Abstract
We have cloned a cDNA encoding a Phe-Met-Arg-Phe-NH2 (FMRFamide)-gated Na+ channel from nervous tissue of the pond snail Helisoma trivolvis (HtFaNaC) and expressed the channel in Xenopus oocytes. The deduced amino acid sequence of the protein expressed by HtFaNaC is 65 % identical to that of the FMRFamide-gated channel cloned from Helix aspersa (HaFaNaC).
HtFaNaC expressed in oocytes was less sensitive to FMRFamide (EC50 = 70 μM) than HaFaNaC (EC50 = 2 μM). The two had a similar selectivity for Na+. The amplitude of the FMRFamide response of HtFaNaC was increased by reducing the extracellular concentration of divalent cations.
The conductance of the two channels was similar, but the mean open time of unitary events was shorter for expressed HtFaNaC compared to expressed HaFaNaC. Each channel was susceptible to peptide block by high agonist concentrations.
In marked contrast to HaFaNaC and other amiloride-sensitive Na+ channels, amiloride, and the related drugs benzamil and 5-(N-ethyl-N-isopropyl)-amiloride (EIPA), enhanced the FMRFamide response in oocytes expressing HtFaNaC cRNA. The potentiating effects of EIPA and benzamil were greater than those of amiloride. Unitary current analysis showed that with such drugs, there was channel blockade as well as an increased probability of channel opening.
The similar permeability of the oocyte-expressed HtFaNaC and the Helisoma neuronal channel, and the susceptibility of both to agonist blockade and blockade by divalent cations, suggest that the channels are the same. However, neuronal channels were less susceptible to enhancement by amiloride analogues and in some patches were more sensitive to FMRFamide than expressed HtFaNaC.
The fast depolarization activated by the neuropeptide FMRFamide in Helixaspersa (subsequently referred to here as Helix) neurones is mediated by a ligand-gated ion channel. This response is notable not only because it is uniquely gated by a peptide, but also because the channel is selective for Na+ and blocked by amiloride (Cottrell et al. 1990; Green et al. 1994). The cDNA of a channel with similar properties (FaNaC) was cloned from Helix neurones and expressed in Xenopus oocytes (Lingueglia et al. 1995). FaNaC (subsequently referred to here as HaFaNaC) is similar in structure to the amiloride-sensitive epithelial Na+ channel (ENaC) and the degenerins of Caenorhabditiselegans (see North, 1996). Available data suggest that the functional FMRFamide-gated channel is a homotetramer (Coscoy et al. 1998). More recently, similar amiloride-sensitive channels have been cloned from mammalian nervous tissue, some of which can be activated by H+ (see Waldmann & Lazdunski, 1998; Chen et al. 1998).
To help define functional domains in this new class of ligand-gated ion channel, we have cloned the cDNA encoding a FMRFamide-gated channel subunit (HtFaNaC) from the pond snail Helisomatrivolvis (subsequently referred to here as Helisoma) and expressed the protein in Xenopus oocytes. The deduced amino acid sequence of the Helisoma channel shows many similarities to that of HaFaNaC but there are differences in agonist sensitivity. The properties of the expressed HtFaNaC clone are similar to those of the neuronal FMRFamide-gated channel of the giant dopamine neurone (GDN) and the large serotonin neurone (LSN), which are located in the pedal ganglia of Helisoma (Harris & Cottrell, 1995).
During the course of the work we observed that amiloride, benzamil and ethylisopropylamiloride (EIPA), which are known to block epithelial Na+ channels and related channels (see e.g. Benos et al. 1997), had an unusual potentiating effect on FMRFamide-activated currents of HtFaNaC expressed in Xenopus oocytes. This led to studies designed to compare the effects of amiloride and EIPA in more detail at the whole-cell and unitary current level on expressed HtFaNaC and also on Helisoma neurones, the results of which are included here.
METHODS
Cloning of the FMRFamide-gated channel from Helisoma
Two degenerate oligonucleotides similar to those designed by Lingueglia et al. (1995) were used in a PCR to amplify a fragment of a FMRFamide-gated channel cDNA from a fractionated Helisoma neuronal cDNA library (constructed by Dr Erno Vreugdenhil and generously contributed by Drs Andrew Bullock and Garry Hauser). The product was cloned into pGEM-T (Promega) and sequenced using the Sequenase (Amersham) version of the dideoxy method; the Big Dye kit (Perkin-Elmer) was later used to prepare samples for automated sequencing. The 5′ and 3′ termini were amplified from a single library fraction by two rounds of PCR incorporating primers specific for the insert and the vector; the second reaction amplified a sample of the first reaction product using nested primers. The three resulting fragments encompassed 3.6 kb of a cDNA that contained a complete open reading frame with high similarity to the Helix clone HaFaNaC. The full open reading frame was then amplified from two additional library fractions and from an independent reverse transcription reaction, using RNA extracted from ganglia of Helisoma, in order to confirm the cDNA sequence and prepare a full-length clone. Specific primers containing Bsa I and Not I sites were used to amplify the full open reading frame; the resulting products were digested with Bsa I and Not I, then ligated into the pXENEX1 vector (Jeziorski et al. 1998) cut with Nco I and Not I. The inclusion of a Bsa I site at the 5′ end of the clone allowed generation of an overhang complementary to an Nco I site without disrupting the coding of the second amino acid in the open reading frame (Patton et al. 1997). The sequences of the new clones were compared to that of the original clone to establish a consensus cDNA sequence. A construct corresponding to the consensus sequence was created in pXENEX1 by minor repair of a PCR-induced error in one clone.
Expression in Xenopus oocytes
The Helisoma FMRFamide-gated channel construct (pHtFaNaC) was linearized with Hin dIII, then purified and used to generate cRNA with the T7 version of the mMessage mMachine in vitro transcription kit (Ambion). The cRNA was denatured, electrophoresed on a denaturing formamide gel and stained with SYBR Green (Molecular Probes) to determine its concentration and purity. An ovary was removed from Xenopus (obtained from Xenopus 1, The Northside, Ann Arbor, MI, USA or from Blades Biological, Cowden, Edenbridge, Kent TN8 7DX, UK) after the frog had been killed by prolonged immersion in MS-222 followed by double pithing. The ovary was then treated with 2 mg ml−1 collagenase (Sigma Type II or Type I) for 50 min to remove the follicular cell layer. RNA (0.5-5 ng) was subsequently injected into each oocyte. Oocytes were stored at 17-18°C in ND96 (see below) containing: 5 % horse serum, 2.5 mM sodium pyruvate, 100 units ml−1 penicillin and 100 μg ml−1 streptomycin (Sigma). Recordings were made 2-5 days after injection.
Solutions and drugs
Patch- and voltage-clamp experiments on oocytes were done using an external solution containing (mM): 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2 and 5 Hepes, adjusted to pH 7.4 with NaOH (ND96 solution). For Helisoma neurones, the external solution consisted of (mM): 51 NaCl, 1.7 KCl, 1.5 MgCl2, 4.1 CaCl2 and 10 Hepes, with the pH adjusted to 7.3 with NaOH. For patch-clamp experiments on oocytes, a hyperosmotic solution was used to remove the vitelline membrane. The solution consisted of (mM): 200 potassium aspartate, 20 KCl, 1 MgCl2, 10 EGTA and 10 Hepes, adjusted to pH 7.4 with KOH. For outside-out patch recordings the pipette solution contained (mM): 100 CsF or NaF (for Xenopus oocytes), 54 CsF or NaF (for Helisoma neurones), 3 NaCl, 1 MgCl2, 5 EGTA and 10 Hepes, adjusted to pH 7.4 with CsOH or NaOH.
