Abstract
This paper asks how the decline in maximal O2 uptake rate (V̇O2,max) with age is related to the properties of a key muscle group involved in physical activity – the quadriceps muscles. Maximal oxygen consumption on a cycle ergometer was examined in nine adult (mean age 38.8 years) and 39 elderly subjects (mean age 68.8 years) and compared with the oxidative capacity and volume of the quadriceps.
V̇O2,max declined with age between 25 and 80 years and the increment in oxygen consumption from unloaded cycling to V̇O2,max (ΔV̇O2) in the elderly was 45 % of the adult value.
The cross-sectional areas of the primary muscles involved in cycling – the hamstrings, gluteus maximus and quadriceps – were all lower in the elderly group. The quadriceps volume was reduced in the elderly to 67 % of the adult value. Oxidative capacity per quadriceps volume was reduced to 53 % of the adult value. The product of oxidative capacity and muscle volume – the quadriceps oxidative capacity – was 36 % of the adult value in the elderly.
Quadriceps oxidative capacity was linearly correlated with ΔV̇O2 among the subjects with the slope indicating that the quadriceps represented 36 % of the V̇O2 increase during cycling.
The decline in quadriceps oxidative capacity with age resulted from reductions in both muscle volume and oxidative capacity per volume in the elderly and appears to be an important determinant of the age-related reduction in ΔV̇O2 and V̇O2,max found in this study.
Aerobic performance declines with age as shown by the decline in oxygen uptake and power output by the legs at maximal oxygen uptake rate (V̇O2,max) (Coggan et al. 1993; McCully et al. 1993; Proctor & Joyner, 1997). Cardiovascular limitations have been suggested to account for the decline in V̇O2,max with age in trained subjects based on a reduction in the elderly of blood flow during submaximal exercise (see Proctor et al. 1998) and in V̇O2,max per muscle volume (Proctor & Joyner, 1997). However, Seals et al. (1984) found an increase in V̇O2,max in previously untrained subjects after endurance training without apparent increase in cardiac output. This result suggests that the peripheral capacity for oxygen consumption in untrained elderly subjects is important in addition to central cardiovascular factors in the age-related drop in aerobic performance.
Evidence for the role of the muscles themselves in the reduction in V̇O2,max is muscle atrophy (Conley et al. 1995; Fleg & Lakatta, 1988; Frontera et al. 1991; Phillips et al. 1992; Proctor & Joyner, 1997) and the reduction in oxidative properties with age (Houmard et al. 1998). Recent in vivo measurements of whole muscle by magnetic resonance (MR) indicate significant age-related declines in oxidative properties per muscle volume (Coggan et al. 1993; McCully et al. 1993; Conley et al. 2000). These in vivo whole muscle determinations point to a significant loss of muscle oxidative capacity that, combined with the observed reduction in muscle mass, may account for the reduction in V̇O2,max with age.
The purpose of this study was to determine how the decline in V̇O2,max is related to the changes in muscle properties with age. A maximal aerobic test on a cycle ergometer was used to determine V̇O2,max. Magnetic resonance was used to determine the in vivo size and oxidative capacity of the quadriceps muscle. These measurements were used to determine the contributions of muscle size and oxidative capacity to the age-related reduction in V̇O2,max.
METHODS
Subjects
The subjects’ characteristics and magnetic resonance methods have been reported in the accompanying paper (Conley et al. 2000). Briefly, the adult group consisted of nine subjects (6 males, 3 females; aged 38.8 ± 7.9 years, mean ±s.d.) and the elderly group consisted of 40 subjects (18 male, 22 female; aged 68.8 ± 5.9 years). All subjects voluntarily gave informed, written consent and the study was undertaken in accordance with the Declaration of Helsinki and approved by the University of Washington Human Subjects Review Committee.
V̇O2,max test
Ergometry procedures
Subjects completed maximal cycle ergometry (Medgraphics CPE 2000, St Paul, MN, USA) to obtain values for V̇O2,max and the corresponding values for leg power output (Pmax), respiratory exchange ratio (RERmax), and heart rate (HRmax) at V̇O2,max. Twelve elderly subjects were tested on two occasions less than 3 weeks apart and the remaining 28 elderly and the nine adult subjects were tested once. The protocol consisted of a brief familiarization on the cycle and a 1 min pre-exercise period during which resting metabolic data were collected. A 1 min period of unloaded cycling was next and then at minute 2 the ramp increase in workload began at a fixed rate of 10, 12 or 14 W min−1. The rate was chosen based on the subject's reported fitness level and body mass. The tests were completed within 7-15 min. Subjects maintained a constant pedalling rate of 60 cycles min−1. For the elderly subjects, a physician continuously oversaw a 12 lead electrocardiogram (Quinton 650, Seattle, WA, USA) to monitor heart rate and any cardiac abnormalities during the test. Blood pressure was measured manually every 2 min during the exercise. Criteria for test termination included subject fatigue, signs and symptoms of exercise intolerance, electrocardiogram changes and abnormal blood pressure or respiratory response in accordance with American College of Sports Medicine guidelines (ACSM, 1991).
