Abstract
Synovial fluid drains out of joints through an interstitial pathway. Hyaluronan, the major polysaccharide of synovial fluid, attenuates this fluid drainage; it creates a graded opposition to outflow that increases with pressure (outflow ‘buffering’). This has been attributed to size-related molecular reflection at the interstitium-fluid interface. Chain length is reduced in inflammatory arthritis. We therefore investigated the dependence of outflow buffering on hyaluronan chain length.
Hyaluronan molecules of mean molecular mass ≈2200, 530, 300 and 90 kDa and concentration 3.6 mg ml−1 were infused into the knees of anaesthetized rabbits, with Ringer solution as control in the contralateral joint. Trans-synovial drainage rate was recorded at known joint pressures. Pressure was raised in steps every 30–60 min (range 2–24 cmH2O).
With hyaluronan-90 and hyaluronan-300 the fluid drainage rate was reduced relative to Ringer solution (P < 0.001, ANOVA) but increased steeply with pressure. The opposition to outflow, defined as the pressure required to drive unit outflow, did not increase with pressure, i.e. there was no outflow buffering.
With hyaluronan-530 and hyaluronan-2000 the fluid drainage rate became relatively insensitive to pressure, causing a near plateau of flow. Opposition to outflow increased markedly with pressure, by up to 3.3 times over the explored pressures.
Hyaluronan concentration in the joint cavity increased over the drainage period, indicating partial reflection of hyaluronan by synovial interstitium. Reflected fractions were 0.12, 0.33, 0.25 and 0.79 for hyaluronan-90, -300, -530 and -2200, respectively.
Thus the flow-buffering effect of hyaluronan depended on chain length, and shortening the chains reduced the degree of molecular reflection. The latter should reduce the concentration polarization at the tissue interface, and hence the local osmotic pressure opposing fluid drainage. In rheumatoid arthritis the reduced chain length will facilitate the escape of hyaluronan and fluid.
The volume of synovial fluid in most healthy joints is very small, namely ∼50 μl in the rabbit knee and 300 μl in the dog knee. Its turnover time, however, is an order of magnitude faster than that of the general interstitial fluid pool, with a turnover time of ∼2 h in rabbit knee. The maintenance of a small volume of synovial fluid in the face of rapid turnover implies a close coupling between drainage rate and input rate, since even a small, sustained imbalance would quickly lead to joint swelling or fluid depletion. Sustained flexion is a particular threat to volume homeostasis because it raises intra-articular pressure, driving fluid out of the joint cavity (Levick et al. 1999). Hyaluronan in joint fluid partly counters this threat by ‘buffering’ the fluid drainage rate. That is to say, hyaluronan generates a dynamic, graded opposition to fluid loss that increases as joint pressure is increased (McDonald & Levick, 1995). As a result the drainage rate attains a plateau, and the plateau flow is very low, namely 3–5 μl min−1 in the rabbit knee.
The buffering of outflow by hyaluronan is thought to arise as follows. The flexible hyaluronan chain, stiffened by H-bonds and expanded by internal electrostatic repulsion, encompasses a very large, roughly spherical domain of solvent of radius 100–200 nm (Fujii et al. 1996; Gribbon et al. 1999). Adjacent molecular domains overlap at ≥ 1 mg ml−1, which causes chain-chain entanglement and other interactions. These create a quasi-infinite, dynamic network of loosely linked polymer chains (Cowman et al. 1998; Wik & Wik, 1998; Scott & Heatley, 1999). As a result, hyaluronan molecules do not escape through the interstitial drainage pores in the synovial lining (radius 30–90 nm) as easily as water or albumin, and hyaluronan is partially reflected during fluid drainage (Coleman et al. 1997; Scott et al. 1998). It has been suggested that the reflected hyaluronan forms a concentrated layer at the tissue-fluid interface (a ‘concentration polarization’ boundary layer), and that this layer exerts sufficient osmotic pressure to oppose fluid escape. Since the boundary layer concentration increases when drainage rate is increased, the osmotic opposition to fluid drainage from the cavity should increase too, in conformity with the experimental observations. Numerical analysis and work in vitro have supported this mechanistic hypothesis (Barry et al. 1996; Coleman et al. 1999).
The buffering action of hyaluronan has been evaluated at concentrations ranging from those in normal joints (up to 4 mg ml−1) to those in severe inflammatory arthropathies (down to 0.2 mg ml−1). Reductions in concentration cause loss of outflow buffering, in conformity with the osmotic boundary layer hypothesis (Scott et al. 2000a).
Severe arthropathies are associated with a reduction in both concentration and chain length of hyaluronan (Balazs et al. 1967; Dahl et al. 1985). The reduced chain length is attributed to the synthesis of shorter chains (Castor & Dorstewitz, 1966; Vuorio et al. 1982; Dahl & Husby, 1985) and/or cleavage by free oxygen radicals (McCord, 1974; Greenwald & Moak, 1986; Baker et al. 1989; Halliwell, 1995; Haubeck et al. 1995; Schenck et al. 1995). Chain length, as well as concentration, can be expected to determine the drainage-attenuating action of hyaluronan, because shorter chains occupy smaller volume domains and should therefore be less well reflected by the interstitial pores. In support of this, early results from our laboratory using human umbilical hyaluronan of subnormal size, namely of weight-average molecular mass ∼0.7 × 106 Da (McDonald & Levick, 1995), indicated weaker buffering than did later results with rooster comb hyaluronan of ∼2 × 106 Da (Coleman et al. 1999). The aim of the present investigation was, therefore, to examine directly the role of chain length in the buffering of fluid drainage by hyaluronan, using a set of ‘tailor-made’ chain lengths constructed from rooster comb hyaluronan. A preliminary report has been published (Scott et al. 1999).
METHODS
Materials
Rooster comb hyaluronan (Sigma Chemical Co.) was used throughout at 3.6 g l−1, a concentration characteristic of rabbit and young human knee joints (Balazs, 1982; Price et al. 1996). The vehicle was Baxter Ringer solution (147 mm Na+, 4 mm K+, 2 mm Ca2+, 156 mm Cl−, pH 7.2; Baxter Healthcare Ltd, Thetford, Norfolk, UK) adjusted to pH 7.4 with drops of NaOH solution.