FMRFamide (Sigma), FLRFamide (Sigma), D-tubocurarine (Sigma) and benzamil (Sigma) were prepared as stock solutions in distilled deionized water and frozen. Amiloride (Sigma) was similarly prepared, but used the same day. EIPA (Sigma) was prepared as a 20 mM stock solution in DMSO. Working dilutions of all drugs were made in ND96 for oocyte recordings, or Helisoma saline for neuronal recordings.
Electrophysiological recordings
Whole-oocyte current recordings
Two-electrode voltage-clamp experiments were made with oocytes placed in a small bath that allowed continuous exchange of the physiological solution. Low resistance (< 1 MΩ) microelectrodes filled with 3 M KCl were used with a Warner Oocyte Voltage-Clamp amplifier or an Axoclamp-2B amplifier (Axon Instruments). Flow through the bath was varied from 3 to 6 ml min−1 in different experiments. Test solutions were applied in 1 ml samples and then, on most occasions, immediately washed from the bath. An interval of 5 min was used between additions of low to moderate concentrations of peptide solutions; longer periods (10-15 min) were required for recovery from the highest concentrations. The effects of amiloride, benzamil and EIPA were tested by adding 3-6 ml of the required dilution in ND96 solution immediately before addition of the peptide solution. In some cases the peptide solution contained the same concentration of amiloride being tested; in other cases the peptide solution added did not contain amiloride. Similar effects of each drug were observed with the two methods of application. Responses were recorded digitally either with a Gateway PC and Axon pCLAMP software, or using a Macintosh LCII with a MacLab 4S interface and Macintosh Scope software.
Intracellular recordings from Helisoma neurones
Recordings were made from two identified neurones (the GDN and the LSN) located in the pedal ganglia of Helisoma. A description of the location of these neurones is given in Harris & Cottrell (1995). Ganglia were exposed to 0.1 % trypsin, then the neurones were dissected free from connective tissue and, after further brief exposure to the trypsin solution, thoroughly washed. Currents from perikarya were recorded intracellularly using 1 M potassium acetate-filled microelectrodes and the discontinuous single electrode voltage-clamp method with an Axoclamp-2B. No significant difference was noted between whole-cell and unitary currents recorded from the GDN or the LSN neurones. FMRFamide was transiently applied to whole neurones by pressure ejection from a microelectrode (150-200 kPa). The recording chamber was perfused throughout all experiments with physiological solution. Drug solutions were added to the bath, or applied locally to the neurone immediately prior to FMRFamide.
Outside-out patch recordings from oocyte and neuronal membranes
Unitary currents were recorded in patches from oocytes or neurones using standard techniques with an Axopatch 200 (Axon Instruments) integrating amplifier. Pipettes were pulled from borosilicate glass, fire polished to a final tip diameter of less than 1 μm, then filled with patch pipette solution (see above for composition). They had resistances of 7-10 MΩ and readily formed seals on the oocyte membrane with resistances of 10-20 GΩ. Analog data recordings were filtered at 500 Hz and digitized at 400 μs intervals. For the neuronal patches, recordings were further filtered at 500 Hz with a digital Gaussian filter. FMRFamide was transiently applied to patches by pressure ejection (150-200 kPa). More prolonged applications were also made to patches using a Warner SF-77A Perfusion Fast-Step perfusion system (Warner Instrument Corporation, Hamden, CT, USA), or by local leakage from a separate blunt-tipped micropipette.
RESULTS
Molecular biology
We used a PCR-based cloning strategy to isolate a cDNA that encodes a FMRFamide-gated channel subunit from a Helisoma library as described in Methods. The cDNA was 3607 bp in length and contained an open reading frame encoding 622 amino acids, resulting in a calculated molecular mass of 70.4 kDa for the protein. The 5′ end of the cDNA differed at two sites in a second cDNA clone. An insert of 173 bp was found 152 bp downstream of the 5′ terminus of the alternative clone. This insert, which was not flanked by consensus intron-exon boundaries, was far upstream of the open reading frame. A second fragment of 95 bp, lying 20 bp upstream of the apparent start methionine and bounded by the GT and AG residues that signify a possible unprocessed intron, was deleted in the alternative cDNA. Deletion of this fragment, which contains an in-frame stop codon, produced in one clone an extension of the open reading frame 45 codons upstream to an earlier methionine codon. However, additional clones possessing this 95 bp deletion, generated by PCR from a library fraction, contained an in-frame stop codon between the two methionines. We therefore assumed that the downstream methionine, which corresponds closely to the start site proposed for the Helix FMRFamide channel and yields a protein of similar length, represents the true amino terminus of the Helisoma channel subunit. The deduced amino acid sequence of HtFaNaC is shown in Fig. 1, where it is aligned with that for HaFaNaC and two related sequences.
Figure 1. Alignment of the peptide sequence of HtFaNaC, the FMRFamide-gated channel from Helisoma, with the sequence of the Helix channel, HaFaNaC.
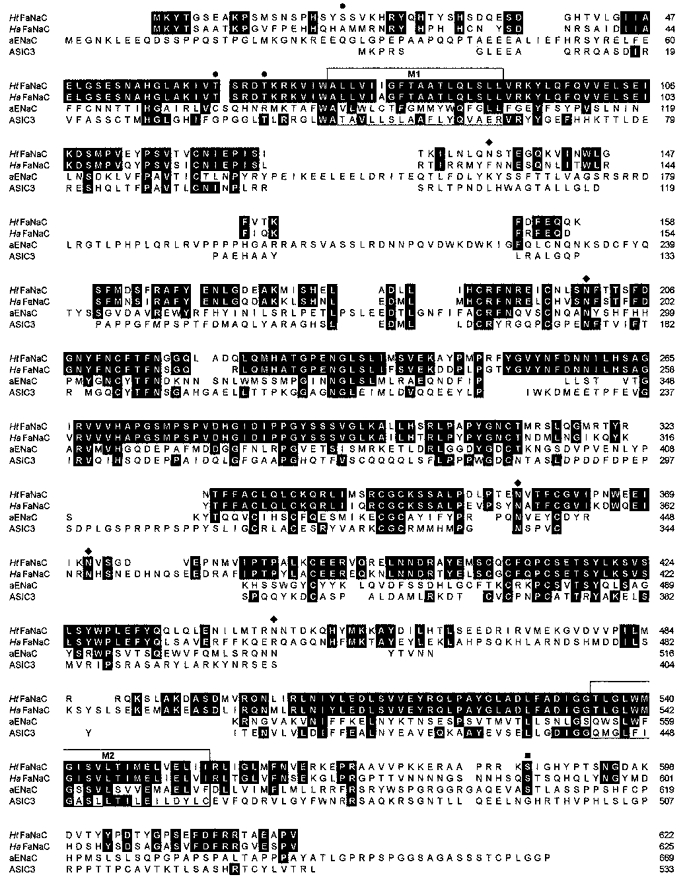
Also shown are two representative amiloride-sensitive Na+ channels: the α-subunit of the human epithelial sodium channel αENaC (aENaC; accession no. AAD28355) and the rat acid-sensing channel ASIC3 (also known as DRASIC; accession no. AF069328). Residues that are identical among the sequences shown are shaded in black. The membrane-spanning regions M1 and M2 are boxed. Potential external N-linked glycosylation sites, ♦. Sites for potential phosphorylation by protein kinase C, •; and by protein kinase A, ▪. The alignment was completed using ClustalW (Thompson et al. 1994). The accession numbers for HaFaNaC and HtFaNaC are X92113 and AF 254118, respectively.
