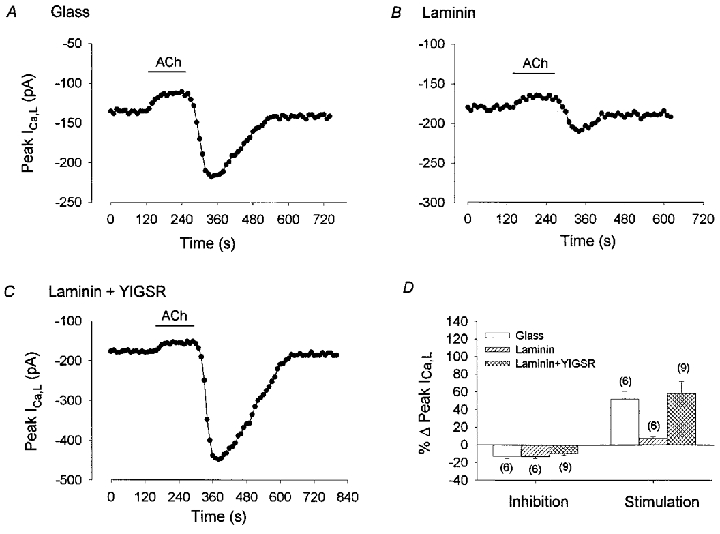
A, B and C, consecutive measurements of peak ICa,L. A, on glass, ACh induced a typical inhibition and rebound stimulation of ICa,L. B, on laminin, ACh exposure inhibited ICa,L and ACh withdrawal elicited an attenuated stimulation of ICa,L. C, in cells exposed to YIGSR and then plated on laminin (Laminin + YIGSR), stimulation of ICa,L elicited by ACh withdrawal was restored. D, bar graph summarizing ACh-induced inhibition and rebound stimulation of ICa,L in the 3 groups. The numbers in parentheses indicate the number of cells studied in each group.
Cardiomyocyte integrins are known to interact with the actin-based cytoskeleton at focal adhesions and costameres. To determine the involvement of the actin cytoskeleton we tested the effects of cytochalasin D, a compound that prevents actin polymerization (Carter, 1967). As shown in Fig. 6A-C, cells from the same hearts were plated on either glass (A), laminin (B) or laminin plus 20 μm cytochalasin D in the external solution (C). On glass (A), exposure to and withdrawal of ACh elicited typical inhibition followed by rebound stimulation of ICa,L (+67 %), respectively. As expected, on laminin (B), ACh-induced inhibition was followed by an attenuated stimulation of ICa,L (+13 %) elicited by ACh withdrawal. On laminin plus cytochalasin D (C), ACh-induced inhibition of ICa,L was followed by a restored stimulation of ICa,L (+63 %), similar to that recorded in cells on glass. The bar graph in Fig. 6D summarizes data from each cell group. ACh-induced inhibition of ICa,L was not different among the three groups. However, stimulation of ICa,L elicited by ACh withdrawal was not different between cells plated on laminin plus cytochalasin D (53 ± 6 %) and those plated on glass (54 ± 9 %), whereas the response of cells on laminin (9 ± 4 %) was significantly smaller than either of the other two groups (P = 0.05). To examine the potential role of the actin cytoskeleton further, additional experiments were performed using latrunculin A, another agent which causes disassembly of the actin cytoskeleton but via a mechanism different from cytochalasin D (Alpin & Juliano, 1999). Qualitatively similar results were obtained using the same protocol in which cells were plated on either glass (+49 %; n = 2), laminin (10 %; n = 2) or laminin plus 1 μm latrunculin A (+38 %; n = 2). These findings support the idea that the actin-based cytoskeleton is an integral component in the laminin- integrin signalling mechanism that attenuates adenylate cyclase activity and thereby the stimulation of ICa,L elicited by ACh withdrawal.
Figure 6. Effects of 1 μm ACh on basal ICa,L recorded from atrial cells plated on glass (A), laminin (B) or laminin plus 20 μm cytochalasin D (C).