Sonication of hyaluronan to specified average chain length
Controlled, progressive cleavage of hyaluronan chains can be induced by ultrasound (Orvisky et al. 1993). Preliminary studies defined the relation between sonication time, ultrasound intensity and reduction in chain length. Samples comprising 10 ml of 3.6 mg ml−1 rooster comb hyaluronan of weight-average molecular mass ∼2.2 × 106 Da were sonicated at amplitudes of 14 or 22 μm for up to 1200 s in a Soniprep (MSE Scientific Instruments, Crawley, UK) and analysed by size-exclusion high performance liquid chromatography (HPLC; see below). The column retention time, Rmin (in minutes), was found to increase as a negative exponential function of sonication time, S (in seconds), indicating progressive, limited cleavage of the chains. Our results were well described by the expression Rmin=k1+k2(1 – e−S/z), where k1= 7.25 and 7.33, k2= 1.55 and 1.53, and z= 408 and 250 for 14 and 22 μm, respectively (correlation r2= 0.99). These expressions were used to predict the times needed to generate hyaluronan molecules of chain lengths from ∼100 to 2200 kDa for infusion in vivo. Sonicated products were checked by HPLC.
Hyaluronan analysis by HPLC
Mean molecular masses and concentrations were determined by HPLC (Waters Ltd, Watford, UK), using a size-exclusion TosoHaas TSK G6000 PWXL column (Anachem Ltd, Luton, UK) and absorbance at 206 nm (Beaty et al. 1985). Hyaluronan standards of average molecular mass 210, 790, 900, 1200, 2000, 3900 and 5500 kDa were generously donated by Dr O. Wik (New Pharmacia, Uppsala, Sweden). Since the standard molecular masses were determined by light scattering, the HPLC-determined values are taken to represent the weight-average molecular mass, Mw. The column retention time was found to be related to Mw by Rmin= 16.22 – 1.416 log M (correlation r2= 0.99).
Viscometric evaluation of molecular domain volume, radius and critical overlap concentration
The relative viscosity ηr of each sonicate was measured in an Ostwald viscometer at 23–26°C at concentrations (C) of 0.9-3.6 mg ml−1, with Ringer solution as the reference fluid. Intrinsic viscosity [η] was determined by linear extrapolation of the plot of reduced viscosity, (ηr– 1)/C, versus C to zero concentration. Intrinsic viscosity represents the space occupied by a gram of solute at infinite dilution, and is a sensitive index of chain length.
The quasi-spherical domain of solvent occupied by a flexible polymer chain is usually characterized by the radius of gyration Rg (root mean square of segment distances from molecular centre of gravity). Rg was determined from the measurements of [η] and molecular mass M using the relation Rg3=M[η]/8.84NA, where NA is Avogadro's number (Flory, 1971).
The ‘critical concentration’C* at which adjacent molecular domains overlap was defined by de Gennes (1979) as the concentration at which the number of chain segments per unit volume of solution is the same as the number of chain segments per unit volume of molecular domain at extreme dilution. This definition, in combination with Flory's model, gives the simple expression C*= 2.1/[η]. The latter was used to evaluate C* for each sonicate from its intrinsic viscosity.
Physiological methods in vivo
Trans-synovial fluid drainage from the cavity of rabbit knees was measured at controlled, incremental intra-articular pressures in the presence of 3.6 mg ml−1 hyaluronan of nominal molecular mass 90 kDa (7 rabbits), 300 kDa (5 rabbits), 530 kDa (6 rabbits) and 2200 kDa (12 rabbits). The last of these were a subset of the results reported by Coleman et al. (1999). Contralateral knees were infused with Ringer solution as a control.
Animal preparation and measurement of pressure and volume drainage rate
New Zealand White rabbits weighing 2–3 kg were anaesthetized with 30 mg kg−1 sodium pentobarbitone plus 500 mg kg−1 urethane i.v. and tracheostomized. Anaesthesia of sufficient depth to abolish the corneal blink reflex was maintained by 15 mg sodium pentobarbitone plus 250 mg urethane i.v. every 30 min. The infusion and recording systems were as described by Coleman et al. (1999). Briefly, an intra-articular cannula connected to a water-calibrated differential pressure transducer measured intra-articular fluid pressure Pj. A second intra-articular cannula was connected to an infusion reservoir, the height of which controlled Pj. Flow of solution into the joint cavity from the reservoir, Q̇in, was recorded by a photoelectric drop counter. An initial infusion raised Pj from a subatmospheric pressure in extensions to ∼2-3 cmH2O to generate a measurable trans-synovial drainage rate. Pj was increased in steps of ∼2-4 cmH2O by raising the infusion reservoir at 30–60 min intervals, at which times the flows were in a steady state. At the end of each period the net trans-synovial drainage rate, Q̇s, was calculated from Q̇in by subtracting the volumetric creep of the cavity walls as described previously (Coleman et al. 1999). Experiments continued to ∼24 cmH2O, which is in the range found in arthritic effusions.
Procedures conformed to UK legislation. Animals were killed by an overdose of i.v. sodium pentobarbitone at the end of the experiment.
Measurement of hyaluronan reflection by the synovial lining
At the end of the experiment samples were aspirated from the infusion line and from the joint cavity after mixing the joint fluid by a series of flexion-extension cycles. Hyaluronan concentrations and molecular masses were analysed by HPLC. The hyaluronan reflected fraction, namely (mass in filtrand – mass in filtrate)/mass in filtrand, was calculated from the increase in the intra-articular concentration and cumulative fluid drainage during the experiment, with corrections for the slight secretion of hyaluronan de novo and the small quantity of endogenous hyaluronan, as described by Scott et al. (1998).
Statistical methods
Slopes (conductances) were fitted by linear regression analysis. Sets of paired conductances were compared by Student's paired t test. Since Pj varied a little between experiments, flows were interpolated to standard pressures at 2.5 cmH2O intervals by linear interpolation between the two bounding measurements to enable comparisons at identical pressures. Flows were compared by two-way analysis of variance (ANOVA), with repeated measures and Bonferroni's post hoc test as appropriate. P < 0.05 was accepted as a significant difference. Means are followed by s.e.m. throughout.
RESULTS
Average molecular masses after sonication
Sonication caused a graded reduction in the average chain length (Fig. 1). The spread of the HPLC peak showed little change, indicating that polydispersity was not much affected. The products used for infusion in vivo had weight-average molecular masses of 88 ± 6 kDa (n= 7), 305 ± 6 kDa (n= 4), 527 ± 56 kDa (n= 4) and 2230 ± 47 kDa (n= 16). Since unit disaccharide mass is 379 Da the average chains comprised ∼232, 805, 1390 and 5884 disaccharides, respectively. Chain lengths are given in Table 1. For convenience these preparations are referred to as hyaluronan-90, hyaluronan-300, hyaluronan-530 and hyaluronan-2200, respectively, based on rounded molecular masses. Hyaluronan-660 refers to umbilical hyaluronan of mass ∼662 kDa as used by McDonald & Levick (1995).
Figure 1. Relation between sonication period and average molecular mass of infused hyaluronan.
