Abstract
We employed the whole-cell recording technique in conjunction with fluorometry to measure cytosolic Ca2+ concentration ([Ca2+]i) and exocytosis (capacitance measurement) in single, identified rat gonadotrophs.
Direct activation of G-protein (via intracellular dialysis of non-hydrolysable analogues of GTP, but not of GDP) triggered a slow rise in capacitance even in the presence of a fast intracellular Ca2+ chelator.
The broad-spectrum kinase inhibitors H7 and staurosporine did not prevent this Ca2+-independent exocytosis, ruling out the involvement of the cAMP and PKC pathways.
AlF4−, a potent stimulator of heterotrimeric G-proteins, failed to stimulate any exocytosis when the intracellular Ca2+ store was depleted, implicating the involvement of AlF4−-insensitive G-protein(s).
Maximal stimulation of Ca2+-independent exocytosis by GTP analogues did not reduce the number of readily releasable granules that were available subsequently for Ca2+-dependent release.
The last finding raises the possibility that the G-protein-stimulated Ca2+-independent exocytosis may regulate a pool of granules that is distinct from the Ca2+-dependent pool.
In rat pituitary gonadotrophs, stimulation of gonadotropin-releasing hormone (GnRH) receptors activates the Gq-coupled phosphoinositide pathway that in turn triggers rhythmic release of Ca2+ from the IP3-sensitive store (Tse & Hille, 1992; Kukuljan et al. 1992). The GnRH-triggered [Ca2+]i oscillation is closely accompanied by exocytosis and the inclusion of intracellular Ca2+ buffer strongly suppresses exocytosis, indicating that the GnRH-stimulated exocytosis is Ca2+ dependent (Tse et al. 1993). However, in permeabilized sheep gonadotrophs in which [Ca2+]i was strongly buffered, activation of G-protein by non-hydrolysable analogues of GTP has been reported to stimulate luteinizing hormone (LH) release at resting or low [Ca2+]i and this action has been attributed to the combined activation of the cAMP and protein kinase C (PKC) pathways (Macrae et al. 1990; van der Merwe et al. 1991). In rat gonadotrophs, on the other hand, cAMP was reported to have negligible effect on stimulation of LH secretion (Turgeon & Waring, 1986; Billiard et al. 1997) but the activation of the PKC pathway can trigger exocytosis even when [Ca2+]i remains near the resting level (Billiard et al. 1997). Here, we investigated whether G-protein activation in rat gonadotrophs could directly affect exocytosis independently of [Ca2+]i elevation. Our results indicate that G-protein activation can directly stimulate a component of exocytosis that is independent of [Ca2+]i elevation, as well as the activation of the cAMP or PKC pathway. This G-protein-mediated and Ca2+-independent exocytosis appears to be acting on a pool of vesicles or granules that is distinct from the Ca2+-dependent pool. Preliminary data of this work have been published in an abstract (Tse & Tse, 1997).
METHODS
Cell preparation
The anterior lobe of the pituitary gland was removed from male Sprague-Dawley rats (age 5-6 weeks) that had been killed with halothane in accordance with the standards of the Canadian Council on Animal Care. Anterior pituitary glands were dissociated enzymatically using collagenase and trypsin as previously described (Tse & Hille, 1994). Single gonadotrophs were identified from the heterogeneous population by reverse haemolytic plaque assay (Smith et al. 1984), using polyclonal antibodies to LH (kindly provided by Dr J. D. Neill, University of Alabama, Birmingham). The procedures were similar to those described previously (Tse & Hille, 1994). The cells were maintained under standard culture conditions in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 % (v/v) horse serum, 50 μg ml−1 penicillin G and 50 μg ml−1 streptomycin (all from Gibco, Grand Island, NY, USA). Recordings were performed on cells cultured for 2-4 days.
Chemicals and solutions
Stock of GnRH (Peninsula Laboratories, Belmont, CA, USA) was dissolved in 0.1 M acetic acid, lyophilized, and kept at -20°C. Indo-1 (Calbiochem, La Jolla, CA, USA) and indo-1 FF (Texas Fluorescence Labs, Austin, TX, USA) were kept as stock solutions in distilled water at -20°C. Guanosine 5′-triphosphate (GTP-γ-S), guanylyl-5′-imidodiphosphate (GppNHP), guanosine 5′-O-(2-thiodiphosphate) (GDP-β-S), 2,5-di-(t-butyl)-1,4-hydroquinone (BHQ), staurosporine and pertussis toxin were obtained from Calbiochem. EGTA, apamin, 1-(5-isoquinolinesulfonyl)-2-methylpiperazine HCl (H7) and BAPTA were obtained from Sigma. DM-nitrophen and caged GTP-γ-S were obtained from Molecular Probes (Eugene, OR, USA).
The standard bath solution contained (mM): 150 NaCl, 10 Hepes, 8 glucose, 2.5 KCl, 2 CaCl2 and 1 MgCl2 (pH 7.4). In experiments where extracellular Ca2+ was removed, Ca2+ was omitted from the standard bath solution but 3 mM MgCl2 and 1 mM Na-EGTA were added. In experiments involving flash photolysis of caged Ca2+-DM-nitrophen, apamin (0.4 μM) was included in the standard bath solution to inhibit the SK type Ca2+-activated K+ current (Tse & Hille, 1992). The standard pipette solution contained (mM): 120 potassium aspartate, 20 KCl, 20 K-Hepes, 1 MgCl2, 2 Na2ATP, 0.1 Na4GTP and 0.1 indo-1 (pH 7.4). In experiments where [Ca2+]i was elevated via flash photolysis of caged Ca2+, the pipette solution contained (mM): 70 caesium aspartate, 40 Cs-Hepes, 20 tetraethylammonium-Cl, 0.1 Na4GTP, 0.1 indo-1 FF and 6.5-8 DM-nitrophen (∼90 % saturated with Ca2+) (pH 7.4). As the purity of DM-nitrophen varied slightly between batches, the loading of each patch of DM-nitrophen with Ca2+ was determined empirically. In experiments involving pertussis toxin treatment, cells were incubated with pertussis toxin (100 ng ml−1) in DMEM at 37°C for 20-24 h before recording.