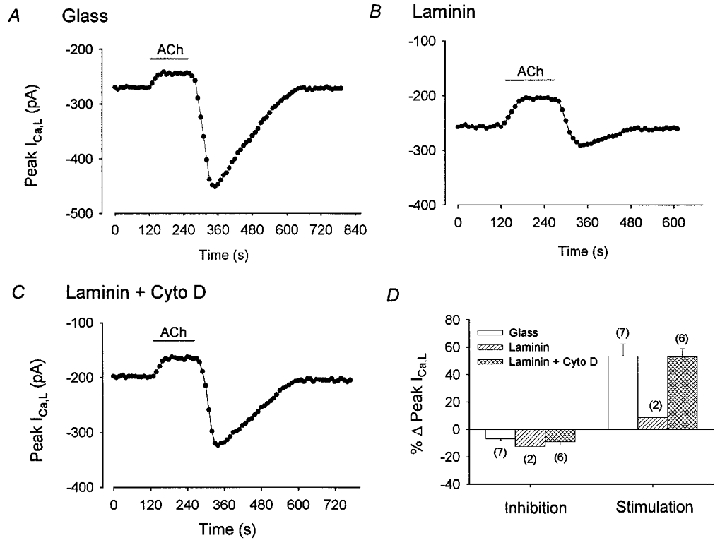
A, B and C, consecutive measurements of peak ICa,L. On glass, ACh induced a typical inhibition and rebound stimulation of ICa,L (A). On laminin, ACh exposure inhibited ICa,L and ACh withdrawal elicited an attenuated stimulation of ICa,L (B). Addition of cytochalasin D to the external solution of cells plated on laminin, restored the rebound stimulation of ICa,L elicited by ACh withdrawal. D, bar graph summarizing ACh-induced inhibition and rebound stimulation of ICa,L in the 3 groups. The numbers in parentheses indicate the number of cells studied in each group.
DISCUSSION
The present findings demonstrate that the ECM glycoprotein laminin can alter cholinergic cell signalling mechanisms that regulate ICa,L in adult atrial myocytes. laminin acts via binding to αβ1 integrin receptors in association with the actin-based cytoskeleton to attenuate adenylate cyclase activity. As a result, laminin inhibits NO signalling mechanisms that stimulate ICa,L via increases in cAMP. We believe that this report is the first to demonstrate that an ECM protein, laminin, acting via integrin-mediated signalling, can modulate autonomic regulation of Ca2+ channels in adult cardiac myocytes.
Changes in intracellular signalling and/or cytoskeletal elements can alter the affinity of integrin binding to ECM proteins, so-called ‘inside-out’ signalling (Hynes, 1992; Schwartz et al. 1995; Dedhar, 1999). The present study demonstrates the converse, where integrin binding to an ECM protein alters intracellular cell signalling mechanisms, so-called ‘outside-in’ signalling. Laminin significantly attenuated and in some cells abolished the stimulation of ICa,L typically elicited by ACh withdrawal while it exerted no discernable effect on ACh-induced inhibition of ICa,L. In atrial myocytes ACh regulates basal ICa,L by simultaneously activating two signalling pathways mediated by Gi-proteins: (1) inhibition of adenylate cyclase-cAMP signalling, and (2) activation of NO-cGMP-induced inhibition of PDE3 (Wang & Lipsius, 1995; Wang et al. 1998). Inhibition of adenylate cyclase-cAMP is primarily responsible for ACh-induced inhibition of basal ICa,L. Upon withdrawal of ACh, adenylate cyclase activity presumably recovers at a time when PDE3 activity is still inhibited, resulting in elevation of cAMP (Linden, 1987) and stimulation of ICa,L above control. The present experiments demonstrate that laminin significantly attenuates adenylate cyclase activity, thereby inhibiting the NO signalling cascade responsible for rebound stimulation of ICa,L. Thus, in atrial cells plated on laminin the stimulatory effects of NO, milrinone, IBMX and forskolin on ICa,L were significantly attenuated. Each of these agents stimulates ICa,L by indirectly or directly elevating cAMP. In turn, cAMP acts via protein kinase A (PKA) to phosphorylate sites on or near the Ca2+ channels. The ability of cAMP-dependent PKA to target phosphorylation sites depends on their association with A-kinase anchoring proteins (AKAPs) (Glantz et al. 1992, 1993; Gao et al. 1997; Yang et al. 1998; Gray et al. 1998). It seems unlikely that laminin is acting via alterations in PKA-AKAP targeting because the stimulatory effect of exogenous cAMP, which acts via PKA to stimulate ICa,L, was unaffected by laminin. Likewise, the finding that sub-maximal stimulation of ICa,L by exogenous cAMP is not different between cells on glass and laminin indicates that laminin does not alter PDE activities to degrade cAMP or phosphatase activities to dephosphorylate Ca2+ channel sites. Moreover, direct