Abstract
Collagenase dispersal of strips of rabbit urethra yielded, in addition to normal spindle-shaped smooth muscle cells, a small proportion of branched cells which resembled the interstitial cells of Cajal dispersed from canine colon. These were clearly distinguishable from smooth muscle in their appearance under the phase-contrast microscope, their immunohistochemistry and their ultrastructure. They had abundant vimentin filaments but no myosin, a discontinuous basal lamina, sparse rough endoplasmic reticulum, many mitochondria and a well-developed smooth endoplasmic reticulum.
Interstitial cells were non-contractile but exhibited regular spontaneous depolarisations in current clamp. These could be increased in frequency by noradrenaline and blocked by perfusion with calcium-free solution. In voltage clamp they showed abundant calcium-activated chloride current and spontaneous transient inward currents which could be blocked by chloride channel blockers.
The majority of smooth muscle cells were vigorously contractile when stimulated but did not show spontaneous electrical activity in current clamp. In voltage clamp, smooth muscle cells showed very little calcium-activated chloride current.
We conclude that there are specialised pacemaking cells in the rabbit urethra that may be responsible for initiating the slow waves recorded from smooth muscle cells in the intact syncitium.
The smooth muscle of the urethra is capable of playing an important role in continence by generating sufficient tone to prevent voiding of urine from the bladder (Bridgewater et al. 1993). This tone appears to be, at least in part, myogenic in nature and is associated with the generation of spontaneous transient depolarisations and large regularly occurring slow waves (Hashitani et al. 1996; Hashitani & Edwards, 1999). These events appear to be due to spontaneous release of calcium from intracellular stores and this in turn activates a calcium-activated chloride current which provides the depolarising current responsible for slow-wave activation. The above studies were carried out by impaling the urethras of rabbits or guinea-pigs with sharp electrodes so it was not possible to determine the exact source of the electrical activity that was being measured. Hashitani et al. (1996) drew attention to the similarities of the slow-wave activity in urethra to that of the gastrointestinal tract where spontaneous activity originates in specialised pacemaker cells or interstitial cells of Cajal (ICC). They noted that no such cells had been found in urethra. However, in the same year, Smet et al. (1996) demonstrated that the human bladder and urethra had interstitial cells which bore a striking resemblance to the ICC in the digestive tract (Thuneberg et al. 1982; Sanders, 1996). The observations by Smet et al. (1996) leave open the intriguing possibility that specialised pacemaker cells may be important in controlling the activity of smooth muscle types which do not normally exhibit regular phasic contractions.
The purpose of the present study was to search for such specialised pacemaking cells in rabbit urethra.
METHODS
The bladder and urethra were removed from both male and female rabbits immediately after they had been killed by lethal injection of pentobarbitone. The most proximal 1 cm of the urethra was removed and placed in Krebs solution and from this strips were dissected for cell dispersal or for histochemical studies.
Cell dispersal
Strips of proximal urethra 0.5 cm in width were cut into 1 mm3 pieces and stored in Hanks’ Ca2+-free solution for 30 min before being incubated in an enzyme medium containing (per 5 ml of Hanks’ Ca2+-free solution): 15 mg collagenase (Sigma type 1a), 1 mg protease (Sigma type XXIV), 10 mg bovine serum albumin (Sigma) and 10 mg trypsin inhibitor (Sigma), for approximately 5 min at 37°C. They were then placed in Hanks’ Ca2+-free solution and stirred for a further 5–10 min to release single relaxed cells. These were either used immediately in electrophysiological experiments or plated on glass coverslips for histochemical experiments.
Immunohistochemistry
Cells were fixed in 4 % paraformaldehyde in PBS for 2 min, washed in PBS (4 × 15 min) at 4°C and then incubated in bovine serum albumin (1 % in PBS) for 1 h at room temperature. They were then incubated overnight at 4°C in primary antibody (anti-vimentin or anti-smooth muscle myosin, Sigma). The antibodies were diluted 1:100 (anti-vimentin) or 1:200 (anti-myosin) in PBS containing 0.3 % Triton X-100. Immunoreactivity was detected by incubating the tissues at room temperature for 1 h in fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (anti-goat and anti-mouse, Sigma) diluted 1:100 in PBS. Controls were prepared in a similar manner but the primary antibody was omitted from the first incubation solution. In some experiments three different c-kit antibodies were used: ACK-2 (Gibco BRL), Ab-3 (Neomarkers) and M-14 (Santa Cruz Biotechnology Inc.), but none of these yielded positive results. Specimens were examined using a BioRad MRC 600 confocal microscope. Three-dimensional shadow projections were constructed from the resulting stacks using a Silicon Graphics workstation running Imaris software (Bitplane AG).