Gas and lactate analysis
Expired air was analysed using zirconia fuel cell O2 and CO2 analysers (Medgraphics). The gas analysers were calibrated with gas mixtures of known O2 and CO2 concentrations. The pneumotachograph was adjusted with a calibrated 3 l syringe. Data were averaged over an eight breath period (typically 10-20 s) and reported every 30 s. Blood samples of 1 ml each were drawn from a venous catheter located on the back of a heated hand. Samples were taken as closely as possible to 80 % of predicted V̇O2,max, at V̇O2,max, at 3 min and at 5 min after stopping exercise. Lactate was determined with an automated analyser (Model 1500, Yellow Springs Instruments, Yellow Springs, OH, USA) and the highest level of the last three samples is reported as the maximal level.
Reproducibility criteria
The V̇O2,max was defined over the last 30 s of the test during which all subjects had a RERmax > 1.1. The reproducibility of the physiological responses was tested on a subset of 12 elderly subjects who repeated the performance test. V̇O2,max, HRmax and Pmax were found not to differ significantly between the two tests with mean values ±s.d. for tests 1 and 2, respectively, as follows: V̇O2,max, 22.9 ± 6.5 and 23.8 ± 6.2 ml (kg min)−1; HRmax, 166 ± 10.9 and 163 ± 12.1 beats min−1; and Pmax, 113 ± 40.7 and 114 ± 39.2 W. There was a significant correlation between tests in V̇O2,max (r2 = 0.92), HRmax (r2 = 0.58), and Pmax (r2 = 0.96). For these 12 subjects, the second test was used in our analysis except in two subjects where a clear V̇O2 plateau was apparent in the first but not in the second test. Blood samples were drawn for analysis of lactate only during the second test.
Maximal V̇O2 criteria
We analysed the ergometer test for all subjects except for the sole case with a mouthpiece leak. Eighty-five percent of the subjects (41/48) met either a V̇O2 plateau or a blood lactate criterion for maximal V̇O2. A plateau or change in V̇O2 of < 100 ml min−1 over the last 60 s (Howley et al. 1995) was found in 5/9 adult and 29/39 elderly subjects. A lactate level > 8 mM was found in two adult and five elderly subjects not showing a V̇O2 plateau. The remaining 15 % of the subjects who did not achieve the standard V̇O2,max criteria nonetheless had an RERmax > 1.15 and accumulated lactate during the test: [lactate]max–[lactate]rest≥ 3.7 mM (no lactate sample in one adult subject). Four of five elderly subjects in this group had a HRmax at the age-predicted value (i.e. 220 – age), and the final subject not meeting the HRmax criterion had a final lactate of 7.6 mM.
Imaging muscle area and volume
A standard T1-weighted magnetic resonance sequence was used to image the thigh and obtain cross-sectional areas (CSA) of the quadriceps, as previously described (Jubrias et al. 1997). T1-weighted images enabled us to distinguish intramuscular fat and omit it from the calculation of contractile tissue area. We determined muscle CSA and volume with standard stereological techniques (Weibel, 1979). Gadeberg et al. (1999) provide a detailed error analysis of the Cavalieri method for analysing muscle area from MR images.
Statistics
The reproducibility study compared experiments using Student's paired two-tailed t test. Group data are reported as means ±s.e.m. and evaluated for differences with Student's unpaired two-tailed t test. Standard linear regression methods were used for correlation analysis. Statistical data are reported when a significance level of 0.05 has been reached.
RESULTS
Our experimental approach was to measure V̇O2,max on the cycle ergometer in the adult vs. elderly groups. In separate experiments on the same subjects we determined the oxidative capacity of the quadriceps using magnetic resonance methods (Conley et al. 2000). These data were used to assess the relationship of the change in muscle properties to the decline in V̇O2,max with age.