measurements of cAMP indicate that basal, forskolin-stimulated and IBMX-stimulated cAMP levels in cells plated on laminin are significantly depressed compared with glass-plated cells. In the light of each of these considerations, the present findings can be interpreted to indicate that laminin attenuates basal adenylate cyclase activity. We therefore conclude that laminin inhibits the ability of NO signalling to elevate cAMP and stimulate ICa,L by attenuating adenylate cyclase activity. This same mechanism prevents a significant rise in cAMP and subsequent stimulation of ICa,L typically elicited by withdrawal of ACh.
The present experiments indicate that laminin acts via binding to β1 integrin receptors to inhibit stimulation of ICa,L elicited by ACh withdrawal. Thus, cells plated on an antibody raised against the β1 integrin subunit mimicked the response of cells plated on laminin. These findings are consistent with β1 integrins being the primary binding proteins for laminin (Terracio et al. 1991; Ruoslahti, 1991). In addition, exposure of cells to YIGSR, a synthetic pentapeptide which binds specifically to laminin-binding sites used for cell attachment and integrin clustering (Iwamoto et al. 1987), prevented the inhibitory effects of laminin.
It is well recognized that integrin signalling is intimately associated with changes in the actin-based cytoskeleton. The present findings show that both cytochalasin D and latrunculin A, two agents that cause disassembly of the actin cytoskeleton, prevented the inhibitory effects of laminin. Although both agents destabilize the actin cytoskeleton, they act via distinctly different mechanisms. Cytochalasin D caps the growing end of actin filaments and inhibits direct integrin-mediated tyrosine phosphorylation of the focal adhesion proteins FAK and paxillin, whereas latrunculin A sequesters actin monomers (Aplin & Juliano, 1999). Both agents leave the microtubule network intact. The fact that both agents, acting via different mechanisms, elicited similar results supports the importance of the actin-based cytoskeleton in the laminin-dependent integrin-mediated signalling mechanism. However, the precise role of the actin cytoskeleton in the present findings is open to interpretation. Alterations in the actin-based cytoskeleton may have altered cytoskeletal and/or signalling elements necessary for integrin-mediated inhibition of adenylate cyclase. In other words, the actin cytoskeleton and integrin-mediated signalling mechanisms may converge on a common signal transduction pathway that leads to inhibition of adenylate cyclase. In this case, both factors are necessary for the laminin-dependent effects. Disruption of actin microfilament have been shown to alter various signal transduction (Jamney, 1998) and cytosketelal molecules (Miyamoto et al. 1995) as well as integrin binding to the ECM (Gumbiner, 1996). Alternatively, laminin-dependent integrin signalling may have acted through alterations in the actin-based cytoskeleton which in turn directly alter adenylate cyclase activity. Studies in various cell systems have shown that plating cells on ECM proteins for time periods comparable to those used in the present experiments induces integrin-mediated changes in cytoskeleton assembly and/or function (Hilenski et al. 1989; Burridge et al. 1992; Clark et al. 1998). Moreover, numerous reports indicate that integrins mediate changes, through both structural and regulatory components of focal adhesion complexes, in the assembly and distribution of the actin cytoskeleton (Schwartz et al. 1995; Miyamoto et al. 1995).