Electronmicroscopy
Cells from the dispersal were centrifuged for 5 min at 1000 r.p.m. in 1.5 ml Eppendorf tubes and the pellets fixed for 40 min at 4°C in 3 % glutaraldehyde in 0.1 M cacodylate buffer. After overnight washing in several changes of buffer the pellet was dehydrated in graded alcohols, transferred to propylene oxide and infiltrated with epoxy resin. After embedding for 36 h in the oven, 70 nm sections were cut with a diamond knife and stained with uranyl acetate and lead citrate.
Physiological studies
Recordings were made using the amphotericin B perforated patch method (Rae et al. 1991) except that we did not include methanesulphonate in the pipette solution. This means that the cell would be hypertonic to both pipette and bath. Nevertheless we did not observe any swelling in the relatively short time taken to do the experiments reported below. After gigaseals were obtained the series resistance fell over a 10–15 min period to 10–15 MΩ and remained stable for up to 1 h. Data were corrected for junction potentials of -3 mV in PSS and +2 mV in both low chloride and iodide solutions according to the method described by Neher (1992). Voltage-clamp commands were delivered with an Axopatch-1D patch-clamp amplifier (Axon Instruments) and currents were recorded by means of a 12-bit AD/DA converter (Labmaster, Scientific Solutions) interfaced to an Intel computer running pCLAMP software (Axon Instruments). During experiments the dish containing the cells was superfused with bath solution. In addition, the cell under study was continuously superfused by means of a close delivery system consisting of a pipette (tip diameter, 200 μm) placed approximately 300 μm away. This could be switched, with a dead space time of around 10 s, to a solution containing a drug. All experiments were carried out at 37°C.
The solutions used were of the following composition (mM): (1) Hanks’ Ca2+-free solution: 141 Na+, 5.8 K+, 130.3 Cl−, 15.5 HCO3−, 0.34 HPO42−, 0.44 H2PO4−, 10 dextrose, 2.9 sucrose and 10 Hepes, pH adjusted to 7.4 with NaOH; (2) PSS: 130 Na+, 5.8 K+, 135 Cl−, 4.16 HCO3−, 0.34 HPO42−, 0.44 H2PO4−, 1.8 Ca2+, 0.9 Mg2+, 0.4 SO42−, 10 dextrose, 2.9 sucrose and 10 Hepes, pH adjusted to 7.4 with NaOH; (3) low chloride solution: 130 Na+, 86 glutamate, 5.8 K+, 49 Cl−, 4.16 HCO3−, 0.34 HPO42−, 0.44 H2PO4−, 1.8 Ca2+, 0.9 Mg2+, 0.4 SO42−, 10 dextrose, 2.9 sucrose and 10 Hepes, pH adjusted to 7.4 with NaOH; (4) Cs+ pipette solution: 133 Cs+, 1 Mg2+, 135 Cl−, 0.5 EGTA and 10 Hepes, pH adjusted to 7.2 with CsOH; and (5) K+ pipette solution: 133 K+, 1 Mg2+, 135 Cl−, 0.5 EGTA and 10 Hepes, pH adjusted to 7.2 with KOH.
The following drugs were used: anthracene-9-carboxylic acid (A-9-C, Sigma), niflumic acid (Sigma), nifedipine (Bayer) and noradrenaline bitartrate (Winthrop Laboratories). Data are presented as the mean ±s.e.m. unless otherwise stated, and statistical differences were compared using Student's paired t test, taking the P < 0.05 level as significant.
