Abstract
Na+-activated potassium channels (KNa channels) were studied in inside-out patches from guinea-pig ventricular myocytes at potentials between -100 and +80 mV. External K+ () was set to 140 mM. For inwardly directed currents with 105 mM internal K+ (), the unitary current-voltage relationship was fitted by the constant field equation with a potassium permeability coefficient, PK, of 3.72 × 10−13 cm3 s−1. The slope conductance (-100 to -10 mV) was 194 ± 4.5 pS (mean ± s.d., n= 4) with 105 mM (35 mM ) but it decreased to 181 ± 5.6 pS (n= 5) in 70 mM (70 mM ).
KNa channels were activated by internal Na+ in a concentration-dependent fashion. With 4 mM , maximal activation was recorded with 100 mM (open probability, Po, about 0.78); half-maximal activation required about 35 mM . When was increased to 70 mM, half-maximal activation shifted to about 70 mM .
With set to 105 mM, channel activity was markedly inhibited when was increased from 35 to 105 mM. Channel openings were abolished with 210 mM .
The inhibitory effect of internal K+ was also observed at more physiological conditions of osmolarity, ionic strength and chloride concentration. With 35 mM and 4 mM , Po was 0.48 ± 0.10 (n= 6); when was increased to 35 mM, Po was reduced to 0.04 ± 0.05 (n= 7, P= 0.001).
The relationship between Po and concentration at different levels of is well described by a modified Michaelis-Menten equation for competitive inhibition; the Hill coefficients were 4 for the Po- relationship and 1.2 for the Po- relationship. It is suggested that Na+ and K+ compete for a superficial site on the channel's permeation pathway.
KNa channels would be most likely to be activated in vivo when an increase in is accompanied by a decrease of .
Increases in potassium conductance activated by high levels of intracellular sodium () have been reported in many different preparations. These include guinea-pig ventricular myocytes (Kameyama et al. 1984) as well as neurones from crayfish (Hartung, 1985), quail trigeminal ganglia (Bader et al. 1985), chick brainstem (Dryer et al. 1989), cat neocortex (Schwindt et al. 1989), rat olfactory bulb (Egan et al. 1992b), rat motoneurones (Safronov & Vogel, 1996), rat dorsal root ganglia (Bischoff et al. 1998) and frog spinal chord (Dale, 1993). In addition, sodium-gated potassium channels (KNa channels) are present in Xenopus oocytes (Egan et al. 1992a), insect neurosecretory cells (Grolleau & Lapied, 1994) and frog taste cells (Miyamoto et al. 1996). In some preparations there is a sufficiently high density of voltage-gated sodium channels that the Na+ accumulated during trains of action potentials may contribute to KNa channel activation. The correlation between the numbers of voltage- and sodium-gated channels in individual membrane patches in Xenopus myelinated axons (Koh et al. 1994) supports this idea. In neurones there is enough KNa channel activation at physiological levels of to suggest that the sodium-activated potassium current may contribute to the resting membrane conductance (Haimann et al. 1990; Bischoff et al. 1998).
In cardiac preparations KNa channels appear to require 20 mM for activation, the response showing no saturation below 100 mM (Kameyama et al. 1984). Guinea-pig myocytes have an intracellular sodium activity of about 7 mM (Rodrigo & Chapman, 1990), which may increase by over 30 % during increased heart rate (Cohen et al. 1982). Nevertheless the relative insensitivity of the channels to suggests that activation occurs (if at all) only during pathological conditions.
The KNa channel binding site requires the co-operative action of at least two Na+ to bring about activation (Kameyama et al. 1984; Haimann et al. 1990) but otherwise little is known about the characteristics of the site of action. We therefore undertook a study of the effect of Na+ in the presence of different concentrations of intracellular potassium (). We report here that K+ at the internal membrane surface competitively inhibits activation of KNa channels in inside-out patches from guinea-pig ventricular myocytes. This may have some physiological significance, as an increase in in vivo is likely to coincide with a decrease in .
METHODS
Preparation
Adult guinea-pigs were killed by cervical dislocation. The heart was removed and perfused retrogradely via the aorta using a Langendorff apparatus. Single ventricular myocytes were prepared by enzymatic dissociation as described previously (Mitra & Morad, 1985; Rodrigo & Chapman, 1990) except that 40 mM taurine was included in the Tyrode solution used for the final perfusion. Myocytes were kept at room temperature (18-23°C) and used within 12 h of isolation.
Recording conditions and analysis
Currents through KNa channels were recorded using the inside-out configuration of the patch clamp technique (Hamill et al. 1981). Pipettes were made from borosilicate glass capillaries (Clark Electromedical Ltd) and had tip resistances of 3-10 MΩ when filled with pipette solution. We used a modified technique to excise inside-out patches; after seal formation, instead of removing a patch of membrane from the myocyte by withdrawing the pipette, the cell was dislodged and washed away by rapid superfusion of standard internal solution through the perfusion system (see Niu & Meech, 1998). One advantage of this approach was that although the cell was maintained in normal bathing medium, after patch isolation the inside surface of the plasma membrane was immediately exposed to a solution with an appropriate internal composition. By preparing detached patches in this way, KNa channel activity was stable for periods of up to 30 min. Membrane potentials were corrected for the calculated loss of junction potential between the patch pipette and bathing solution upon seal formation (Barry & Lynch, 1991).
KNa channel currents were recorded using an Axopatch-1D patch clamp amplifier and digitised via a TL-1 interface. For multi-channel analysis, currents were filtered at 0.5-1 kHz and digitised at 2-10 kHz. For single channel analysis the currents were filtered at 2 kHz and sampled at 20 kHz. All experiments were conducted at room temperature (18-23°C). Channel open probability (Po) was calculated as:
| (1) |
where N is the number of channels in the membrane patch and tj is the time spent at each current level (j= 1, 2,…N). The total duration of the recording, T, was at least 15 s. The 50 % open/closed level was used to mark the beginning and end of each transition. Subconductance states, which were observed close to the full open and closed levels, were both rare and brief (i.e. less than 1 % of open time); they were ignored in this analysis.