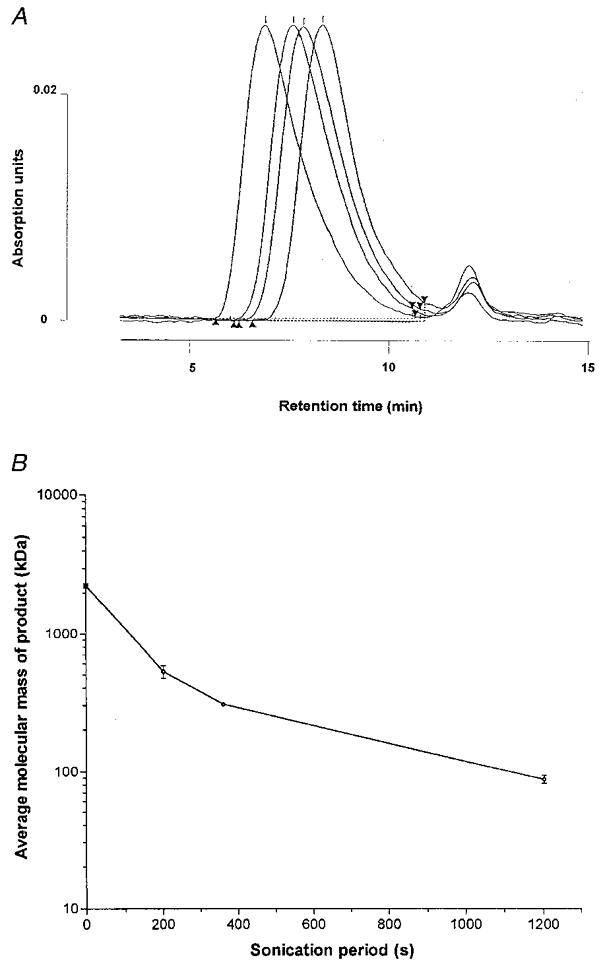
A, exclusion column elution profiles for starting material (left) and progressively sonicated products (3.6 mg ml−1). B, relation between sonication time and hyaluronan size. Samples of weight-average molecular mass (Mw) 2230, 527, 305 and 88 kDa were produced by 0 s sonication, 200 s sonication at 14 μm, 360 s sonication at 14 μm, and 1200 s sonication at 22 μm, respectively.
Table 1.
Average molecular parameters of infused hyaluronan molecules
| Hyaluronan-90 | Hyaluronan-300 | Hyaluronan-530 | Hyaluronan-660a | Hyaluronan-2200 | |
|---|---|---|---|---|---|
| Mw (kDa) | 88 ± 6 | 305 ± 6 | 527 ± 56 | 662 | 2230 ± 47 |
| Chain length (nm)b | 220 | 765 | 1320 | 1659 | 5590 |
| D20(10−7 cm2 s−1)c | 1.93 | 0.82 | 0.56 | 0.48 | 0.21 |
| [η](ml g−1) | 524 | 625 | 873 | 1456 | 2953 |
| RK(nm) | 20 | 33 | 44 | 57 | 107 |
| C*(mg ml−1) | 4.0 | 3.4 | 2.4 | 1.4 | 0.7 |
| Reflected fraction | 0.12 | 0.33 | 0.25 | – | 0.79 |
Mw, weight-average molecular mass; D20, diffusion coefficient; [η], intrinsic viscosity; Rg, radius of gyration; C*, critical overlap concentration.
Median of estimates for umbilical hyaluronan from McDonald & Levick (1995).
Unit disaccharide mass = 379 Da, length = 0.95 nm.
Extrapolated from Laurent et al. (1960) using D20= 41.79M0·6867.
Viscosity, domain radius and overlap concentration of sonicates
The relative viscosities of 3.6 mg ml−1 solutions in the Ostwald viscometer were 3.46 for hyaluronan-90, 5.18 for hyaluronan-300, 9.36 for hyaluronan-530 and 107 for hyaluronan-2230. The solvent volume occupied by a gram of hyaluronan, [η], increased with chain length from 524 ml g−1 for hyaluronan-90 to 2953 ml g−1 for hyaluronan-2200 (Table 1). The Flory radius of gyration of the smallest sonicate, 20 nm, was less than a fifth of that of the starting material, 107 nm. The de Gennes overlap concentration fell with increasing molecular size from 4 mg ml−1 for hyaluronan-90, through 3.4 mg ml−1 for hyaluronan-300 and 2.4 mg ml−1 for hyaluronan-530, to 0.7 mg ml−1 for hyaluronan-2200. Since the infused concentration was 3.6 mg ml−1 throughout, hyaluronan-2200 and hyaluronan-530 were well above de Genne's overlap concentration, hyaluronan-300 was on the borderline and hyaluronan-90 was a little below overlap concentration.
Effect of hyaluronan-90 on fluid drainage
Hyaluronan-90 caused moderate reductions in the rate of fluid escape, namely by up to 40 % compared with the Ringer controls (P < 0.0003, ANOVA; Fig. 2). Hyaluronan-90 failed to induce the quasi-plateau of flow that is characteristic of physiological chain lengths, and the shape of the pressure-flow relation was similar to that for Ringer solution. The latter steepens with pressure at 7–12 cmH2O (yield pressure), with little or inconsistent curvature above or below this (Edlund, 1949; Levick, 1984). Four out of seven joints containing hyaluronan-90 showed a steepening with pressure, but the slopes were always depressed relative to the control joints. The average of seven conductances (slopes) with Ringer solution below yield pressure, 1.33 ± 0.36 μl min−1 cmH2O−1, was reduced by hyaluronan-90 to 0.62 ± 0.22 μl min−1 cmH2O−1 (P= 0.054, n= 7, paired t test). Similarly, the average of seven conductances with Ringer solution above yield pressure, 2.22 ± 0.50 μl min−1 cmH2O−1, was reduced by hyaluronan-90 to 1.11 ± 0.17 μl min−1 cmH2O−1 (P= 0.085, n= 7, paired t test).
Figure 2. Effect of hyaluronan-90 on fluid drainage from joint cavity.