Measurement of [Ca2+]i
[Ca2+]i was measured fluorometrically using the ratiometric Ca2+ indicator indo-1 or indo-1 FF. The Ca2+ indicator was dialysed into the cell via the whole-cell patch pipette. Details of the instrumentation and procedures of the [Ca2+]i measurement were as described previously (Tse & Hille, 1994; Tse & Tse, 1998). Briefly, indo-1 or indo-1 FF in single gonadotrophs was excited by 365 nm (band-pass filtered) light delivered from a HBO 100 W mercury lamp via a × 40, 1.3 NA UV fluor oil objective (Nikon). Photon counts were collected at 405 and 500 nm by two photomultiplier tubes (Hamamatsu H3460-04) and then translated into logic signals counted simultaneously by a CYCTM-10 counter card (Cyber Research Inc., Branford, CT, USA) on an IBM-compatible computer. [Ca2+]i was calculated from the ratio (R) of fluorescence at 405 and 500 nm, using the equation of Grynkiewicz et al. (1985):
| (1) |
where Rmin is the fluorescence ratio of Ca2+-free indicator and Rmax is the ratio of Ca2+-bound indicator. K* is a constant that was determined empirically. Calibrations were determined from groups of single gonadotrophs (n = 5-8) dialysed with one of the three pipette solutions as described previously (Tse & Tse, 1998). For indo-1 measurement, Rmin was measured in cells loaded with (mM): 52 potassium aspartate, 10 KCl, 50 K-EGTA, 0.1 indo-1 and 50 K-Hepes (pH 7.4); and Rmax was measured in cells loaded with (mM): 136 potassium aspartate, 15 CaCl2, 0.1 indo-1 and 50 K-Hepes (pH 7.4). K* was calculated from eqn (1) using R values obtained from cells loaded with (mM): 60 potassium aspartate, 50 K-Hepes, 20 K-EGTA, 15 CaCl2 and 0.1 indo-1 (pH 7.4), which had a calculated free Ca2+ concentration of 212 nM at 24°C (Blinks et al. 1982). For all indo-1 measurements reported here, the values of Rmin, Rmax and K* were 0.403, 5.05 and 2.62 μM, respectively. In experiments involving flash photolysis of caged Ca2+-DM-nitrophen, [Ca2+]i was measured with the low affinity Ca2+ indicator indo-1 FF. For these measurements, Rmax was measured in cells loaded with (mM): 109 potassium aspartate, 25 CaCl2, 10 DM-nitrophen, 0.1 indo-1 FF and 40 K-Hepes (pH 7.4). K* was calculated from eqn (1) using R values obtained from cells loaded with (mM): 70 diglycolic acid, 15 potassium aspartate, 50 K-Hepes, 10 CaCl2 and 0.1 indo-1 FF (pH 7.4), which had a free Ca2+ concentration of 24 μM at 24°C (measured with a Ca2+ electrode). For all indo-1 FF measurements reported here, the values of Rmax and K* were 2.56 and 79.2 μM, respectively. Rmin was obtained individually for each cell as the average of 5-10 measurements taken immediately before the flash and the values ranged from 0.21 to 0.25.
Electrophysiological recording
Single gonadotrophs were voltage clamped with the whole-cell, gigaseal method (Hamill et al. 1981) using an EPC-9 patch clamp amplifier. Changes in cell capacitance (Cm) during intracellular dialysis of GTP analogues were measured with the capacitance-tracking feature of the EPC-9 amplifier (Tse et al. 1997). During flash photolysis of caged Ca2+, the rapid increase in Cm was measured at high temporal resolution with a dual-phase lock-in amplifier (Tse et al. 1997). Values of [Ca2+]i and Cm were first recorded on VCR tapes with a NeuroData PCM recorder and digitized later. The pipettes were made from haematocrit glass (VWR Scientific Canada Ltd, London, Ontario, Canada) and the resistance was 2-4 MΩ after filling and 5-10 MΩ during whole-cell recording. Recordings were done at room temperature (22-25°C). In all the experiments reported here, the cell membrane potential was held at -70 mV, a potential at which most voltage-gated channels in the gonadotrophs were closed (Tse et al. 1993). Values given in the text are means ±s.e.m. To photolyse caged molecules, a UV flash from a modified XF-10 xenon flash lamp (Hi-Tech Ltd, Salisbury, UK) was delivered to the cell via a fused silica focusing lens which replaced the microscope's condenser (Tse et al. 1997).
RESULTS
Activation of G-protein(s) triggers Ca2+-independent exocytosis
Figure 1 shows a simultaneous measurement of [Ca2+]i and cell membrane capacitance (Cm) in a gonadotroph during G-protein activation. In order to bypass the GnRH receptors and directly activate G-protein, we have employed GTP-γ-S, a non-hydrolysable analogue of GTP. In this experiment, the cell was recorded with 100 μM caged GTP-γ-S in the pipette solution. About 3 min after whole-cell recording had begun, a UV flash was delivered (at time zero) to photolyse the caged molecule and release GTP-γ-S. Shortly following the flash (∼10 s), Cm started to rise, reflecting exocytosis. Cm continued to rise and at ∼100 s after the UV flash, the cumulative increase in Cm reached ∼0.09 pF. This increase in Cm was subsequently followed by a decrease, reflecting endocytosis. Although GTP-γ-S has been postulated to inhibit some types of endocytosis (Carter et al. 1993; Takei et al. 1995), endocytosis could still occur in gonadotrophs even in the continued presence of GTP-γ-S (see also Fig. 6B). Note that the exocytosis in Fig. 1 is not accompanied by any change in [Ca2+]i. [Ca2+]i remained near its resting level (∼50 nM) throughout the recording period. A similar result was observed in three other cells. In all the cells examined, exocytosis started with a delay of 10-20 s after the delivery of the UV flash. This delay cannot be due to a slow release of GTP-γ-S because the kinetics of release of GTP-γ-S from the cage during flash photolysis is rapid (milliseconds). Instead, the delay suggests that the GTP-γ-S effect in the gonadotrophs is intrinsically slow.
Figure 1. Flash photolysis of caged GTP-γ-S triggers slow exocytosis in gonadotrophs without [Ca2+]i elevation.
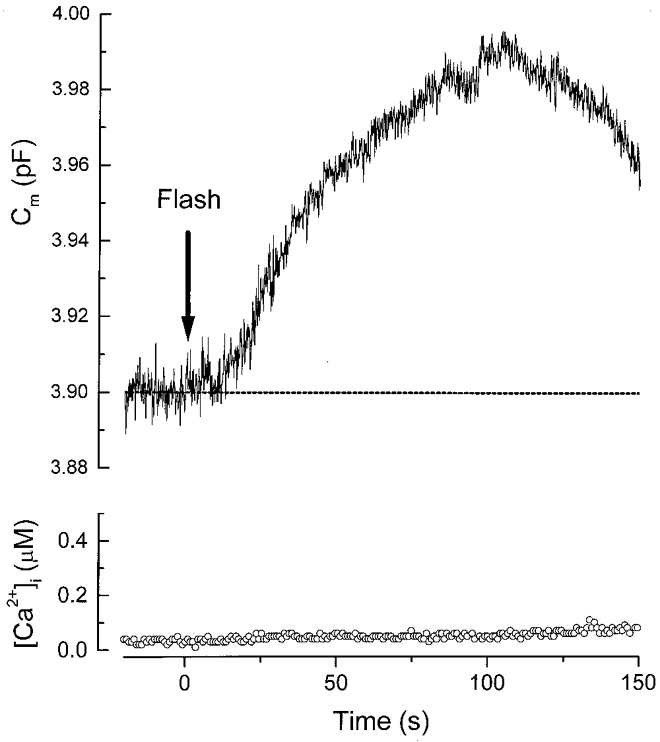
The pipette solution contained 100 μM caged GTP-γ-S and 100 μM indo-1. A UV flash was delivered at time zero (indicated by the arrow). Note that there was a delay of ≈10 s before the membrane capacitance (Cm) started to rise.
Figure 6. Multiple components of exocytosis in a gonadotroph.