Solutions
Myocytes were maintained in normal saline (mM): NaCl, 140; KCl, 5.4; MgCl2, 1; CaCl2, 2; and Hepes, 5, at pH 7.25. Seals were obtained with patch pipettes that contained (mM): KCl, 140; CaCl2, 1; and Hepes, 5, at pH 7.25 (adjusted with KOH). Once detached, the internal surface of the membrane patch was perfused with a standard internal solution composed of (mM): KCl + NaCl, 140; EGTA, 5; ATP, 2-5; and Hepes, 10, at pH 7.25 (adjusted with KOH). Test solutions are specified in the text; in some cases they were hyperosmotic to the standard solution; in other cases the osmolarity was made the same as the standard solution using either glucose or N-methyl-D-glucamine-HCl (NMDG-HCl). All chemicals were obtained from Sigma Chemical Co. Ltd.
RESULTS
During single channel recordings of KNa channel activity from guinea-pig ventricular myocytes, we observed two other classes of potassium channel: the ATP-sensitive potassium channel (KATP) and the inwardly rectifying potassium channel (KIR). KATP channel activity was inhibited by including ATP (at least 2 mM) in the perfusion solution (see Niu & Meech, 1998) but interference from KIR was almost inevitable. However, KIR channels had a smaller unitary conductance than KNa channels, and their kinetics were significantly different. This meant that their activity could be excluded easily and had little impact on the analysis of KNa channel properties. Furthermore, KIR channels were generally less stable than KNa channels and appeared to undergo rundown within 2-5 min after excision of the inside-out patch.
KNa channels were present in about 10 % of inside-out patches, and in most cases one to two channels were observed. Consistent with previously published work, the channels often had multiple subconductance states and their activity was significantly affected by changes in Na+ concentration at the internal membrane surface. Figure 1A shows sections of a continuous recording from an inside-out patch containing two KNa channels. Channel currents were recorded at different holding potentials in the presence of 140 mM ; the internal concentrations were 70 mM K+ and 70 mM Na+ (left column), or 105 mM K+ and 35 mM Na+ (right column). KNa channel activity was markedly reduced when the patch was perfused with the 105 mM , 35 mM solution. The unitary current-voltage relationship (Fig. 1B) showed a significant inward rectification, which became even more marked as was increased from 35 to 70 mM. The lines drawn through the data were calculated from the constant field equation (Goldman, 1943; Hodgkin & Katz, 1949) and are equivalent to a conductance of 199 pS in 140 mM external and internal K+. The potassium permeability coefficient, PK, was taken as 3.72 × 10−13 cm3 s−1 and the PNa/PK ratio was set at 0.02 (see Kameyama et al. 1984; Wang et al. 1991). The mean slope conductance of the fully opened channel was 194 ± 4.5 pS (n= 4) when measured with 140 mM and 105 mM (35 mM ) for inward currents in the range -100 to -10 mV. The slope conductance of the inward current decreased slightly to 181 ± 5.6 pS (n= 5) in 70 mM (70 mM ). The values shown are means ±s.d. throughout.
Figure 1. Effect of membrane voltage on KNa channel currents.
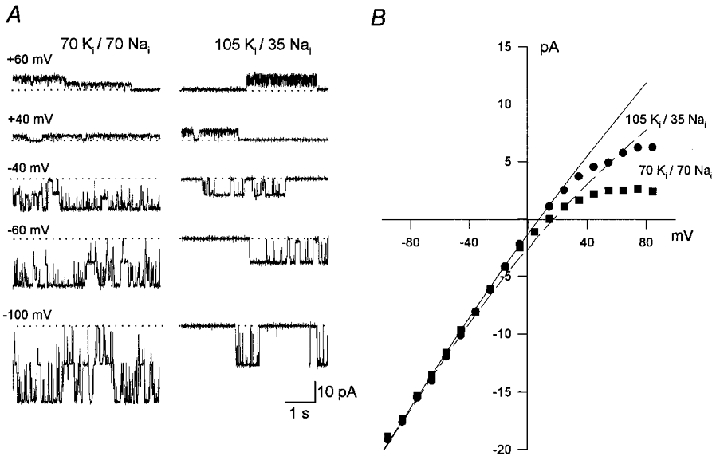
A, current traces from an inside-out patch containing two active KNa channels recorded at potentials between +60 and -100 mV in the presence of 140 mM . Internal solutions were 70 mM , 70 mM (left) and 105 mM , 35 mM (right). The dotted lines indicate the current level recorded with both channels closed. B, the unitary current-voltage relationship in 70 mM , 70 mM (▪) and 105 mM , 35 mM (•). The lines drawn through the data were calculated from the constant field equation (Goldman, 1943; Hodgkin & Katz, 1949). The permeability coefficient, PK, was taken as 3.72 × 10−13 cm3 s−1 and the PNa/PK ratio was 0.02 (see Kameyama et al. 1984; Wang et al. 1991). Deviation from the expected relationship at membrane potentials more positive than +30 mV is due to channel block by (Wang et al. 1991).
Inhibitory effect of intracellular K+
With set to 105 mM there was a high level of KNa channel activity in inside-out membrane patches exposed to 35 mM (Fig. 2, top trace) but the open probability was markedly reduced upon switching to a solution with 105 mM . Channel activity was abolished completely in the presence of 210 mM but it returned to control levels once the bathing solution was returned to 35 mM (Fig. 2, bottom trace). Similar, fully reversible, effects were observed in three additional patches. Because was set to 105 mM, the different test solutions were hyperosmotic. Thus the reduction in open probability could be the result of the greater osmolarity of the internal solutions, or their greater ionic strength and higher chloride concentration. Figure 3 shows that none of these factors are involved because in 35 mM the high open probability was retained even when 350 mM glucose (top trace), 175 mM lithium chloride (n= 2; middle trace), or 175 mM NMDG-HCl (n= 4; bottom trace) was added to the test solution. Thus, increased concentrations of appear to inhibit KNa channel activity in a fully reversible fashion.