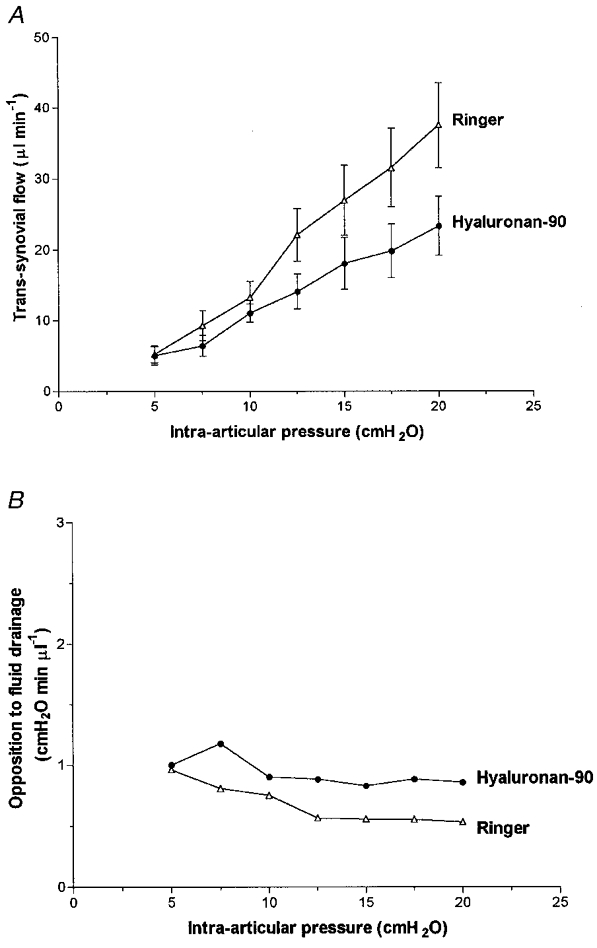
Hyaluronan-90 average mass 88 kDa. A, effect on pressure- flow relation (mean flow ±s.e.m., n= 7). Contralateral joint received Ringer solution. B, effect on average opposition to outflow (pressure needed to drive unit flow across lining).
In analysing the above effects it is helpful to calculate the ‘opposition to outflow’ at each point along the curve. Opposition to outflow is defined here as the pressure required to generate unit outflow, i.e. Pj/Q̇s. As noted in the Introduction, long-chain hyaluronan causes the opposition to outflow to increase with pressure. Rearrangement of the equation for flow across a membrane shows that the opposition term Pj/Q̇s depends on four factors, namely the osmotic pressure across the membrane, the solute reflection coefficient, the membrane resistance and the intramembrane fluid viscosity (Coleman et al. 1999).
In the Ringer-infused joints the opposition almost halved as pressure was raised (Fig. 2B). This was expected, because synovial membrane resistance is known to fall with pressure, the effective osmotic pressure of Ringer solution across interstitium is zero and its relative viscosity is unity (Edlund, 1949; Levick et al. 1999). In the contralateral joints hyaluronan-90 increased the opposition to outflow by 56 % on average compared with Ringer solution (P < 0.0001, ANOVA). However, the opposition showed a slight downward trend with pressure overall (negative regression slope; P= 0.08), and was marginally lower at 20 cmH2O than at 5 cmH2O, namely 1.04 ± 0.23 and 1.29 ± 0.22 cmH2O min μl−1, respectively. This contrasted sharply with the effect of chains of ≥ 530 kDa (see below).
Effect of hyaluronan-300
Hyaluronan-300 reduced the fluid drainage rate to 54–57 % of the Ringer control (P < 0.0001, ANOVA; Fig. 3). Reductions at a given pressure reached individual significance at > 12.5 cmH2O (P < 0.01, Bonferroni test). In no case did hyaluronan-300 induce a flow plateau; a steepening with pressure was seen in 3/5 joints and the relation was virtually linear in the other two joints. The average of the slopes for Ringer solution below yield pressure, 1.02 ± 0.46 μl min−1 cmH2O−1, was almost halved by hyaluronan-300 to 0.58 ± 0.21 μl min−1 cmH2O−1 (P= 0.41, n= 5, paired t test). The average slope for Ringer solution above yield pressure, 2.52 ± 0.45 μl min−1 cmH2O−1, was reduced by hyaluronan-300 to 1.73 ± 0.56 μl min−1 cmH2O−1 (P= 0.06, n= 5, paired t test). The effects of hyaluronan-300 were thus broadly similar to those of hyaluronan-90.
Figure 3. Effect of hyaluronan-300 on fluid drainage from joint cavity.
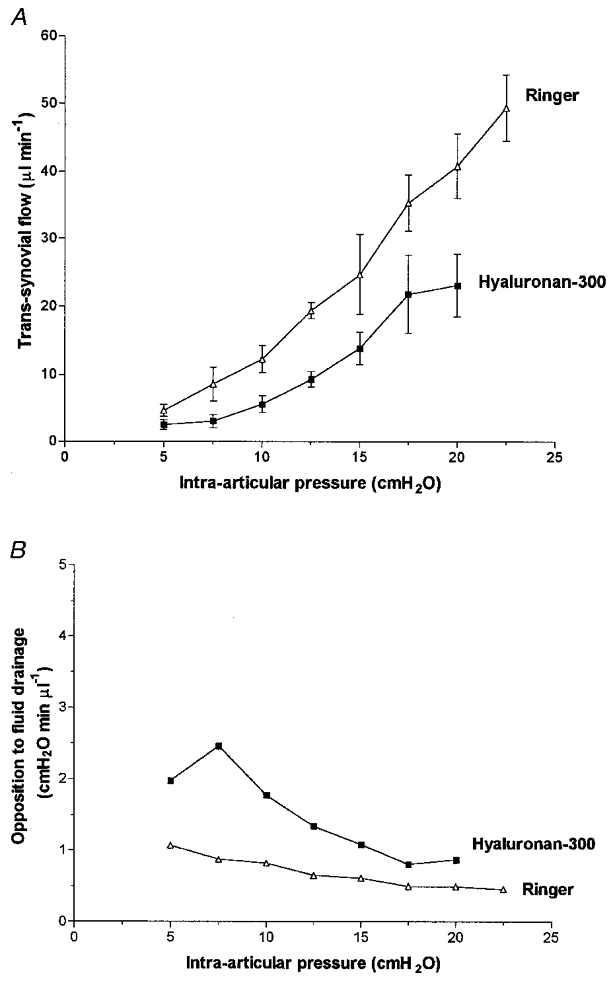
Hyaluronan-300 average mass 305 kDa. A, effect on pressure-flow relation (mean flow ±s.e.m., n= 5). B, effect on opposition to outflow.
In the five Ringer-infused joints the opposition to outflow fell by 54 % as pressure was raised (Fig. 3B). In the contralateral joints hyaluronan-300 raised the opposition to outflow (P= 0.04, ANOVA) but, as with hyaluronan-90, the opposition showed an overall downward trend with pressure (negative regression slope; P= 0.004), falling by 56 % between 5 and 20 cmH2O.