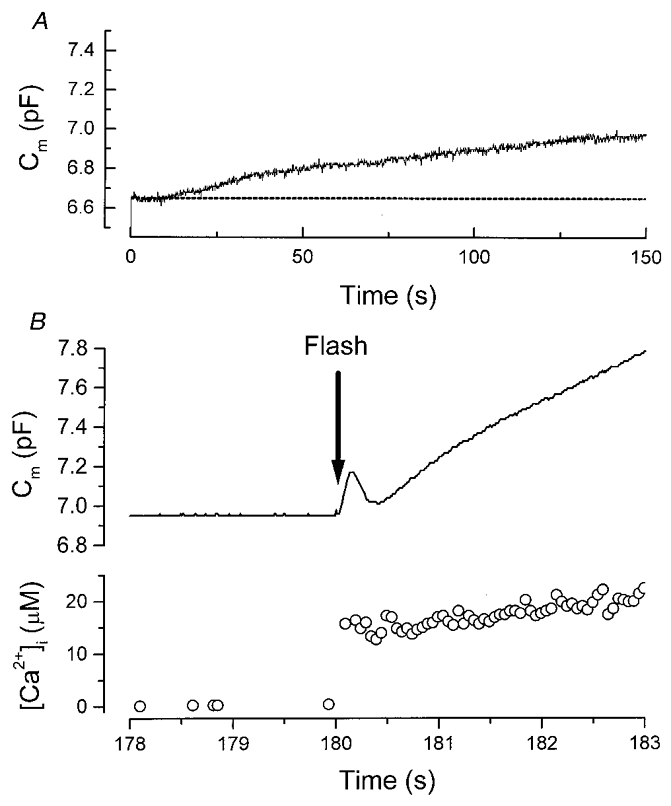
A, slow Ca2+-independent exocytosis triggered by intracellular GTP-γ-S. The pipette solution contained GTP-γ-S (50 μM), 100 μM indo-1 FF and the caged Ca2+ compound (DM-nitrophen). Before the delivery of the UV flash, DM-nitrophen was a strong Ca2+ chelator, thus the resting [Ca2+]i was buffered to a low level. B, fast Ca2+-dependent exocytosis triggered by the rapid [Ca2+]i elevation in the same cell as shown in A. At 180 s after whole-cell recording began, a UV flash (denoted by the arrow) was delivered to photolyse caged Ca2+. [Ca2+]i rose rapidly to ≈15 μM and triggered at least two kinetically distinct phases of exocytosis. The fastest burst reflected the exhaustion of a pool of most readily releasable granules.
Although flash photolysis of caged GTP-γ-S has the advantage of inducing rapid elevation in the concentration of GTP-γ-S inside the cell, the fraction of caged GTP-γ-S photolysed by each flash cannot be controlled precisely. The uncertainty about the concentration of GTP-γ-S creates difficulties in making comparisons among cells. Therefore, for the rest of the experiments in this study, a known concentration of non-hydrolysable GTP analogue was dialysed into the cell via the whole-cell pipette.
To examine whether the GTP-γ-S-triggered exocytosis was indeed independent of any rise in [Ca2+]i, we included 10 mM BAPTA in the whole-cell pipette solution to buffer [Ca2+]i to a low concentration. In all cells recorded with BAPTA, [Ca2+]i (data not shown) remained near zero (∼Rmin of indo-1) throughout the experiment. Figure 2A shows that during the first 25 s of the whole-cell recording, Cm remained near the resting level. Subsequently, Cm started to rise. This cell was recorded with 200 μM GTP-γ-S in the pipette solution. The delay observed here is in part due to the time taken for sufficient GTP-γ-S to diffuse into the cell. In a previous study (Tse & Hille, 1994), we have shown that the diffusion of indo-1 (MW = 750), a molecule which is comparable in size to GTP-γ-S (MW = 563), into a gonadotroph (typically ∼5 pF) has a time constant of ∼65 s (pipette series resistance of 6 MΩ). Thus, under our current experimental conditions, the diffusion of GTP-γ-S into the cell should reach an equilibrium within 200-300 s. Figure 2A shows that Cm continued to rise slowly and appeared to reach a plateau after ∼5-6 min. In this and all other cells recorded with GTP analogues (e.g. Fig. 3A), Cm remained near a plateau level during the time course of recording (∼8-10 min). This probably reflects a saturation of the effect of GTP-γ-S. Alternatively, it may reflect a balance between exocytosis and endocytosis in the cell. Nevertheless, Fig. 2A shows that the cumulative Cm increase triggered by 200 μM GTP-γ-S reached ∼0.4 pF. Note that this stimulatory action of GTP-γ-S could be observed even in the absence of extracellular Ca2+ (see Fig. 5C). Thus the GTP-γ-S-triggered exocytosis is independent of a rise in [Ca2+]i or the presence of extracellular Ca2+. On the other hand, in cells recorded with a pipette solution containing 10 mM BAPTA but no GTP-γ-S, Cm remained near its resting level for > 5 min (e.g. Fig. 2B; n = 5). Figure 2C shows that the amplitude of the GTP-γ-S-triggered Cm rise was dependent on the concentration of GTP-γ-S. In cells stimulated with 50, 200 or 500 μM GTP-γ-S in the pipette solution, the cumulative increase in Cm was 0.259 ± 0.047 pF (n = 13), 0.502 ± 0.09 pF (n = 4) and 0.549 ± 0.08 pF (n = 9), respectively. Note that increasing the concentration of GTP-γ-S from 200 to 500 μM caused little additional increase in Cm and the maximal increase in Cm was ∼0.5 pF. In rat gonadotrophs, the total number of granules present in a single cell is estimated to be ∼10 000 (Tse et al. 1993) and there are few if any microvesicles (Tse et al. 1997). Using flash photolysis of caged Ca2+, we have previously shown that only ∼140 granules are readily releasable (Tse et al. 1997). The average diameter of the granules in gonadotrophs is ∼250 nm (Tse et al. 1997), and each granule is expected to have a capacitance of 1.9 fF. Therefore, a Cm increase of 500 fF by GTP-γ-S reflects the exocytosis of ∼260 granules, almost 2-fold the size of the pool of readily releasable granules.
Figure 2. The GTP-γ-S-triggered exocytosis is Ca2+ independent.
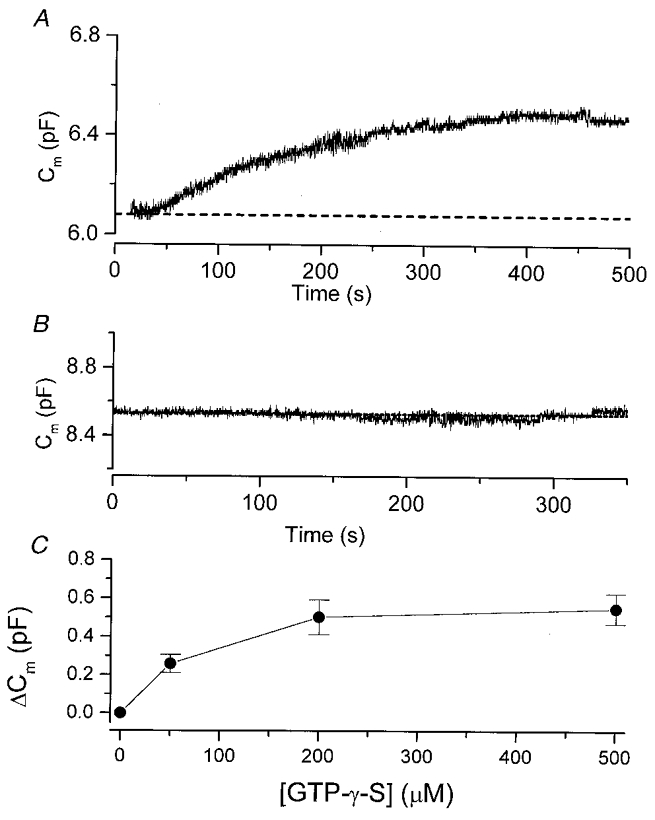
A, intracellular dialysis of GTP-γ-S triggered exocytosis even in the presence of intracellular Ca2+ chelator. The pipette solution contained 200 μM GTP-γ-S and 10 mM BAPTA. At time zero, the whole-cell recording began. B, no exocytosis could be observed when the pipette solution contained BAPTA but no GTP-γ-S. C, the amount of exocytosis is dependent on the concentration of GTP-γ-S. The pipette solution contained 0, 50, 200 or 500 μM GTP-γ-S. Each data point is generated from 4-13 cells. Note that at [GTP-γ-S] > 200 μM, the effect of GTP-γ-S appeared to saturate at ≈0.5 pF.
Figure 3. The GTP-γ-S response is due to G-protein activation.
