Abstract
The contribution of low voltage-activated (LVA) T-type Ca2+ channels and four different types of high voltage-activated (HVA) Ca2+ channel to exocytosis, and the relationship between calcium influx and exocytosis during action potentials (APs) were studied in pituitary melanotropes.
Selective HVA Ca2+ channel blockers reduced exocytosis, monitored by membrane capacitance measurements, proportional to the reduction in Ca2+ influx. The efficacy of Ca2+ in stimulating exocytosis did not change in the presence of the Ca2+ channel blockers, indicating that all HVA Ca2+ channels act together in stimulating exocytosis.
The relationship between Ca2+ influx and exocytosis during the AP was examined using APs recorded from spontaneously active melanotropes as command templates under voltage clamp. Under voltage clamp, multiphasic Ca2+ currents were activated over the entire duration of the APs, i.e. during the rising phase as well as the plateau phase. The maximum amplitude of the Ca2+ current coincided with the peak of the AP.
The relationship between Ca2+ entry and exocytosis was linear for the different phases of the AP. Also, the influx of Ca2+ through LVA T-type channels stimulated exocytosis with the same efficacy as through the HVA channels.
APs of increasing duration (∼50 to ∼300 ms) evoked increasing amounts of exocytosis. The number of entering Ca2+ ions and the capacitance change were linearly related to AP duration, resulting in a fixed relationship between Ca2+ entry and exocytosis.
The results show that Ca2+ ions, entering a melanotrope, couple with equal strength to exocytosis regardless of the channel type involved. We suggest that the linear relationship between Ca2+ entry and secretion observed under physiological conditions (during APs), results from the equal strength with which LVA and HVA channels in melanotropes couple to exocytosis. This guarantees that secretion takes place over the entire duration of the AP.
Release of neurotransmitters and hormones from nerve terminals and neuroendocrine cells is controlled by the influx of Ca2+ through voltage-gated Ca2+ channels. The natural stimulus for opening Ca2+ channels and triggering exocytosis is the action potential (AP). The strength of the coupling between an AP and exocytosis depends on the spatial organization of Ca2+ channels and vesicles. Release-ready vesicles located in the close vicinity of Ca2+ channels are likely to be released with little delay when an AP is fired. This is the case in fast synapses where N- and P-type Ca2+ channels are located close to the release sites and actually bind to the fusion peptides syntaxin and/or synaptotagmin (Bennett et al. 1992; Sheng et al. 1996). On the other hand, vesicles that are further away are released with a considerable delay, due to a longer diffusion distance for Ca2+.
In the neuroendocrine chromaffin cells, the majority of the large dense-cored vesicles (LDCVs) are thought to be docked far away from Ca2+ channels (200-300 nm), because the delay between stimulation and secretion is rather long and exocytosis persists after the stimulus has ended (Chow et al. 1992, 1994, 1996; Klingauf & Neher, 1997). Moreover, the concentration of intracellular Ca2+ chelator that affects exocytosis is low (Chow et al. 1996). This distance results in a weak coupling between the AP and exocytosis (Zhou & Misler, 1995). However, a small population of LDCVs are docked within 30 nm from the Ca2+ channel. The release of these vesicles is strongly coupled to the AP, and they are rapidly released upon firing (Klingauf & Neher, 1997; Elhamdani et al. 1998). In calf chromaffin cells, these ‘near-by’ vesicles are specifically docked near the L-type facilitation Ca2+ channel (Artalejo et al. 1991, 1994). As a result, Ca2+ entry through this channel stimulates exocytosis about 5 times more effectively than Ca2+ entry through other types of Ca2+ channel. A similar strong coupling between L-type channels and exocytosis exists in pancreatic β-cells (Bokvist et al. 1995).
In melanotropes of the rat, the influx of Ca2+ through Ca2+ channels causes the release of predocked LDCVs (Thomas et al. 1990; Parsons et al. 1995). Exocytosis starts with a delay (Thomas et al. 1993b) and low concentrations of Ca2+ buffers block exocytosis, suggesting a large distance between Ca2+ channels and LDCVs (Mansvelder & Kits, 1998). Nevertheless, we observed a stringent coupling between depolarization and secretion in melanotropes. Exocytosis was observed upon very short depolarizations (2-5 ms), as well as upon the final stimulus in a train of 25 depolarizations of 40 ms. This indicates that in melanotropes different channel types are able to sustain secretion, and that APs should be robustly coupled to secretion. The present paper puts these two predictions to the test. Thus, we first addressed the role of the different Ca2+ channel types in melanotropes in exocytosis and we analysed the efficacy with which the various channel types couple to secretion. Second, we examined the relation between calcium influx and exocytosis during the AP and addressed the question of whether the shape and duration of the APs influence the strength of excitation-secretion coupling.
Melanotropes express five different types of Ca2+ channel: L-, N-, P-, Q- and T-type (Keja et al. 1991; Stack & Suprenant, 1991; Ciranna et al. 1996; Mansvelder et al. 1996). To investigate the coupling of each of these channel types to exocytosis we examined the effects of specific Ca2+ channel blockers on secretion, assessed by capacitance measurements. To study the coupling of APs to secretion, we recorded APs from spontaneously active cells. Subsequently we stimulated melanotropes under voltage clamp with APs which covered the range of shapes and durations encountered, and measured the resulting changes in membrane capacitance.
Part of this work has appeared in abstract form (Mansvelder & Kits, 1999).
METHODS
Cell culture
Pituitary melanotropes of male Wistar rats (200-300 g, Harlan CPB, The Netherlands) were isolated as described previously (Keja et al. 1991). Animals were killed by swift decapitation using a guillotine, without the use of anaesthetics. Killing procedures were approved by the ethical committee concerning animal experiments. The cells were cultured on poly-L-lysine-coated coverslips (7 mm × 7 mm) at a density of 0.1 intermediate lobe per coverslip. The culture medium consisted of Biorich I (Flow) supplemented with 26.2 mm NaHCO3, 5 % Ultroser G (Gibco), 200 U ml−1 penicillin G (Sigma), 50 μg ml−1 streptomycin (Sigma) and 1 μm cytosine arabinosine (Sigma). Cells were maintained in a 37°C incubator with a humidified atmosphere comprising 5 % CO2 in air. Recordings were made up to 4 days after isolation.
Recording solutions
Coverslips bearing melanotropes were transferred to a recording chamber containing ∼0.5 ml external solution. The external solution for the experiments determining the pharmacological sensitivity of the whole-cell calcium current consisted of (mM): TEACl 142, glucose 10, CaCl2 5, Hepes 10 and 4-aminopyridine (4-AP) 1; pH adjusted to 7.4 with TEAOH. The internal solution for these experiments contained (mM): CsCl 145, MgCl2 2, EGTA (Sigma) 0.1, Hepes 10 and MgATP 2; pH adjusted to 7.4 with CsOH. For recording spontaneous electrical activity of melanotropes the external solution contained (mM): NaCl 145, MgCl2 1.2, CaCl2 2.4, KCl 3, glucose 10 and Hepes 10; pH adjusted to 7.4 with NaOH. The internal solution for these experiments contained (mM): KCl 135, MgCl2 2, CaCl2 1, Hepes 10, EGTA 11, MgATP 2 and GTP Tris salt 0.1; pH adjusted to 7.4 with KOH (free Ca2+ concentration, 3.8 nM). The external solution for measuring the calcium current and changes in membrane capacitance elicited by AP templates contained (mM): TEACl 145.75, glucose 10, CaCl2 2.5, Hepes 10 and 4-AP 1; pH 7.4 with TEAOH. The internal solution for these experiments contained (mM): CsCl 145, MgCl2 2, EGTA 0.1, Hepes 10 and MgATP 2; pH adjusted to 7.4 with CsOH (nominal zero calcium). The recording chamber was continuously perfused at a rate of ∼1.5 ml min−1, driven by a pump, while the bath volume was kept constant by continuous suction. All experiments were carried out at a temperature of 32–34°C.