Figure 2. Effect of on KNa channel activity.
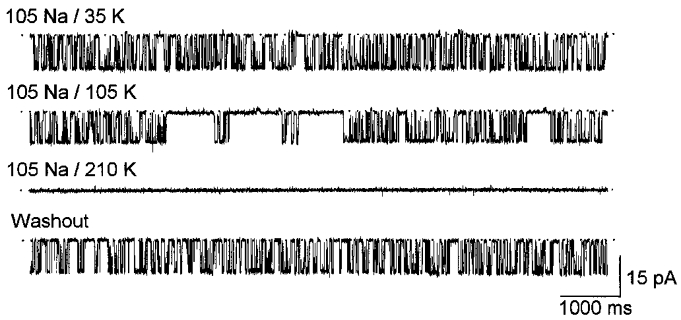
Single KNa channel inward currents recorded from an inside-out membrane patch with 105 mM . The high open probability seen with 35 mM (top trace) was markedly reduced upon increasing to 105 mM and the channel was fully inhibited with at 210 mM (middle traces). The channel activity recovered to the control level after was returned to 35 mM (bottom trace). Note that because was set to 105 mM the test solutions were hyperosmotic. The membrane potential was held at -60 mV.
Figure 3. Effect of osmotic strength, internal Li+ and NMDG-HCl on KNa channel activity.
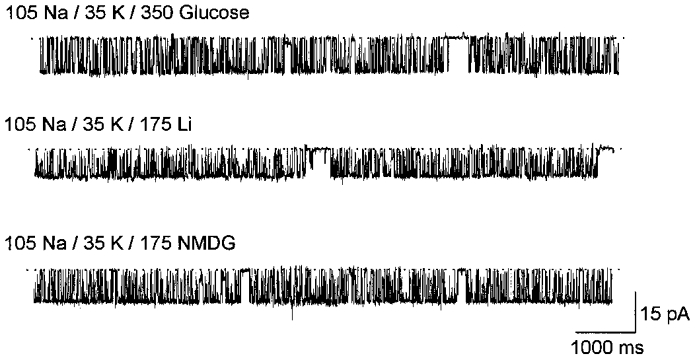
Single KNa channel inward currents recorded from an inside-out membrane patch with 105 mM and 35 mM . Top trace, high open probability with hyperosmotic solution (containing 350 mM glucose) at the internal surface. Middle trace, open probability was unaffected by the addition of 175 mM LiCl in place of glucose. Bottom trace, open probability was unaffected by the addition of 175 mM NMDG-HCl in place of glucose. The membrane potential was held at -60 mV.
Internal K+ was also inhibitory at lower concentrations and at a more physiological osmolarity (maintained using NMDG-HCl). Figure 4 shows KNa channel activity with set to 35 mM. Upon raising from 4 mM (upper trace) to 35 mM (lower trace) there was a clear reduction in the number of channel openings. The bar chart in Fig. 4 shows the open probability in the two solutions; the open probability was 0.48 ± 0.10 (n= 6) with 4 mM but became significantly smaller (0.04 ± 0.05; n= 7; P= 0.001, unpaired t test) upon raising to 35 mM.
Figure 4. The effect of on KNa channel activity at physiological osmolarity.
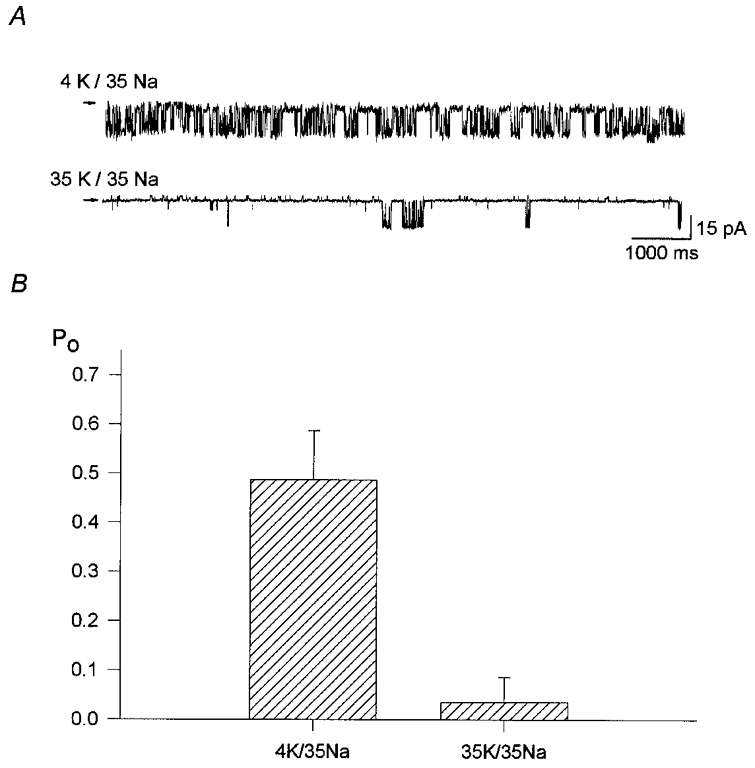
A, examples of inward currents recorded from single KNa channels in inside-out membrane patches with 35 mM . The high channel activity recorded with 4 mM (upper trace) was in contrast to the low number of openings seen with 35 mM (lower trace; different patch). Changes in osmolarity were corrected with NMDG-HCl and the membrane potential was held at -60 mV. B, bar graph to show that the open probability (Po) was reduced from 0.48 ± 0.10 (n= 6) to 0.04 ± 0.05 (n= 7, P= 0.001) upon raising the internal potassium concentration from 4 to 35 mM in the presence of 35 mM . Values are means ±s.d.; P value is from Student's unpaired t test.
From the data presented thus far, it is possible that internal Na+ relieved the KNa channel from inhibition by internal K+. To determine whether internal Na+ was essential for KNa channel activation, we exposed the channel to a K+-Na+-free internal solution containing 105 mM NMDG-HCl (see Fig. 5). Although there was an almost immediate reduction in open probability upon switching to the K+-Na+-free solution, there was a delay of 12 s before channel opening was completely abolished. Upon returning to 105 mM , the increase in open probability followed an exponential time course with a time constant of about 1.2 s. The same result was observed for the two other patches tested.