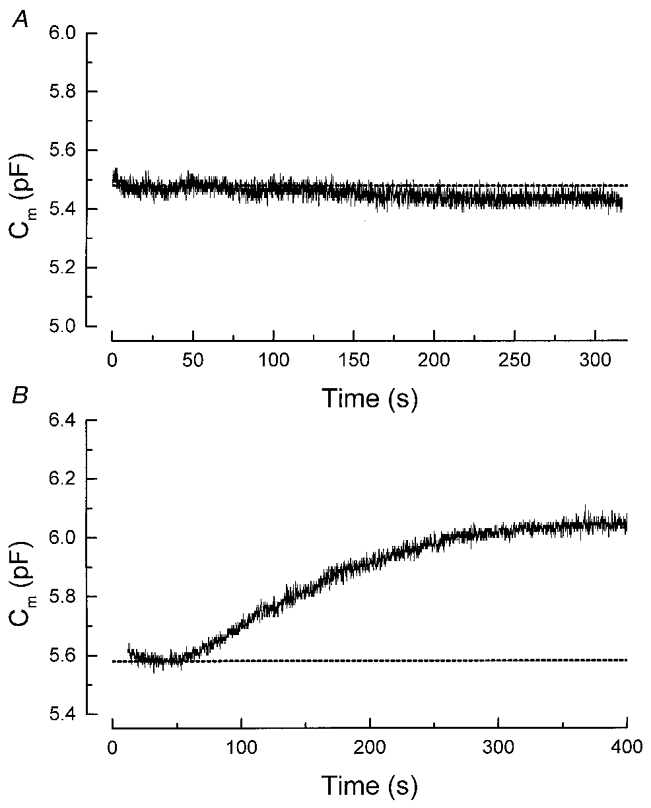
A, intracellular dialysis of GDP-β-S, a non-hydrolysable analogue of GDP, failed to mimic the GTP-γ-S response. The pipette solution contained 1 mM GDP-β-S and 10 mM BAPTA. B, intracellular dialysis of another non-hydrolysable GTP analogue, GppNHP, also triggered slow Ca2+-independent exocytosis. The pipette solution contained 500 μM GppNHP and 10 mM BAPTA.
Figure 5. The G-protein action does not involve activation of heterotrimeric G-proteins.
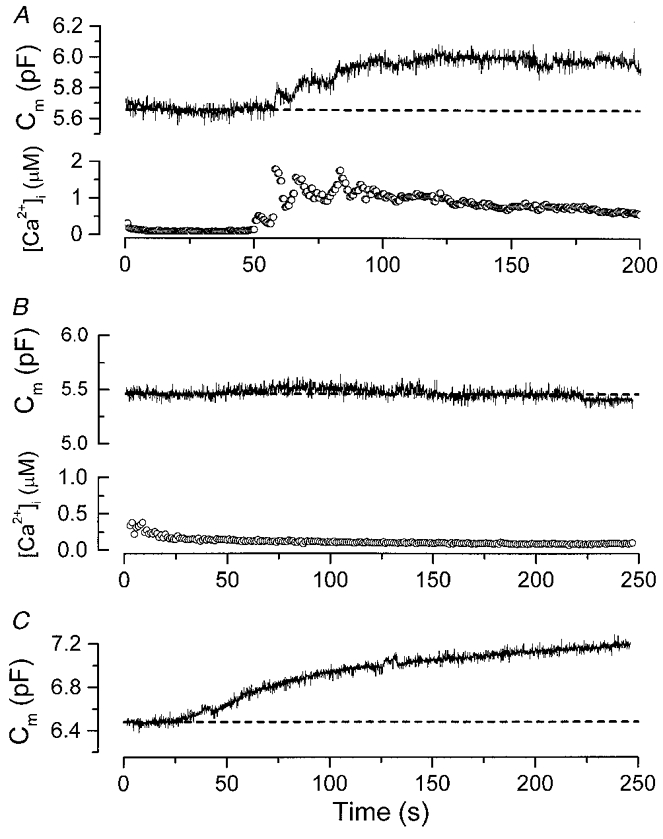
A, AlF4−, a potent activator of heterotrimeric G-proteins, stimulated [Ca2+]i oscillation which was accompanied by Ca2+-dependent exocytosis. The pipette solution contained AlF4− (100 μM) and 100 μM indo-1 but no BAPTA. B, when the intracellular Ca2+ store was depleted, AlF4− failed to trigger any exocytosis. To deplete the intracellular Ca2+ store, the cell was bathed continuously in a solution containing 1 mM EGTA (no added Ca2+) plus the SERCA inhibitor BHQ (1 μM). It was then challenged with GnRH (100 nM) for 3 min. About 5 min following the removal of GnRH, the whole-cell recording began. Note that [Ca2+]i remained near 100-200 nM throughout the time course of the recording, suggesting that most of the intracellular Ca2+ store was depleted by this experimental manipulation. Same pipette solution as in A. C, the depletion of the intracellular Ca2+ store does not affect the GTP-γ-S triggered Ca2+-independent exocytosis. The cell was subjected to the same experimental procedure as described in B. Intracellular dialysis of GTP-γ-S still triggered robust exocytosis. The pipette solution contained 500 μM GTP-γ-S and 10 mM BAPTA. Note that in both B and C, the cells were continuously bathed in a Ca2+-free solution containing BHQ (1 μM).
The effect of GTP-γ-S may be mediated by at least two distinct mechanisms: the binding of GTP-γ-S to a G-protein and thus directly activating the G-protein or the inhibition of a GTPase. To distinguish between these two mechanisms, we examined the action of a non-hydrolysable analogue of GDP (GDP-β-S). Similar to GTP-γ-S, GDP-β-S can inhibit GTPase activities. Thus a mimicking of the GTP-γ-S effect by GDP-β-S will imply an inhibition of GTPase. On the other hand, GDP-β-S cannot activate G-protein and a failure of GDP-β-S to mimic the action of GTP-γ-S will indicate an involvement of G-protein activation. Figure 3A shows that in cells recorded with 1 mM GDP-β-S for > 5 min, no significant increase in Cm could be detected (n = 4). This result suggests that the GTP-γ-S-triggered exocytosis is mediated by the stimulation of a G-protein rather than via GTPase inhibition. Consistent with this, in cells recorded with 500 μM GppNHP (another non-hydrolysable analogue of GTP), an increase in Cm could be observed following the diffusion of GppNHP into the cell (Fig. 3B). In six cells examined with GppNHP (500 μM), the mean cumulative increase in membrane capacitance (at 240-300 s after whole-cell recording) was 0.478 ± 0.109 pF, closely resembling that triggered by 500 μM GTP-γ-S (0.549 ± 0.08 pF; n = 9).