Findings in other cell systems also indicate a close association between cytosketelal components and adenylate cyclase activity (Zor, 1983). For example, early work in rat erythrocytes showed that adenylate cyclase possesses a hydrophobic as well as hydrophilic domain, allowing the enzyme to associate with the lipid bilayer and with cytoskeletal elements, respectively (Sahyoun et al. 1981). In mouse S49 lymphoma cells mechanical force transmitted through the actin cytoskeleton increases adenylate cyclase activity (Watson, 1990). In neural membranes (Rasenick et al. 1981; Rasenick & Wang, 1988) and S49 lymphoma cells (Leiber et al. 1993; Jasper et al. 1995), interventions that disrupt microtubule and/or myofilament assembly enhance Gs-protein-mediated activation of adenylate cyclase activity. Similar interventions were without affect on Gi-mediated regulation of adenylate cyclase. This latter finding is consistent with the present results that ACh-induced inhibition of ICa,L, which is mediated via Gi-proteins, is unaffected by laminin, cytochalasin D or latrunculin A. Possible Gs-protein-mediated changes in adenylate cyclase activity do not appear to play a role in the present findings. Thus, the fact that the effects of forskolin, which directly stimulates adenylate cyclase independently of G-protein activation, were attenuated by laminin indicates a direct change in the enzyme itself. Moreover, stimulation of ICa,L elicited by ACh withdrawal does not involve regulation of adenylate cyclase via Gs-protein signalling. This does not, however, preclude the possibility that additional changes involving Gs-proteins may occur in the signalling pathway proximal to adenylate cyclase.
The finding that laminin alters adenylate cyclase-mediated signalling may be relevant to parallel changes in cardiac function that occur during normal development. Thus, in rabbit ventricular myocytes (Osaka & Joyner, 1992) and membranes (Kumar et al. 1994) forskolin stimulates ICa,L and adenylate cyclase activity, respectively, to a greater extent in newborns than in adults. Moreover, basal adenylate cyclase activity is higher in newborn than adult whole ventricles and myocytes (Kumar et al. 1994). In rat heart, the spatial distribution of laminin increases throughout development (Price et al. 1992), and cell attachment to laminin and the expression of β1 integrins increases from neonatal to adult myocytes (Terracio et al. 1991). The present results, therefore, suggest the possibility that developmental changes in laminin-integrin cell signalling may mediate, at least in part, developmental changes in adenylate cyclase-mediated signalling mechanisms. Moreover, due to their well developed expression of β1 integrins, adult cardiac myocytes may be expected to be quite sensitive to laminin-mediated changes in cell signalling. As freshly isolated myocytes maintain laminin on their cell surface (Lundgren et al. 1988), plating cells on laminin may enhance β1 integrin receptor-binding, thereby exaggerating a signalling mechanism that occurs during normal cardiac development.
Another important aspect of the present study is its potential role in disease processes such as cardiac hypertrophy and failure. Ventricular pressure overload is associated with increases in ECM proteins, and changes in integrin-binding proteins (Terracio et al. 1991) and integrin-mediated signalling pathways that are intimately associated with cytoskeletal proteins (Kuppuswamy et al. 1997). In addition, in rat ventricular myocytes adenovirus-mediated over-expression of β1 integrins augmented the hypertrophic response, suggesting that integrin signalling is an important factor in the development of cardiac hypertrophy (Ross et al. 1998). Based on the present findings, we speculate that in heart disease over-expression of β1 integrins may alter adenylate cyclase-mediated regulation of ion channel function. In fact, failing human myocardium exhibits a decrease in the catalytic subunit activity of adenylate cyclase (Bristow & Feldman, 1992). Finally, the present results indicate that laboratory methodologies which use laminin as a substrate for cell attachment should not ignore the potential effects that the ECM-integrin- cytoskeleton complex may exert on intracellular signalling mechanisms.