The amino acid sequence of HtFaNaC is 65 % identical to that of HaFaNaC. The similarity between the two proteins is high in the two proposed transmembrane segments (M1 and M2), where only three conservative substitutions are found in the Helisoma channel (see Fig. 1). The putative pore-forming region on the extracellular side of M2 is highly conserved in the Helisoma protein channels and other, discrete, regions of the extracellular domain are identical or very similar. Fourteen extracellular cysteines are conserved between the two proteins; thirteen of these cysteines are found in all members of the amiloride-sensitive sodium channel superfamily (e.g. as shown for αENaC and ASIC3). The extracellular domain contains five consensus sites for N-linked glycosylation, of which four are conserved with the Helix protein. Three sites for potential phosphorylation by protein kinase C are found near the N-terminus, and one potential cAMP-dependent protein kinase site lies near the C-terminus. Although most of the N- and C-terminal domains are only weakly conserved between the two proteins, a region of more than 25 amino acids immediately preceding M1 is identical in the two.
Response of HtFaNaC expressed in Xenopus oocytes to FMRFamide
Inward current responses to FMRFamide, which did not readily desensitize on prolonged exposure to the peptide, were observed in oocytes injected with HtFaNaC, but not in uninjected eggs, or eggs injected with water (Fig. 2A). Responses were detected 1-2 days after injection of HtFaNaC cRNA. The dose-response relationship for FMRFamide is shown in Fig. 2B. The EC50 value for FMRFamide was about 70 μM, which is considerably higher than the corresponding value of 2 μM for HaFaNaC (Lingueglia et al. 1995; Zhainazarov & Cottrell, 1998). The response to Phe-Leu-Arg-Phe-NH2 (FLRFamide) was very small, being observed only at doses of 100 μM and above. Such high concentrations can cause pronounced channel block (Green & Cottrell, 1999). For this reason, and because such large amounts of peptide were required, a detailed study of the effect of FLRFamide was not made on intact oocytes.
Figure 2. Responses to FMRFamide of intact oocytes, and outside-out patches taken from oocytes, injected with HtFaNaC cRNA.
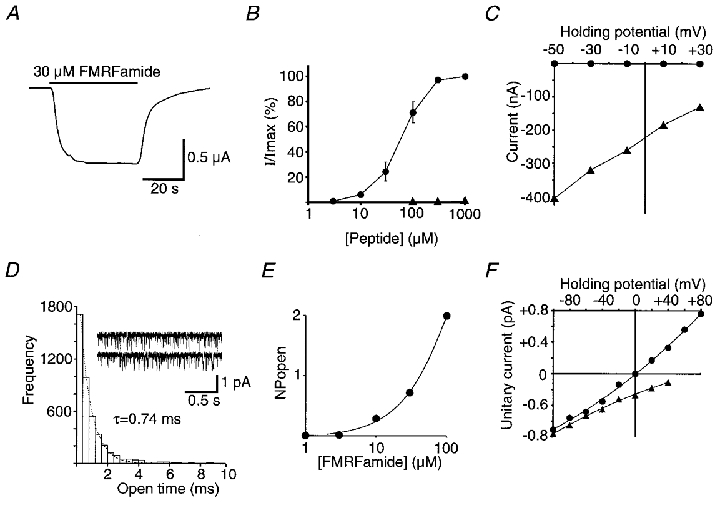
A, example of a response showing little desensitization to the applied FMRFamide. B, dose-response relationship of HtFaNaC to FMRFamide (•). Also shown are data from responses obtained from one oocyte with FLRFamide (▴). I/Imax is the amplitude of the response at a given concentration divided by the maximum response to FMRFamide. The points for FMRFamide represent the mean of responses from six experiments with different oocytes. The EC50 value for FMRFamide is approximately 70 μM. Error bars correspond to the s.e.m.C, relationship between the holding potential and the amplitude of the FMRFamide response of HtFaNaC recorded in the normal oocyte physiological solution (▴), and in physiological solution with all the Na+ replaced with K+ (•). All the points in this graph were obtained from one oocyte and demonstrate a very much higher permeability of the channel to Na+ than to K+. D, the open time frequency distribution of the unitary currents activated by 10 μM FMRFamide recorded from an outside-out patch of an oocyte that had been injected with HtFaNaC cRNA. The data were fitted with a single exponential distribution (dotted line), with τ = 0.74 ms. Example traces from the same patch are also shown. The patch potential was -100 mV. In this and all subsequent unitary current recordings, the inward currents are shown as downward deflections from the baseline using the standard convention. E, an incomplete dose-response curve showing the relationship between FMRFamide concentration and level of channel activity in a patch assessed by the summed products of the number of channels open in the patch and the probability of that number of channels being open (NPopen). The patch contained at least four channels. The holding potential was -100 mV. F, the dependency of the amplitude of the unitary currents on the holding potential of the membrane patch. In normal extracellular physiological solution and with CsF in the recording pipette (▴), the currents were inward in sign at +40 mV, suggesting a preferential permeability of the channel to Na+. With the recording electrode filled with NaF to give the same concentration of Na+ across the patch (•), the currents reversed at 0 mV, confirming selective permeability to Na+.
Like the Helix clone, expressed HtFaNaC showed preferential permeability for Na+ compared to K+ (Fig. 2C). Further, using symmetrical Na+ solution, the FMRFamide-gated unitary currents reversed at 0 mV, compared with a positive extrapolated potential with normal physiological solutions (and see below). The inward current response therefore appears to be mainly due to an increase in Na+ permeability. Neither Ca2+ nor Mg2 appears to contribute to the FMRFamide response because FMRFamide did not evoke an inward current in physiological solution containing Ca2+ and Mg2 when Na+ had been replaced by K+. Furthermore, FMRFamide-activated unitary currents reversed at 0 mV with symmetrical Na+ solution but normal levels of divalent cations (see e.g. Fig. 2C and F). Ca2+ and Mg2 did, however, reduce the amplitude of FMRFamide-activated unitary currents (see below).
FMRFamide activated unitary currents in outside-out patches of membrane from oocytes injected with HtFaNaC cRNA. Examples of unitary currents evoked by 30 μM FMRFamide are shown in Fig. 2D. The time constant of the open time distribution was 0.74 ms. The partial dose- response relationship for a single oocyte patch containing four or more active channels, shown in Fig. 2E, was similar to the dose-response curve for the whole oocyte. Agonist blockade by FMRFamide, which was sometimes even detected at 30 μM FMRFamide (see e.g. Fig. 4A), made determination of NPopen (number of active channels × open probability) values impracticable at higher concentrations. There was no difference in the unitary current when the patch pipette contained KCl or CsCl, but when the patch pipette contained high Na+ (symmetrical high Na+) the unitary currents reversed at 0 mV, providing further evidence that the channels were Na+ selective (Fig. 2F). The conductance of expressed HtFaNaC was 6.8 pS at negative holding potentials with symmetrical high Na+, corresponding closely with the value of 6.1 pS for expressed HaFaNaC measured under similar conditions (not shown).