The G-protein action is not mediated via the PKC or cAMP pathway
The experiments above strongly suggest that the effect of non-hydrolysable analogues of GTP (GTP-γ-S or GppNHP) on exocytosis is mediated by the activation of G-proteins. The non-hydrolysable analogue of GTP employed here will stimulate all heterotrimeric G-proteins in the gonadotrophs. These will include Gs which is coupled to the cAMP pathway and Gq which is coupled to the phospholipase C and PKC pathway. To examine whether the G-protein-triggered Ca2+-independent exocytosis observed here is caused by the activation of protein kinases, we employed two broad-spectrum protein kinase inhibitors, H7 and staurosporine. H7 inhibits cAMP-dependent kinase and PKC with Ki values of 3.0 and 6.0 μM, respectively (Hidaka et al. 1984). Staurosporine, a more potent inhibitor, inhibits these two kinases with Ki values of 8.2 and 2.7 nM (Takahashi et al. 1990). In rat pituitary cells, both of these inhibitors have been reported to inhibit phorbol ester-mediated LH release with an IC50 of 1.7 μM for H7 and 44 nM for staurosporine (Thomson et al. 1993). Figure 4A shows a gonadotroph that had been treated with H7 (50 μM) for 15-25 min before whole-cell recording began. Intracellular dialysis of GppNHP still triggered robust Ca2+-independent exocytosis. In six cells treated with H7, the mean increase in capacitance triggered by GppNHP (500 μM) was 0.425 ± 0.070 pF, similar to that of the controls (0.478 ± 0.109 pF; n = 6). Figure 4B shows that pretreatment with staurosporine (200 nM) for 15 min also failed to inhibit the GppNHP response. In seven cells treated with staurosporine, the mean increase in membrane capacitance triggered by GppNHP (500 μM) was 0.471 ± 0.101 pF. At the concentrations employed here, H7 and staurosporine would inhibit the cAMP-dependent kinase as well as PKC (Hidaka et al. 1984; Takahashi et al. 1990). Thus it is unlikely that these protein kinases are involved in the G-protein-triggered Ca2+-independent exocytosis.
Figure 4. Second messenger pathway.
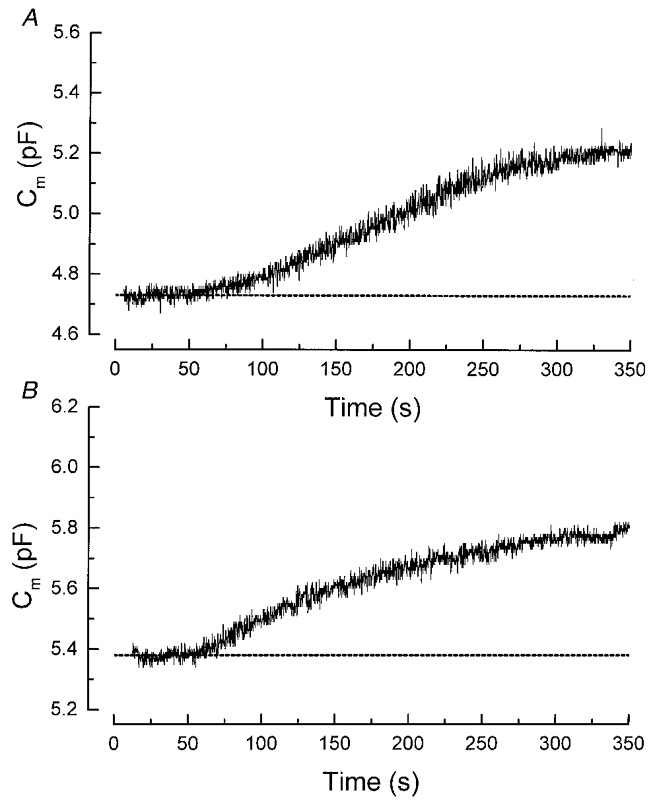
A, the broad spectrum kinase inhibitor H7 failed to inhibit the G-protein-triggered exocytosis. The cell had been exposed to the kinase inhibitor H7 (50 μM) in the bath for 20 min before whole-cell recording began. Intracellular dialysis of GppNHP still triggered robust exocytosis. B, the PKC inhibitor staurosporine (200 nM) did not prevent the G-protein stimulation of exocytosis. The cell was exposed to staurosporine in the bath for 15 min before the establishment of the whole-cell configuration. In both A and B, the pipette solution contained 500 μM GppNHP and 10 mM BAPTA.
To examine further the involvement of a second messenger pathway underlying the G-protein response, we asked whether AlF4−, a potent activator of all heterotrimeric G-proteins (including Gs, Gq, Gi/Go) can stimulate Ca2+-independent exocytosis. Since most Ca2+ chelators, including BAPTA, bind strongly to Al3+ and can reduce the effect of AlF4−, we omitted BAPTA from the pipette solution in this series of experiments. As expected from the stimulatory action of AlF4− on Gq which in turn leads to the generation of IP3 and activation of PKC, Fig. 5A shows that the diffusion of AlF4− (100 μM; generated as AlFx− where x = 3-5 as described in Okano et al. 1993) into the gonadotroph stimulated [Ca2+]i oscillations that were accompanied by Ca2+-dependent exocytosis (n = 3). While this result shows that AlF4− indeed stimulates heterotrimeric G-proteins in gonadotrophs, it is difficult to separate the Ca2+-independent component from the Ca2+-dependent component of exocytosis. In order to study the Ca2+-independent component of exocytosis in isolation, we reduced intracellular Ca2+ release in gonadotrophs by depleting the intracellular Ca2+ stores. In a previous study (Tse et al. 1994), we have shown that during GnRH stimulation, the sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) has an essential role in the reuptake of Ca2+ into the intracellular stores. Here, we exploited this property to deplete the intracellular stores by exposing the cell to the SERCA inhibitor BHQ (1 μM), in the absence of extracellular Ca2+. The cell was then challenged with GnRH (100 nM) for 3 min to stimulate intracellular Ca2+ release. Following GnRH removal, the cell was exposed continuously to BHQ in the absence of extracellular Ca2+ for another 5 min before whole-cell recording began. Under such experimental condition, intracellular AlF4− failed to elicit any [Ca2+]i rise or exocytosis (Fig. 5B; n = 5). To examine whether the experimental manipulation to deplete intracellular Ca2+ stores inhibited Ca2+-independent exocytosis, the same experiments were repeated in cells recorded with GTP-γ-S and BAPTA in the whole-cell pipette. Figure 5C shows that even following the depletion of intracellular Ca2+ stores, GTP-γ-S can still trigger robust Ca2+-independent exocytosis (n = 6). Since AlF4− activates both Gs and Gq, the failure of AlF4− to trigger Ca2+-independent exocytosis in gonadotrophs further confirms that neither the cAMP nor the PKC pathway is involved. In addition, this result suggests that the pertussis toxin-sensitive heterotrimeric G-proteins Gi and Go are not involved in the GTP-γ-S-triggered Ca2+-independent exocytosis. Consistent with this, GTP-γ-S could still trigger Ca2+-independent exocytosis in gonadotrophs treated with pertussis toxin (n = 5; data not shown). Note that for the experiment shown in Fig. 5C, the cell was bathed in a Ca2+-free bath solution. Thus GTP-γ-S can stimulate exocytosis even when the intracellular Ca2+ stores were depleted and in the absence of extracellular Ca2+ (n = 6).
G-protein may regulate a pool of granules that is distinct from the Ca2+-dependent pool
The saturation of the GTP-γ-S-triggered exocytosis at ∼0.5 pF (Fig. 2C) raised the possibility that the persistent stimulation of G-protein might eventually exhaust the pool of readily releasable granules. In a previous study (Tse et al. 1997), we have shown that in gonadotrophs, rapid elevation of [Ca2+]i beyond 15 μM triggered kinetically distinct phases of exocytosis. The fastest kinetic phase reflects the exhaustion of a pool of readily releasable granules. We examined whether the same pool of readily releasable granules was exhausted by the G-protein-triggered Ca2+-independent exocytosis. Any reduction in the amplitude of the fastest Ca2+-dependent exocytic burst will suggest that the G-protein-stimulated Ca2+-independent exocytosis has partially exhausted the pool of readily releasable granules. In this series of experiments, the pipette solution contained a caged Ca2+ compound (DM-nitrophen) and GTP-γ-S (50 or 500 μM). After the establishment of whole-cell recording, the G-protein-stimulated exocytosis was monitored for 3-4 min. A UV flash was then delivered to elevate [Ca2+]i rapidly to trigger Ca2+-dependent exocytosis. An example of such an experiment is shown in Fig. 6. In this cell, at 150 s after whole-cell recording began, the cumulative increase in membrane capacitance stimulated by GTP-γ-S (50 μM) was ∼0.2 pF. At 180 s after whole-cell recording, a UV flash was delivered to photolyse the caged Ca2+. [Ca2+]i rose rapidly to ∼15 μM and triggered two distinct kinetic phases of exocytosis: an initial fast exocytic burst followed by a rapid endocytosis, and a slower phase of exocytosis. The rapid endocytosis was seen in about half of the cells recorded with 50 μM (8/13 cells) or 500 μM GTP-γ-S (5/12 cells) as well as in control cells (8/15 cells). This suggests that the rapid endocytosis in gonadotrophs is unaffected by GTP-γ-S. As the fastest exocytic burst reflected the exhaustion of the most readily releasable pool of granules, the size of the pool of readily releasable granules can be measured from the amplitude of this burst (Tse et al. 1997).