V̇O2,max test
Our first question concerns how maximum oxygen uptake rate (V̇O2,max) changed with age (Fig. 1). Table 1 shows that the elderly group was significantly below the adult group in V̇O2,max and in the difference between unloaded and maximum cycling oxygen uptake rate (ΔV̇O2). We use the increment in oxygen uptake rate with exercise (ΔV̇O2) because it eliminates the unloaded cycling V̇O2 to which the quadriceps makes a minimal contribution. The ΔV̇O2 of the elderly group was less than half the value of the adult group. The two groups showed no signifcant difference in V̇O2,rest on the cycle or unloaded cycling, resting lactate level (elderly, 1.10 ± 0.05 mM; adult, 0.97 ± 0.12 mM) or peak lactate level (elderly, 7.4 ± 0.4 mM, n = 39; adult, 8.3 ± 0.8 mM, n = 6).
Figure 1. Maximal oxygen uptake rate (V̇O2,max) as a function of age.
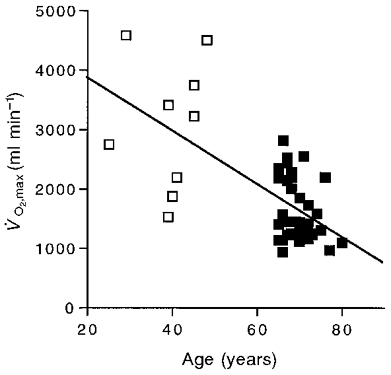
□, adult subjects; ▪, elderly subjects. The regression equation for all subjects is: y = -44.1 x = 4724.6, r2 = 0.42.
Table 1.
Oxygen uptake in the aerobic performance test
| Group | Age(years) | V̇O2,rest(ml min−1) | V̇O2,unloaded(ml min−1) | V̇O2,max(ml min−1) | ΔV̇O2(ml min−1) |
|---|---|---|---|---|---|
| Adult (n = 9) | 39 | 374 ± 51 | 566 ± 65 | 3091 ± 415 | 2525 ± 335 |
| Elderly (n = 39) | 69 | 324 ± 14 | 491 ± 26 | 1624 ± 83* | 1137 ± 69* |
| E/A × 100 | — | 87% | 87% | 52% | 45% |
Values are means ± S.E.M. Variables: V̇O2,rest, resting V̇O2; V̇O2,unloaded, V̇O2 whilst cycling unloaded; V̇O2,max, maximal V̇O2; ΔV̇O2, V̇O2,max–V̇O2,unloaded; E/A × 100, elderly/adult value expressed as a percentage.
Significant difference between the elderly and the adult means.
Muscle properties
Our second question concerns how the size and oxidative capacity of the quadriceps differed between our elderly and adult subjects. Table 2 shows that the quadriceps volume (VQ) was significantly lower in the elderly vs. adult group. Figure 2 shows a significant decline of VQ with age.
Table 2.
Quadriceps volume and energetic capacities
| Group | VQ(ml) | Oxidative capacity/VQ(mM ATP s−1) | Quadriceps oxidative capacity (mmol ATP s−1) |
|---|---|---|---|
| Adult (n = 9) | 3560 ± 220 | 1.16 ± 0.15 | 4.27 ± 0.75 |
| Elderly (n = 39) | 2370 ± 120* | 0.61 ± 0.04* | 1.50 ± 0.15* |
| E/A × 100 | 67% | 53% | 36% |
Values are means ± s.e.m. Variables: VQ, quadriceps volume; E/A × 100 as in Table 1.
Significant difference between the elderly and the adult means.
Figure 2. Quadriceps volume (VQ) as a function of age.
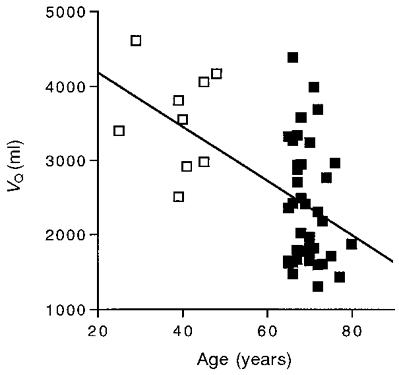
Symbols as in Fig. 1. The regression equation for all subjects is: y = - 37.4 x = 4973 (r2 = 0.28).
The size of the quadriceps relative to the other major muscles involved in cycling was determined from MR images. These images covered enough of the volume of the quadriceps, hamstrings and gluteus to yield the cross-sectional areas (CSA) shown in Fig. 3. The ratio of the quadriceps CSA to the total CSA of the three muscles was significantly smaller in the elderly group (0.44 ± 0.01, n = 14) than in the adult group (0.49 ± 0.017, n = 6; P = 0.025). Thus the quadriceps relative to the other major cycling muscles represents about half the total CSA in the adults, but is a significantly smaller fraction of the total in the elderly.