Membrane potential recordings
Electrodes were pulled on a Flaming-Brown P-87 (Sutter Instruments, UK) horizontal microelectrode puller from thick-walled Clark GC-150 borosilicate glass (Clark Electromedical Instruments, UK). To reduce the pipette capacitance, the tips of the electrodes were covered with Sylgard. The impedance of the electrodes after fire polishing was 5–10 MΩ. Membrane voltages were monitored in the ‘fast I-clamp’ mode of an Axopatch 200A amplifier (Axon Instruments), filtered at 1 kHz (4-pole low-pass Bessel filter on the Axopatch 200A) and digitized with a Digidata 1200 interface (Axon Instruments) at 6 kHz. The pipette capacitance was compensated for. Membrane voltages were recorded in the whole-cell configuration. Data were stored and analysed with pCLAMP 6 software (Axon Instruments).
Capacitance measurements
Electrodes were pulled in the same manner as for the membrane potential recordings, and the tips of the electrodes were also covered with Sylgard. Impedance of the electrodes after fire polishing was 2.5-4 MΩ. The whole-cell membrane current was monitored and digitized with an EPC 9 amplifier (HEKA, Lambrecht Germany). Capacitance measurements were made using PULSE software running on an IBM-compatible computer. The membrane capacitance, access conductance and membrane conductance were calculated according to the Lindau-Neher technique (Lindau & Neher, 1988; for review, see Gilles, 1995), implemented as the ‘Sine + D.C.’ feature of the PULSE lock-in module. A sine wave of 1000 Hz, 40 mV peak-to-peak, was added to a holding potential of -80 mV. The reversal potential of the lock-in module was set to 0 mV. The membrane current was filtered at 2 kHz by the Bessel filter of the EPC 9 and then sampled at 10 kHz. The membrane capacitance, access conductance and membrane conductance were calculated at 1 kHz.
Cells that generated a peak calcium current smaller than -50 pA upon the step depolarization from -80 mV to +10 mV were left out of the analysis. The number of Ca2+ ions that entered the cell during a step depolarization or during an AP was determined using the following equation:
where t is time, F is Faraday's constant (96 485 C mol−1) and NA is Avogadro's constant (6.022 × 1023 mol−1). Tail currents were included in this integration. Leak currents were not corrected for.
Data analysis
The amount of exocytosis was calculated as the difference between the mean of 100 membrane capacitance samples before and the first 10 samples following a particular depolarizing pulse or AP template. The amount of endocytosis was calculated as the difference between the first membrane capacitance sample after a pulse and the mean of the last five samples before the next pulse. The numerical values of exocytosis and endocytosis thus obtained were corrected for transient capacitance changes (ΔCt, amounting to 2.0 ± 0.29 fF and -1.1 ± 0.21 fF, respectively) that are not related to Ca2+ influx (Horrigan & Bookman, 1994; Mansvelder & Kits, 1998). ΔCt was determined by depolarizing the cell in the presence of Ni2+ (40 μm) and Cd2+ (100 μm) to block all calcium currents (n= 4 cells, not shown). The statistical significance of differences of means was determined with Student's t test, using Systat software (Evanson, IL, USA). Means mentioned in the text are given with standard errors of the mean (s.e.m.) unless mentioned otherwise. Error bars presented signify s.e.m.
Pharmacology
Calcium channel blockers were applied with pressure ejection from a glass pipette. Nimodipine was purchased from RBI (Natick, MA, USA), ω-conotoxin GVIA (ω-CgTx GVIA), ω-agatoxin TK (ω-AgTx) and ω-conotoxin MVIIC (ω-CgTx MVIIC) were obtained from Alamone Labs (Jerusalem, Israel). The toxins were stored as stock solutions at -20°C in small aliquots for single use, and diluted to their final concentrations in external medium immediately before the experiments started. Nimodipine was stored as a stock solution of 10−2 M in methanol at -20°C and only diluted to the final concentration in external solution immediately before the experiments. The amount of methanol was below 0.1 %, which did not affect calcium currents.
RESULTS
Pharmacology of the Ca2+ currents
Our first goal in this study was to examine the contribution of different Ca2+ channel types to exocytosis at 33°C. Therefore, we first determined the pharmacological sensitivity of the whole-cell Ca2+ current at 33°C by measuring the amplitude and the kinetics of the block induced by specific blockers of the various HVA Ca2+ channels. Ca2+ currents were stable for at least 7 min of recording, before rundown began (n= 7, not shown). Control applications of bath solution did not reduce the Ca2+ current (n= 4, not shown). The mean peak Ca2+ current elicited by short step depolarizations from -80 mV to +10 mV at 33°C was -174 ± 75 pA (mean ±s.d.; n= 60). We used four drugs to block the different HVA Ca2+ currents found in these cells (Mansvelder et al. 1996; Ciranna et al. 1996; Fig. 1). All drugs took 2–3 min to establish a steady-state level of block (Fig. 1A-D). L-type Ca2+ channels constituted 21 ± 4 % of the whole-cell Ca2+ current at 33°C (n= 8), N-type channels constituted 30 ± 2 % (n= 6), P-type channels constituted 28 ± 3 % (n= 10) and Q-type channels constituted 25 ± 4 % (n= 7). At 33°C the L-type current was non-inactivating (see insets of Fig. 1A), whereas the N-, P- and Q-type currents were all inactivating (see insets of Fig. 1B-D). These results differ somewhat from previous data obtained at room temperature and with Ba2+ as the charge carrier. Under these conditions, the contributions were 35 % for the L-type, 26 % for the N-type and 31 % for the combined P- and Q-type currents (Mansvelder et al. 1996). The differences in percentages between these conditions might result from different temperature dependencies, or from differences in the relative conductance of Ca2+ and Ba2+ for different channel types.
Figure 1. Kinetics of block of the whole-cell HVA Ca2+ current by different blockers.
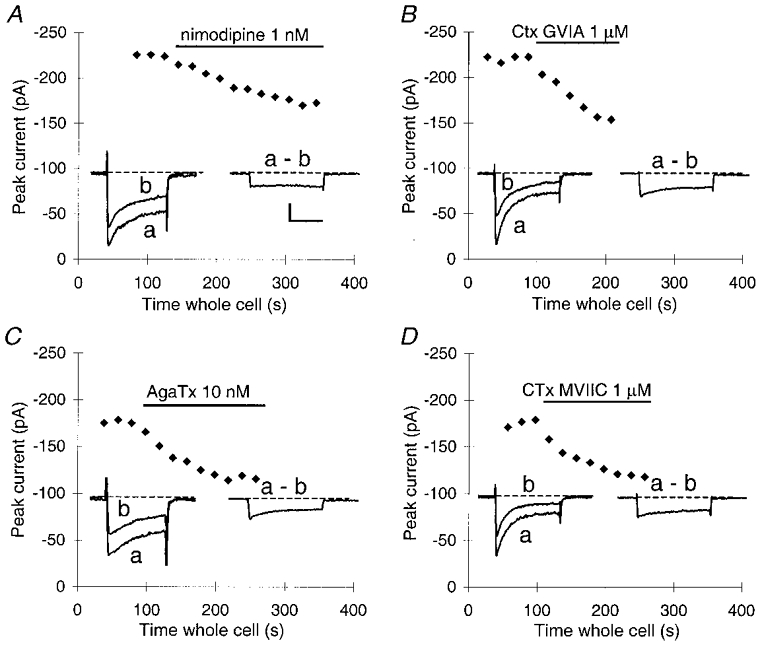
Block was induced by 1 nM nimodipine (A), 1 μmω-CgTx GVIA (B), 10 nM ω-AgTx TK (C) and 1 μmω-CgTx MVIIC (D). Every data point represents the peak inward current reached during a 40 ms depolarization from -80 mV to +10 mV (holding potential, -80 mV). To allow Ca2+ channels to recover from inactivation, the interval between depolarizations was 20 s. All drugs were applied after three or four stable control pulses. Bars indicate the timing of drug applications. Left insets, example traces in the absence (a) and presence (b) of the Ca2+ channel blocker. Right insets, means of the current blocked by the Ca2+ channel blocker.
In separate experiments we confirmed earlier data of Keja et al. (1992) that melanotropes express LVA T-type channels. At 33°C, a transient current response was obtained upon stepping from -80 mV to -40 mV, which was completely blocked by 40 μm Ni2+ (n= 3, data not shown).