The results from these experiments are summarized in Fig. 7. Since gonadotrophs vary in size, we normalized the cumulative exocytosis and the amplitude of the fast exocytic burst to the initial membrane capacitance of individual cells. The normalized data were plotted as the percentage increase in cell surface area. In cells recorded with 50 μM GTP-γ-S, the G-protein-stimulated cumulative exocytosis (before the UV flash) was 4.36 ± 0.91 % (n = 13). The mean size of the pool of readily releasable granules from these cells was 3.61 ± 0.18 %, closely resembling the value of the control cells (3.56 + 0.22 %; n = 15). In cells recorded with 500 μM GTP-γ-S, the G-protein-stimulated cumulative exocytosis was 8.09 ± 1.67 % (n = 12), almost 2-fold larger than that triggered by 50 μM GTP-γ-S. However, even in these cells, the mean size of the pool of readily releasable granules (3.76 ± 0.36 %) was similar to that of the control cells.
Figure 7. The size of the pool of most readily releasable granules during Ca2+-dependent exocytosis is not affected by the G-protein-triggered Ca2+-independent exocytosis.
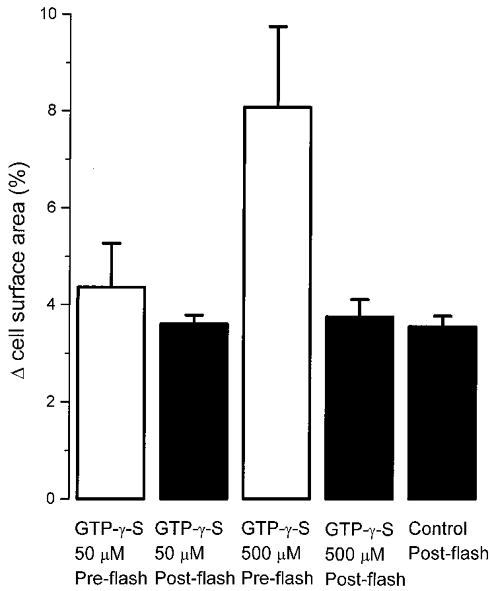
Plot of the cumulative exocytosis triggered (in 3-4 min) by GTP-γ-S (50 or 500 μM) before the delivery of the UV flash (Pre-flash) and the size of the readily releasable pool (Post-flash; measured as the amplitude of the fastest exocytic burst following [Ca2+]i elevation). Exocytosis was normalized to the initial capacitance of individual cells and plotted as the percentage increase in cell surface area. A two-tailed Student's t test showed that the post-flash increases in cell surface area in cells stimulated with 50 and 500 μM GTP-γ-S were not statistically different from control cells (P = 0.87 and 0.62). Thus GTP-γ-S has no significant effect on the size of the readily releasable pool. The experimental procedure is as described in Fig. 6. In control cells, the pipette solution contained no added GTP-γ-S. Since no increase in membrane capacitance was detected in control cells before the UV flash, the value of the pre-flash for control cells was not included here. The mean value for each group of cells was generated from 12-15 cells.
DISCUSSION
G-protein directly stimulates exocytosis
The general phenomenon that non-hydrolysable GTP analogues stimulate Ca2+-independent exocytosis has been reported in a variety of cell types including melanotrophs (Okano et al. 1993), chromaffin cells (Burgoyne & Handel, 1994) and mast cells (Fernandez et al. 1984). However, the mechanism(s) involved in different cell types may not be identical. For example, in gonadotrophs and melanotrophs, both GTP-γ-S and GppNHP trigger robust Ca2+-independent exocytosis. In contrast, GppNHP was reported to be far more effective than GTP-γ-S in triggering capacitance increase (Burgoyne & Handel, 1994) as well as in stimulating noradrenaline release in chromaffin cells (Ahnert-Hilger et al. 1992). This discrepancy probably reflects fundamental differences in the regulation of the exocytic machinery of different cell types.
Several lines of evidence suggest that the action of GTP analogues on gonadotrophs in this study cannot be due to the activation of cAMP or PKC pathways via heterotrimeric G-proteins. First, a previous study has shown that the PKC-mediated LH secretion from rat gonadotrophs could be completely abolished by intracellular BAPTA (Billiard et al. 1997). In contrast, the GTP analogue-stimulated exocytosis in this study persisted even in the absence of extracellular Ca2+ (Fig. 5C) and when [Ca2+]i was buffered to a very low concentration by intracellular BAPTA (Fig. 3A). Second, treatment with the broad-spectrum protein kinase inhibitors H7 (50 μM) or staurosporine (200 nM) failed to prevent the action of the GTP analogues (Fig. 4). At similar concentrations, these protein kinase inhibitors have been shown to be effective in inhibiting PKC-mediated LH release (Thomson et al. 1993) or PKC-mediated enhancement of Ca2+-activated K+ current in rat gonadotrophs (Tse et al. 1995). Third, in the absence of a [Ca2+]i rise, AlF4−, a potent stimulator of heterotrimeric G-proteins, including Gq and Gs, failed to mimic the action of GTP analogues (Fig. 5). The non-involvement of Gs was further supported by a previous study showing that cholera toxin, a potent stimulator of Gs, was an ineffective LH secretagogue in rat gonadotrophs (Hawes et al. 1993). Overall, our results suggest that the exocytosis examined here is equally well stimulated by GTP-γ-S or GppNHP, and is independent of any rise in [Ca2+]i, phosphorylation or AlF4−-sensitive G-proteins. To our knowledge, the triggering of exocytosis under all of the above conditions has been reported only in rat melanotrophs (Okano et al. 1993). Our results contrast with those in mast cells where the GTP-γ-S-stimulated exocytosis is mediated via the activation of an AlF4−-sensitive heterotrimeric G-protein, Gi3 (Aridor et al. 1993). Although monomeric G-proteins were previously thought to be insensitive to AlF4−, recent reports have suggested that AlF4− can affect the interactions between some monomeric G-proteins and their GTPase-activating proteins (of Ras and Rho) or effectors (of Rho) (Mittal et al. 1996; Hoffman et al. 1998). Thus our observation also rules out the involvement of any monomeric G-proteins that can be activated by AlF4−. Ca2+-independent exocytosis triggered by intracellular dialysis of short peptides derived from the putative effector domain of the monomeric G-protein Rab3a have been reported in mast cells (Oberhauser et al. 1992), pancreatic cells (Padfield et al. 1992) and chromaffin cells (Senyshyn et al. 1992). However, interpretation of these data has been complicated by the observations that some of these peptides (even when the sequences are scrambled; MacLean et al. 1993) may stimulate heterotrimeric G-proteins directly, perhaps because they have a structure similar to mastoparan (Law et al. 1993). In addition, a study (Johannes et al. 1994) has suggested that the GTP-bound form of Rab3a has a negative regulatory role in Ca2+-dependent exocytosis. In the anterior pituitary gland, Rab3b has been shown to be the dominant Rab protein, and the inhibition of Rab3b expression in lactotrophs (via antisense oligonucleotides) reduces Ca2+-dependent exocytosis (Lledo et al. 1993). Further experiments are needed to determine whether Rab proteins or other monomeric G-proteins mediate the G-protein-stimulated Ca2+-independent exocytosis in gonadotrophs.