Acknowledgments
We thank Ms Rachel Gulling and Holly Gray for their expert technical assistance in isolating myocytes and fabricating micropipettes, and Mr Alan Ferguson for his expert technical assistance with the cAMP measurements. This work was supported by NIH grants HL27652 (S.L.L.) and HL34328 (A.M.S.).
References
- Aplin AE, Juliano RL. Integrin and cytoskeletal regulation of growth factor signalling to the MAP kinase pathway. Journal of Cell Science. 1999;112:695–706. doi: 10.1242/jcs.112.5.695. [DOI] [PubMed] [Google Scholar]
- Borg TK, Terracio L. Cellular adhesion to artificial substrates and long term culture of adult cardiac myocytes. In: Clark WA, Decker RS, Borg TK, editors. Biology of Isolated Adult Cardiac Myocytes. New York: Elsevier; 1988. pp. 14–24. [Google Scholar]
- Borg TK, Xuehui M, Hilenski L, Vinson N, Terracio L. The role of the extracellular matrix on myofibrillogenesis in vitro. In: Clark EB, Takao A, editors. Developmental Cardiology: Morphogenesis and Function. Mt Kisco, New York: Futura; 1990. pp. 175–190. [Google Scholar]
- Bristow MR, Feldman AM. Changes in the receptor-G protein-adenylyl cyclase system in heart failure from various types of heart muscle disease. Basic Research in Cardiology. 1992;87:15–35. doi: 10.1007/978-3-642-72474-9_2. [DOI] [PubMed] [Google Scholar]
- Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. Journal of Cell Biology. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter SB. Effects of cytochalasins on mammalian cells. Nature. 1967;213:261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the Rho family of GTPases. Journal of Cell Biology. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedhar S. Integrins and signal transduction. Current Opinion in Hematology. 1999;6:37–43. doi: 10.1097/00062752-199901000-00007. [DOI] [PubMed] [Google Scholar]
- Ehara T, Mitsuiye T. Transient increase in the slow inward current following acetylcholine removal in catecholamine-treated guinea-pig Purkinje fibers. Japanese The Journal of Physiology. 1984;34:775–779. doi: 10.2170/jjphysiol.34.775. [DOI] [PubMed] [Google Scholar]
- Endoh M, Blinks JR. Effects of endogenous neurotransmitters on calcium transients in mammalian atrial muscle. In: Fleming WW, Graefe K-H, Langer SZ, Weiner N, editors. Neuronal and Extraneuronal Events in Autonomic Pharmacology. New York: Raven Press; 1984. pp. 221–230. [Google Scholar]
- Gao T, Yatani A, Dell'Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- Glantz SB, Amat JA, Rubin CS. cAMP signalling in neurons: patterns of neuronal expression and intracellular localization for a novel protein, AKAP 150, that anchors the regulatory subunit of cAMP-dependent protein kinase IIβ. Molecular Biology of the Cell. 1992;3:1215–1228. doi: 10.1091/mbc.3.11.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz SB, Li Y, Rubin CS. Characterization of distinct tethering and intracellular targeting domains in AKAP75, a protein that links cAMP-dependent protein kinase IIβ to the cytoskeleton. Journal of Biological Chemistry. 1993;268:12796–12804. [PubMed] [Google Scholar]
- Gray PC, Johnson BD, Westenbroek RE, Hays LG, Yates JRI, Scheuer T, Catterall WA, Murphy BJ. Primary structure and function of an A kinase anchoring protein associated with calcium channels. Neuron. 1998;20:1017–1026. doi: 10.1016/s0896-6273(00)80482-1. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison SA, Reifsnyder DH, Gallis B, Cadd GG, Beavo JA. Isolation and characterization of bovine cardiac muscle cGMP-inhibited phosphodiesterase: a receptor for new cardiotonic drugs. Molecular Pharmacology. 1986;29:506–514. [PubMed] [Google Scholar]