Figure 4. Agonist block, and block by divalent cations of the FMRFamide response of oocyte-expressed HtFaNaC and the neuronal channel.
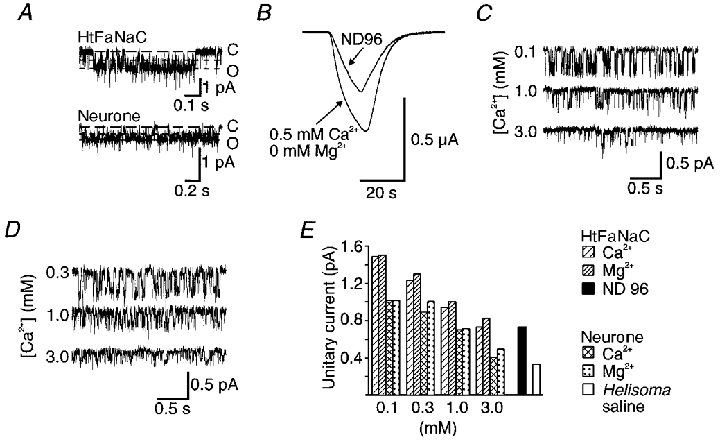
A, flickery block of FMRFamide-induce unitary currents in outside-out patches of oocyte expressing HtFaNaC and of the neurone. In each case, the long-dash line indicates the fully closed level (C), while the short-dash lines indicate the fully open level (O) and the subconductance to which the majority of transitions occur. In these recordings, the concentration of FMRFamide was 30 μM. B, an example of the influence of reduced divalent cation levels on the intact oocyte response of HtFaNaC to FMRFamide. The amplitude of the FMRFamide response was markedly increased with 0.5 mM Ca2+ and nominally 0 Mg2+ ND96 solution. A similar effect was observed in more than 10 preparations. C and D, FMRFamide-activated unitary currents recorded, respectively, from an oocyte injected with HtFaNaC and a neurone. Recordings are shown for three different external concentrations of Ca2+ and with no Mg2+. The holding potential in A, C and D was -100 mV. E, histogram showing the amplitude of the FMRFamide-activated unitary currents recorded from HtFaNaC and the neurone. Data are shown for each with four different concentrations of Ca2+ or Mg2+ from 0.1 to 3 mM, and also for the respective standard external solution.
Response of Helisoma neurones to FMRFamide
The fast inward current response elicited by FMRFamide was studied on the GDN and LSN. These neurones responded to locally applied FMRFamide with a biphasic response comprising a fast inward current and a slower outward current. The inward current could be observed in isolation when the neurone was voltage clamped at the K+ equilibrium potential, approximately -80 to -100 mV (see Cottrell et al. 1984; Colombiaoni et al. 1995). Repeated application of FMRFamide resulted in some desensitization of the inward current response (Fig. 3A), but the degree of desensitization was variable. As with Helix neurones, 100 μM D-tubocurarine, known to block many other ligand-gated responses (see Carpenter et al. 1977; Green et al. 1994), was without effect on the FMRFamide-response (not shown).
Figure 3. Recordngs of FMRFamide-activated whole-cell currents, and unitary currents in outside-out patches, from Helisoma neurones.
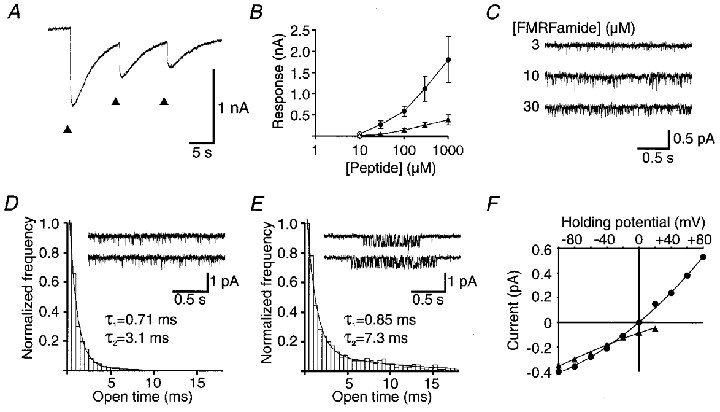
A, inward current responses were observed in isolation when recordings were made at the K+ equilibrium potential (approximately -100 mV). Repeated application of FMRFamide (arrowheads) resulted in partial desensitization, as with the response in Helix neurones (see Green et al. 1994). B, dose-response relationship for FMRFamide (circles) and FLRFamide (triangles). For filled symbols (with s.e.m. bars), n = 3-6 for both FMRFamide and FLRFamide; for open symbols, n = 1 or 2. C, unitary currents activated by 3, 10 or 30 μM FMRFamide in an outside-out patch from the GDN. The holding potential was -100 mV. D and E, open time frequency distribution histograms of the unitary currents activated by 10 μM FMRFamide recorded from neuronal outside-out patches, illustrating the two modes of channel opening encountered in neuronal patches. The data of the mode of activity represented in the inset in D were fitted with the sum of two time constants: 0.71 ms (97 %) and 3.1 ms (3 %). Data of the type shown in the inset in E were fitted by the sum of two exponential components: 0.85 ms (92 %) and 7.3 ms (8 %). F, current-voltage relationship for unitary currrents recorded with CsF (▴) or NaF (•) in the recording pipette solutions, similar to Fig. 2F.
The relationship between peptide concentration and response amplitude is shown in Fig. 3B, and example traces with three different FMRFamide concentrations tested on unitary current activity on another patch are shown in Fig. 3C. The log dose-response curve (Fig. 3B) was similar to that observed with the oocyte-expressed HtFaNaC clone. However, FMRFamide, and to a lesser extent FLRFamide, could occasionally activate unitary currents in neuronal patches at concentrations as low as 1 μM. This suggests that some local factor(s) can influence the activity level of the neuronal channel. Furthermore, FMRFamide was observed to activate two different modes of unitary current activity on the neurone: isolated brief openings (as in Fig. 3D) and prolonged clusters of activity (as in Fig. 3E). Frequency distribution histograms of open times showed the presence of a brief, major component with τ = 0.71 ms and a minor, slower component which was more prominent in recordings that included the prolonged clusters of activity. A persistent downregulation of channel activity was also seen in the neuronal patches after application of 10 μM FMRFamide or higher concentrations, suggesting a process of partial desensitization.
With high Na+ in the patch pipette, at the same concentration as in the extracellular solution (i.e. symmetical high Na+ solution), the unitary current response reversed at 0 mV, as with expressed HtFaNaC. The relationship between the amplitude of FMRFamide-activated unitary currents and holding potential in normal physiological solution, and in symmetrical high Na+ solution, is shown in Fig. 3F. The permeability for the neuronal channel was calculated to be 2.06e−14 cm3 s−1 compared with 2.01e−14 cm3 s−1 for HtFaNaC expressed in oocytes, assuming complete selectivity for Na+.