RESULTS
Our first clue that there were cells which were morphologically distinct from normal smooth muscle cells was that dispersal of segments of proximal urethra yielded cells such as those shown in Fig. 1a, b and d. These were variable in their morphology, ranging from the long thin type bearing many lateral spiny projections (Fig. 1a) through a bipolar type with varying numbers of branches at each end (Fig. 1d) to the type that had an enlarged central region with many radiating branches one of which was usually bigger than the others (two such cells are shown in Fig. 1b). The size of these cells (maximum dimension) ranged from 35 to 140 μm with a mean (±s.d.) of 84 ± 28 μm (n= 22). This contrasted with the generally more regular dimensions of relaxed smooth muscle cells which ranged in length from 112 to 154 μm with a mean (±s.d.) of 135 ± 13 μm (n= 12). Despite the morphological heterogeneity of the branched cells they had very similar electrophysiological properties and were clearly distinguishable from smooth muscle cells (Fig. 1c) in their appearance under the phase-contrast microscope and in their electrophysiology. Many of these cells (particularly those of the type shown in Fig. 1a) bore a striking resemblance to the cells isolated by Langton et al. (1989) from canine proximal colon. Under phase contrast they appeared darker and thinner than smooth muscle cells and had four or more lateral spiny projections in contrast to the typical spindle shape of the smooth muscle cells (Fig. 1c). These observations prompted us to take a closer look at the morphology of the various cell types. ICC can be distinguished from smooth muscle cells in that the former have abundant vimentin intermediate filaments but no myosin while the latter have abundant myosin but very little vimentin (Rumessen & Thuneberg, 1996). When the cells were stained with anti-vimentin antibody and viewed by confocal microscopy, smooth muscle cells were almost invisible while cells such as those shown in the shadow projections in Fig. 1e and f were clearly imaged. Again a variety of cell shapes was encountered of which two examples are shown. The cell shown in Fig. 1e was bipolar in appearance with a centrally placed nucleus and between two and three branches at each end. The cell in Fig. 1f, on the other hand, had a multipolar appearance with many branches radiating from an expanded central region containing the nucleus. Few vimentin fibres were found in the central nuclear region and thus the shadow projection appears hollow in this region. Cells such as those in Fig. 1e and f were invisible when the anti-myosin antibody was used while smooth muscle cells could be clearly imaged (Fig. 1g). These smooth muscle cells had the characteristic spindle shape similar to their appearance under phase contrast (Fig. 1c). No evidence was found of immuno-reactivity to c-kit antibodies even though three different antibodies were used. When dispersed cells were examined under the electron microscope the various cell types could be clearly distinguished. Most of the dispersal consisted of smooth muscle cells (Fig. 1h and i) which typically had their cytoplasm packed with actin and myosin filaments with very little evidence of intermediate filaments; they had dense bodies, numerous caveolae and a continuous basal lamina. In some dispersals small numbers of fibroblasts were found (Fig. 1j) and these could easily be distinguished from interstitial cells by the abundance and characteristic arrangement of rough endoplasmic reticulum, their lack of caveolae and scarcity of smooth endoplasmic reticulum and intermediate filaments. Interstitial cells (Fig. 1k and l) had an incomplete basal lamina, abundant caveolae, a cytoplasm containing many mitochondria and abundant intermediate (10 nm) filaments, a well-developed smooth endoplasmic reticulum and a rather sparse rough endoplasmic reticulum.
Figure 1. Morphology of cells dispersed from rabbit urethra.
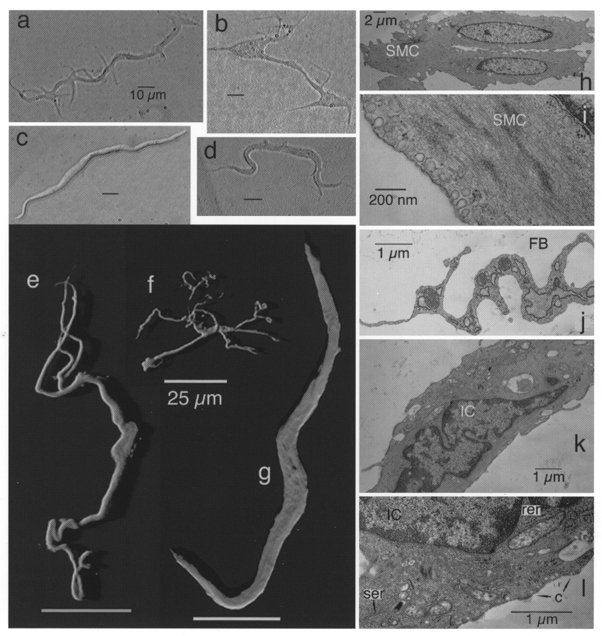
Left panel, interstitial cells (a, b and d) and a typical smooth muscle cell (c) under phase contrast. Shadow projections of confocal stacks of cells which had been incubated with anti-vimentin antibody (e and f) or with anti-myosin antibody (g) are also shown. Right panel, electron micrographs of smooth muscle cells (SMC; h and i), part of a fibroblast (FB; j) and interstitial cells (IC; k and l). (Note caveolae, c, and smooth, ser, and rough endoplasmic reticulum, rer, in l.)