Figure 3. Quadriceps cross-sectional area as a function of the sum of the cross-sectional areas of the quadriceps, gluteus and hamstring muscles.
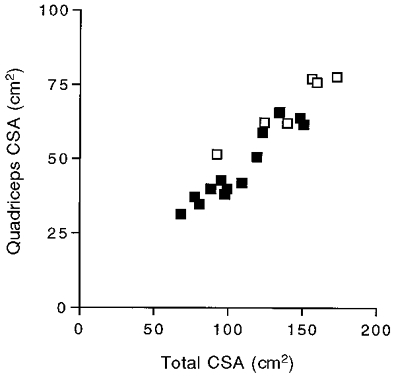
Symbols as in Fig. 1.
Quadriceps oxidative capacity
Table 2 shows a significant drop in quadriceps volume and oxidative capacity/VQ in the elderly compared to the adult group. Also shown is the decline in the product of these two properties which yields the oxidative capacity of the whole quadriceps (hereafter termed quadriceps oxidative capacity). Both muscle properties in the elderly are greater than half the adult value, while the product of the two – the quadriceps oxidative capacity – in the elderly subjects is only 36 % of the adult value. This relative decline in quadriceps oxidative capacity is shown in Fig. 4. Removal of the highest point in Fig. 4 did not eliminate the significant difference between the two groups (P = 0.0002, Student's t test) or the significant correlation between quadriceps oxidative capacity and age (P = 0.0001).
Figure 4. Quadriceps oxidative capacity as a function of age.
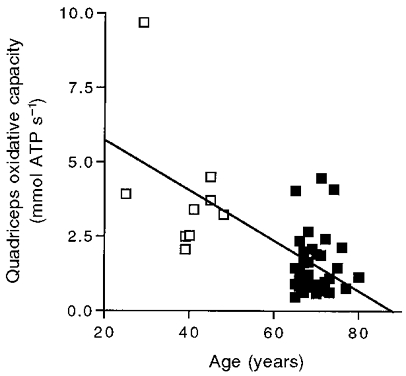
Symbols are as in Fig. 1. The regression equation for all subjects is: y = -0.086 x = 7.498 (r2 = 0.44).
ΔV̇O2vs. muscle properties
Our final question concerns how the loss of muscle oxidative capacity is related to the decline in V̇O2,max with age. Figure 5 shows quadriceps oxidative capacity vs.ΔV̇O2 for each subject. This figure demonstrates a proportionality of the two variables, although there is a significantly lower quadriceps oxidative capacity/ΔV̇O2 in the elderly (0.30 ± 0.02) compared with the adult group (0.38 ± 0.04).
Figure 5. Quadriceps oxidative capacity as a function of the increment in oxygen consumption between unloaded cycling and V̇O2,max (ΔV̇O2).
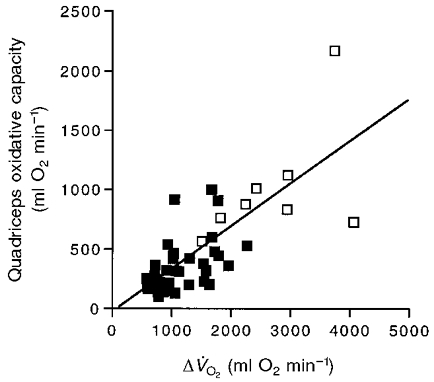
Symbols are as in Fig. 1. The regression equation for all subjects is: y = 0.36 x– 46.97 (r2 = 0.57).
DISCUSSION
The key result of this study is the correspondence of the decline in quadriceps oxidative capacity to the decline in V̇O2,max with age. This correspondence was discerned using MR determinations in combination with cycle ergometric measurements of the aerobic limit. These measurements showed that the reductions in muscle size and oxidative capacity/VQ together – rather than any single property – resulted in a decline in quadriceps oxidative capacity proportional to the loss of V̇O2,max between the adult and elderly groups.
This comparison of quadriceps properties and V̇O2,max depends on several assumptions: (1) that the metabolic response measured by MR is reflected in the pulmonary oxygen uptake underlying our V̇O2,max determination, (2) that electrical stimulation and the V̇O2,max test fully recruit the fibres of the quadriceps, and (3) that the MR determinations reasonably estimate the quadriceps oxidative capacity.