Contribution of different Ca2+ channel types to exocytosis
Next, we determined the role of each of the different Ca2+ channel types in secretion in melanotropes. To this end, we first examined the effects of the HVA Ca2+ channel blockers on changes in membrane capacitance (ΔCm). Exocytosis in melanotropes is a multiphasic process involving different pools of vesicles, which differ from each other in release readiness (Thomas et al. 1993a; Parsons et al. 1995). The rate of release of these pools ranges from tens of milliseconds up to several seconds. We wanted to study the role of Ca2+ channels in the release of the predocked, immediately releasable pool of vesicles, because this only depends on the distance between the Ca2+ channels and vesicles, and not on additional priming steps. The closer a channel is to the vesicles, the more efficiently that Ca2+ channel couples to exocytosis.
Exocytosis of this small group of predocked release-ready vesicles in melanotropes is complete in ∼50 ms at 30–34°C (Thomas et al. 1993a). To induce the release of vesicles only from this pool, we used step depolarizations to +10 mV of 40 ms. Prior to the step depolarizations the cells were exposed for 3 min to either bath solution alone or bath solution containing one of the Ca2+ channel blockers. Under control conditions, the step depolarization elicited a Ca2+ current of -173 ± 12 pA which induced a ΔCm of 17.1 ± 2.1 fF (n= 18, Figs 2A, and 3A and B). This confirms earlier data from melanotropes (Mansvelder & Kits, 1998) that only a small fraction of the total immediately releasable pool (250 fF; Parsons et al. 1995) is released by a 40 ms depolarization. In cells that were exposed to a Ca2+ channel blocker, both the Ca2+ current and the increase in capacitance in response to step depolarizations were reduced (Fig. 2A). The effects of the blockers on ΔCm were proportional to the effects on the Ca2+ current (Fig. 3A and B). These data show that all HVA Ca2+ channel types contribute to exocytosis in melanotropes.
Figure 2. All classes of Ca2+ channel contribute to release, as indicated by selective activation of T-type channels or application of selective blockers of HVA channels.
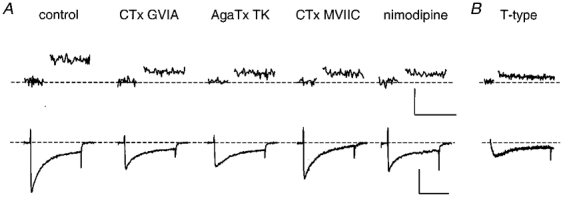
A, upper traces, changes in Cm in response to a step depolarization of 40 ms. The control Cm response and the responses in the presence of the Ca2+ channel blockers were evoked by depolarizations from -80 mV to +10 mV. Lower traces, Ca2+ currents evoked by the step depolarizations. In the presence of blockers, the Ca2+ currents were reduced. Single example traces are shown. B, upper trace, the Cm change in response to only T-type current activation evoked by a 40 ms step depolarization from -80 mV to -40 mV. Lower trace, example of the T-type current response at -40 mV. Application of either control solution or solutions containing one of the blockers was started 3 min before the step depolarizations were applied. Each point in the Cm traces is a consecutive mean of 10 Cm samples with a 1 ms time resolution. Thus, the resulting time resolution of the Cm traces shown here is 10 ms per sample. Each cell was subjected to only one set of five step depolarizations (1 Hz), because a second train of depolarizations always evoked less exocytosis than the first train. The traces shown here are the means of three single traces obtained from one cell per group. Cm measurements were interrupted during the depolarizations.
Figure 3. All classes of calcium channel couple with equal efficency to exocytosis.
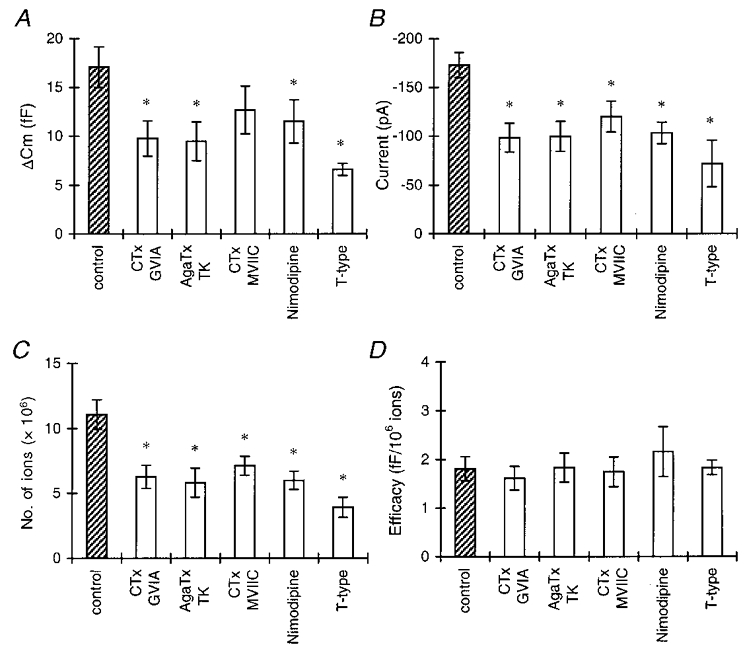
A, the amount of exocytosis per step depolarization for control conditions (total HVA current), selective activation of the T-type current, and the HVA current in the presence of selective HVA Ca2+ channel blockers. First, the mean ΔCm elicited by five step depolarizations was calculated per cell, then the mean for the total group was calculated. Asterisks in A-C indicate statistical differences from the control group ( P < 0.05). B, peak amplitudes of the control Ca2+ current, the T-type current, and the HVA current in the presence of different Ca2+ channel blockers. C, number of Ca2+ ions entering the cell during a step depolarization, calculated as described in Methods. D, efficacy of Ca2+ ions in stimulating exocytosis. ΔCm was divided by the number of Ca2+ ions entering the cell for each depolarizing pulse. For each cell, the mean efficacy for five pulses was calculated. Shown are the means of a number of cells (see text) per group. There was no significant difference between the control group and the groups with the Ca2+ channel blockers. (Stimulation protocols are as described for Fig. 2.)
This conclusion is substantiated when we compare the efficacy of Ca2+ in stimulating exocytosis (Fig. 3D). The reduction of the Ca2+ current implies a decrease in the number of Ca2+ ions that enter the cell during the depolarization (Fig. 3C). Under control conditions the efficacy for the HVA channels, defined as the change in Cm per 106 Ca2+ ions entering the cell, was 1.8 ± 0.25 fF (106 Ca2+ ions)−1. In the presence of blockers the efficacy was not significantly different from that of the control group (Fig. 3D; P > 0.5). Thus, the efficacy of Ca2+ in stimulating exocytosis is constant for all conditions and is not affected if the entry of Ca2+ through a certain type of Ca2+ channel is blocked. From these data, we conclude that all HVA Ca2+ channel types couple to exocytosis with a similar strength.
To establish whether the LVA T-type channels also contribute to release, we recorded the capacitance change upon a 40 ms step to -40 mV. This step depolarization, which only evoked a T-type current response (see above), did induce an increase in Cm (Figs 2B and 3). In six cells, the mean ΔCm was 8.6 ± 0.61 fF, and the efficacy was 1.8 ± 0.15 fF (106 Ca2+ ions)−1. Thus T-type channels also couple to exocytosis in melanotropes and do so with a similar efficacy to that of HVA channels.
APs in melanotropes
Ca2+ channels play an important role in the endogenous electrical activity of melanotropes, since the major part of the AP depends on the activation of voltage-gated Ca2+ channels (Douglas & Taraskevich, 1980, 1982; Williams et al. 1990). Since all HVA Ca2+ channels, despite their differences in activation and inactivation kinetics and voltage dependencies, couple with equal efficiency to exocytosis, a robust and constant relation between calcium influx and exocytosis during the AP is expected. This was specifically tested by asking whether the shape and duration of the APs influence the strength of excitation-secretion coupling.