Ca2+-independent exocytosis may affect a distinct pool of granules
Our study also shows that the G-protein stimulated-Ca2+-independent exocytosis is different from the Ca2+-dependent exocytosis in several aspects. First, the G-protein-stimulated exocytosis has a slow onset. Even in experiments involving flash photolysis of caged GTP-γ-S, where the concentration of GTP-γ-S in the cell rose within milliseconds, exocytosis was triggered only at tens of seconds following the delivery of the UV flash (Fig. 1). This slow onset may reflect the time of G-protein turnover in the cell. In contrast, Ca2+-dependent exocytosis was triggered within tens of milliseconds following the photolysis of caged Ca2+ (Tse et al. 1997; see also Fig. 6B). Second, the rate of G-protein-stimulated exocytosis is slow. Even in cells stimulated with 500 μM GTP-γ-S or GppNHP, the maximum rate of exocytosis was only ∼6 fF s−1. A similar slow rate of exocytosis was also observed in chromaffin cells (Burgoyne & Handel, 1994) and melanotrophs (Okano et al. 1993) stimulated with GTP analogues. In contrast, the rate of Ca2+-dependent exocytosis in gonadotrophs could range from several femtofarads per second (at [Ca2+]i of 1-2 μM) to several thousand femtofarads per second (at [Ca2+]i of > 20 μM; Tse et al. 1997). Third, during maximal stimulation by GTP analogues, the total exocytosis triggered via the Ca2+-independent process is almost twice the pool size of the readily releasable granules that is available for Ca2+-dependent release (Fig. 7). However, subsequent stimulation of Ca2+-dependent exocytosis still elicited an exocytic burst that was similar to that of the control cells (Fig. 7). This raises the possibility that G-protein-stimulated Ca2+-independent exocytosis may affect a pool of granules that is distinct from the Ca2+-dependent pool.
Since the capacitance measurement monitors only changes in cell surface area during exocytosis, it is not certain whether the G-protein-stimulated Ca2+-independent exocytosis observed here indeed reflects exocytosis of granules containing LH and/or follicle stimulating hormone (FSH) in rat gonadotrophs. Discrepancies between the time course of the rapid phase of capacitance increase (triggered by flash photolysis of caged Ca2+) and amperometry signals (reflecting fusion of granules containing oxidizable products) have been reported in mast cells and chromaffin cells (Oberhauser et al. 1996), and this has led to the suggestion that capacitance increase may not always reflect fusion of granules containing oxidizable products. Yet, another study on chromaffin cells has shown that the rapid increase in capacitance triggered by flash photolysis of caged Ca2+ (when [Ca2+]i < 100 μM) is always accompanied by amperometry signals (Xu et al. 1998). In mast cells, the GTP-γ-S-triggered increase in capacitance was accompanied by amperometry signals, indicating GTP-γ-S indeed caused exocytosis of granules containing oxidizable products (Oberhauser et al. 1996). Since LH and FSH cannot be detected by amperometry, similar experiments cannot be performed here. Considering that microvesicles are rarely seen in gonadotrophs (Tse et al. 1997), the Ca2+-independent exocytosis probably came from fusion of granules. However, it is not clear whether every granule present in gonadotrophs contained LH and/or FSH. Nevertheless, in permeabilized sheep gonadotrophs, GTP-γ-S has been reported to stimulate LH release even when [Ca2+]i was buffered below 100 nM (van der Merwe et al. 1991). Therefore, it is likely that in rat gonadotrophs, LH/FSH release indeed occurred during GTP-γ-S stimulation. Since GnRH induced negligible exocytosis when [Ca2+]i was buffered (Tse et al. 1993), the G-protein-stimulated Ca2+-independent pathway probably contributes little to secretion during GnRH stimulation. However, gonadotrophs exhibit basal release of LH/FSH. The basal release was slow and manyfold smaller that that triggered by GnRH stimulation but persisted in the presence of voltage-gated Ca2+ channel blockers, intracellular Ca2+ buffers or removal of extracellular Ca2+ (Chang et al. 1988; Billard et al. 1997). Since the G-protein-stimulated Ca2+-independent pathway triggers slow exocytosis, it is possible that this pathway may have a role in the basal release of LH/FSH.
Acknowledgments
We thank Drs J. D. Neill for the gift of antibodies and W. F. Dryden for comments, and Mrs C. Shiu for technical assistance. This work was supported by the Canadian Medical Research Council and the Alberta Heritage Foundation for Medical Research (AHFMR).
F.W.T. and A.T. contributed equally to this work.
References
- Ahnert-Hilger G, Wegenhorst U, Stecher B, Spicher K, Rosenthal W, Gratz M. Exocytosis from permeabilized bovine adrenal chromaffin cells is differently modulated by guanosine 5′-[γ-thio]triphosphate and guanosine 5′-[βγ-imido]triphosphate. Biochemical Journal. 1992;284:321–326. doi: 10.1042/bj2840321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Rajmilevich G, Beaven MA, Sagi-Eisenberg R. Activation of exocytosis by the heterotrimeric G protein Gi3. Science. 1993;262:1569–1572. doi: 10.1126/science.7504324. [DOI] [PubMed] [Google Scholar]
- Billiard J, Koh D, Babcock DF, Hille B. Protein kinase C as a signal for exocytosis. Proceedings of the National Academy of Sciences of the USA. 1997;94:12192–12197. doi: 10.1073/pnas.94.22.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks JR, Wier WG, Hess P, Prendergast FG. Measurement of Ca2+ concentrations in living cells. Progress in Biophysics and Molecular Biology. 1982;40:1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Handel SE. Activation of exocytosis by GTP analogues in adrenal chromaffin cells revealed by patch-clamp capacitance meaurement. FEBS Letters. 1994;344:139–142. doi: 10.1016/0014-5793(94)00361-0. [DOI] [PubMed] [Google Scholar]
- Carter LL, Redelmeier TE, Woolenweber LA, Schmid SL. Multiple GTP-binding proteins participate in clathrin-coated vesicle-mediated endocytosis. Journal of Cell Biology. 1993;120:37–45. doi: 10.1083/jcb.120.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JP, Stojilkovic SS, Graeter JS, Catt K. Gonadotropin-releasing hormone stimulates luteinizing hormone secretion by extracellular caclium-dependent and -independent mechanisms. Endocrinology. 1988;123:87–97. doi: 10.1210/endo-123-1-87. [DOI] [PubMed] [Google Scholar]
- Fernandez JM, Neher E, Gomperts BD. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. Nature. 1984;312:453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hawes BE, Barnes S, Conn P. Cholera toxin and pertussis toxin provoke differential effects on luteinizing hormone release, inositol phosphate production, and gonadotropin-releasing hormone (GnRH) receptor binding in the gonadotrope: evidence for multiple guanyl nucleotide binding proteins in GnRH action. Endocrinology. 1993;132:2124–2130. doi: 10.1210/endo.132.5.8386608. [DOI] [PubMed] [Google Scholar]