- Hilenski LL, Terracio L, Sawyer R, Borg TK. Effects of extracellular matrix on cytoskeletal and myofibrillar organization in vitro. Scanning Microscopy. 1989;3:535–548. [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. Journal of General Physiology. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Robey FA, Graf J, Sasaki M, Kleinman HK, Yamada Y, Martin GR. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987;238:1132–1134. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- Janmey PA. The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiological Reviews. 1998;78:763–781. doi: 10.1152/physrev.1998.78.3.763. [DOI] [PubMed] [Google Scholar]
- Jasper JR, Post SR, Desai KH, Insel PA, Bernstein D. Colchicine and cytochalasin B enhance cyclic AMP accumulation via postreceptor actions. Journal of Pharmacology and Experimental Therapeutics. 1995;274:937–942. [PubMed] [Google Scholar]
- Kirstein M, Rivet-Bastide M, Hatem S, Benardeau A, Mercadier J-J, Fischmeister R. Nitric oxide regulates the calcium current in isolated human atrial myocytes. Journal of Clinical Investigation. 1995;95:794–802. doi: 10.1172/JCI117729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Joyner RW, Hartzell HC, Ellingsen D, Rishi F, Eaton DC, Lu C, Akita T. Postnatal changes in the G-proteins, cyclic nucleotides and adenylyl cyclase activity in rabbit heart cells. Journal of Molecular and Cellular Cardiology. 1994;26:1537–1550. doi: 10.1006/jmcc.1994.1174. [DOI] [PubMed] [Google Scholar]
- Kuppuswamy D, Kerr C, Narishige T, Kasi VS, Menick DR, Cooper GI. Association of tyrosine-phosphorylated c-Src with the cytoskeleton of hypertrophying myocardium. Journal of Biological Chemistry. 1997;272:4500–4508. doi: 10.1074/jbc.272.7.4500. [DOI] [PubMed] [Google Scholar]
- Leiber D, Jasper JR, Alousi AA, Martin J, Bernstein D, Insel PA. Alteration in Gs-mediated signal transduction in S49 lymphoma cells treated with inhibitors of microtubules. Journal of Biological Chemistry. 1993;268:3833–3837. [PubMed] [Google Scholar]
- Linden J. Enhanced cAMP accumulation after termination of cholinergic action in the heart. FASEB Journal. 1987;1:119–124. doi: 10.1096/fasebj.1.2.2440752. [DOI] [PubMed] [Google Scholar]
- Lundgren E, Gullberg D, Rubin K, Borg TK, Terracio MJ, Terracio L. In vitro studies on adult cardiac myocytes: attachment and biosynthesis of collagen type IV and laminin. Journal of Cellular Physiology. 1988;136:43–53. doi: 10.1002/jcp.1041360106. [DOI] [PubMed] [Google Scholar]
- Maragos CM, Morley D, Wink DA, Dunams TM, Saavedra JE, Hoffman A, Bove AA, Isaac L, Hrabie JA, Keefer LK. Complexes of NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. Journal of Medicinal Chemistry. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. Journal of Cell Biology. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Noma A. Autonomic regulation of cardiac chloride current. Japanese Journal of Physiology. 1994;44:S193–198. [PubMed] [Google Scholar]
- Osaka T, Joyner RW. Developmental changes in the β-adrenergic modulation of calcium currents in rabbit ventricular cells. Circulation Research. 1992;70:104–115. doi: 10.1161/01.res.70.1.104. [DOI] [PubMed] [Google Scholar]
- Price RL, Nakagawa M, Terracio L, Borg TK. Ultrastructural localization of laminin on in vivo embryonic, neonatal, and adult rat cardiac myocytes and in early rat embryos raised in whole-embryo culture. Journal of Histochemistry and Cytochemistry. 1992;40:1373–1381. doi: 10.1177/40.9.1506674. [DOI] [PubMed] [Google Scholar]
- Rasenick MM, Stein PJ, Bitensky MW. The regulatory subunit of adenylate cyclase interacts with cytoskeletal components. Nature. 1981;294:560–562. doi: 10.1038/294560a0. [DOI] [PubMed] [Google Scholar]