We made membrane potential recordings from 49 melanotropes to obtain endogenously generated APs that could be used in voltage-clamp experiments as physiological stimuli to induce exocytosis. The resting membrane potential ranged from -35 to -60 mV, with a mean of -49 ± 6 mV (±s.d.), which is in accordance with earlier reports (Williams et al. 1989; Stack & Suprenant, 1991; Lee, 1996). Usually the membrane potential was quite unstable, showing small depolarizations of 5–10 mV. Twenty-two cells (∼45 %) were spontaneously active at 33°C, generating APs (Fig. 4A). Mostly, the AP patterns were irregular. Spontaneous activity declined with recording time, and cells became silent after ∼5 min, possibly due to washout of a soluble component, essential for activity. The duration of the APs ranged from 50 to 300 ms (mean ±s.d., 135 ± 43 ms, measured at ∼10 mV above resting membrane potential). This is in the range of previously reported values for stimulated APs (15-180 ms; Douglas & Taraskevich, 1978, 1980). In contrast to stimulated APs, the peak value of the spontaneous APs never exceeded 0 mV in our measurements. The maximal membrane potential that was reached during an AP was -14 ± 6 mV (mean ±s.d.), resulting in an AP amplitude of ∼35 mV. The frequency and patterning of AP firing differed widely between cells.
Figure 4. AP recordings from melanotropes firing spontaneously.
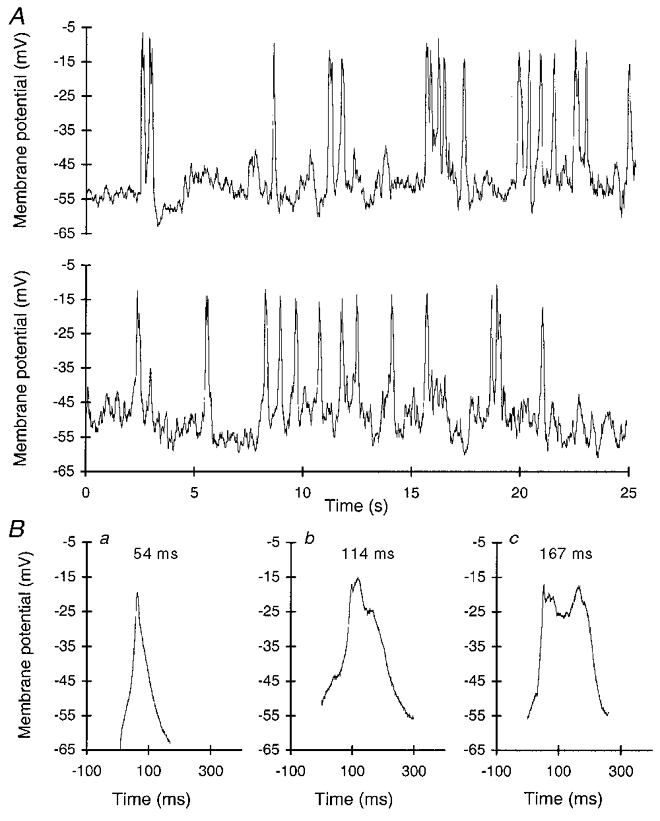
A, example of the spontaneous activity of a melanotrope (consecutive traces). B, APs that were selected to be used as templates for stimulation in voltage-clamp experiments. Durations at half-maximal amplitude are given above the traces.
Since there was considerable variation in the duration of the APs, we selected three AP waveforms that differed widely in shape and duration to cover this variation (Fig. 4B). The AP waveforms had similar peak amplitudes. The shortest waveform lasted 54 ms at half-maximal amplitude (Fig. 4Ba). The AP waveform of intermediate duration lasted 114 ms at half-maximal amplitude (Fig. 4Bb). The waveform of longest duration lasted 167 ms at half-maximal amplitude (Fig. 4Bc). These AP waveforms were slightly modified to be used as templates in voltage-clamp experiments for the following reasons. (1) To be able to monitor the membrane capacitance at high amplitude and time resolution, a sine wave of 40 mV amplitude was used. Reliable capacitance recordings require that the membrane conductance remains constant during the entire sine wave (Neher & Marty, 1982; Gilles, 1995). To achieve this a holding potential of -80 mV was used, around which the sine wave fluctuates between -60 and -100 mV. Within this voltage range no Ca2+ channels were activated. The APs were adjusted to slowly ramp from -80 mV to about the original resting membrane potential, without activating any Ca2+ current. (2) We wanted to measure the membrane capacitance as soon as the waveform ended. The use of the PULSE software allowed us to measure the membrane capacitance with only a 5 ms delay. It required us, however, to re-construct the APs as a series of ramps.
The efficacy of different AP waveforms in eliciting exocytosis
To determine how much exocytosis was elicited by each of the three AP waveforms, we stimulated cells 5 times in succession at 1 Hz with one of the AP waveforms (Fig. 5A). We monitored the calcium current during the AP waveform (Fig. 5B) and we recorded the membrane capacitance before and immediately after each imposed AP waveform (Fig. 5C). The Ca2+ current elicited by the AP waveform was in all cases multiphasic, partly due to the successive activation of different types of Ca2+ channel at increasingly depolarized membrane potentials. In most cells, a local maximum was reached by the Ca2+ current during the rising phase of the AP waveform at approximately -50 mV (Fig. 5B). This Ca2+ current solely flows through the LVA T-type channels, since all current at membrane potentials up to -40 mV is blocked by 40 μm Ni2+ (see above and Keja et al. 1992). The absolute maximum of the Ca2+ current (-44 ± 14 pA, mean ±s.d.) coincided with the peak of the AP waveform. This maximum is significantly lower than the -175 pA that is reached when cells are stimulated with a step depolarization (see above). During the decay of the AP waveform, the Ca2+ current deactivated. The duration of the Ca2+ current increased with increasing AP waveform duration.
Figure 5. Exocytosis increases with increasing AP duration.
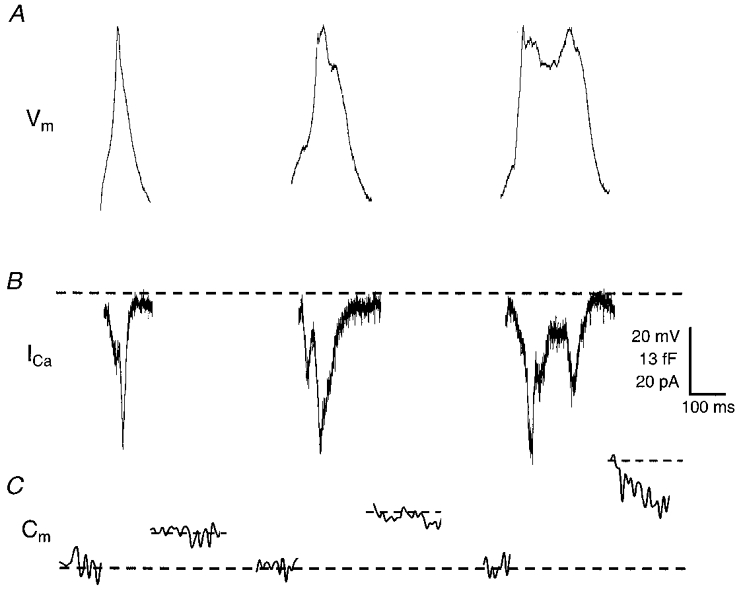
A, AP templates used with the PULSE software. For technical reasons, AP waveforms had to start from a holding potential of -80 mV (see text for explanation). B, example traces of the calcium currents elicited by the APs depicted in A. The peak currents coincided with the peak membrane voltages reached during the AP. C, examples of the membrane capacitance traces associated with the various AP templates. Cm measurements were interrupted during stimulation with the AP templates. Cm traces shown are the means of three such traces obtained from single cells. Note the different time scales in B and C.