PCr dynamics and pulmonary V̇O2
The dynamics of PCr generates the signals activating mitochondrial oxygen uptake (see Meyer, 1988; Conley et al. 2000). Several studies have shown a close association between these physiological responses of muscle and the whole body pulmonary oxygen uptake. Simultaneous measurements of the PCr dynamics in the quadriceps and pulmonary V̇O2 show the same kinetics (Rossiter et al. 1999). A similar correspondence has been found in separate MR and V̇O2 measurements in the plantar flexors (McCreary et al. 1996) and in the PCr dynamics after plantar flexor exercise vs. the V̇O2 kinetics in cycling (Barstow et al. 1994). These studies confirm that the change in pulmonary V̇O2 with exercise reflects an increase in muscle V̇O2 resulting from changes in PCr level.
Fibre recruitment in the MR and V̇O2,max determinations
All of the fibres of the quadriceps appear to be activated in both the measurements of oxidative capacity by MR and the V̇O2,max test on the ergometer. Full recruitment of the quadriceps during cycling at levels close to V̇O2,max is indicated by glycogen depletion measurements (see Saltin & Gollnick, 1983) and by the increase in mitochondrial volume density (Vv(mt,f)) in all fibre types after endurance training (Howald et al. 1985). Complete recruitment of the quadriceps in the MR determination is attained by electrical stimulation at an intensity that evokes a maximal EMG response (Blei et al. 1993). Full recruitment of the fibres of the quadriceps group in both determinations permit us to compare the MR and V̇O2,max test results.
MR determination of quadriceps oxidative capacity
The PCr recovery time course following exercise is an index of the oxidative properties of muscle (McCully et al. 1993; Paganini et al. 1997). The resting PCr level along with the PCr dynamics provide the information necessary to estimate oxidative capacity in the muscle. We found this estimate to correspond with the oxidative capacity estimated from Vv(mt,f) of the quadriceps in adults (Conley et al. 2000). The quadriceps appears to work at its oxidative capacity during cycling at V̇O2,max since the PCr level drops to near zero and no increase in V̇O2 is possible with increases in work (Richardson et al. 1995). In addition, Hoppeler et al. (1985) found that the increase in oxygen consumption predicted from the rise in Vv(mt,f) in quadriceps muscle accounted for the elevation in V̇O2,max after cycle endurance training in young subjects. Thus direct measurement of quadriceps oxygen uptake and the changes in mitochondrial properties with training indicate that the quadriceps operates at its oxidative capacity during cycling at V̇O2,max in adult muscle. We can now ask how the changes in quadriceps properties relate to the decline in V̇O2,max with age.
Decline in aerobic performance and muscle properties with age
The well-established decline in V̇O2,max with age is shown in Fig. 1, and the resulting drop in the increment in V̇O2 between unloaded cycling and maximal uptake rate (ΔV̇O2) appears in Table 1. Babcock et al. (1994) reported that the V̇O2 time course from rest to submaximal exercise is slowed as a function of age. A slowing of PCr recovery after exercise with age has also been reported (McCully et al. 1993; Conley et al. 2000). These time course changes indicate that a decline in whole body and muscle oxidative properties in part underlies the reduction in V̇O2,max with age. This decline in muscle oxidative properties is accompanied by a well documented (see Jubrias et al. 1997) drop in muscle volume (VQ, Fig. 2). Unique to this study is our finding that the product of VQ (Fig. 2) and oxidative capacity/VQ (Conley et al. 2000) – quadriceps oxidative capacity – also drops as a function of age (Fig. 4). Table 2 shows that the quadriceps oxidative capacity dropped in the elderly relatively more than either muscle property alone and was a slightly smaller percentage of the adult value (36 %) than was ΔV̇O2 (45 %).
These results emphasize the importance of measuring both muscle volume and oxidative properties in studying the link between muscle properties and V̇O2,max. Many muscles show a smaller change in their oxidative properties with age than does V̇O2,max (see Houmard et al. 1998). This study demonstrates that both VQ and oxidative capacity/VQ decline less with age than does V̇O2,max (Tables 1 and 2). However, a key point of this study is that the size of the muscle along with the volume-specific oxidative properties are important determinants of the capacity for oxygen uptake. Thus an individual can have the same quadriceps oxidative capacity with a large VQ and low oxidative capacity/VQ and vice versa. This point is illustrated by the considerable individual variation (low correlation) of either VQ (r2 = 0.28; Fig. 2) or oxidative capacity/VQ (r2 = 0.34; Conley et al. 2000) alone with age, but a higher correlation of the product of the two, quadriceps oxidative capacity (r2 = 0.44; Fig. 4), with age and with V̇O2,max (r2 = 0.57; Fig. 5).
Does the loss of ΔV̇O2 reflect the decline in muscle properties with age?