Figure 5. Effect of Na+-K+-free internal solution on KNa channel activity.
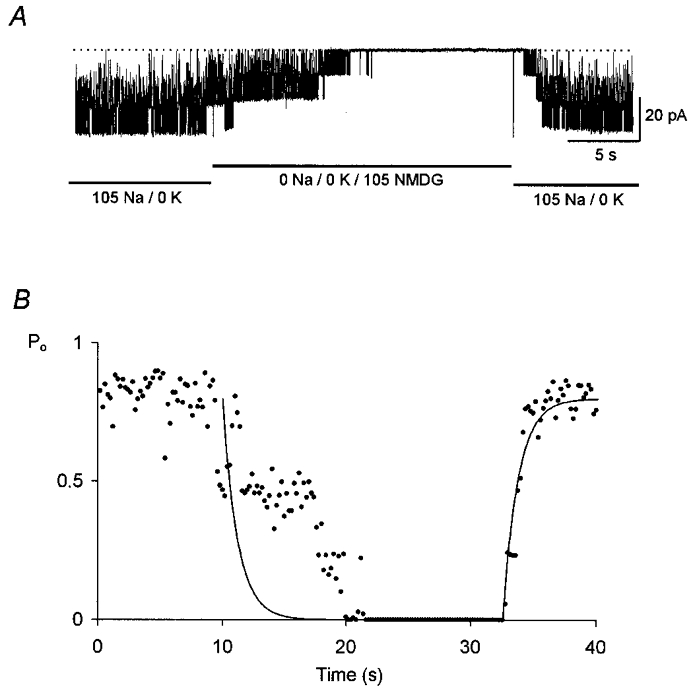
A, KNa channel inward currents recorded from an inside-out membrane patch with 105 mM , 0 mM . Three levels of unitary current are evident. Upon switching abruptly to a Na+-K+-free solution containing 105 mM NMDG-HCl, channel activity declined over a period of 12 s and was finally abolished. Activity quickly recovered when the patch was returned to the control solution. The membrane potential was held at -60 mV. B, change in Po with time. The fitted line has a time constant of 1.2 s.
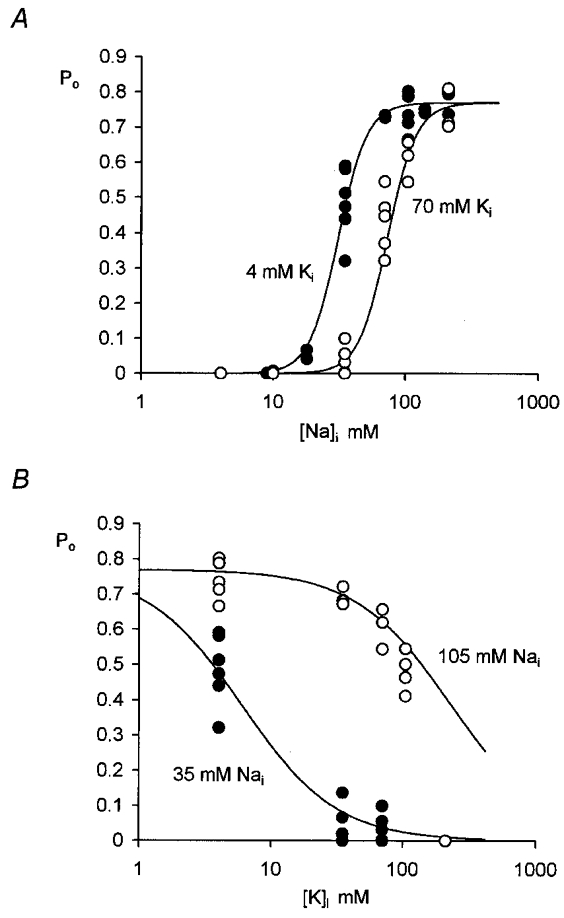
While internal Na+ was obviously necessary for KNa activation, it is clear that there was also an interaction with internal K+. To examine the inhibitory effect of K+ on the sodium dependency in more detail, we first set at 4 mM and measured the channel open probability in different concentrations of (see Fig. 6A, filled circles). For concentrations below 140 mM, NaCl was replaced by equimolar concentrations of NMDG-HCl. When we compared the effect of in the presence of 70 mM (Fig. 6A, open circles) the maximum open probability appeared to be as great but the data were displaced towards the right, i.e. the half-maximal open probability was observed at higher values of . In Fig. 6B the effect of different concentrations of on channel open probability is compared in the presence of 105 mM (open circles) and 35 mM (filled circles) . In Fig. 6 lines through the data points were drawn according to the following modified Michaelis-Menten equation, which can be derived assuming that there is a competitive interaction between and :
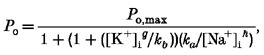 |
(2) |
where ka and kb are dissociation constants, [Na+]i and [K+]i are the sodium and potassium ion concentrations at the intracellular surface of the membrane, and g and h represent Hill coefficients. The data were fitted by eye with the ka:kb ratio set to 1.75 × 105; the Hill coefficients g and h were 1.2 and 4.0; the maximum open probability (Po,max) was 0.78.
Figure 6. Effect of and on KNa channel open probability.
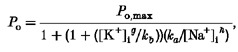 |
Single channel kinetics
Patches with a single active KNa channel were studied at -60 mV with 105 mM . Figure 7 shows the distributions of open and closed times in 4 mM (left) and 105 mM (right). Although obtained under slightly different conditions, the data confirm the findings of Mistry et al. (1997) and demonstrate the presence of two open and at least four closed states. The time constants obtained in the two studies are in broad agreement. In Fig. 7 the increase in from 4 to 105 mM had little effect on the open time constants or on the two shorter closed time constants but the longer closed time constants increased by factors of about 4 and 3. This matches the finding by Mistry et al. (1997) who showed that the time constants of these same closed states increased as the concentration was decreased (with NaCl being replaced by Tris-HCl).