In addition to their striking differences in morphology and immunohistochemical properties there were equally clear differences between the two cell types in contractile and electrical properties. Thus the interstitial cells were non-contractile although they exhibited regular spontaneous depolarisations in current-clamp mode. In contrast, smooth muscle cells were vigorously contractile when stimulated with depolarising current but did not usually exhibit spontaneous electrical activity. In the total of 265 cells studied, 120 out of 143 interstitial cells showed spontaneous electrical activity (84 %) while only 4 out of 122 of the smooth muscle cells did (3.3 %). On the other hand none of the interstitial cells was found to contract either spontaneously or in response to depolarising current while more than 90 % of the smooth muscle cells contracted in response to depolarising current pulses. Figure 2A shows a series of spontaneous depolarisations in an interstitial cell in current-clamp mode. Under these conditions smooth muscle cells rarely exhibited spontaneous electrical activity but, as the record shown in Fig. 2B demonstrates, it was possible to elicit an action potential by applying a depolarising pulse to the quiescent cell. A clue as to why spontaneous activity occurs in interstitial cells but does so very rarely in smooth muscle cells is given in the records shown in Fig. 2C and D. These voltage-clamp experiments were conducted using caesium pipette solution to block potassium currents and focus on the inward currents evoked by depolarisation. When interstitial cells were held at a potential of -60 mV and stepped from -80 to +50 mV in 10 mV steps of 500 ms duration, currents such as those shown in Fig. 2C were elicited. Steps to -10 mV evoked fast inward current which peaked within 50 ms and this was followed by a more slowly developing inward current. The fast current peaked at 0 mV and reversed about +40 mV while the slow current reversed at 0 mV to give a series of large outward currents. On stepping back to -80 mV large slowly decaying tail currents were observed. When a similar protocol was applied to the smooth muscle cell the results were quite different (Fig. 2D). The fast inward current was clearly evident but the slowly developing inward current was less apparent. Equally the slowly decaying tails which were so prominent in the experiment shown in Fig. 2C were less apparent. When the results of 26 (interstitial cells) and 21 (smooth muscle cells) such experiments were summarised the graphs shown in Fig. 2E and F were obtained. The peak fast inward current was measured within the first 50 ms of the test potential and plotted as the open circles. Mean current activated at potentials positive to -50 mV, peaked at 0 mV and reversed at +40 mV. The pattern of activation and reversal of the fast inward current was typical of L-type calcium current (ICa). This was confirmed by applying 10 μm nifedipine, which blocked the fast current in both cell types (Fig. 2G and H). In contrast the slowly developing current (measured just before the end of the test potential) was evident in the interstitial cells but much less so in the smooth muscle cells. This current activated at potentials positive to -50 mV and reversed at 0 mV (the calculated chloride equilibrium potential, ECl, under these experimental conditions). This current was also sensitive to 1 μm nifedipine, being almost abolished at all voltages studied.
Figure 2. Electrical properties of interstitial and smooth muscle cells.
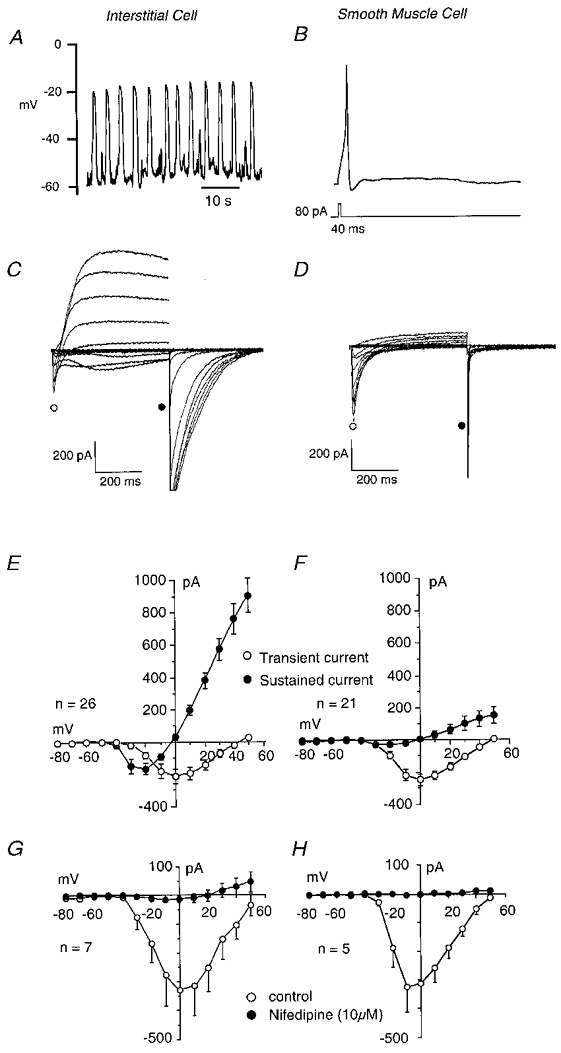
Interstitial cells showed regular ‘slow-wave’ depolarisations in current clamp (A) while smooth muscle cells were usually quiescent, although they could produce an action potential in response to depolarising current (B). Under voltage clamp, interstitial cells exhibited both L-type calcium currents and calcium-activated chloride currents (C) while the smooth muscle cells showed predominantly L-type calcium currents (D). E and F show summaries of the currents measured in 26 interstitial and 21 smooth muscle cells. Nifedipine (10 μm) blocked ICa both in interstitial (G) and in smooth muscle cells (H).