Effects of hyaluronan-530 and hyaluronan-660
Hyaluronan-530 reduced the fluid drainage rate markedly (P < 0.0001, ANOVA). Reductions at a given pressure reached individual significance at > 12.5 cmH2O (P < 0.05, Bonferroni test), and the drainage rate was a third of the Ringer control value at the highest pressure. Unlike the shorter chains, hyaluronan-530 caused a striking change in the shape of the pressure-flow relation. In all five joints the relation developed a virtual plateau at ≥ 12.5 cmH2O, with the flows fixed at ∼10 μl min−1 (Fig. 4). The average of the slopes of the quasi-plateau, 0.05 ± 0.04 μl min−1 cmH2O−1, was 3 % of that for Ringer solution over the same pressure range, namely 1.96 ± 0.30 μl min−1 cmH2O−1 (P < 0.01, paired t test). Over the lower pressure range the reduction in slope was less striking, namely 0.34 ± 0.20 μl min−1 cmH2O−1 for hyaluronan-530 compared with 1.01 ± 0.29 μl min−1 cmH2O−1 for Ringer solution (P= 0.3, t test).
Figure 4. Effect of hyaluronan-530 on fluid drainage from joint cavity.

Hyaluronan-530 average mass 527 kDa. A, effect on pressure-flow relation, with development of a near plateau (mean flow ±s.e.m., n= 6). Dashed line shows data for umbilical hyaluronan of average molecular mass 660 kDa (n= 5; McDonald & Levick, 1995). B, effect on opposition to outflow; opposition increases with pressure (‘buffering’ of outflow).
As well as increasing the opposition to outflow relative to Ringer solution (P < 0.001, ANOVA), hyaluronan-530 caused the opposition to increase as a function of pressure at ≥ 12.5 cmH2O (Fig. 3B). The opposition had increased by 69 % at 22.5 cmH2O. This contrasted with the decrease in opposition with pressure in the contralateral Ringer-infused joints and in joints infused with hyaluronan-330 or hyaluronan-90 (Figs 2B and 3B).
Figure 4 also shows for comparison some results from our laboratory using hyaluronan-660 (McDonald & Levick, 1995). The plateauing curves generated by hyaluronan-530 and hyaluronan-660 are of similar form but the flows in the presence of hyaluronan-660 were, overall, lower than with hyaluronan-530, plateauing out at just under 8 μl min−1 (P < 0.0001, ANOVA).
Effect of hyaluronan-2200
Hyaluronan-2200 greatly attenuated the fluid drainage rate when compared with Ringer solution (P < 0.0001, ANOVA). The flows reached a virtual plateau at ∼3.6 μl min−1 (Fig. 5). The plateau flow was approximately one-third of that for hyaluronan-530 solution (P < 0.0001, ANOVA) and was 8 % of the Ringer drainage rate at the highest pressure. The average of the slopes of the quasi-plateau, 0.013 ± 0.016 μl min−1 cmH2O−1, was 0.5 % of the Ringer slope (2.30 ± 0.05 μl min−1 cmH2O−1; P < 0.01, t test) and approximately one-quarter of the slope obtained with hyaluronan-530 (P= 0.12, t test).
Figure 5. Effect of hyaluronan-2200 on fluid drainage from joint cavity.
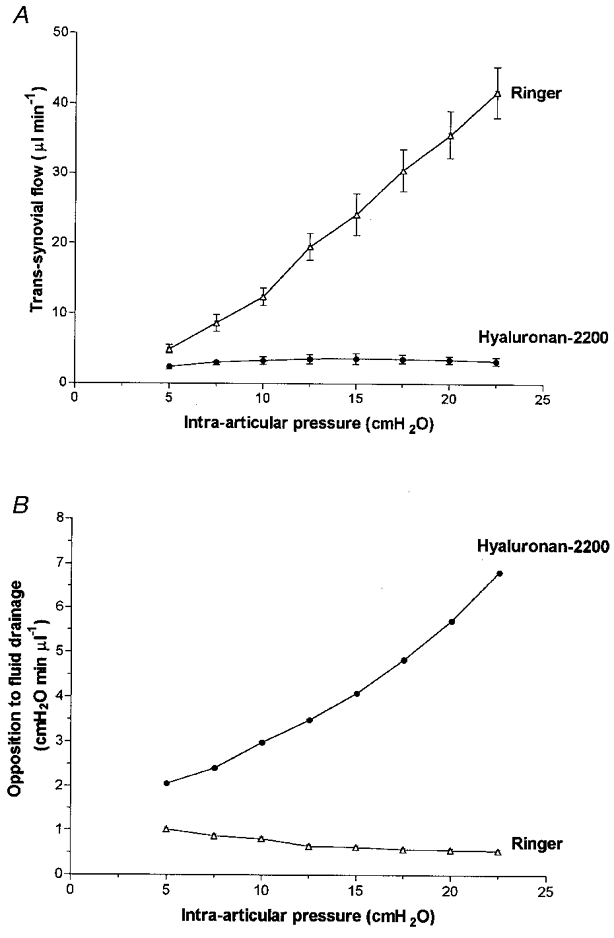
Hyaluronan-2200 average mass 2230 kDa. A, effect on pressure-flow relation (mean flow ±s.e.m., n= 12). B, effect on opposition to outflow.
The opposition to outflow increased steeply with pressure over the entire pressure range in the presence of hyaluronan-2200 (Fig. 5B). The 3.3-fold rise in opposition with pressure contrasted with the smaller, 1.7-fold rise with hyaluronan-530 and the declining opposition in the joints containing hyaluronan-330, hyaluronan-90 or Ringer solution.
Molecular mass and concentration of aspirated hyaluronan solutions
Analysis of the aspirated fluid showed that the average molecular mass of the intra-articular hyaluronan at the end of an experiment was not significantly different from that infused (P= 0.5, n= 15, paired t test). The values were as follows: infused hyaluronan 87.8 ± 6.4 kDa, aspirated hyaluronan 77.5 ± 6.1 kDa; infused hyaluronan 304.7 ± 6.3 kDa, aspirated hyaluronan 349.2 ± 7.4 kDa; infused hyaluronan 526.9 ± 55.7 kDa, aspirated hyaluronan 544.8 ± 24.6 kDa. Likewise, an earlier study of hyaluronan-2200 showed that the infused and aspirated molecular masses, namely 2267 ± 84 and 2252 ± 73 kDa, respectively, were not significantly different (P= 0.8, n= 16, paired t test; Scott et al. 1998). Thus the average intra-articular chain length was not distorted significantly by factors such as endogenous hyaluronan admixture, intra-articular degradation or hyaluronan secretion de novo over 3–5 h (5-6 μg h−1, Coleman et al. 1997).