Figure 7. The effect of on open and closed times.
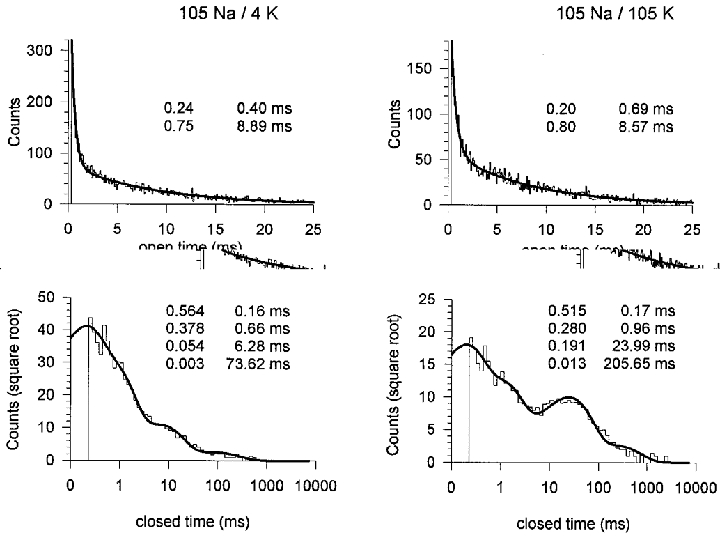
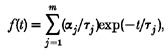 |
DISCUSSION
KNa channel ‘rundown’
KNa channels recorded from detached inside-out membrane patches are particularly susceptible to a gradual loss of activity (or ‘rundown’) that is not seen in cell-attached patches (Egan et al. 1992b; Dryer, 1993). Egan et al. (1992b) suggest that the loss of some diffusible intracellular substance might reduce the sensitivity of the channel to sodium. Using techniques of myocyte preparation similar to those used here, Rodrigo (1993) found that in newly excised patches Po was about 0.3 with 150 mM and that it declined to 0.1 after 30 min. In cell-attached patches, activity remained constant for up to 140 min with Po about 0.7 ( concentration was calculated to be 150 mM). In our hands high open probabilities could be obtained within seconds of patch isolation; most channels showed little rundown of activity in recordings lasting up to 30 min. The technique of patch isolation used (see Methods) has already proved effective in reducing KATP channel rundown (Niu & Meech, 1998) and it seems to be equally valuable here. In other respects the KNa channels appeared to have properties identical to those reported previously. For example, for inward currents the unitary current-membrane potential relationship was well fitted by the Goldman-Hodgkin-Katz equation with PK equal to 3.72 × 10−13 cm3 s−1 (see Fig. 1 and Kameyama et al. 1984; Wang et al. 1991). A similar, though slightly lower value for PK (3.17 × 10−13 cm3 s)−1 has been reported for KNa channels in quail trigeminal neurones (Haimann et al. 1990).
Inhibition of KNa channel activity by internal K+
The main conclusion to be drawn from this work is that internal potassium ions have an inhibitory effect on the activity of KNa channels in guinea-pig ventricular myocytes. Increasing from 4 to 70 mM increased the concentration of needed for half-activation from about 35 mM to nearer 70 mM (see relationship between Po and in Fig. 6A). The latter value is close to that obtained in the same preparation by Kameyama et al. (1984) with equal to 40 mM or more. In quail trigeminal neurones, half-activation occurred in the range 25-30 mM in the presence of either 75 mM (Haimann et al. 1990) or 50 mM (Haimann et al. 1992). With physiological concentrations of a lower sensitivity to was reported so that here too Na+ and K+ may interact competitively (Haimann et al. 1992).
In a model to account for the channel kinetics in guinea-pig ventricular myocytes, Mistry et al. (1997) suggested that the presence of four closed states (see also Fig. 7) was consistent with the binding of 2 or 3 sodium ions before KNa channels entered one of two open states. All the available data are consistent with the duration of the two longest closed states being dependent on the : ratio. We considered the possibility that the role of Na+ was simply to displace K+ from an inhibitory site. If so, 0 mM might permit activation even in the absence of . As Fig. 5 shows, the channel remained active for up to 12 s in Na+-K+-free solution, the change in Po with time suggesting that the decline in activation had both fast and slow phases. Perhaps Na+ is firmly bound at the activation site so that there is no requirement for when the channel makes transitions between its shorter open and closed states. However, reopening after prolonged closure is evident even after some seconds in Na+-free solution so the possibility remains that incoming K+ had access to an inhibitory site.
Hill coefficient
One explanation for the steep relationship between Po and concentration is that the binding of more than one sodium ion is necessary to open the channel. For Fig. 6A, was kept constant at either 4 or 70 mM, the ionic strength and osmolarity of the different solutions being maintained by replacing NaCl with equimolar NMDG-HCl. The Po data were fitted by a modified Michaelis-Menten equation for competitive inhibition (eqn (2)). The Hill coefficients required to provide a satisfactory fit suggest that, while only 1-2 potassium ions are necessary for inhibition, at least 4 sodium ions are needed for activation of the channel. A similar high value, 4.8, has been reported for the effect of internal Na+ on KNa channels in the spinal neurones of the frog embryo (Dale, 1993), but values in the region of 2-3 are more generally reported (see Haimann et al. 1990; Rodrigo & Chapman, 1990). The Hill coefficient of 2.8 reported by Kameyama et al. (1984) was obtained in experiments where NaCl was replaced by equimolar KCl and it is likely that the inhibitory effect of increased concentration contributed to this low value. A similar explanation can account for the results of Veldkamp et al. (1994), who reported Po values of 0.73 in 135 mM and 0.62 in 100 mM .