All three AP waveforms induced exocytosis, but the amount of exocytosis increased with increasing AP waveform duration (Fig. 5C). Upon stimulation with the short AP waveform, Cm increased by 5.4 ± 0.9 fF (n= 12, Fig. 6A). The intermediate and the long AP waveforms induced a change in Cm of 12.1 ± 1.2 fF (n= 10) and 18.1 ± 2.3 fF (n= 8), respectively. As can be seen from Fig. 6A, Cm increased linearly with AP waveform duration. The number of Ca2+ ions that entered the cell during the AP waveforms also increased linearly with the AP waveform duration (Fig. 6B). This was not due to a change in the peak amplitude of the Ca2+ current, since this was the same for all three AP waveforms (Figs 5B and 6C). Rather, it was caused by the increase in duration of the Ca2+ current. The number of Ca2+ ions entering the cell per AP waveform increased from 4.7 (± 0.6) × 106 during the short AP waveform to 15.6 (± 2.4) × 106 during the long AP waveform. As a result, the efficacy of Ca2+ in stimulating exocytosis, i.e. ΔCm per 106 Ca2+ ions, was constant for the three different APs, and amounted to ∼1.2 fF (106 Ca2+ ions)−1 (Fig. 6D). This is well in accordance with the efficacy found with step depolarizations (Mansvelder & Kits, 1998; Kits et al. 1999; this study). Thus, the equal efficacy of the different types of Ca2+ channel in inducing exocytosis in these cells results in a linear relation between AP duration and exocytosis.
Figure 6. The efficacy of Ca2+ ions in stimulating exocytosis is the same for different APs.
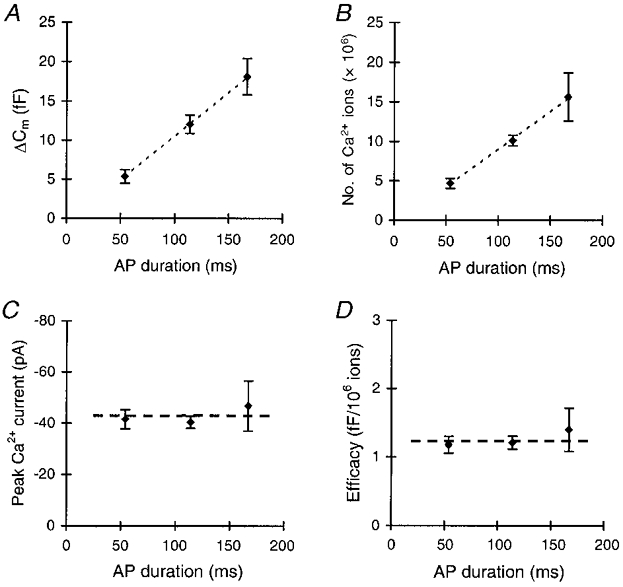
A, the mean increase in Cm per AP increases with increasing AP duration. Each cell was subjected once to stimulation with five successive APs at 1 Hz. The mean Cm response to these stimulations was calculated for each cell, and these means were averaged for a number of different cells. For each AP waveform different cells were taken. B, the number of Ca2+ ions that entered the cell during the AP increased with increasing AP duration. Dashed lines in A and B are linear regression lines. C, the peak Ca2+ currents during the APs were the same for the different AP waveforms. D, the efficacy of Ca2+ ions in stimulating exocytosis, expressed as the ratio of capacitance change per 106 Ca2+ ions, was constant for the different AP waveforms (n= 8–12). The duration of the idealized APs, as expressed on the horizontal axis in A-D, was measured at the half-maximal amplitude.
The efficacy during different phases of the AP
Finally, we examined whether the linearity of the stimulus-secretion coupling also holds during an AP. Due to the different biophysical properties of the five different types of Ca2+ channel (Keja et al. 1992; Keja & Kits, 1994; Mansvelder et al. 1996), it is likely that the types of channel that are activated during the rising phase of the AP differ from the types of channel that are activated during the plateau phase. Furthermore, with the ongoing depolarization at the plateau, only the non-inactivating Ca2+ channels will remain activated. Moreover, the driving force for Ca2+ during the rising phase will be different from the driving force during the plateau phase. As a result, the different phases of the AP will most probably generate different Ca2+ concentration profiles under the cell membrane. This may affect the efficacy of Ca2+ in stimulating exocytosis during different parts of the long AP.
We stimulated melanotropes with increasing fractions of the long AP waveform (Fig. 7A 1–4). The first fraction we used consisted of the first half of the rising phase, up to -45 mV (Fig. 7A1). During this voltage trajectory, only LVA T-type channels are activated (see above). The LVA current stimulated exocytosis, albeit weakly (Fig. 7C 1). On average, the LVA current had an amplitude of -23 ± 1.9 pA, which elicited a ΔCm of 2.2 ± 0.89 fF (n= 7, Fig. 8A and C). We then monitored exocytosis stimulated with the complete rising phase of the AP waveform (up to -17 mV). The rising phase activated an extra component of the Ca2+ current, yielding a significantly larger peak current (-45 ± 7.3 pA; P < 0.01, Fig. 4C). The amount of exocytosis elicited was also increased to 6.7 ± 1.91 fF (Fig. 7A2-C 2). This trend continued when stimulus templates of longer duration were used (Fig. 7A3-C 3 and A4-C 4).
Figure 7. Different phases of the AP contribute equally to exocytosis.
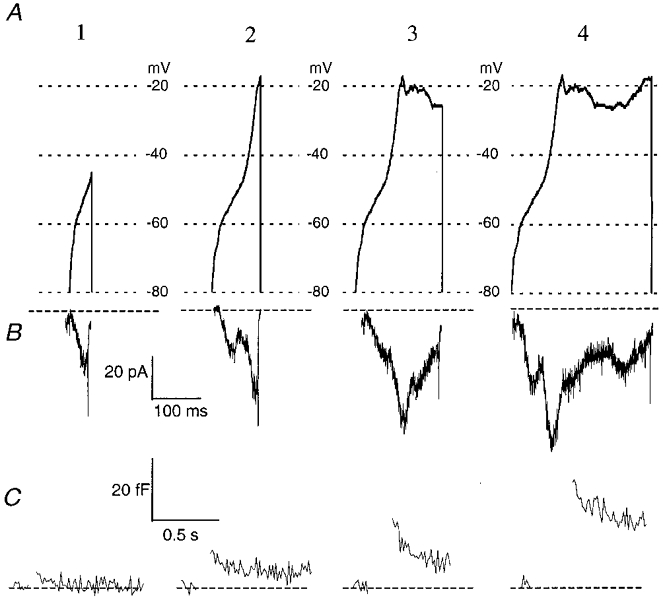
A, stimulus templates of the different parts of the AP. Template 1 is the first half of the rising phase up to a voltage of -45 mV. Template 2 comprises the complete rising phase. The last template (4) is the complete double AP without the repolarizing phase. All templates ended with an immediate step back to the rest holding potential of -80 mV. B, examples of the Ca2+ currents evoked by the templates shown in A. As in Fig. 2, different peaks can be distinguished in the traces. Trace 1 shows the current carried by LVA T-type channels, since the maximal voltage during stimulation was -45 mV. In the other traces, HVA components were also present. C, changes in Cm evoked by the Ca2+ currents in B. With increasing pulse duration, ΔCm also increased.
Figure 8. The efficacy in stimulating exocytosis is the same for different phases of the AP.
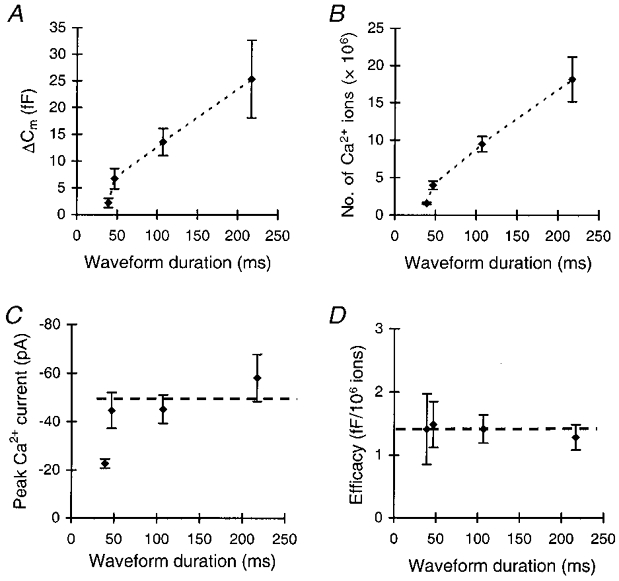
A, mean capacitance change evoked by different phases of the AP, plotted as ΔCm against waveform duration, measured at the half-maximal amplitude. Each cell was subjected to one train of five stimuli. B, the number of Ca2+ ions entering the cell during a stimulus for the different stimulus templates. Note the very similar patterns of increase in the number of entering ions and in the ΔCm in B and A. C, maximal amplitude of the Ca2+ currents during the stimulations. Asterisks denote a significant difference from the peak current in the other groups at P < 0.05. D, the efficacy of Ca2+ ions in stimulating exocytosis for the different stimulus templates, expressed as the capacitance change per 106 Ca2+ ions (n= 7 for each template).