Effect of divalent cations on the FMRFamide response of expressed HtFaNaC and the Helisoma neuronal channel
Reducing the extracellular concentrations of Ca2+ and/or Mg2+ increased the amplitude of FMRFamide current responses recorded from intact oocytes expressing HtFaNaC (Fig. 4B). The amplitude of unitary FMRFamide currents was seen to be progressively decreased with increased concentrations of Ca2+ and/or Mg2+ from 0.1 to 3 mM (Fig. 4C and E), suggesting that expressed HtFaNaC is sensitive to blockade by extracellular divalent cations. There was also an enhancement in the unitary current amplitude of neuronal channels evoked by FMRFamide with reduced extracellular levels of Ca2+ and/or Mg2+ (Fig. 4D and E). Similar effects have been observed with some related channels (Schild et al. 1997) and oocyte-expressed HaFaNaC and neuronal Helix FMRFamide-gated channels (K. A. Green & G. A. Cottrell, manuscript in preparation).
Effect of amiloride, benzamil and EIPA on HtFaNaC
Unexpectedly, it was found that amiloride markedly potentiated the whole-oocyte response to FMRFamide of oocytes injected with HtFaNaC cRNA (Fig. 5A). The enhancement was completely reversed with washing. The threshold for amiloride enhancement was about 10 μM. Benzamil and EIPA (Fig. 5B) also potentiated the effect of FMRFamide on oocytes expressing HtFaNaC. The threshold for benzamil was 1-5 μM (not shown). At 20 μM, EIPA produced a shift to the left in the dose-response curve of about 1 log unit bringing the EC50 of FMRFamide much closer to 2 μM, the corresponding EC50 for HaFaNaC (Fig. 5C). EIPA was the most potent of the drugs tested in enhancing the action of FMRFamide. Enhancement occurred at sub-micromolar concentations; the effect markedly increased with higher concentrations of EIPA (Fig. 5D). The potentiating effect of each of these drugs could also be observed with FMRFamide-gated unitary currents (Figs 6 and 7A and B). Each increased the probability of channel opening, but EIPA and benzamil could be seen to induce a change in the mode of channel opening from a state with a low probability of openings to one with bursts of longer openings. Each drug also exhibited a blocking effect on the FMRFamide response, which was clearly seen in outside-out patch recordings with for example EIPA (Fig. 7A and C). The overall effect observed in both whole-oocyte and patch recordings therefore appeared to be a combination of two separate phenomena, blockade and potentiation, each of which varied in potency with the three drugs tested. For example amiloride was a relatively effective blocker but poor enhancer, whereas EIPA appeared to be a less effective blocker, but was potent in enhancing the action of FMRFamide.
Figure 5. Potentiation by amiloride and EIPA of FMRFamide-activated currents recorded from whole oocytes injected with HtFaNaC cRNA.
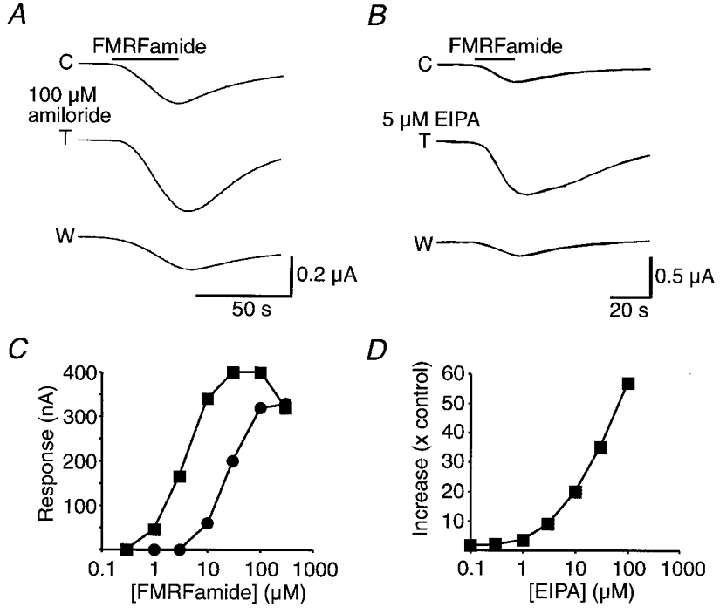
A, effect of 100 μM amiloride on the current response of an oocyte evoked by 10 μM FMRFamide. The oocyte was voltage clamped at -60 mV. C, control response to FMRFamide; T, response in the presence of 100 μM amiloride; and W, response after washing. B, effect of 5 μM EIPA on the current response of another oocyte evoked by 30 μM FMRFamide. The oocyte was voltage clamped at -60 mV. C, control response to FMRFamide; T, response in the presence of 5 μM EIPA; and W, response after washing. C, dose-response relationship for FMRFamide-activated currents recorded from an oocyte in the absence (•) and presence (▪) of 20 μM EIPA. All the recordings were made from the same injected oocyte. D, relationship between the increase in amplitude of the response to 10 μM FMRFamide and EIPA concentration. The response to 10 μM FMRFamide was 30 nA at the outset of the experiment. With increasing concentrations of EIPA the size of the response was markedly potentiated, reaching 57 times the amplitude of the response recorded to FMRFamide alone at the outset. The amplitude of responses to FMRFamide alone, made intermittently throughout the experiment, varied between 20 and 50 nA. NB 100 μM EIPA evoked an inward current of about 150 nA both in oocytes injected with HtFaNaC cRNA and in uninjected oocytes. Lower concentrations of EIPA did not produce any significant inward current.
Figure 6. Enhancement of unitary FMRFamide-activated currents recorded in patches from oocytes injected with HtFaNaC cRNA.
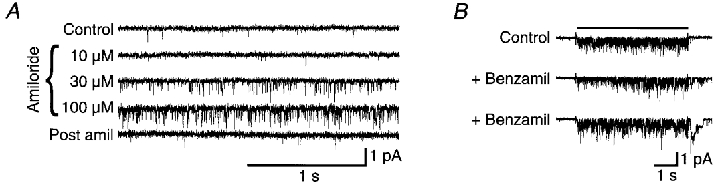
A, unitary currents activated by 10 μM FMRFamide alone (Control and Post amil), and in its combined presence with 10, 30 or 100 μM amiloride. B, unitary currents activated by short (5 s) duration applications of 10 μM FMRFamide, and by two consecutive 5 s applications of 10 μM FMRFamide together with 3 μM benzamil.
Figure 7. Simultaneous stimulation and block of unitary current activity recorded in a patch from an oocyte injected with HtFaNaC cRNA.
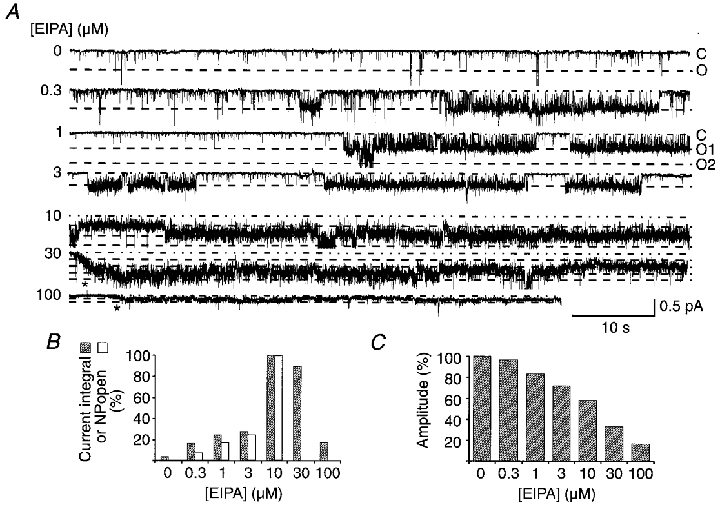
A, unitary currents activated by 30 μM FMRFamide with co-application of 0-100 μM EIPA. For each trace a dot-dash line indicates the base current level, whilst the dashed lines represent successive unitary current opening levels. FMRFamide was present throughout except for the 30 and 100 μM traces, where the start of application is indicated by an asterisk below the recordings. B, unitary current activity as measured by either the integrated current per unit time or the NPopen value (which could only be estimated at < = 10 μM EIPA), plotted against the EIPA concentration. Values are percentage of activity with 10 μM EIPA. C, histogram of unitary current amplitude plotted as a percentage of amplitudes measured for 30 μM FMRFamide in the absence of EIPA. The holding potential was -100 mV.