Mechanism of KNa channel activation
Permeant ions determine channel selectivity
In many K+ channels the presence of potassium ions at binding sites within the pore seems to be essential for maintenance of selectivity. Under K+-free conditions such channels permit a substantial Na+ current (Zhu & Ikeda, 1993; Callahan & Korn, 1994; Korn & Ikeda, 1995; Starkus et al. 1997). K+ channels that undergo C-type inactivation also appear to pass through an intermediate state with an increased Na+ permeability before entering the non-conducting inactivated state (Kiss et al. 1999). Changing a single amino acid residue in the S6 segment of the channel decreases the ability of K+ to block the Na+ current (Ogielska & Aldrich, 1998). In the present study on KNa channels the 13 pS decrease in the mean inward slope conductance when was reduced from 105 to 70 mM (see Results) might also indicate some form of K+-modulated permeation.
Intracellular ions and gating
In small conductance calcium-activated potassium channels (KCa) an increase in Po follows Ca2+ binding to a calmodulin-like receptor site (Xia et al. 1998). Functional studies show that activation by different divalent ions depends on their crystalline radius, as expected for a calcium-binding protein (Meech, 1980; Meech & Thomas, 1980). The receptor site responsible for sodium activation of the KNa channel has been more difficult to pin down. Activation of the KNa channel could follow the binding of Na+ to a receptor site on or within the channel itself, or binding might occur some distance away and produce a more widespread change in the channel environment. KNa channels show up to 12 subconductance states (Sanguinetti, 1990; Mistry et al. 1996) and Sanguinetti (1990) has suggested that the channel is actually a complex of 12 identical pores each of which gates independently of an over-riding main gate. One could imagine the cell membrane as a matrix of fixed charges with which Na+ interacts to cause the conformation change necessary to open the 12-pore complex. The binding site itself need not be selective; the specificity of activation might lie in the conformation change that the cation brings about.
To envision a more discrete receptor we might compare its selectivity to that of other Na+-binding sites. For example, H+ and Li+ can substitute for both Na+ and K+ during Na+-K+ active transport (Dunham & Hoffman, 1978) whereas the mammalian KNa channel is insensitive to Li+ and blocked by H+ (Veldkamp et al. 1994). The enzyme dialkylglycine decarboxylase, which is activated by K+ and inhibited by Na+ (Aaslestad et al. 1968), contains an example of a more selective receptor. An increase in from 75 to 130 mM (while reducing from 15 to 0 mM) substitutes Na+ for K+ at a site located near the reaction centre of the enzyme (site 1; Toney et al. 1993). The protein is too rigid to accept Na+ without an additional water molecule that appears to cause a slight expansion of the structure around the metal ion. This in turn reorientates two amino acid residues within the reaction centre and produces the switch from the active to inactive form of the enzyme (Hohenester et al. 1994).
The KNa channel site for which K+ and Na+ compete appears to bind 1-2 potassium or 4 sodium ions (see Fig. 6). The crystal radius of K+ is 1.33 Å while that of Na+ is 0.95 Å (Pauling, 1927, 1960), so K+ has a volume about 2.7 times the volume of Na+. Although it would be possible to pack 4 sodium ions in the same space as 1.5 potassium ions, electrostatic repulsion makes such close packing improbable and the receptor site must be large enough to accept the shell of water molecules that shield the individual ion charges.
Site of Na+-K+ competition
In a model to account for the crystal structure of the K+ channel from Streptomyceslividans (Doyle et al. 1998), there is a 10 Å diameter water-filled cavity mid-way across the membrane which is large enough to accept all 4 sodium ions together with their associated water molecules. The cavity is accessible to cations from the cell cytoplasm, their position being determined in part by the electrostatic field set up by the negative charges associated with the COOH-terminals of the four α-helices of the selectivity filter (Roux & MacKinnon, 1999). Small movements of these pore helices may bring about subconductance states by altering the throughput of K+ (Perozo et al. 1999). The presence of 4 or more sodium ions in this space might be all that is required to initiate activation. The induced strain on the pore helices might promote an initial entry of external K+ into the narrow external pore, the temporary presence of a single potassium ion at a binding site within the pore being sufficient to hold it open transiently once the Na+ is flushed away by the entering K+.
Estimates of the size of the internal opening (the internal pore) of voltage-gated K+ channels, based on the access of large tetra-N-alkylammonium ions (French & Shoukimas, 1981), suggest a diameter of at least 12 Å; in inwardly rectifying K+ channels the opening may be even larger (Lu et al. 1999). Assuming that the conductivity of the channel remains constant all the way across the membrane, most of the potential field will be associated with the high resistance external pore. Nevertheless up to 25 % of the field is across the internal pore and the outward movement of 4 sodium ions through it should be markedly affected by membrane voltage. A difficulty with placing the Na+ activation site within the central cavity is that Po for KNa channels has little or no voltage dependence (between -40 and +20 mV, Kameyama et al. 1984; between -80 and -20 mV, Wang et al. 1981; between -70 and -30 mV, Mistry et al. 1997). Hence the simplest explanation for the requirement for 4 activating sodium ions is that each ion binds to a separate channel subunit at some superficial site and that displacement of one sodium ion by a single potassium ion is sufficient to inhibit the process. The binding site appears to have a high affinity for K+, Na+ binding possibly being accompanied by water, to bring about the conformation change (c.f. dialkylglycine decarboxylase, above).
Physiological significance
The physiological significance of the KNa channel in cardiac myocytes is unknown. The channels are inactive in normal saline because the cellular sodium concentration is less than 10 mM. However, they are significantly activated in intact guinea-pig ventricular myocytes when the concentration is increased by superfusion with Ca2+-Mg2+-free solution (Rodrigo & Chapman, 1990). In addition, Luk & Carmeliet (1990) found that KNa channel open probability increased when the Na+-K+ pump was blocked by ouabain. Under some experimental conditions, however, it is internal K+ that changes not internal Na+. In stimulated canine ventricular muscle, decreased to about 80 mM during hypoxia in acidic, glucose-free solution whereas was not significantly altered (Nakaya et al. 1985). Overall the evidence indicates that KNa channels would be activated during pathological conditions, such as prolonged ischaemia, Ca2+ paradox and digitalis toxicity when the increase of internal Na+ is accompanied by a decrease of internal K+.