Although slowly developing tails were not prominent in smooth muscle cells, some current could be measured. To assess the relative amounts of chloride current in each cell type, tail current was measured 100 ms after the end of a depolarising pulse to 0 mV. The mean tail current in 111 smooth muscle cells was -18.7 ± 3.29 pA and in 60 interstitial cells it was -363 ± 57 pA.
Effect of low Cl− solutions and Cl− channel blockers
The results described so far strongly suggest that the slow current found in the interstitial cells is a calcium-activated chloride current (ICl(Ca)), based on its calcium dependence and reversal at the Cl− equilibrium potential. This conclusion was further supported by the evidence shown in Fig. 3A. To determine whether the current was carried by chloride ions, the effect of reducing the bath concentration of Cl− on tail current reversal potential was studied. An example of the protocol used is shown in the top panel of Fig. 3A. This involved loading the cell with Ca2+ by means of a 500 ms step to 0 mV and then applying a 200 ms voltage ramp from -50 to +50 mV. This gave a tail current which reversed at -3 mV (when corrected for junction potential), close to the calculated value for ECl (0 mV). The procedure was then repeated a few seconds after reducing [Cl−]o to 49 mm (calculated ECl=+27 mV). This had the effect not only of shifting the reversal potential of the tail current to +17 mV (+19 mV when corrected for junction potential), but also of inducing a slow inward current during the step to 0 mV. These observations are consistent with both the slow inward current and the tail current passing through Cl− channels. In six such experiments the mean reversal potential was 0 ± 1.4 mV for 135 mm[Cl−]o and 20.3 ± 1.3 mV for 49 mm[Cl−]o (P < 0.05). When the bath chloride was replaced with 135 mm I− the slow inward current became outward and the tail reversed at -26 mV suggesting that the channels were more permeable to iodide than to chloride. In five such experiments the mean reversal potential after iodide replacement was -27.8 ± 2.8 mV. Capacitative current was compensated for in all of the above experiments.
Figure 3. Calcium-activated chloride current in interstitial cells.
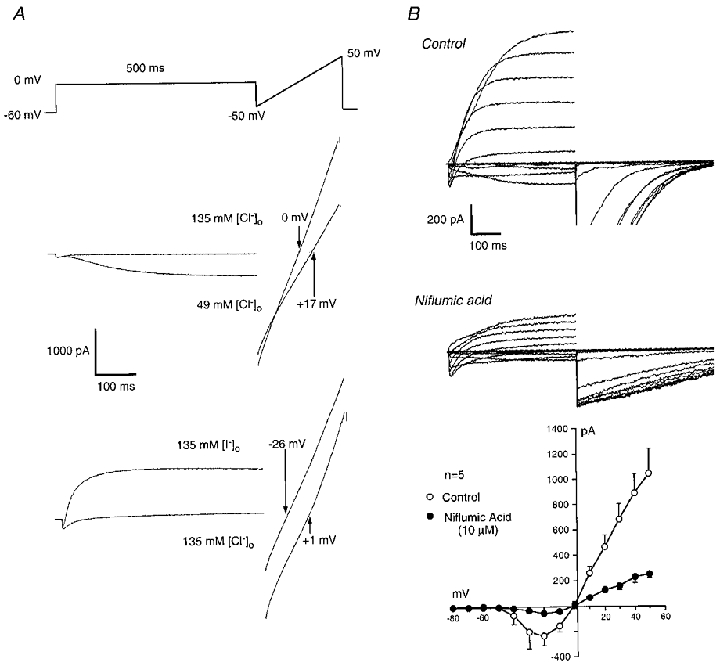
A, the reversal potential of the tail current (corrected for capacitative current) was examined by stepping from a holding potential of -60 to 0 mV to load the cell with calcium. At the end of a 500 ms pulse the potential was ramped for 200 ms from -50 to +50 mV (top panel). The effect of reducing [Cl−]o to 49 mm (middle panel) or of replacing it with 135 mm I− (bottom panel) is shown. The shift in each case is consistent with the current being carried through Cl− channels. B, when interstitial cells were held at a potential of -60 mV and stepped from -80 to +50 mV in 10 mV steps of 500 ms duration, currents such as those shown in the top panel were elicited. Niflumic acid (10 μm) significantly reduced both the inward and outward components of ICl(Ca) while there was little effect on ICa (middle panel). The bottom panel shows summary I-V plots for chloride currents in control (○) and in the presence of 10 μm niflumic acid (•).