- Hidaka H, Inagaki M, Kawamoto S, Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984;23:5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hoffman GR, Nasser N, Oswald RE, Cerione RA. Fluoride activation of the Rho family GTP-binding protein Cdc42Hs. Journal of Biological Chemistry. 1998;273:4392–4399. doi: 10.1074/jbc.273.8.4392. [DOI] [PubMed] [Google Scholar]
- Johannes L, Lledo P-M, Roa M, Vincent J-D, Henry J-P, Darchen F. The GTPase Rab3a negatively controls calcium-dependent exocytosis in neuroendocrine cells. EMBO Journal. 1994;13:2029–2037. doi: 10.1002/j.1460-2075.1994.tb06476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukuljan M, Stojikovic SS, Rojas E, Catt KJ. Apamin-sensitive potassium channels mediate agonist-induced oscillations of membrane potential in pituitary gonadotrophs. FEBS Letters. 1992;301:19–22. doi: 10.1016/0014-5793(92)80201-q. [DOI] [PubMed] [Google Scholar]
- Law GJ, Northrop AJ, Mason WT. Rab3-peptide stimulates exocytosis from mast cells via a pertussis toxin-sensitive mechanism. FEBS Letters. 1993;333:56–60. doi: 10.1016/0014-5793(93)80374-4. [DOI] [PubMed] [Google Scholar]
- Lledo P-M, Vernier P, Vincent J-D, Mason WT, Zorec R. Inhibition of rab3B expression attenuates Ca2+-dependent exocytosis in rat anterior pituitary cells. Nature. 1993;364:540–544. doi: 10.1038/364540a0. [DOI] [PubMed] [Google Scholar]
- Maclean CM, Law GJ, Edwardson JM. Stimulation of exocytotic membrane fusion by modified peptides of the rab3 effector domain: re-evaluation of the role of rab3 in regulated exocytosis. Biochemical Journal. 1993;294:325–328. doi: 10.1042/bj2940325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae MB, Davidson JS, Millar RP, van der Merwe PA. Cyclic AMP stimulates luteinizing-hormone (lutropin) exocytosis in permeabilized sheep anterior-pituitary cells. Biochemical Journal. 1990;271:635–639. doi: 10.1042/bj2710635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal R, Ahmadian MR, Goody RS, Wittinghofer A. A formation of transition-state analog of the Ras GTPase reaction by Ras-GDP, tertrafluoroaluminate, and GTPase-activating proteins. Science. 1996;273:115–117. doi: 10.1126/science.273.5271.115. [DOI] [PubMed] [Google Scholar]
- Oberhauser AF, Robinson IM, Fernandez JM. Simultaneous capacitance and amperometric measurements of exocytosis: a comparison. Biophysical Journal. 1996;71:1131–1139. doi: 10.1016/S0006-3495(96)79315-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser AF, Monck JR, Balch WE, Fernandez JM. Exocytotic fusion is activated by rab3a peptides. Nature. 1992;360:270–273. doi: 10.1038/360270a0. [DOI] [PubMed] [Google Scholar]
- Okano K, Monck JR, Fernandez JM. GTPγS stimulates exocytosis in patch-clamped rat melanotrophs. Neuron. 1993;11:165–172. doi: 10.1016/0896-6273(93)90280-5. [DOI] [PubMed] [Google Scholar]
- Padfield PJ, Balch WE, Jamieson JD. A synthetic peptide of the rab3a effector domain stimulates amylase release from permeabilized pancreatic acini. Proceedings of the National Academy of Sciences of the USA. 1992;89:1656–1660. doi: 10.1073/pnas.89.5.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senyshyn J, Balch WE, Holz RW. Synthetic peptides of the effector-binding domain of rab enhance secretion from digitonin-permeabilized chromaffin cells. FEBS Letters. 1992;309:41–46. doi: 10.1016/0014-5793(92)80735-y. [DOI] [PubMed] [Google Scholar]
- Smith PE, Frawley LS, Neill D. Detection of LH release from individual pituitary cells by the reverse hemolytic plaque assay. Endocrinology. 1984;115:2484–2486. doi: 10.1210/endo-115-6-2484. [DOI] [PubMed] [Google Scholar]
- Takahashi I, Kobayashi E, Nakano H, Murakata C, Saitoh H, Suzuki K, Tamaoki T. Potent selective inhibition of 7-o-methyl UCN-01 against protein kinase C. Journal of Pharmcology and Experimental Therapeutics. 1990;255:1218–1221. [PubMed] [Google Scholar]
- Takei K, McPherson PS, Schmid SL, Decamilli P. Tubular membrane invaginations coated by dynamin rings are induced by GTP-γS in nerve terminals. Nature. 1995;374:186–190. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- Thomson FJ, Johnson MS, Mitchell R, Wolbers WB, Ison AJ, MacEwan DJ. The differential effects of protein kinase C activators and inhibitors on rat anterior pituitary hormone release. Molecular and Cellular Endocrinology. 1993;94:223–234. doi: 10.1016/0303-7207(93)90171-f. [DOI] [PubMed] [Google Scholar]
- Tse A, Hille B. GnRH-induced Ca oscillations and rhythmic hyperpolarizations of pituitary gonadotropes. Science. 1992;255:462–464. doi: 10.1126/science.1734523. [DOI] [PubMed] [Google Scholar]
- Tse A, Hille B. Patch clamping studies on identified pituitary gonadotropes in vitro. In: Levine JE, editor. Pulsatility in Neuroendocrine Systems, Methods in Neurosciences. Vol. 20. Orlando, FL, USA: Academic Press; 1994. pp. 85–99. [Google Scholar]
- Tse A, Tse FW. G-protein activation stimulates Ca2+-independent exocytosis in pituitary gonadotrophs. Society for Neuroscience Abstracts. 1997;23:95. [Google Scholar]
- Tse A, Tse FW. α-Adrenergic stimulation of cytosolic Ca2+ oscillations and exocytosis in identified rat corticotrophs. The Journal of Physiology. 1998;512:385–393. doi: 10.1111/j.1469-7793.1998.385be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse A, Tse FW, Almers W, Hille B. Rhythmic exocytosis stimulated by GnRH-induced calcium oscillations in rat gonadotropes. Science. 1993;260:82–84. doi: 10.1126/science.8385366. [DOI] [PubMed] [Google Scholar]
- Tse A, Tse FW, Hille B. Calcium homeostasis in identified rat gonadotrophs. The Journal of Physiology. 1994;477:511–525. doi: 10.1113/jphysiol.1994.sp020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse A, Tse FW, Hille B. Modulation of Ca2+ oscillation and apamin-sensitive, Ca2+-activated K+ current in rat gonadotropes. Pflügers Archiv. 1995;430:645–651. doi: 10.1007/BF00386158. [DOI] [PubMed] [Google Scholar]
- Tse FW, Tse A, Hille B, Horstmann H, Almers W. Local Ca2+ release from internal stores controls exocytosis in pituitary gonadotrophs. Neuron. 1997;18:121–132. doi: 10.1016/s0896-6273(01)80051-9. [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Waring DW. cAMP augmentation of secretagogue-induced luteinizing hormone secretion. American Journal of Physiology. 1986;250:E62–68. doi: 10.1152/ajpendo.1986.250.1.E62. [DOI] [PubMed] [Google Scholar]
- van der Merwe PA, Millar RP, Wakefield IK, Davidson JS. Inhibition of luteinizing-hormone exocytosis by guanosine 5′-[γ-thio]triphosphate reveals involvement of a GTP-binding protein distal to second messenger generation. Biochemical Journal. 1991;275:399–405. doi: 10.1042/bj2750399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Binz T, Niemann H, Neher E. Multiple kinetic components of exocytosis distinguished by neurotoxin sensitivity. Nature Neuroscience. 1998;1:192–200. doi: 10.1038/642. [DOI] [PubMed] [Google Scholar]