Acknowledgments
The late Professor R. A. Chapman, who died in December 1995, initiated this work on KNa channels. It was funded by a grant to Professor Chapman from the British Heart Foundation. X. W. Niu also acknowledges the support of grant NHLBI HL-57414 (J. C. Makielski PI) which permitted completion of the study. The authors thank Jonathan Makielski for his advice and support and for his comments on the manuscript. Mrs Valerie Buswell and Mr Mike Rickard provided excellent technical assistance.
References
- Aaslestad HG, Bouis PJ, Jr, Phillips AT, Larson AD. In: Pyridoxal Catalysis: Enzymes and Model Systems. Snell EE, Braunstein AE, Severin ES, Torchinsky YM, editors. New York: John Wiley & Son, Inc.; 1968. pp. 479–490. [Google Scholar]
- Bader CR, Bernheim L, Bertrand D. Sodium-activated potassium current in cultured avian neurones. Nature. 1985;317:540–542. doi: 10.1038/317540a0. [DOI] [PubMed] [Google Scholar]
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. Journal of Membrane Biology. 1991;121:101–117. doi: 10.1007/BF01870526. Published erratum appears in Journal of Membrane Biology 1992 125, 286. [DOI] [PubMed] [Google Scholar]
- Bischoff U, Vogel W, Safronov BV. Na+-activated K+ channels in small dorsal root ganglion neurones of rat. The Journal of Physiology. 1998;510:743–754. doi: 10.1111/j.1469-7793.1998.743bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan MJ, Korn SJ. Permeation of Na+ through a delayed rectifier K+ channel in chick dorsal root ganglion neurons. Journal of General Physiology. 1994;104:747–771. doi: 10.1085/jgp.104.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen CJ, Fozzard HA, Sheu SS. Increase in intracellular sodium ion activity during stimulation in mammalian cardiac muscle. Circulation Research. 1982;50:651–662. doi: 10.1161/01.res.50.5.651. [DOI] [PubMed] [Google Scholar]
- Dale N. A large, sustained Na+- and voltage-dependent K+ current in spinal neurons of the frog embryo. The Journal of Physiology. 1993;462:349–372. doi: 10.1113/jphysiol.1993.sp019559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Dryer SE. Properties of single Na+-activated K+ channels in cultured central neurons of the chick embryo. Neuroscience Letters. 1993;149:133–136. doi: 10.1016/0304-3940(93)90754-9. [DOI] [PubMed] [Google Scholar]
- Dryer SE, Fujii JT, Martin AR. A Na+-activated K+ current in cultured brain stem neurones from chicks. The Journal of Physiology. 1989;410:283–296. doi: 10.1113/jphysiol.1989.sp017533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham PB, Hoffman JF. Na and K transport in red blood cells. In: Andreoli TE, Hoffman JF, Fanestil DD, editors. Physiology of Membrane Disorders. New York: Plenum Press; 1978. pp. 255–272. [Google Scholar]
- Egan TM, Dagan D, Kupper J, Levitan IB. Na+-activated K+ channels are widely distributed in rat CNS and in Xenopus oocytes. Brain Research. 1992a;584:319–321. doi: 10.1016/0006-8993(92)90913-t. [DOI] [PubMed] [Google Scholar]
- Egan TM, Dagan D, Kupper J, Levitan IB. Properties and rundown of sodium-activated potassium channels in rat olfactory bulb neurons. Journal of Neuroscience. 1992b;12:1964–1976. doi: 10.1523/JNEUROSCI.12-05-01964.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French RJ, Shoukimas JJ. Blockage of squid axon potassium conductance by internal tetra-N-alkylammonium ions of various sizes. Biophysical Journal. 1981;34:271–291. doi: 10.1016/S0006-3495(81)84849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman DE. Potential, impedance and rectification in membranes. Journal of General Physiology. 1943;27:37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grolleau F, Lapied B. Transient Na+-activated K+ current in beating pacemaker-isolated adult insect neurosecretory cells (dum neurones) Neuroscience Letters. 1994;167:46–50. doi: 10.1016/0304-3940(94)91024-3. [DOI] [PubMed] [Google Scholar]
- Haimann C, Bernheim L, Bertrand D, Bader CR. Potassium current activated by intracellular sodium in quail trigeminal ganglion neurons. Journal of General Physiology. 1990;95:961–979. doi: 10.1085/jgp.95.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimann C, Magistretti J, Pozzi B. Sodium-activated potassium current in sensory neurons: a comparison of cell-attached and cell-free single-channel activities. Pflügers Archiv. 1992;422:287–294. doi: 10.1007/BF00376215. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartung K. Potentiation of a transient outward current by Na+ influx in crayfish neurones. Pflügers Archiv. 1985;404:41–44. doi: 10.1007/BF00581488. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Katz B. The effect of sodium ions on the electrical activity of the giant axon of the squid. The Journal of Physiology. 1949;108:37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenester E, Keller JW, Jansonius JN. An alkali metal ion size-dependent switch in the active site structure of dialkylglycine decarboxylase. Biochemistry. 1994;33:13561–13570. doi: 10.1021/bi00250a008. [DOI] [PubMed] [Google Scholar]
- Kameyama M, Kakei M, Sato R, Shibasaki T, Matsuda H, Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 1984;309:354–356. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- Kiss L, LoTurco J, Korn SJ. Contribution of the selectivity filter to inactivation in potassium channels. Biophysical Journal. 1999;76:253–263. doi: 10.1016/S0006-3495(99)77194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh D-S, Jonas P, Vogel W. Na+-activated K+ channels localized in the nodal region of myelinated axons of Xenopus. The Journal of Physiology. 1994;479:183–197. doi: 10.1113/jphysiol.1994.sp020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn SJ, Ikeda SR. Permeation selectivity by competition in a delayed rectifier potassium channel. Science. 1995;269:410–412. doi: 10.1126/science.7618108. [DOI] [PubMed] [Google Scholar]