The concentration of hyaluronan in the aspirated fluid was greater than that in the infusate in every case for hyaluronan-90, hyaluronan-300 and hyaluronan-530 (P < 0.01, n= 15, paired t test). Concentrations increased by 22 ± 3, 44 ± 11 and 25 ± 7 %, respectively. We have reported previously that hyaluronan-2200 is concentrated by 26 ± 8 % (P= 0.02, n= 6, paired t test; Scott et al. 1998). The reflected fractions, calculated from the mean concentration increase and filtered volume (see Methods), were 0.12, 0.33, 0.25 and 0.79 for hyaluronan-90, hyaluronan-300, hyaluronan-530 and hyaluronan-2200, respectively. The relation between reflected fraction and radius of the polymer domain is plotted in Fig. 6. The observations reinforce a previous study of the size dependence of hyaluronan permeation by Brown et al. (1991). The latter group found that the half-life of labelled hyaluronan in rabbit knees decreased as molecular mass was reduced from 6000 to 90 kDa.
Figure 6. Relation between size of molecular domain and fraction of molecules reflected during drainage through synovial interstitium.
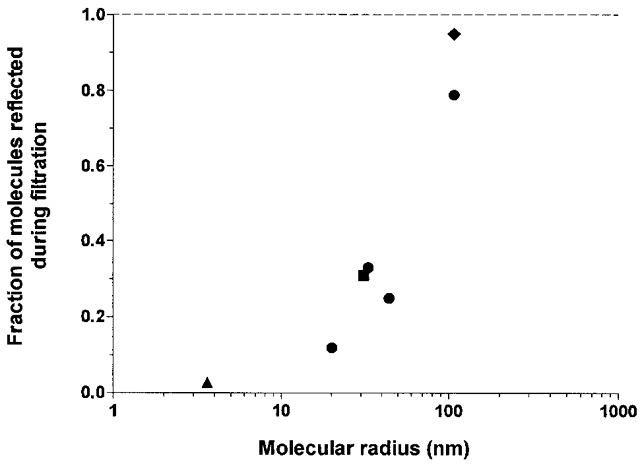
The abscissa is the Flory radius of gyration Rg for the polysaccharides. The dashed line of value 1.0 represents the upper limit for the reflected fraction. •, results for hyaluronan-90 to -2200. Additional data: ♦, hyaluronan-2200 at 2 mg ml−1 (Scott et al. 1998); ▪, dextran of molecular mass 2 × 106 Da (Scott et al. 2000b); ▴, albumin (J. R. Levick & S. Sabaratnam, unpublished results).
DISCUSSION
Chain length is clearly a major determinant of the effect of synovial fluid hyaluronan on fluid drainage through synovial interstitium. The effect of hyaluronan changed strikingly between 305 and 527 kDa. Hyaluronan of mass ≥ 527 kDa caused outflow buffering, i.e. increasing opposition to drainage with pressure, whereas hyaluronan of mass ≤ 305 kDa reduced drainage without outflow buffering, which profoundly altered the shape of the pressure-flow relation compared to longer chains. Studies of transperitoneal drainage show that hyaluronan of mass 85 or 280 kDa has only a small effect on transperitoneal flow whereas 500 and 4000 kDa hyaluronan markedly attenuates drainage (Wang et al. 1999).
Mechanisms underlying the effect of chain length
Reduction of hyaluronan concentration, like reduction of chain length, can abolish outflow buffering (Scott et al. 2000a). The effect of reduced concentration can be explained largely by a fall in the osmotic pressure of the concentration polarization layer that forms during fluid drainage. There are several reasons for arguing that shortened chains should likewise produce a less concentrated polarization layer, as follows. (i) Short polymer chains are less well reflected by a porous membrane than are long chains, as is evident in Fig. 6 and as shown in vitro by Munch et al. (1979). This is in keeping with the change in hydrodynamic radius of hyaluronan with chain length; the hydrodynamic radius falls from ∼103 nm at 2000 kDa to ∼14 nm at 90 kDa (Fujii et al. 1996). A hydrodynamic radius of 14 nm is smaller than the equivalent pore radius of 30–90 nm for synovial interstitium. Lindholm et al. (1996) by contrast observed no difference between the half-lives of radiolabelled 600 and 2500 kDa hyaluronan in equine joints; but the drainage rates and pressures were not recorded, and may have been low. (ii) Experiments with infused dextran of mass 2000 kDa support the argument that molecular domain volume is crucial to outflow buffering. Dextran-2000 fails to buffer the trans-synovial flow despite its large mass (Scott et al. 2000b). Its reflected fraction of 0.31 and Rg of 31–34 nm are very similar to those of hyaluronan-300 (0.33 and 33 nm, respectively), which likewise fails to buffer outflow. (iii) Chain length affects diffusivity, both directly and through chain-chain interactions. The diffusion coefficient increases ∼9-fold as chain length is reduced from 2200 to 88 kDa (Table 1). Increased diffusivity will greatly reduce the degree of concentration polarization, as shown by the theoretical polarization curves in Fig. 9A of Coleman et al. (1999). The combined effect of increased back-diffusion in the concentration polarization layer and reduced reflection at the interface is to reduce the interfacial hyaluronan concentration to a point where its colloid osmotic pressure is little more than in the bulk phase, namely 1 cmH2O.
The sensitivity of outflow buffering to chain length may relate partly to the degree of chain-chain interaction. The critical concentration C* for overlap of adjacent molecular domains depends on the chain length. Consequently, the infused hyaluronan molecules of mass 2200–530 kDa were above C* (Table 1), whereas those of mass 300–90 kDa were below C*. Hyaluronan-300 and hyaluronan-90 were not, therefore, in the mutually entangled, quasi-continuous network state that characterized the longer chains. Moreover, measurable chain-chain interactions are often found to begin only at a concentration greater than C*, referred to here as C**. For rooster comb hyaluronan C** was 1.9 ×C* (Scott et al. 2000a). Hyaluronan-90 and hyaluronan-300 were well below C** while hyaluronan-660 and hyaluronan-2200 were well above it.
Effect of low molecular weight hyaluronan (≤ 305 kDa)
Although the smaller hyaluronan chains failed to generate outflow buffering, they did reduce outflow substantially, namely by almost half compared with Ringer solution. This could be due to the increased viscosity of the interstitial fluid as non-reflected hyaluronan chains permeate the interstitial void. The bulk phase viscosity of hyaluronan-90 and hyaluronan-300 solutions reduced the macroscopic flow in the viscometer capillary tube by 71% and 81%, respectively, relative to Ringer solution. The viscosity of the intramembrane fluid, however, is probably less than in the bulk phase, due partly to the partial reflection of hyaluronan at the tissue-fluid interface and partly to the phenomenon of anomalous viscous flow in narrow channels (Levick, 1994).