The effect of two chloride channel blockers was also examined. Figure 3B shows the effect of 10 μm niflumic acid on currents evoked in interstitial cells by the stepping protocol described in Fig. 2. It is clear that both the inward and outward components of ICl(Ca) were reduced while there was little effect on ICa (peak ICa in control conditions was -289 ± 45 pA and in 10 μm niflumic acid it was -272 ± 71 pA, n= 5, n.s.). The tail currents recorded on stepping back to -80 mV were also reduced in amplitude but prolonged in duration. The latter effect is likely to be due to niflumic acid acting as an open channel blocker (Hogg et al. 1994). A summary of the effect of the drug on ICl(Ca) in five cells is shown in the bottom panel of Fig. 3B where it is clear that ICl(Ca) was greatly depressed at all voltages. The effects of 1 mm A-9-C were similar to those of 10 μm niflumic acid. In five such experiments slow inward current before drug addition was -94 ± 27 pA (at -20 mV, after 500 ms) while outward current was 609 ± 144 pA (at +50 mV, after 500 ms), and these were reduced to -42 ± 15 and 102 ± 19 pA, respectively, in the presence of 1 mm A-9-C (P < 0.05).
Spontaneous activity in interstitial cells
When interstitial cells were voltage clamped at -60 mV, spontaneous transient inward currents (STICs) such as those in Fig. 4A were observed. Niflumic acid both slowed the frequency of firing of the STICs and reduced their amplitude. In five such experiments mean STIC amplitude was -359 ± 67 pA (frequency, 8.8 ± 0.8 min−1) before and -86 ± 32 pA (frequency, 5.0 ± 2.3 min−1) in the presence of 10 μm niflumic acid (P < 0.05). STICs reversed at ECl, and were also inhibited by 1 mm A-9-C (mean amplitude was -670 ± 173 pA before and -122 ± 32 pA in A-9-C, n= 9). Noradrenaline is the major excitatory transmitter in the rabbit urethra (Andersson, 1993; Creed et al. 1997) so it was of interest to examine its effects on the two cell types. The regular spontaneous depolarisations found in current-clamped interstitial cells (Fig. 4B) were increased in frequency in the presence of 10 μm noradrenaline. In five such experiments frequency increased from a mean of 7.0 ± 1.8 to 15.4 ± 2.7 min−1. Spontaneous depolarisations were abolished by perfusion with Ca2+-free Hanks’ solution (Fig. 4C) suggesting that they were due to rhythmic activation of ICl(Ca). Smooth muscle cells, on the other hand, showed no electrical response to noradrenaline.
Figure 4. Spontaneous activity in interstitial cells.
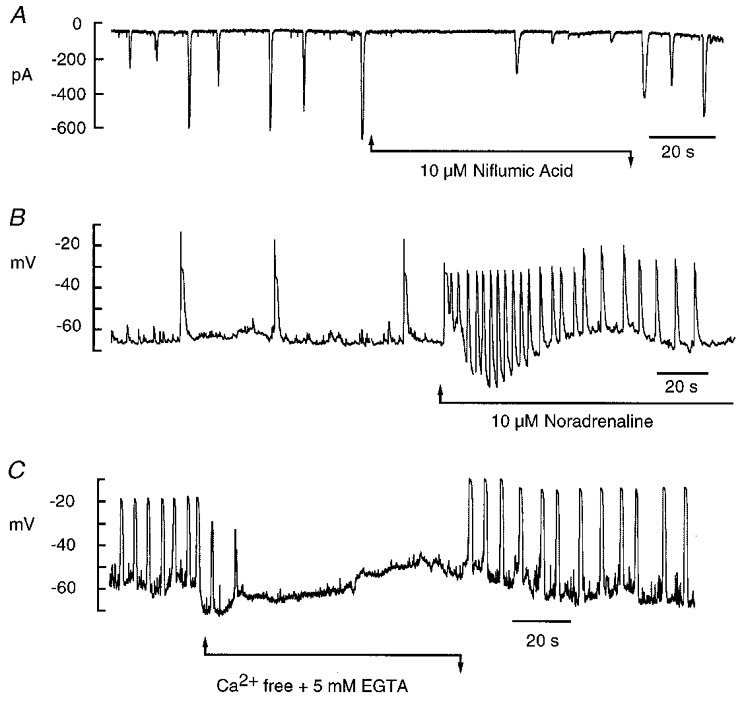
A, in voltage-clamp mode interstitial cells typically showed spontaneous transient inward currents which could be reduced in both amplitude and frequency by 10 μm niflumic acid. B, in current-clamp mode the interstitial cells fired spontaneous depolarisations at a frequency of about 1.5 min−1 and this was increased to 18 min−1 in the presence of 10 μm noradrenaline. C, when the interstitial cells were perfused with Ca2+-free Hanks’ solution containing 5 mm EGTA, spontaneous depolarisations were abolished.