- Lu T, Nguyen B, Zhang X, Yang J. Architecture of a K+ channel inner pore revealed by stoichiometric covalent modification. Neuron. 1999;22:571–580. doi: 10.1016/s0896-6273(00)80711-4. [DOI] [PubMed] [Google Scholar]
- Luk H-N, Carmeliet E. Na+-activated K+ current in cardiac cells: rectification, open probability, block and role in digitalis toxicity. Pflügers Archiv. 1990;416:766–768. doi: 10.1007/BF00370627. [DOI] [PubMed] [Google Scholar]
- Meech RW. Ca++-activated K+ conductance. In: Kester J, Byrne JH, editors. Molluscan Nerve Cells: from Biophysics to Behavior. New York: Cold Spring Harbor Laboratory; 1980. pp. 93–103. [Google Scholar]
- Meech RW, Thomas RC. Effect of measured calcium chloride injections on the membrane potential and internal pH of snail neurones. The Journal of Physiology. 1980;298:111–129. doi: 10.1113/jphysiol.1980.sp013070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry DK, Tripathi O, Chapman RA. The occurrence of stable subconductance levels in Na+-activated K+ channels in excised membrane patches from guinea-pig ventricular myocytes. Experimental Physiology. 1996;81:899–907. doi: 10.1113/expphysiol.1996.sp003991. [DOI] [PubMed] [Google Scholar]
- Mistry DK, Tripathi O, Chapman RA. Kinetic properties of unitary Na+-dependent K+ channels in inside-out patches from isolated guinea-pig ventricular myocytes. The Journal of Physiology. 1997;500:39–50. doi: 10.1113/jphysiol.1997.sp021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. American Journal of Physiology. 1985;249:H1056–1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Fujiyama R, Okada Y, Sato T. Properties of Na+-dependent K+ conductance in the apical membrane of frog taste cells. Brain Research. 1996;715:79–85. doi: 10.1016/0006-8993(95)01551-5. [DOI] [PubMed] [Google Scholar]
- Nakaya H, Kimura S, Kanno M. Intracellular K+ and Na+ activities under hypoxia, acidosis, and no glucose in dog hearts. American Journal of Physiology. 1985;249:H1078–1085. doi: 10.1152/ajpheart.1985.249.6.H1078. [DOI] [PubMed] [Google Scholar]
- Niu XW, Meech RW. The effect of polyamines on KATP channels in guinea-pig ventricular myocytes. The Journal of Physiology. 1998;508:401–411. doi: 10.1111/j.1469-7793.1998.401bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogielska EM, Aldrich RW. A mutation in S6 of Shaker potassium channels decreases the K+ affinity of an ion binding site revealing ion-ion interactions in the pore. Journal of General Physiology. 1998;112:243–257. doi: 10.1085/jgp.112.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling L. The sizes of ions and the structure of ionic crystals. Journal of the American Chemical Society. 1927;49:769–790. [Google Scholar]
- Pauling L. The Nature of the Chemical Bond. 3. Ithaca, NY, USA: Cornell University Press; 1960. [Google Scholar]
- Perozo E, Cortes DM, Cuello LG. Structural rearrangements underlying K+-channel activation gating. Science. 1999;285:73–78. doi: 10.1126/science.285.5424.73. [DOI] [PubMed] [Google Scholar]
- Rodrigo GC. The Na+-dependence of Na+-activated K+-channels (IK(Na)) in guinea pig ventricular myocytes, is different in excised inside/out patches and cell attached patches. Pflügers Archiv. 1993;422:530–532. doi: 10.1007/BF00375082. [DOI] [PubMed] [Google Scholar]
- Rodrigo GC, Chapman RA. A sodium-activated potassium current in intact ventricular myocytes isolated from the guinea-pig heart. Experimental Physiology. 1990;75:839–842. doi: 10.1113/expphysiol.1990.sp003465. [DOI] [PubMed] [Google Scholar]
- Roux B, MacKinnon R. The cavity and pore helices in the KcsA K+ channel: electrostatic stabilization of monovalent cations. Science. 1999;285:100–102. doi: 10.1126/science.285.5424.100. [DOI] [PubMed] [Google Scholar]
- Safronov BV, Vogel W. Properties and functions of Na+-activated K+ channels in the soma of rat motoneurones. The Journal of Physiology. 1996;497:727–734. doi: 10.1113/jphysiol.1996.sp021803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC. -activated and ATP-sensitive K+ channels in the heart. Progress in Clinical and Biological Research. 1990;334:85–109. [PubMed] [Google Scholar]
- Schwindt PC, Spain WJ, Crill WE. Long-lasting reduction of excitability by a sodium-dependent potassium current in cat neocortical neurons. Journal of Neurophysiology. 1989;61:233–244. doi: 10.1152/jn.1989.61.2.233. [DOI] [PubMed] [Google Scholar]
- Starkus JG, Kuschel L, Rayner MD, Heinemann SH. Ion conduction through C-type inactivated Shaker channels. Journal of General Physiology. 1997;110:539–550. doi: 10.1085/jgp.110.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toney MD, Hohenester E, Cowan SW, Jansonius JN. Dialkylglycine decarboxylase structure: bifunctional active site and alkali metal sites. Science. 1993;261:756–759. doi: 10.1126/science.8342040. [DOI] [PubMed] [Google Scholar]
- Veldkamp MW, Vereecke J, Carmeliet E. Effects of intracellular sodium and hydrogen ion on the sodium activated potassium channel in isolated patches from guinea pig ventricular myocytes. Cardiovascular Research. 1994;28:1036–1041. doi: 10.1093/cvr/28.7.1036. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kimitsuki T, Noma A. Conductance properties of the Na+-activated K+ channel in guinea-pig ventricular cells. The Journal of Physiology. 1991;433:241–257. doi: 10.1113/jphysiol.1991.sp018424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X-M, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Ikeda SR. Anomalous permeation of Na+ through a putative K+ channel in rat superior cervical ganglion neurones. The Journal of Physiology. 1993;468:441–461. doi: 10.1113/jphysiol.1993.sp019781. [DOI] [PMC free article] [PubMed] [Google Scholar]