Pathophysiological significance
Inflammatory arthritis is associated with a fall in hyaluronan concentration and chain length, as described in the Introduction. Normal human hyaluronan, of molecular mass 6000–7000 kDa (Dahl et al. 1985; Lee & Cowman, 1994), is bigger than rabbit hyaluronan, which has a mass of 2400–2900 kDa. The subnormal average molecular masses in human arthritis span a wide range, namely 1284–4800 kDa (Balazs et al. 1967; Kofoed & Barcelo, 1979; Bjelle et al. 1982; Dahl et al. 1985), and individual values as low as 849 kDa have been reported in acute synovitis (Praest et al. 1997). Even so, such chains would still generate outflow buffering according to the results in Fig. 4, unless the reflective properties of the lining are also impaired. The latter has never been studied directly; however, the intra-articular half-life of 3H-labelled hyaluronan in sheep hock joints is approximately halved during acute synovitis (Fraser et al. 1993), and one of several possible explanations for this could be increased synovial lining permeability.
Based on the present results and those of Scott et al. (2000a), it seems likely that the fall in hyaluronan concentration in human arthritis, often to < 1 mg ml−1, will have a greater effect on fluid drainage than does the fall in molecular size. Reduced chain length and reduced reflection may contribute, however, to the rise in plasma hyaluronan concentration observed in rheumatoid arthritis after exercise (Engström-Laurent & Hallgren, 1987). Exercise causes cyclical rises in intra-articular pressure and thus promotes drainage from the joint cavity. Hyaluronan chain length also influences synovial cellular activities and lubrication, as reviewed by Strachan et al. (1990).
Acknowledgments
This work was supported by Wellcome Trust grant 039033/Z/93.
References
- Baker MS, Green SP, Lowther DA. Changes in the viscosity of hyaluronic acid after exposure to a myeloperoxidase-derived oxidant. Arthritis and Rheumatism. 1989;32:461–471. doi: 10.1002/anr.1780320416. [DOI] [PubMed] [Google Scholar]
- Balazs EA. The physical properties of synovial fluid and the special role of hyaluronic acid. In: Helfet AJ, editor. Disorders of the Knee. Philadelphia: Lippincott; 1982. pp. 61–75. [Google Scholar]
- Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritic human fluids. Arthritis and Rheumatism. 1967;10:357–376. doi: 10.1002/art.1780100407. [DOI] [PubMed] [Google Scholar]
- Barry SI, Gowman LM, Ethier CR. Obtaining the concentration-dependent diffusion coefficient from ultrafiltration experiments: application to hyaluronate. Biopolymers. 1996;39:1–11. [Google Scholar]
- Beaty NB, Tew WP, Mello RJ. Relative molecular weight and concentration determination of sodium hyaluronate solutions by gel-exclusion high-performance liquid chromatography. Analytical Biochemistry. 1985;146:387–395. doi: 10.1016/0003-2697(85)90287-8. [DOI] [PubMed] [Google Scholar]
- Bjelle A, Anderson T, Granath K. Molecular weight distribution of hyaluronic acid of human synovial fluid in rheumatic diseases. Scandinavian Journal of Rheumatology. 1982;12:133–138. doi: 10.3109/03009748309102899. [DOI] [PubMed] [Google Scholar]
- Brown TJ, Laurent UBG, Fraser JRE. Turnover of hyaluronan in synovial joints: elimination of labelled hyaluronan from the knee joint of the rabbit. Experimental Physiology. 1991;76:125–134. doi: 10.1113/expphysiol.1991.sp003474. [DOI] [PubMed] [Google Scholar]
- Castor CW, Dorstewitz EL. Abnormalities of connective tissue cells cultured from patients with rheumatoid arthritis. I. Relative unresponsiveness of rheumatoid synovial cells to hydrocortisone. Journal of Laboratory and Clinical Medicine. 1966;68:306–313. [PubMed] [Google Scholar]
- Coleman PJ, Scott D, Mason RM, Levick JR. Characterization of the effect of high molecular weight hyaluronan on trans-synovial flow in rabbit knees. The Journal of Physiology. 1999;514:265–282. doi: 10.1111/j.1469-7793.1999.265af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PJ, Scott D, Ray J, Mason RM, Levick JR. Hyaluronan secretion into synovial cavity of rabbit knees and comparison with albumin turnover. The Journal of Physiology. 1997;503:645–657. doi: 10.1111/j.1469-7793.1997.645bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman MK, Liu J, Li M, Hittner DM, Kim JS. Hyaluronan interactions: self, water, ions. In: Laurent TC, editor. The Chemistry, Biology and Medical Applications of Hyaluronan and its Derivatives. London: Portland Press; 1998. pp. 17–24. [Google Scholar]
- Dahl IMS, Husby G. Hyaluronic acid production in vitro by synovial lining cells from normal and rheumatoid joints. Annals of the Rheumatic Diseases. 1985;44:647–657. doi: 10.1136/ard.44.10.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl LB, Dahl IM, Engström-Laurent A, Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Annals of the Rheumatic Diseases. 1985;44:817–822. doi: 10.1136/ard.44.12.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennes P-G. Scaling Concepts in Polymer Physics. Ithaca and London: Cornell University Press; 1979. [Google Scholar]
- Edlund T. Studies on the absorption of colloids and fluid from rabbit knee joints. Acta Physiologica Scandinavica. 1949;18(suppl. 62):1–108. [Google Scholar]
- Engström-Laurent A, Hällgren R. Circulating hyaluronic acid levels vary with physical activity in healthy subjects and in rheumatoid arthritis patients. Arthritis and Rheumatism. 1987;30:1333–1338. doi: 10.1002/art.1780301203. [DOI] [PubMed] [Google Scholar]
- Flory PJ. Principles of Polymer Chemistry. Ithaca, NY, USA: Cornell University Press; 1971. [Google Scholar]
- Fraser JRE, Kimpton WG, Pierscionek BK, Cahill RNP. The kinetics of hyaluronan in normal and acutely inflamed synovial joints: observations with experimental arthritis in sheep. Seminars in Arthritis and Rheumatism. 1993;22(suppl. 1):9–17. doi: 10.1016/s0049-0172(10)80015-0. [DOI] [PubMed] [Google Scholar]
- Fujii K, Kawata M, Kobayashi Y, Okamoto A, Nishinari K. Effects of addition of hyaluronate segments with different chain lengths on the viscoelasticity of hyaluronic acid solutions. Biopolymers. 1996;38:583–591. doi: 10.1002/(SICI)1097-0282(199605)38:5%3C583::AID-BIP4%3E3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Greenwald RA, Moak SA. Degradation of hyaluronic acid by polymorphonuclear leukocytes. Inflammation. 1986;10:15–30. doi: 10.1007/BF00916037. [DOI] [PubMed] [Google Scholar]