Figure 5 shows that quadriceps oxidative capacity varies linearly with ΔV̇O2. The slope of this plot indicates that the quadriceps are responsible for on average 0.36 of the increment in oxygen uptake above low load cycling. An independent estimate of the oxygen uptake of the quadriceps at V̇O2,max comes from the work accomplished by this muscle group along with the percentage contribution of the legs to the total V̇O2,max. The quadriceps generate 0.35 of the total work by the legs during cycling at the pedalling rate (60 r.p.m.) and the range of leg power outputs found in this study (120-240 W; Ericson, 1988). With the legs consuming 0.85 of the oxygen used during cycling at V̇O2,max (Harms et al. 1997), 0.35 of the leg work production yields a 0.30 contribution (i.e. 0.35 × 0.85 = 0.3) of quadriceps oxygen uptake to V̇O2,max. These calculations confirm our estimate of the quadriceps quantitative contribution to ΔV̇O2 and the important role that the loss of quadriceps oxidative capacity has in the decline in V̇O2,max with age.
Central vs. peripheral limitations to V̇O2,max
The slightly larger loss of quadriceps oxidative capacity compared with ΔV̇O2 between the two groups argues against a significant central cardiovascular limitation to V̇O2,max. Proctor & Joyner (1997) suggested that the significantly reduced V̇O2,max per appendicular muscle mass in trained elderly compared with trained young was due to lower maximal O2 delivery. Evidence for an O2 delivery limitation to V̇O2,max has been reported for young, trained subjects (Richardson et al. 1999). However, two recent studies demonstrated that blood flow and oxygen delivery were not limiting to V̇O2,max in either trained (Pedersen et al. 1999) or untrained (Cardus et al. 1998) young subjects. Both studies that failed to find an O2 delivery limitation suggested a muscle mitochondrial limitation to V̇O2,max in these subjects. Thus cardiovascular limitations may be restricted to highly trained subjects and may not be present in the general population.
Our data are from recreationally active elderly and show a smaller relative quadriceps oxidative capacity (36 %) than V̇O2,max (52 %) or ΔV̇O2 (45 %) compared to adults. This greater decline in peripheral oxidative capacity than of whole body oxygen consumption may reflect a larger decline in the properties of the quadriceps relative to other muscles involved in cycling. Reimers et al. (1998) found that age-related atrophy does not affect all muscles equally. A greater atrophy of the quadriceps relative to other muscles is evident in the smaller fraction of this muscle group of the total CSA of the cycling muscles in the elderly (0.44) compared with adults (0.49). Confirming the differential ageing effects on muscle groups is the Chilibeck et al. (1996) finding of a significant decline in V̇O2 kinetics during cycling but not during plantar flexion in the elderly. They suggested that this difference reflects a smaller chronic use of the quadriceps than the plantar flexors during walking. We found that the age-related change in quadriceps muscle properties resulted in greater loss of quadriceps oxidative capacity than ΔV̇O2. A greater loss of muscle vs. whole body capacity is opposite to the expectation of an increased cardiovascular limitation to V̇O2,max with age. This result points to the important role of skeletal muscle oxidative capacity in the loss of whole body maximal oxygen uptake with age.
Conclusions
Figure 6 summarizes the key cellular and muscle factors contributing to the decline in V̇O2,max with age. First, we have reported that the oxidative capacity per quadriceps volume declined because of losses of both mitochondrial content and function (oxidative capacity per Vv(mt,f); Conley et al. 2000). Figure 6A shows that similar relative declines in oxidative capacity per Vv(mt,f) and Vv(mt,f) itself are responsible for this loss in oxidative capacity/VQ, as shown by the decrease in the size of the box. A second major change was the volume of quadriceps muscle. Figure 6B shows that the loss of oxidative capacity/VQ and of VQ contributed nearly equally to the reduction of quadriceps oxidative capacity with age. The result of these muscle changes is a relative decline in the quadriceps oxidative capacity close to that found for V̇O2,max between age groups. The significant contribution of the quadriceps oxidative capacity to V̇O2,max indicates that the reductions in muscle properties in the elderly are important determinants of the age-related reduction in ΔV̇O2 and V̇O2,max found in this study.
Figure 6. Change in muscle properties between adult (□) and elderly (▪) groups.