DISCUSSION
Spontaneous electrical activity has been observed in strips of circular smooth muscle from the rabbit urethra (Hashitani et al. 1996) and in longitudinal strips of the guinea-pig urethra (Hashitani & Edwards, 1999). It has always been assumed, however, that this was an inherent property of the smooth muscle cells themselves and that no specialised pacemaker cells were necessary to initiate the activity. Here we demonstrate for the first time that, at least in the rabbit urethra, there are specialised pacemaking cells which are excitable, non-contractile, and contain abundant vimentin but no myosin filaments. They show many of the features found in ICC, which are thought to be pacemaking cells in the gastrointestinal tract (Thuneberg, 1982; Rumessen & Thuneberg, 1996). Thus they have a well-developed smooth endoplasmic reticulum, an incomplete basal lamina and abundant caveolae intracellulares. Their appearance under phase contrast is remarkably similar to ICC isolated from canine colon (Langton et al. 1989) but their electrophysiology appears to be quite different. Thus ICC of the gastrointestinal tract do not appear to possess a calcium-activated chloride current (Malysz et al. 1995; Horowitz et al. 1999) although this was a prominent feature of the interstitial cells of the rabbit urethra. However, the findings of the present study do fit well with other studies of urethral smooth muscle. Hashitani et al. (1996) recorded regular depolarisations in the circular muscle of the rabbit urethra using sharp electrodes. These ‘slow waves’ were blocked in low chloride solutions and by chloride channel blockers such as niflumic acid and DIDS. They were increased in frequency by noradrenaline but inhibited by BAPTA-AM, cyclopiazonic acid and caffeine suggesting that calcium release was necessary for their generation. Since these authors knew of no evidence for the existence of interstitial cells in urethra they assumed that the above activity was generated by the smooth muscle cells themselves. However, the results of the present study suggest that the spontaneous slow waves recorded by Hashitani et al. (1996) in the intact syncitium may indeed have originated in specialised pacemaker cells and then conducted to the bulk smooth muscle. The effects of chloride channel blockers and of the agents that inhibit calcium release from stores in the above study and in that by Hashitani & Edwards (1986) in guinea-pig urethra are consistent with this interpretation. Equally, however, the results of the present study do not exclude the possibility that urethral smooth muscle cells may be important for pacemaking in the rabbit as they appear to be in sheep (Cotton et al. 1997). Even though only 4 out of 122 smooth muscle cells were capable of initiating spontaneous activity this could still represent a significant proportion (perhaps 3–5 %) of the cells in the rabbit urethra.
Spontaneous electrical activity which has its basis in the activation of chloride currents by the rhythmical release of calcium has been observed in other smooth muscle types such as portal vein (Wang et al. 1992) and lymphatic smooth muscle (Van Helden, 1993). However, while a smooth muscle ‘heart’ such as the lymph duct could be expected to have pacemaker cells that control its rhythmic beating, it is harder to explain why a tonic organ such as the urethra should also possess pacemaker cells. What is their role in a tissue whose only purpose is to remain contracted most of the time until deliberately relaxed by inhibitory nerves to allow the voiding of urine? We would like to speculate that tonic contraction in urethral smooth muscle is ‘frequency modulated’ rather than ‘amplitude modulated’, that tone is normally controlled by the constant firing of pacemaking cells the activity of which is conducted asynchronously to the bulk smooth muscle, rather as the asynchronous recruitment of muscle units produces sustained contraction in skeletal muscle. Neuromodulation of tone in such a system would be very simple and economical. The release of small amounts of inhibitory transmitter would slow down or stop pacemaker firing causing the muscle to relax while excitatory transmitter would increase pacemaker frequency and thus tone. Interstitial cells are known to mediate the response to nerves in the gastrointestinal tract (Sanders, 1996; Wang et al. 1999; Vannuchi, 1999) so a similar pattern of innervation may well exist in the urethra. This would represent a rather elegant means of controlling urethral tone. There is currently no direct evidence to support such a speculation but the observations of Smet et al. (1996) are interesting in this context. These authors drew attention to the existence of specialised cells in guinea-pig bladder and urethra which bear some resemblance to the interstitial cells we describe in the present study. Thus they had long processes, contained vimentin intermediate filaments and demonstrated an intense induction of cGMP immunoreactivity in response to sodium nitroprusside. The authors did not ascribe any pacemaking properties to the cells but suggested that they might be important in mediating the effects of neurally released nitric oxide. If the interstitial cells we have described above are preferentially innervated the implications are most interesting and worthy of further study.