- Gribbon P, Heng BC, Hardingham TE. The molecular basis of the solution properties of hyaluronan investigated by confocal fluorescence recovery after photobleaching. Biophysical Journal. 1999;77:2210–2216. doi: 10.1016/S0006-3495(99)77061-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Oxygen radicals, nitric oxide and human inflammatory joint disease. Annals of the Rheumatic Diseases. 1995;54:505–510. doi: 10.1136/ard.54.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubeck HD, Kock R, Fischer D-C, Leur EV, Hoffmeister K, Greiling H. Transforming growth factor β1, a major stimulator of hyaluronan synthesis in human synovial lining cells. Arthritis and Rheumatism. 1995;38:669–677. doi: 10.1002/art.1780380515. [DOI] [PubMed] [Google Scholar]
- Kofoed JA, Barcelo AC. The synovial fluid hyaluronic acid in rheumatoid arthritis. Experentia. 1979;34:1545–1546. doi: 10.1007/BF02034662. [DOI] [PubMed] [Google Scholar]
- Laurent TC, Ryan M, Pietruszkiewicz A. Fractionation of hyaluronic acid. The polydispersity of hyaluronic acid from the bovine vitreous body. Biochimica et Biophysica Acta. 1960;42:476–485. doi: 10.1016/0006-3002(60)90826-x. [DOI] [PubMed] [Google Scholar]
- Lee HG, Cowman MK. An agarose gel electrophoretic method for analysis of hyaluronan molecular weight distribution. Analytical Biochemistry. 1994;219:278–287. doi: 10.1006/abio.1994.1267. [DOI] [PubMed] [Google Scholar]
- Levick JR. Blood flow and mass transport in synovial joints. In: Renkin EM, Michel CC, editors. Handbook of Physiology, section 2, The Cardiovascular System, The Microcirculation. IV. Bethesda: American Physiological Society; 1984. pp. 917–947. [Google Scholar]
- Levick JR. An analysis of the interaction between extravascular plasma protein, interstitial flow and capillary filtration; application to synovium. Microvascular Research. 1994;47:90–125. doi: 10.1006/mvre.1994.1007. [DOI] [PubMed] [Google Scholar]
- Levick JR, Mason RM, Coleman PJ, Scott D. Physiology of synovial fluid and trans-synovial flow. In: Archer CW, Benjamin M, Caterson B, Ralphs JR, editors. Biology of the Synovial Joint. Amsterdam: Harwood Academic Publishers; 1999. pp. 235–252. [Google Scholar]
- Lindholm A, Roneus B, Lindblad G, Jones B. Hyaluronan turnover in the synovial fluid in metacarpophalangeal and middle carpal joints in standardbred horses. Acta Veterinaria Scandinavica. 1996;37:147–151. doi: 10.1186/BF03548107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974;185:529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- McDonald JN, Levick JR. Effect of intra-articular hyaluronan on pressure-flow relation across synovium in anaesthetized rabbits. The Journal of Physiology. 1995;485:179–193. doi: 10.1113/jphysiol.1995.sp020722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch WD, Zestar LP, Anderson JL. Rejection of polyelectrolytes from microporous membranes. Journal of Membrane Science. 1979;5:77–102. [Google Scholar]
- Orvisky E, Soltés L, Chabrecek P, Novák I, Stancikova M. Size exclusion chromatographic characterization of sodium hyaluronate fractions prepared by high energetic sonication. Chromatographia. 1993;37:20–21. [Google Scholar]
- Praest BM, Greiling H, Kock R. Assay of synovial fluid parameters: hyaluronan concentration as a potential marker for joint diseases. Clinica Chimica Acta. 1997;266:117–128. doi: 10.1016/s0009-8981(97)00122-8. [DOI] [PubMed] [Google Scholar]
- Price FM, Levick JR, Mason RM. Glycosaminoglycan concentration in synovium and other tissues of rabbit knee in relation to hydraulic resistance. The Journal of Physiology. 1996;495:803–820. doi: 10.1113/jphysiol.1996.sp021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck P, Schneider S, Miehlke R, Prehm P. Synthesis and degradation of hyaluronate by synovia from patients with rheumatoid arthritis. Journal of Rheumatology. 1995;22:400–405. [PubMed] [Google Scholar]
- Scott D, Coleman PJ, Mason RM, Levick JR. Direct evidence for partial reflection of hyaluronan molecules by the synovial lining of joints. The Journal of Physiology. 1998;508:619–623. doi: 10.1111/j.1469-7793.1998.619bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D, Coleman PJ, Mason RM, Levick JR. Fluid buffering effect of hyaluronan in synovial joints depends on chain length. Journal of Vascular Research. 1999;36:325. [Google Scholar]
- Scott D, Coleman PJ, Mason RM, Levick JR. Concentration-dependence of interstitial flow buffering by hyaluronan in synovial joints. Microvascular Research. 2000a;59:345–353. doi: 10.1006/mvre.1999.2231. [DOI] [PubMed] [Google Scholar]
- Scott D, Coleman PJ, Mason RM, Levick JR. Action of polysaccharides of differing molecular structure but similar mass on fluid drainage through the synovial interstitial pathway. The Journal of Physiology. 2000b;523.P:143P. doi: 10.1111/j.1469-7793.2000.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JE, Heatley F. Hyaluronan forms specific stable tertiary structures in aqueous solution: a 13C NMR study. Proceedings of the National Academy of Sciences of the USA. 1999;96:4850–4855. doi: 10.1073/pnas.96.9.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan RK, Smith P, Gardner DL. Hyaluronate in rheumatology and orthopaedics: Is there a rôle? Annals of the Rheumatic Diseases. 1990;49:949–952. doi: 10.1136/ard.49.11.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorio E, Einola S, Hakkarainen S, Penttinen S. Synthesis of underpolymerized hyaluronic acid by fibroblasts cultured from rheumatoid and non-rheumatoid synovitis. Rheumatology International. 1982;2:97–102. doi: 10.1007/BF00541160. [DOI] [PubMed] [Google Scholar]
- Wang T, Chen H-H, Heimburger O, Waniewski J, Bergstrom J, Lindholm B. Hyaluronan prevents the decreased net ultrafiltration caused by increased peritoneal dialysate fill volume. Kidney International. 1999;53:496–502. doi: 10.1046/j.1523-1755.1998.00773.x. [DOI] [PubMed] [Google Scholar]
- Wik HB, Wik O. Rheology of hyaluronan. In: Laurent TC, editor. The Chemistry, Biology and Medical Applications of Hyaluronan and its Derivatives. London: Portland Press; 1998. pp. 25–32. [Google Scholar]