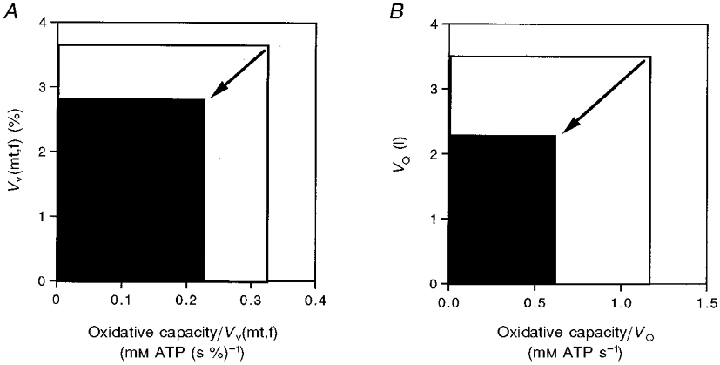
A, decline in volume-specific oxidative capacity as the product of the loss of mitochondrial volume density (Vv(mt,f)) and of mitochondrial capacity (oxidative capacity/Vv(mt,f)). B, decline in whole muscle oxidative capacity as the product of loss of muscle volume (VQ) and volume-specific oxidative capacity (oxidative capacity/VQ).
Acknowledgments
Thanks go to Elizabeth Egan for coordinating these studies. Greg Crowther, Michael Carey, Martin Kushmerick, Ib Odderson and Ron Saxon provided helpful input, advice or support. This work was supported by the University of Washington Clinical Research Center facility grant no. M01-RR00037, and NIH grants AG-10853, AR-41928 and HL-07403.
References
- ACSM. Guidelines for Exercise Testing and Prescription. Philadelphia: Lea & Febiger; 1991. [Google Scholar]
- Babcock MA, Paterson DH, Cunningham DA, Dickinson JR. Exercise on-transient gas exchange kinetics are slowed as a function of age. Medicine and Science in Sports and Exercise. 1994;26:440–446. [PubMed] [Google Scholar]
- Barstow TJ, Buchthal S, Zanconato S, Cooper DM. Muscle energetics and pulmonary oxygen uptake kinetics during moderate exercise. Journal of Applied Physiology. 1994;77:1742–1749. doi: 10.1152/jappl.1994.77.4.1742. [DOI] [PubMed] [Google Scholar]
- Blei ML, Conley KE, Kushmerick MJ. Separate measures of ATP utilization and recovery in human skeletal muscle. The Journal of Physiology. 1993;465:203–222. doi: 10.1113/jphysiol.1993.sp019673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardus J, Marrades RM, Roca J, Barbera JA, Diaz O, Masclans JR, Rodriguez-Roisin R, Wagner PD. Effects of FIO2 on leg VO2 during cycle ergometry in sedentary subjects. Medicine and Science in Sports and Exercise. 1998;30:697–703. doi: 10.1097/00005768-199805000-00009. [DOI] [PubMed] [Google Scholar]
- Chilibeck PD, Paterson DH, Smith WD, Cunningham DA. Cardiorespiratory kinetics during exercise of different muscle groups and mass in old and young. Journal of Applied Physiology. 1996;81:1388–1394. doi: 10.1152/jappl.1996.81.3.1388. [DOI] [PubMed] [Google Scholar]
- Coggan AR, Abduljalil AM, Swanson SC, Earle MS, Farris JW, Mendenhall LA, Robitaille PM. Muscle metabolism during exercise in young and older untrained and endurance-trained men. Journal of Applied Physiology. 1993;75:2125–2133. doi: 10.1152/jappl.1993.75.5.2125. [DOI] [PubMed] [Google Scholar]
- Conley KE, Cress ME, Jubrias SA, Esselman PC, Odderson IR. From muscle properties to human performance using magnetic resonance. Journal of Gerontology. 1995;50:35–40. doi: 10.1093/gerona/50a.special_issue.35. [DOI] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA, Esselman PE. Oxidative capacity and ageing in human muscle. The Journal of Physiology. 2000;526:203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson MO. Mechanical muscular power output and work during ergometer cycling at different work loads and speeds. European Journal of Applied Physiology. 1988;57:382–387. doi: 10.1007/BF00417980. [DOI] [PubMed] [Google Scholar]
- Fleg JL, Lakatta EG. Role of muscle loss in the age-associated reduction in VO2 max. Journal of Applied Physiology. 1988;65:1147–1151. doi: 10.1152/jappl.1988.65.3.1147. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. Journal of Applied Physiology. 1991;71:644–650. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- Gadeberg P, Andersen H, Jakobsen J. Volume of ankle dorsiflexors and plantar flexors determined with stereological techniques. Journal of Applied Physiology. 1999;86:1670–1675. doi: 10.1152/jappl.1999.86.5.1670. [DOI] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. Journal of Applied Physiology. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Howald H, Conley KE, Lindstedt SL, Claassen H, Vock P, Weibel ER. Endurance training in humans: aerobic capacity and structure of skeletal muscle. Journal of Applied Physiology. 1985;59:320–327. doi: 10.1152/jappl.1985.59.2.320. [DOI] [PubMed] [Google Scholar]