When potentiation and channel blockade were seen in outside-out patch recordings, potentiation appeared to develop more slowly than blockade. For example, when the expressed HtFaNaC was exposed to FMRFamide and then to the same concentration of FMRFamide in the presence of benzamil, a reduction in the FMRFamide-activated current was often initially seen (Fig. 6B). This contrasted with the rapid potentiation observed when channels were pre-exposed to benzamil, or the other drugs, and then exposed to FMRFamide in the absence of benzamil. Potentiation usually persisted for several minutes after washing.
Effect of amiloride and related drugs on neuronal FMRFamide responses
Amiloride and related drugs both blocked and enhanced neuronal responses to FMRFamide, but the enhancing effect was less obvious than with HtFaNaC expressed in oocytes. Only blockade of the FMRFamide response was observed with amiloride at the whole-cell level (Fig. 8A), but at the unitary current level, amiloride was seen to increase the probability of channel opening in the presence of FMRFamide (Fig. 8D). EIPA and benzamil were more effective in producing enhancement. Application of EIPA to intracellularly recorded neurones produced a dose-dependent increase in the amplitude of the response to pressure-applied 300 μM FMRFamide (Fig. 8B and C), with some prolongation of the response. The effect was readily reversed with washing. EIPA was also observed to stimulate the occurrence of high activity clusters of unitary currents (Fig. 8E), as seen with oocyte patches with expressed HtFaNaC. At higher EIPA concentrations, however, the stimulatory effect was less continuous than in oocyte patches, possibly due to a greater susceptibility to desensitization in the neuronal patches. The neuronal channels also showed flickery block at higher EIPA concentrations.
Figure 8. The effect of amiloride analogues on the neuronal responses to FMRFamide.
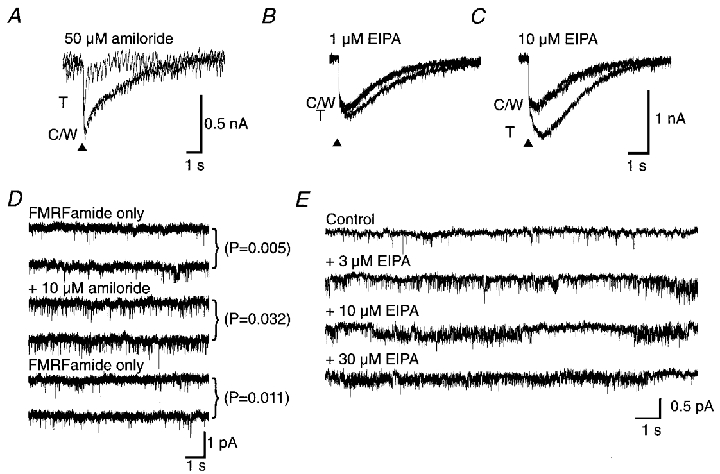
A, the inward current FMRFamide response was blocked by 50 μM amiloride. The superimposed recordings show a control response (C), the test response in the presence of amiloride (T) and the response after washing (W). B and C, enhancement of whole-cell current responses to pressure-applied 300 μM FMRFamide (▴) by 1 and 10 μM EIPA, respectively. C and W indicate the control and wash traces, respectively, and T is the response in the presence of EIPA. D, unlike the whole-neurone response, an enhancing effect of amiloride was observed on unitary currents activated by 10 μM FMRFamide in a patch from the LSN neurone at a holding potential of -100 mV. Two frames are shown for the initial control activity with 10 μM FMRFamide only, the activity in the combined presence of 10 μM FMRFamide and 10 μM amiloride and the final activity after removing the amiloride. Popen was reversibly increased in the presence of amiloride. E, unitary currents activated by 10 μM FMRFamide recorded in a patch from the LSN neurone in the presence of 0, 3, 10 or 30 μM EIPA. Enhancement and blockade are seen. The holding potential was -100 mV throughout.
DISCUSSION
The primary structure of HtFaNaC
The FMRFamide-gated channel of Helix was the first peptide-gated channel to be cloned and heterologously expressed (Lingueglia et al. 1995). Although the channel is a member of the superfamily of amiloride-sensitive Na+ channels, it occupies a branch separate from other superfamily members. Each member of the superfamily contains a large extracellular region, two transmembrane domains (M1 and M2) and intracellular N- and C-terminal regions. The cloning of HtFaNaC from Helisoma now allows us to make more direct inferences about sequences specific to FMRFamide-gated channels. Like HaFaNaC and the other members of the superfamily, the extracellular domain in HtFaNaC is rich in cysteines. In the extracellular domain, the sequence of amino acids just before M2 and of M2 itself are probably important in forming the ion pore in HaFaNaC (Lingueglia et al. 1995), as in other members of the superfamily (see e.g. Waldmann et al. 1995). Evidence has also been presented that the region preceding M1 in ASIC participates in the ion pore (Coscoy et al. 1999). It is notable that these pre-M regions in the FMRFamide-gated channels of Helix and Helisoma are identical. Between the last of the extracellular cysteines and the putative pore-forming domain preceding M2, there are about 50 residues in both FMRFamide-gated channels that are not seen in other members of the superfamily. This sequence of amino acids may represent all or part of the peptide-binding moiety of FMRFamide-gated channels. The weak conservation between the sequences of the two channels in this region is intriguing in view of their differing sensitivity to peptide agonists.
In contrast to the epithelial Na+ channels, which require three distinct subunits to form a fully functional channel (Canessa et al. 1994), the Helix FMRFamide-gated channel subunit is itself sufficient to generate a response in oocytes to FMRFamide comparable to the neuronal response. Similarly, expression of HtFaNaC cRNA alone in oocytes produced a FMRFamide-sensitive current comparable to that of Helisoma neurones. It is thought that the functional FMRFamide-gated channel of Helix is a tetramer of four identical subunits (Coscoy et al. 1998), but it is not yet established that the neuronal channel is homomeric. The Helix subunit (HaFaNaC) contains approximately 15 kDa of glycosylation (Coscoy et al. 1998). The native Helisoma channel may be similarly modified as several consensus sites for N-linked glycosylation are conserved between HtFaNaC and HaFaNaC.
Comparison of the FMRFamide responses of HtFaNaC with those of HaFaNaC
The EC50 value of HtFaNaC expressed in oocytes for FMRFamide was about 70 μM compared to about 2 μM for HaFaNaC, possibly indicating a lower affinity of HtFaNaC. However, the difference in EC50 values could also be explained in terms of differences in open time constants and Popen between the two clones (see Colquhoun, 1998). The amplitudes of the whole-oocyte currents evoked by FMRFamide were usually smaller than those evoked from oocytes injected with a comparable amount of HaFaNaC cRNA. FLRFamide, a peptide formed from the same precursor protein (see Lutz et al. 1992) was only a very weak agonist and its effect on oocyte-expressed HtFaNaC was not studied in detail. FMRFamide, FLRFamide and related peptides have been shown to exert significant channel block at concentrations higher than 10 μM with the Helix channel (Green & Cottrell, 1999); this also occurs with HtFaNaC as well as with the Helisoma neuronal channel. Unlike HaFaNaC, amiloride and related drugs had a pronounced enhancing effect on FMRFamide currents evoked in intact oocytes injected with HtFaNaC cRNA in addition to having a blocking action. These effects are discussed in more detail below.