The amount of exocytosis and the number of entering Ca2+ ions evoked by the three longer waveforms showed a similar pattern of increase with increasing waveform duration (Fig. 8A and B). In both cases the shortest waveform did not conform to this trend. This most probably results from the significantly smaller amplitude of the LVA current during the shortest waveform (Fig. 8C). The number of entering ions was 1.58 (± 0.13) × 106 when only the T-type current was activated and it increased to 18.2 (± 2.98) × 106 upon activation of LVA and HVA currents during the longest stimulus. Figure 8A and B thus clearly shows that over the entire duration of the waveform, the increasing number of entering Ca2+ ions correlates with a concomitant increase in exocytosis. As a result, the efficacy of Ca2+ in stimulating exocytosis was constant for all parts of the AP waveform. It amounted to ∼1.4 fF (106 Ca2+ ions)−1 for all stimulus durations (Fig. 8D). Thus, although different Ca2+ concentration profiles may be generated by the different phases of the AP waveform, exocytosis is always evoked with a constant efficacy. Furthermore, the results show that Ca2+ ions flowing through T-type Ca2+ channels are equally capable of stimulating exocytosis as Ca2+ ions flowing through the HVA Ca2+ channels. Taken together, the data presented in this paper suggest that, in melanotropes, the equal strength with which the Ca2+ channels couple to exocytosis gives rise to a linear stimulus-secretion coupling during normal AP firing. This suggests that, in melanotropes, the duration of the AP effectively controls the amount of hormone secretion.
DISCUSSION
Melanotropes in the intermediate pituitary of the rat secrete hormones from LDCVs upon the influx of Ca2+ through voltage-gated Ca2+ channels. Five different types of Ca2+ channel are expressed by these cells (Mansvelder et al. 1996; Ciranna et al. 1996). In the present study, we examined whether the different channels couple with different efficiencies to exocytosis. We found that blockers of L-, N-, P- and Q-type Ca2+ channels all reduced exocytosis. However, none of them affected the efficacy of Ca2+ ions in stimulating exocytosis, suggesting that HVA Ca2+ channels couple equally efficiently to exocytosis. Also, when we selectively activated the LVA T-type channels, exocytosis was evoked with the same efficacy as when the HVA components were also present. These data show that any influx of Ca2+ ions, regardless of the Ca2+ channel type through which this occurs, will contribute to exocytosis in an equal fashion.
Ca2+ channels and vesicles are not co-localized in melanotropes
In a previous study (Mansvelder & Kits, 1998), we showed that fast Ca2+-dependent exocytosis was affected by sub-millimolar concentrations of Ca2+ buffers. This was taken as evidence that diffusion of Ca2+ to the predocked vesicles causes a delay between channel activity and secretion that allows Ca2+ chelators to interfere with exocytosis. Model studies (Kits et al. 1999) yielded an adequate description of these data when a distance between Ca2+ channels and LDCVs of > 100 nm was assumed. The present study demonstrates that all Ca2+ channel classes couple with a similar strength to exocytosis. This reinforces the hypothesis that, in melanotropes, LDCVs do not co-localize with any specific type of Ca2+ channel, nor with Ca2+ channels in general.
In adult bovine chromaffin cells, secretion of catecholamines starts with a delay of 5–100 ms and persists for tens of milliseconds after Ca2+ entry has stopped, which is mostly attributable to Ca2+ diffusion (Chow et al. 1992, 1994, 1996). Model studies by Klingauf & Neher (1997) showed that these processes were best accommodated by assuming a distance between Ca2+ channels and the majority of vesicles of ∼200-300 nm. Only a small fraction (∼8 %) of the LDCVs had to be located at a distance of ∼30 nm to account for the short delays of ∼5 ms (see also Seward & Nowycky, 1996; Elhamdani et al. 1998). We cannot exclude the possibility that in melanotropes a small fraction of LDCVs are docked within ∼30 nm from a Ca2+ channel, since the ultrafast release of this fraction would probably not be detected with our approach. However, since only ∼5-20 vesicles (assuming 1 fF per LDCV) are released during an AP, this would pertain to only 1–2 vesicles, even if ∼10 % of the vesicles were docked this close to the Ca2+ channels. We conclude that in melanotropes, as in chromaffin cells, the majority of the predocked, rapidly releasable LDCVs do not co-localize with Ca2+ channels.
The absence of co-localization of vesicles and Ca2+ channels indicates that none of the Ca2+ channel types expressed by melanotropes has a leading role in exocytosis. This distinguishes melanotropes from neurons and many other neuroendocrine cells. In different neuroendocrine cell types, preferential docking of release-ready LDCVs near specific types of Ca2+ channel does occur, thereby ensuring the coupling of secretion to these channels. Examples are vasopressinergic neurohypophyseal nerve endings (Wang et al. 1997), mouse pancreatic β-cells (Bokvist et al. 1995), pancreatic A-cells (Gromada et al. 1997) and calf chromaffin cells (Artalejo et al. 1994; Elhamdani et al. 1998). In these preparations, the docking of LDCVs near Ca2+ channels appears to be precisely regulated and gives rise to distinct roles for specific Ca2+ channel types in exocytosis. This mechanism is most probably absent in melanotropes, rendering all Ca2+ channel types equally important in stimulating exocytosis.
A linear relationship between calcium entry and secretion characterizes stimulus-secretion coupling in melanotropes
One of the remarkable features of the release process of LDCVs in melanotropes is the linear relationship between the number of Ca2+ ions that enter the cell and the amount of exocytosis that is induced. This property holds during a train of step depolarizations, with different intracellular EGTA concentrations and with different pulse durations (Mansvelder & Kits, 1998; Kits et al. 1999; this study). Here, we found that exocytosis stimulated by AP waveforms with very different shapes and durations still displayed a constant relation to Ca2+ influx. Most probably, the equal strength with which Ca2+ channels couple to exocytosis results in a linear stimulus-secretion coupling when cells fire APs.
In general, the release of LDCVs from neuroendocrine cells has a third or fourth power relation to either the extracellular or intracellular Ca2+ concentration:
where n is 3 or 4 (Dodge & Rahamimoff, 1967; Augustine & Charlton, 1986; Stanley, 1986; Thomas et al. 1990; Heinemann et al. 1993; Borst & Sakmann, 1996). This power relation between concentration and secretion also exists in melanotropes (Thomas et al. 1990). However, little attention has been given to the question of how this power relation translates to the relation between the number of entering Ca2+ ions and the amount of exocytosis that is stimulated by these ions. Our findings indicate that this relation can have a very stable linearity.
In adrenal chromaffin cells, exocytosis relates to the Ca2+ concentration with a power of 3 (Heinemann et al. 1993), yet the relation between Ca2+ entry and exocytosis is characterized by a power of ∼1.9 (Engisch & Nowycky, 1996). This relation is resistant to a number of different experimental conditions and protocols (Engisch & Nowycky, 1996). In nerve terminals in the posterior pituitary (Hsu & Jackson, 1996) and in the bullfrog sympathetic ganglia (Peng & Zucker, 1993) exocytosis of LDCVs increases linearly with Ca2+ influx. Finally, in the giant axon of the squid, where the amplitude of the postsynaptic current also scales to the third or fourth power of the extracellular Ca2+ concentration (Augustine & Charlton, 1986; Stanley, 1986), the postsynaptic current scales linearly to the duration of the AP and the ensuing presynaptic Ca2+ entry (Augustine, 1990). Evidently, a linear relation between Ca2+ entry and exocytosis in a system where a higher power relation between calcium concentration and exocytosis is found, is not limited to neuroendocrine systems, but also extends to neurons.