- Houmard JA, Weidner ML, Gavigan KE, Tyndall GL, Hickey MS, Alshami A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. Journal of Applied Physiology. 1998;85:1337–1341. doi: 10.1152/jappl.1998.85.4.1337. [DOI] [PubMed] [Google Scholar]
- Howald H, Hoppeler H, Claassen H, Mathieu O, Straub R. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflügers Archiv. 1985;403:369–376. doi: 10.1007/BF00589248. [DOI] [PubMed] [Google Scholar]
- Howley ET, Bassett DRJ, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Medicine and Science in Sports and Exercise. 1995;27:1292–1301. [PubMed] [Google Scholar]
- Jubrias SA, Odderson IR, Esselman PE, Conley KE. Decline in isokinetic force with age: muscle area and specific force. Pflügers Archiv. 1997;434:246–253. doi: 10.1007/s004240050392. [DOI] [PubMed] [Google Scholar]
- McCreary CR, Chilibeck PD, Marsh GD, Paterson DH, Cunningham DA, Thompson RT. Kinetics of pulmonary oxygen uptake and muscle phosphates during moderate-intensity calf exercise. Journal of Applied Physiology. 1996;81:1331–1338. doi: 10.1152/jappl.1996.81.3.1331. [DOI] [PubMed] [Google Scholar]
- McCully KK, Fielding RA, Evans WJ, Leigh JS, Posner JD. Relationship between in vivo and in vitro measurements of metabolism in young and old human calf muscles. Journal of Applied Physiology. 1993;75:813–819. doi: 10.1152/jappl.1993.75.2.813. [DOI] [PubMed] [Google Scholar]
- Meyer RA. A linear model of muscle respiration explains monoexponential phosphocreatine changes. American Journal of Physiology. 1988;254:C548–553. doi: 10.1152/ajpcell.1988.254.4.C548. [DOI] [PubMed] [Google Scholar]
- Paganini AT, Foley JM, Meyer RA. Linear dependence of muscle phosphocreatine kinetics on mitochondrial content. American Journal of Physiology. 1997;272:C501–510. doi: 10.1152/ajpcell.1997.272.2.C501. [DOI] [PubMed] [Google Scholar]
- Pedersen PK, Kiens B, Saltin B. Hyperoxia does not increase peak muscle oxygen uptake in small muscle group exercise. Acta Physiologica Scandinavica. 1999;166:309–318. doi: 10.1046/j.1365-201x.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Phillips SK, Bruce SA, Newton D, Woledge RC. The weakness of old age is not due to failure of muscle activation. Journal of Gerontology. 1992;47:M45–49. doi: 10.1093/geronj/47.2.m45. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Joyner MJ. Skeletal muscle mass and the reduction of V̇O2max in trained older subjects. Journal of Applied Physiology. 1997;82:1411–1415. doi: 10.1152/jappl.1997.82.5.1411. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance- trained men. Journal of Applied Physiology. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- Reimers CD, Harder T, Saxe H. Age-related muscle atrophy does not affect all muscles and can partly be compensated by physical activity: an ultrasound study. Journal of the Neurological Sciences. 1998;159:60–66. doi: 10.1016/s0022-510x(98)00134-8. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J, Wagner PD. Evidence of O2 supply-dependent VO2 max in the exercise-trained human quadriceps. Journal of Applied Physiology. 1999;86:1048–1053. doi: 10.1152/jappl.1999.86.3.1048. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. Journal of Clinical Investigation. 1995;96:1916–1926. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Doyle VL, Howe FA, Griffiths JR, Whipp BJ. Inferences from pulmonary O2 uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans. The Journal of Physiology. 1999;518:921–932. doi: 10.1111/j.1469-7793.1999.0921p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Gollnick PD. Skeletal muscle adaptability: significance for metabolism and performance. In: Peachey LD, Adrian RH, Geiger SR, editors. Handbook of Physiology, section 10, Skeletal Muscle. Washington, DC, USA: American Physiological Society; 1983. pp. 555–631. [Google Scholar]
- Seals DR, Hagberg JM, Hurley BF, Ehsani AA, Holloszy JO. Endurance training in older men and women. I. Cardiovascular responses to exercise. Journal of Applied Physiology. 1984;57:1024–1029. doi: 10.1152/jappl.1984.57.4.1024. [DOI] [PubMed] [Google Scholar]
- Weibel ER. Stereological Methods. London: Academic Press; 1979. [Google Scholar]