Unitary currents from oocyte-expressed HtFaNaC activated by FMRFamide had a conductance similar to that recorded from similarly expressed HaFaNaC (Lingueglia et al. 1995; Zhainazarov & Cottrell, 1998). The similar conductance of the two FaNaCs may reflect their high conservation within the putative pore-forming regions (see above). The major open time constant for the HtFaNaC was 0.71 ms. This was consistently shorter than that of 4.84 ms for HaFaNaC (Zhainazarov & Cottrell, 1998). The difference in open times of HtFaNaC and HaFaNaC probably contributes to the smaller whole-cell FMRFamide responses observed with HtFaNaC compared to HaFaNaC, but other factors are also likely to be important (see Zhainazarov & Cottrell, 1998; Green & Cottrell, 1999). The different gating kinetics may be influenced by differences in the terminal regions. Grunder et al. (1999) have shown that changes in the amino acid sequence immediately preceding M1 can influence gating of ENaCs. The same region is, however, conserved between HtFaNaC and HaFaNaC, as discussed above. Consequently, if differences in the N-terminal region are important, those described by Grunder et al. (1999) cannot explain the differences observed in gating between expressed HtFaNaC and HaFaNaC. The C-terminus is also known to be important in the gating of ENaCs (Fuller et al. 1996), but it remains to be seen if this is also the case for FaNaCs.
Effect of amiloride, benzamil and EIPA on HtFaNaC
The results show that amiloride, benzamil and EIPA can markedly enhance the response to FMRFamide of HtFaNaC expressed in Xenopus oocytes. The predominant effect of each of the amiloride-related drugs on intact oocytes expressing HtFaNaC was potentiation of the FMRFamide-induced current.
At the unitary current level, it could be seen that the stimulatory effects of each drug were complicated by blockade, most probably open-channel blockade, as assessed by shortened open times (see McNicholas & Cannessa, 1997). The enhancing effect appeared to occur independently of channel blockade, because the two effects could be separated in time. This was particularly obvious with benzamil. In the combined presence of FMRFamide with any of the amiloride analogues tested, the net effect on the current response was the result of a balance between the potentiating and inhibitory effects of the drugs. This varied with the different drugs, EIPA and benzamil being more effective than amiloride in enhancing FMRFamide responses.
The potentiating effect of amiloride on FMRFamide responses of HtFaNaC contrasts with the related channel from Helix, where amiloride has so far been observed only to block the FMRFamide response of HaFaNaC (Lingueglia et al. 1995; Cottrell, 1997), as well as the neuronal channel (Green et al. 1994). The effects of benzamil and EIPA on HaFaNaC or on the neuronal response of Helix neurones have not yet been described.
Comparison of response of HtFaNaC expressed in oocytes with neuronal responses of the FMRFamide-gated channel
Although we have not yet established that the HtFaNaC clone obtained from a neuronal cDNA library partly underlies or is solely responsible for the FMRFamide-gated current recorded from the identified Helisoma neurones, GDN and LSN, most aspects of the fast inward current elicited in these neurones by FMRFamide were similar to those of expressed HtFaNaC. Responses of Helisoma neurones to FMRFamide were very similar to those of expressed HtFaNaC. The sensitivity of the whole-neurone response was comparable to that observed with the expressed clone, but occasionally the neuronal unitary responses appeared to be much more sensitive, with responses being observed with 1 μM FMRFamide (see below). Reduction in the extracellular Ca2+ and/or Mg2+ levels increased the amplitude of FMRFamide-activated unitary currents of the neurone and the clone similarly in a dose-dependent manner. Furthermore, the permeability of the expressed HtFaNaC was similar to that of the neuronal channel, as was the mean open time of the preponderant open state recorded. The rate of desensitization of the neuronal response was generally faster than that for expressed HtFaNaC, although it is difficult to quantify the difference between the neurone and oocyte because of the different modes of application necessarily used with each preparation. A similar difference in desensitization has been noted between HaFaNaC and the Helix neuronal response (Cottrell, 1997). Although the Helisoma neuronal response and that of the HtFaNaC were both enhanced and blocked by amiloride, benzamil and EIPA, enhancement was less obvious with the neuronal response than with HtFaNaC. The reason for this discrepancy is not clear; it could relate to differences between the blocking and enhancing activities of each drug on the neuronal channel, or to factors in the natural environment of the channel that increase its initial control responsiveness to FMRFamide, by perhaps evoking a higher activity state (see below).
On some occasions, the neuronal FMRFamide-gated channel did actually show a much higher sensitivity to FMRFamide. The reason for the variation in responsiveness to FMRFamide of the neurone is not known but may relate to different activity states of the channel, as shown in Fig. 3D and E (and K. A. Green & G. A. Cottrell, manuscript in preparation). The pronounced potentiation of the FMRFamide response of expressed HtFaNaC with EIPA and benzamil has drawn attention to one means by which the activity of HtFaNaC can be dramatically enhanced. EIPA may mimic some endogenous factor(s) that normally regulates activity of the channel. If such factors exist, it may be possible to detect them by comparing the effects of extracts of snail ganglia on FMRFamide responses of HtFaNaC and HtFaNaC expressed in Xenopus oocytes.
Do amiloride-like drugs potentiate other ‘amiloride-sensitive’ channels?
Benzamil has been shown to enhance as was well as block the effect of H+ on isolated neurones from rat dorsal root ganglia (Green et al. 2000). Further, although amiloride at 200 μM reduced the fast component of the H+ response of the ASIC3 clone expressed in COS7 cells, the small slow component of the H+ response appeared to be increased in amplitude (Waldmann et al. 1997). This effect of amiloride on the ASIC3 channel was not examined further by these workers, however. Adams et al. (1999) showed that 100 μM amiloride can potentiate a small inward current activated by 100 μM Zn2+ on a mutant channel derived from BNC1 (i.e. BNC1 G430C; BNC1 is also known as MDEG). These workers proposed the existence of two different interaction sites for amiloride. Our data too are best explained in terms of amiloride, EIPA and benzamil each interacting at more than one site on the channel protein because: (a) the enhancing and blocking effects could be separated in time, (b) there was marked variation in the degree of enhancement compared to blockade with each of the different drugs and (c) whereas the enhancing effect is independent of membrane voltage, the blocking effect is voltage dependent being more effective at positive patch-electrode potentials (K. A. Green & G. A. Cottrell, unpublished data; and see McNicholas & Canessa, 1997). The observation that amiloride and related drugs can exert more than one effect on such ENaC/DEG channels may be relevant with respect to the question of whether their persistent anti-hypertensive effect is due to their diuretic action alone (Brody et al. 1994).
Acknowledgments
We thank the Wellcome Trust for financial support and Dr P. A. V. Anderson for reading an early version of the manuscript.