- Rasenick MM, Wang N. Exchange of guanine nucleotides between tubulin and GTP-binding proteins that regulate adenylate cyclase: cytoskeletal modification of neuronal signal transduction. Journal of Neurochemistry. 1988;51:300–311. doi: 10.1111/j.1471-4159.1988.tb04870.x. [DOI] [PubMed] [Google Scholar]
- Ross RR, Pham C, Shai S-Y, Goldhaber JI, Fenczik C, Glembotski CC, Ginsberg MH, Loftus JC. β1 integrins participate in the hypertrophic response of rat ventricular myocytes. Circulation Research. 1998;82:1160–1172. doi: 10.1161/01.res.82.11.1160. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins as receptors for extracellular matrix. In: Hay ED, editor. Cell Biology of Extracellular Matrix. New York: Plenum Press; 1991. pp. 343–363. [Google Scholar]
- Sahyoun NE, Le Vine H, III, Hebdon G M, Hemadah R, Cuatrecasas P. Specific binding of solubilized adenylate cyclase to the erythrocyte cytoskeleton. Proceedings of the National Academy of Sciences of the USA. 1981;78:2359–2362. doi: 10.1073/pnas.78.4.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annual Review of Cell and Developmental Biology. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Terracio L, Rubin K, Gullberg D, Balog E, Carver W, Jyring R, Borg TK. Expression of collagen binding integrins during cardiac development and hypertrophy. Circulation Research. 1991;68:734–744. doi: 10.1161/01.res.68.3.734. [DOI] [PubMed] [Google Scholar]
- Wang YG, Lipsius SL. Acetylcholine elicits a rebound stimulation of Ca2+ current mediated by pertussis toxin-sensitive G protein and cAMP-dependent protein kinase A in atrial myocytes. Circulation Research. 1995;76:634–644. doi: 10.1161/01.res.76.4.634. [DOI] [PubMed] [Google Scholar]
- Wang YG, Lipsius SL. A cellular mechanism contributing to post-vagal tachycardia studied in isolated pacemaker cells from cat right atrium. Circulation Research. 1996;79:109–114. doi: 10.1161/01.res.79.1.109. [DOI] [PubMed] [Google Scholar]
- Wang YG, Rechenmacher CE, Lipsius SL. Nitric oxide signaling mediates stimulation of L-type Ca2+ current elicited by withdrawal of acetylcholine in cat atrial myocytes. Journal of General Physiology. 1998;111:113–125. doi: 10.1085/jgp.111.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YG, Samarel AM, Lipsius SL. Laminin alters signaling mechanisms that regulate L-type Ca2+ current in cat atrial myocytes. Biophysical Journal. 1999;76:24. (abstract) [Google Scholar]
- Watson PA. Direct stimulation of adenylate cyclase by mechanical force in S49 mouse lymphoma cells during hyposmotic swelling. Journal of Biological Chemistry. 1990;265:6569–6575. [PubMed] [Google Scholar]
- Wu J, Vereecke J, Carmeliet E, Lipsius SL. Ionic currents activated during hyperpolarization of single right atrial myocytes from cat heart. Circulation Research. 1991;68:1059–1069. doi: 10.1161/01.res.68.4.1059. [DOI] [PubMed] [Google Scholar]
- Yang J, Drazba JA, Ferguson DG, Bond M. A-kinase anchoring protein 100 (AKAP100) is localized in multiple subcellular compartments in the adult rat heart. Journal of Cell Biology. 1998;142:511–522. doi: 10.1083/jcb.142.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharov SI, Harvey RD. Rebound stimulation of the cAMP-regulated Cl− current by acetylcholine in guinea-pig ventricular myocytes. The Journal of Physiology. 1997;499:105–120. doi: 10.1113/jphysiol.1997.sp021914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zor U. Role of cytoskeletal organization in the regulation of adenylate cyclase-cyclic adenosine monophosphate by hormones. Endocrine Reviews. 1983;4:1–21. doi: 10.1210/edrv-4-1-1. [DOI] [PubMed] [Google Scholar]