How the power relation might translate into linearity
How is it possible that a higher power relation between Ca2+ concentration and exocytosis translates into a linear relation between Ca2+ entry and exocytosis? To explain the linearity in the squid giant presynaptic terminal, Augustine (1990) suggested that Ca2+ might trigger secretion from domains that do not overlap. Increasing the Ca2+ current by increasing the duration of the AP would then trigger increasingly more domains to release. This most probably offers no explanation for the linearity of the relation between Ca2+ entry and exocytosis in the melanotrope, since the distance between Ca2+ channels and LDCVs is large (100-200 nm; Mansvelder & Kits, 1998; Kits et al. 1999). Domains from which secretion is triggered will most probably overlap.
For the melanotrope, we favour an alternative explanation which is based upon buffer saturation occurring locally under the plasma membrane (Kits et al. 1999). Multiple factors (e.g. size and duration of the single channel currents, diffusion and buffer conditions) influence the relation between influx and concentration. Using computer simulations, we found earlier that the relation between entry and exocytosis can be linear, depending on the assumed conditions for diffusion (Kits et al. 1999). Under the assumptions of homogeneous calcium entry and free diffusion of Ca2+ and intracellular calcium buffers in all directions, the efficacy of Ca2+ ions in stimulating exocytosis is not constant, but increases with pulse duration (Kits et al. 1999). The constant relation emanates when an inhomogeneous cytoplasm is assumed, where diffusion of Ca2+ ions and buffers is not free in all directions, for instance due to the presence of intracellular membranes. Mobile Ca2+ buffers become saturated locally and the Ca2+ concentration at the site of exocytosis is to a lesser extent determined by the buffer conditions. Under defined conditions, this results in a linear relation between Ca2+ entry and exocytosis (Kits et al. 1999). Limited diffusion and buffer saturation thus obscure the third power relation between Ca2+ concentration and exocytosis. The present finding that the linear relation bears relevance to the situation where APs stimulate exocytosis may indicate that also during an AP diffusion limited by barriers and buffer saturation primarily determines the relation between Ca2+ entry and secretion. Extrapolating our results to the adrenal chromaffin cell, where the distance between Ca2+ channels and the majority of the vesicles is also large (Klingauf & Neher, 1997), the deviation from the third power factor may suggest that buffer saturation plays a substantial role in stimulus-secretion coupling in these cells as well.
Physiological significance of a linear relationship between Ca2+ entry and exocytosis
Earlier work on melanotropes revealed that APs contain a large Ca2+ current-dependent component (Douglas & Taraskevich, 1980, 1982; Williams et al. 1989). This is confirmed by our findings: Ca2+ currents were activated over the entire duration of the AP. In synaptic terminals, in squid as well as in mammals, where APs have a much shorter duration, the Ca2+ current seems to be predominantly a tail current, reinforced by the increasing driving force during the falling phase of the AP (Augustine, 1990; Borst & Sakmann, 1996). At physiological temperatures, however, the peak of the Ca2+ current can coincide with the peak of the AP (Sabatini & Regehr, 1997). In melanotropes, Ca2+ currents are markedly activated during the rising phase of the AP. Most probably, LVA T-type Ca2+ channels play an important role in initiating the rising phase of the AP, since activation of the Ca2+ current had already started at -60 mV and the first maximum was reached at around -50 mV. By using only the initial phase of the AP to stimulate exocytosis, we found that the influx of Ca2+ through the LVA T-type channels not only serves excitability (Llinás & Yarom, 1981), but also contributes to exocytosis. Interestingly, it stimulated exocytosis with the same efficacy as Ca2+ entry during later phases of the AP.
Unlike in melanotropes, in other neuroendocrine cells such as mouse pancreatic β-cells and rat chromaffin cells, single APs couple poorly to exocytosis (Ämmäläet al. 1993; Zhou & Misler, 1995), while bursts of APs (four or more APs in β-cells) evoked exocytosis consistently. However, APs in the latter cell types only last a few milliseconds instead of 50–300 ms in melanotropes. In calf chromaffin cells (Elhamdani et al. 1998), single fast APs are able to elicit secretion reliably, because LDCVs are docked very close to Ca2+ channels. The slow rising phase of APs in melanotropes, with respect to the fast APs of chromaffin cells, may be well tuned to the large distance between Ca2+ channels and vesicles. With slow AP kinetics, a strict co-localization of channels and vesicles is simply not needed. The equal strength with which all Ca2+ channels couple to exocytosis guarantees that there is exocytosis over the enitre duration of the AP.
Acknowledgments
This work was supported by an NWO-Medical Research Council grant (903-42-008). We are grateful to Jacqueline Leyting-Vermeulen for assistance with preparing the cell culture and to Hans Lodder and Frank Groot for assistance with some of the experiments.
References
- Ämmälä C, Ashcroft FM, Rorsman P. Calcium-independent potentiation of insulin release by cyclic AMP in single β cells. Nature. 1993;363:356–358. doi: 10.1038/363356a0. [DOI] [PubMed] [Google Scholar]
- Artalejo CR, Adams ME, Fox AP. Three types of Ca2+ channel trigger secretion with different efficacies in chromaffin cells. Nature. 1994;367:72–76. doi: 10.1038/367072a0. [DOI] [PubMed] [Google Scholar]
- Artalejo CR, Mogul DJ, Perlman RL, Fox AP. Bovine chromaffin cells exhibit three types of calcium channels: facilitation, induced by large pre-depolarizations or repetitive activity is due to the increased opening probability of a 27 pS channel. The Journal of Physiology. 1991;444:213–240. doi: 10.1113/jphysiol.1991.sp018874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine GJ. Regulation of transmitter release at the squid giant synapse by presynaptic delayed rectifier potassium current. The Journal of Physiology. 1990;431:343–364. doi: 10.1113/jphysiol.1990.sp018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine GJ, Charlton MP. Calcium dependence of presynaptic calcium current and post-synaptic response at the squid giant synapse. The Journal of Physiology. 1986;381:619–640. doi: 10.1113/jphysiol.1986.sp016347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Bokvist K, Eliasson L, Ämmälä C, Renstrom E, Rörsman P. Co-localization of L-type Ca2+ channels and insulin-containing secretory granules and its significance for the initiation of exocytosis in mouse pacreatic B-cells. EMBO Journal. 1995;14:50–57. doi: 10.1002/j.1460-2075.1995.tb06974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- Chow RH, Klingauf J, Heinemann C, Zucker RS, Neher E. Mechanisms determining the time course of secretion in neuroendocrine cells. Neuron. 1996;16:369–376. doi: 10.1016/s0896-6273(00)80054-9. [DOI] [PubMed] [Google Scholar]