References
- Adams CM, Snyder PM, Welsh MJ. Paradoxical stimulation of a DEG/ENaC channel by amiloride. Journal of Biological Chemistry. 1999;274:15500–15504. doi: 10.1074/jbc.274.22.15500. [DOI] [PubMed] [Google Scholar]
- Benos DJ, Awayda MS, Ismailov II, Johnson JP. Structure and function of amiloride-sensitive Na+ channels. Journal of Membrane Biology. 1995;143:1–18. doi: 10.1007/BF00232519. [DOI] [PubMed] [Google Scholar]
- Brody TM, Larner J, Minnemann KP, Neu HC. Human Pharmacology. St Louis, USA: Mosby; 1994. [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger J-D, Rossier BC. Amiloride-sensitive epithelial Na channel is made up of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Carpenter DO, Swann JW, Yarowsky PJ. Effects of curare on responses to different putative neurotransmitters in Aplysia neurones. Journal of Neurobiology. 1977;8:119–132. doi: 10.1002/neu.480080204. [DOI] [PubMed] [Google Scholar]
- Chen C-C, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proceedings of the National Academy of Sciences of the USA. 1998;95:10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombiaoni L, Paupardin-Tritsch D, Vidal PP, Gerschenfeld HM. The neuropeptide FMRF-amide decreases both the Ca2+ conductance and a cyclic 3′,5′-adenosine monophosphate-dependent K+ conductance in identified molluscan neurones. Journal of Neuroscience. 1985;5:2533–2538. doi: 10.1523/JNEUROSCI.05-09-02533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D. Binding, gating, affinity and efficacy: The interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. British Journal of Pharmacology. 1998;125:923–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coscoy S, de Weille JR, Lingueglia E, Lazdunski M. The pre-transmembrane 1 domain of acid-sensing ion channels participates in the ion pore. Journal of Biological Chemistry. 1999;274:10129–10132. doi: 10.1074/jbc.274.15.10129. [DOI] [PubMed] [Google Scholar]
- Coscoy S, Lingueglia E, Lazdunski M, Barbry P. The Phe-Met-Arg-Phe-amide-activated sodium channel is a tetramer. Journal of Biological Chemistry. 1998;273:8317–8322. doi: 10.1074/jbc.273.14.8317. [DOI] [PubMed] [Google Scholar]
- Cottrell GA. The first peptide-gated ion channel. Journal of Experimental Biology. 1997;200:2377–2386. doi: 10.1242/jeb.200.18.2377. [DOI] [PubMed] [Google Scholar]
- Cottrell GA, Davies NW, Green KA. Multiple actions of a molluscan cardio-excitatory neuropeptide and related peptides on identified Helix neurones. The Journal of Physiology. 1984;356:315–333. doi: 10.1113/jphysiol.1984.sp015467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell GA, Green KA, Davies NW. The neuropeptide Phe-Met-Arg-Phe-NH2 (FMRFamide) can activate a ligand-gated ion channel in Helix neurones. Pflügers Archiv. 1990;416:612–614. doi: 10.1007/BF00382698. [DOI] [PubMed] [Google Scholar]
- Fuller CM, Ismailov II, Berdiev BK, Shlyonsky VG, Benos DJ. Kinetic interconversion of rat and bovine homologs of the α subunit of an amiloride-sensitive Na+ channel by C-terminal truncation of the bovine subunit. Journal of Biological Chemistry. 1996;271:26602–26608. doi: 10.1074/jbc.271.43.26602. [DOI] [PubMed] [Google Scholar]
- Green KA, Cottrell GA. Block of the Helix FMRFamide-gated Na+ channel by FMRFamide and its analogues. The Journal of Physiology. 1999;519:47–56. doi: 10.1111/j.1469-7793.1999.0047o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KA, Falconer SWP, Cottrell GA. The neuropeptide Phe-Met-Arg-Phe-NH2 directly gates two ion channels in an identified Helix neurone. Pflügers Archiv. 1994;428:232–240. doi: 10.1007/BF00724502. [DOI] [PubMed] [Google Scholar]
- Green KA, Powell B, Cottrell GA. Amiloride drugs both block and enhance the action of H+ ions on rat dorsal root ganglion neurones. British Journal of Pharmacology. 2000 in the Press. [Google Scholar]
- Grunder S, Fowler N, Gautschi I, Schild L, Rossier RC. Identification of a highly conserved sequence at the N-terminus of the epithelial Na+ channel α subunit involved in gating. Pflügers Archiv. 1999;438:709–715. doi: 10.1007/s004249900119. [DOI] [PubMed] [Google Scholar]
- Harris SJ, Cottrell GA. Properties of an identified dopamine-containing neurone in culture from the snail Helisoma. Experimental Physiology. 1995;80:37–51. doi: 10.1113/expphysiol.1995.sp003833. [DOI] [PubMed] [Google Scholar]
- Jeziorski MC, Greenberg RM, Clark KS, Anderson PA. Cloning and functional expression of a voltage-gated calcium channel α1 subunit from jellyfish. Journal of Biological Chemistry. 1998;273:22792–22799. doi: 10.1074/jbc.273.35.22792. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, Champigny G, Lazdunski M, Barbry P. Cloning of the amiloride-sensitive FMRFamide peptide-gated sodium channel. Nature. 1995;378:730–733. doi: 10.1038/378730a0. [DOI] [PubMed] [Google Scholar]
- Lutz EM, MacDonald M, Hettle S, Price DA, Cottrell GA, Sommerville J. Structure of cDNA clones and genomic DNA encoding FMRFamide-related peptides (FaRPs) in Helix. Molecular and Cellular Neuroscience. 1992;3:373–382. doi: 10.1016/1044-7431(92)90049-8. [DOI] [PubMed] [Google Scholar]
- McNicholas CM, Canessa CM. Diversity of channels generated by different combinations of epithelial sodium channels. Journal of General Physiology. 1997;109:681–692. doi: 10.1085/jgp.109.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Families of ion channels with two hydrophobic segments. Current Opinion in Cell Biology. 1996;8:474–483. doi: 10.1016/s0955-0674(96)80023-8. [DOI] [PubMed] [Google Scholar]
- Patton DE, Silva T, Bezanilla F. RNA editing generates a diverse array of transcripts encoding squid Kv2 K+ channels with altered functional properties. Neuron. 1997;19:711–722. doi: 10.1016/s0896-6273(00)80383-9. [DOI] [PubMed] [Google Scholar]
- Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acids residues in the α, β and γ subunits of the epithelial sodium channel (ENaC) involved in amiloride block and permeation. Journal of General Physiology. 1997;109:15–26. doi: 10.1085/jgp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ, Clustal W. Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, de Weill J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurones. Journal of Biological Chemistry. 1997;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Voilley N, Lazdunski M. Molecular cloning and functional expression of a novel amiloride-sensitive Na+ channel. Journal of Biological Chemistry. 1995;270:27411–27414. doi: 10.1074/jbc.270.46.27411. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Current Opinion in Neurobiology. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- Zhainazarov AB, Cottrell GA. Single-channel currents of a peptide-gated sodium channel expressed in Xenopus oocytes. The Journal of Physiology. 1998;513:19–31. doi: 10.1111/j.1469-7793.1998.019by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhainazarov AB, Cottrell GA. Single-channel currents of a peptide-gated sodium channel expressed in Xenopus oocytes. The Journal of Physiology. 1998;513:19–31. doi: 10.1111/j.1469-7793.1998.019by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