Acknowledgments
The authors wish to thank the European Union and the Queen's University Faculty of Medicine for providing financial support. Gerard Sergeant is in receipt of a graduate award from the European Social Fund.
References
- Andersson KE. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacological Reviews. 1993;45:253–307. [PubMed] [Google Scholar]
- Bridgewater M, MacNeill HF, Brading AF. Regulation of urethral tone in pig urethral smooth muscle. Journal of Urology. 1993;48:347–354. doi: 10.1016/s0022-5347(17)35451-4. [DOI] [PubMed] [Google Scholar]
- Cotton KD, Hollywood MA, McHale NG, Thornbury KD. Ca2+ current and Ca2+-activated chloride current in isolated smooth muscle cells of the sheep urethra. The Journal of Physiology. 1997;505:121–131. doi: 10.1111/j.1469-7793.1997.121bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed KE, Oike M, Ito Y. The electrical properties and responses to nerve stimulation of the proximal urethra of the male rabbit. British Journal of Urology. 1997;79:543–553. doi: 10.1046/j.1464-410x.1997.00105.x. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Edwards FR. Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea-pig urethra. The Journal of Physiology. 1999;514:459–470. doi: 10.1111/j.1469-7793.1999.459ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Van Helden DF, Suzuki H. Properties of spontaneous depolarisations in circular smooth muscle cells of rabbit urethra. British Journal of Pharmacology. 1996;118:1627–1632. doi: 10.1111/j.1476-5381.1996.tb15584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RC, Wang Q, Large WA. Action of niflumic acid on evoked and spontaneous calcium-activated chloride and potassium currents in smooth muscle cells from rabbit portal vein. British Journal of Pharmacology. 1994;112:977–984. doi: 10.1111/j.1476-5381.1994.tb13177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz B, Ward SM, Sanders KM. Cellular and molecular basis for electrical rhythmicity in gastrointestinal muscles. Annual Review of Physiology. 1999;61:19–43. doi: 10.1146/annurev.physiol.61.1.19. [DOI] [PubMed] [Google Scholar]
- Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proceedings of the National Academy of Sciences of the USA. 1989;86:7280–7284. doi: 10.1073/pnas.86.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malysz J, Richardson D, Farraway L, Christen MO, Huizinga JD. Generation of slow wave type action potentials in the mouse small intestine involves a non-L-type calcium channel. Canadian The Journal of Physiology Pharmacology. 1995;73:1502–1511. doi: 10.1139/y95-208. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods in Enzymology. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin-B. Journal of Neuroscience Methods. 1991;37:5–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Rumessen JJ, Thuneberg L. Pacemaker cells in the gastrointestinal tract: interstitial cells of Cajal. Scandinavian Journal of Gastroenterology. 1996;31(suppl. 216):82–94. doi: 10.3109/00365529609094564. [DOI] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Smet PJ, Jonavicius J, Marshall VR, DeVente J. Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience. 1996;71:337–348. doi: 10.1016/0306-4522(95)00453-x. [DOI] [PubMed] [Google Scholar]
- Thuneberg L. Interstitial cells of Cajal: interstitial pacemaker cells. Advances in Anatomy, Embryology and Cell Biology. 1982;71:1–130. [PubMed] [Google Scholar]
- Thuneberg L, Rumessen JJ, Mikkelsen HB. The interstitial cells of Cajal: intestinal pacemaker cells? In: Wienbeck M, editor. Motility of the Digestive Tract. New York: Raven Press; 1982. pp. 115–122. [Google Scholar]
- Van Helden DF. Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. The Journal of Physiology. 1993;471:465–479. doi: 10.1113/jphysiol.1993.sp019910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucchi MG. Receptors in interstitial cells of Cajal: identification and possible physiological roles. Microscopical Research Techniques. 1999;47:325–335. doi: 10.1002/(SICI)1097-0029(19991201)47:5<325::AID-JEMT4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Wang Q, Hogg RC, Large WA. Properties of spontaneous inward currents recorded in smooth muscle cells isolated from the rabbit portal vein. The Journal of Physiology. 1992;451:525–537. doi: 10.1113/jphysiol.1992.sp019177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XY, Sanders KM, Ward SM. Intimate relationship between interstitial cells of Cajal and enteric nerves in the guinea-pig small intestine. Cell and Tissue Research. 1999;295:247–256. doi: 10.1007/s004410051231. [DOI] [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Pacemaker activity in septal structures of canine colonic circular muscle. American Journal of Physiology. 1990;259:G264–273. doi: 10.1152/ajpgi.1990.259.2.G264. [DOI] [PubMed] [Google Scholar]