- Chow RH, Klingauf J, Neher E. Time course of Ca2+ concentration triggering exocytosis in neuroendocrine cells. Proceedings of the National Academy of Sciences of the USA. 1994;91:12765–12769. doi: 10.1073/pnas.91.26.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RH, Von Rüden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Ciranna L, Feltz P, Schlichter R. Selective inhibition of high voltage-activated L-type and Q-type calcium currents by serotonin in rat melanotrophs. The Journal of Physiology. 1996;490:595–609. doi: 10.1113/jphysiol.1996.sp021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge FA, Rahamimoff R. Co-operative action of calcium ions in transmitter release at the neuromuscular junction. The Journal of Physiology. 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas WW, Taraskevich PS. Action potentials in gland cells of rat pituitary pars intermedia: inhibition by dopamine, an inhibitor of MSH secretion. The Journal of Physiology. 1978;285:171–184. doi: 10.1113/jphysiol.1978.sp012565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas WW, Taraskevich PS. Calcium component to action potentials in rat pars intermedia cells. The Journal of Physiology. 1980;309:623–630. doi: 10.1113/jphysiol.1980.sp013530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas WW, Taraskevich PS. Slowing effects of dopamine and calcium-channel blockers on frequency of sodium spikes in rat pars intermedia cells. The Journal of Physiology. 1982;326:201–211. doi: 10.1113/jphysiol.1982.sp014186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhamdani A, Zhou Z, Artalejo CR. Timing of dense-cored vesicle exocytosis depends on the facilitation L-type calcium channel in adrenal chromaffin cells. Journal of Neuroscience. 1998;18:6230–6240. doi: 10.1523/JNEUROSCI.18-16-06230.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engisch KL, Nowycky MC. Calcium dependence of large dense-cored vesicle exocytosis evoked by calcium influx in bovine adrenal chromaffin cells. Journal of Neuroscience. 1996;16:1359–1369. doi: 10.1523/JNEUROSCI.16-04-01359.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles KD. Techniques for membrane capacitance measurements. In: Sakmann B, Neher E, editors. Single-Channel Recording. 2. New York: Plenum Press; 1995. [Google Scholar]
- Gromada J, Bokvist K, Ding W-G, Barg S, Buschard K, Renström E, Rorsman P. Adrenaline stimulates glucagon secretion in pancreatic A-cells by increasing the Ca2+ current and the number of granules close to the L-type Ca2+ channels. Journal of General Physiology. 1997;110:217–228. doi: 10.1085/jgp.110.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann C, Von Rüden L, Chow RH, Neher E. A two-step model of secretion control in neuroendocrine cells. Pflügers Archiv. 1993;424:105–112. doi: 10.1007/BF00374600. [DOI] [PubMed] [Google Scholar]
- Horrigan FT, Bookman RJ. Releasable pools and the kinetics of exocytosis in adrenal chromaffin cells. Neuron. 1994;13:1119–1129. doi: 10.1016/0896-6273(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Hsu S-F, Jackson MB. Rapid exocytosis and endocytosis in nerve terminals of the rat posterior pituitary. The Journal of Physiology. 1996;494:539–553. doi: 10.1113/jphysiol.1996.sp021512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keja JA, Kits KS. Single channel properties of high- and low-voltage-activated calcium channels in rat pituitary melanotropic cells. Journal of Neurophysiology. 1994;71:840–855. doi: 10.1152/jn.1994.71.3.840. [DOI] [PubMed] [Google Scholar]
- Keja JA, Stoof JC, Kits KS. Voltage-activated currents through calcium channels in rat pituitary melanotropic cells. Neuroendocrinology. 1991;53:349–359. doi: 10.1159/000125741. [DOI] [PubMed] [Google Scholar]
- Keja JA, Stoof JC, Kits KS. Dopamine D2 receptor stimulation differentially affects voltage-activated calcium currents in rat pituitary melanotropic cells. The Journal of Physiology. 1992;450:409–435. doi: 10.1113/jphysiol.1992.sp019134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kits KS, De Vlieger TA, Kooi BW, Mansvelder HD. Diffusion barriers limit the effect of mobile calcium buffers on exocytosis of large dense cored vesicles. Biophysical Journal. 1999;76:1693–1705. doi: 10.1016/S0006-3495(99)77328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingauf J, Neher E. Modelling buffered Ca2+ diffusion near the membrane; implications for secretion in neuroendocrine cells. Biophysical Journal. 1997;72:674–690. doi: 10.1016/s0006-3495(97)78704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK. Dopamine D2 receptor regulation of intracellular calcium and membrane capacitance changes in rat melanotrophs. The Journal of Physiology. 1996;495:627–640. doi: 10.1113/jphysiol.1996.sp021621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M, Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflügers Archiv. 1988;411:137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Llinás R, Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurons in vitro. The Journal of Physiology. 1981;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, Kits KS. The relation of exocytosis and rapid endocytosis to calcium entry evoked by short repetitive depolarizing pulses in rat melanotropic cells. Journal of Neuroscience. 1998;18:81–92. doi: 10.1523/JNEUROSCI.18-01-00081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, Kits KS. The control of exocytosis by different types of calcium channels and physiological waveforms. Biophysical Journal. 1999;76:A400. [Google Scholar]
- Mansvelder HD, Stoof JC, Kits KS. Dihydropyridine block of ω-agatoxin IVA and ω-conotoxin GVIA sensitive Ca2+ channels in rat pituitary melanotropic cells. European Journal of Pharmacology. 1996;311:293–304. doi: 10.1016/0014-2999(96)00432-3. [DOI] [PubMed] [Google Scholar]
- Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin. Proceedings of the National Academy of Sciences of the USA. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TD, Coorssen JR, Horstmann H, Almers W. Docked granules, the exocytotic burst, and the need for ATP hydrolysis in endocrine cells. Neuron. 1995;15:1085–1096. doi: 10.1016/0896-6273(95)90097-7. [DOI] [PubMed] [Google Scholar]
- Peng Y-Y, Zucker RS. Release of LHRH is linearly related to the time integral of presynaptic Ca2+ elevation a threshold level in bullfrog sympathetic ganglia. Neuron. 1993;10:465–473. doi: 10.1016/0896-6273(93)90334-n. [DOI] [PubMed] [Google Scholar]
- Sabatini BL, Regehr W. Timing of neurotransmission at fast synapses in the mammalian brain. Nature. 1997;384:170–172. doi: 10.1038/384170a0. [DOI] [PubMed] [Google Scholar]
- Seward EP, Nowycky MC. Kinetics of stimulus-coupled secretion in dialyzed bovine chromaffin cells in response to trains of depolarizing pulses. Journal of Neuroscience. 1996;16:553–562. doi: 10.1523/JNEUROSCI.16-02-00553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z-H, Rettig J, Cook T, Catterall WA. Calcium-dependent interaction of N-type calcium channels with the synaptic core complex. Nature. 1996;379:451–454. doi: 10.1038/379451a0. [DOI] [PubMed] [Google Scholar]
- Stack J, Suprenant A. Dopamine actions on calcium currents, potassium currents and hormone release in rat melanotrophs. The Journal of Physiology. 1991;439:37–58. doi: 10.1113/jphysiol.1991.sp018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EF. Decline in calcium cooperativity as the basis of facilitation at the squid giant synapse. Journal of Neuroscience. 1986;6:782–789. doi: 10.1523/JNEUROSCI.06-03-00782.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Suprenant A, Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990;5:723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- Thomas P, Wong JG, Almers W. Millisecond studies of secretion in single rat pituitary cells stimulated by flash photolysis of caged Ca2+ EMBO Journal. 1993a;12:303–306. doi: 10.1002/j.1460-2075.1993.tb05657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Wong JG, Lee AK, Almers W. A low affinity Ca2+ receptor controls the final steps in peptide secretion from pituitary melanotrophs. Neuron. 1993b;11:93–104. doi: 10.1016/0896-6273(93)90274-u. [DOI] [PubMed] [Google Scholar]
- Wang G, Dayanithi G, Kim S, Hom D, Nadasdi L, Kristipati R, Ramachandran J, Stuenkel EL, Nordmann JJ, Newcomb R, Lemos JR. Role of Q-type Ca2+ channels in vasopressin secretion from neurohypophyseal terminals of the rat. The Journal of Physiology. 1997;502:351–363. doi: 10.1111/j.1469-7793.1997.351bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PJ, MacVicar BA, Pittman QJ. A dopaminergic inhibitory postsynaptic potential mediated by an increased potassium conductance. Neuroscience. 1989;31:673–681. doi: 10.1016/0306-4522(89)90432-6. [DOI] [PubMed] [Google Scholar]
- Williams PJ, MacVicar BA, Pittman QJ. Electrophysiological properties of neuroendocrine cells of the intact rat pars intermedia: multiple calcium currents. Journal of Neuroscience. 1990;10:748–756. doi: 10.1523/JNEUROSCI.10-03-00748.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Misler S. Action potential-induced quantal secretion of catecholamines from rat adrenal chromaffin cells. Journal of Biological Chemistry. 1995;270:3498–3505. [PubMed] [Google Scholar]
- Zucker RS. Exocytosis: A molecular and physiological perspective. Neuron. 1996;17:1049–1055. doi: 10.1016/s0896-6273(00)80238-x. [DOI] [PubMed] [Google Scholar]


