Abstract
The rate at which an isometrically contracting muscle uses energy is thought to be proportional to its twitch speed. In both slow and fast muscles, however, a constant proportion (25-40 %) of the total energy has been found to be used by SR-Ca2+ pumps and the remainder by crossbridges. We examined whether SR-Ca2+ pumps account for a larger proportion of the energy in the fastest vertebrate muscle known (the toadfish swimbladder), and whether the swimbladder muscle utilizes energy at the superfast rate one would predict from its mechanics.
The ATP utilization rates of the SR-Ca2+ pumps and crossbridges were measured using a coupled assay system on fibres skinned with saponin. Surprisingly, despite its superfast twitch speed, the ATP utilization rate of swimbladder was no higher than that of much slower fast-twitch amphibian muscles.
The swimbladder achieves tremendous twitch speeds with a modest steady-state ATP utilization rate by employing two mechanisms: having a small number of attached crossbridges and probably utilizing intracellular Ca2+ buffers (parvalbumin) to spread out the time over which Ca2+ pumping can occur.
Finally, although the total ATP utilization rate was not as rapid as expected, the relative proportions used by SR-Ca2+ pumps and the crossbridges were similar to other muscles.
Powering the large variety of motor activities in which animals engage has required the evolution of different muscle types with widely varying contraction speeds. Hill showed as early as 1938 that the rate at which an active muscle uses energy is proportional to its isometric twitch speed (Hill, 1938, 1950). The mechanisms underlying this relationship appear to be that both the rates of SR-Ca2+ pumping and crossbridge cycling increase to permit faster twitch speeds. Because both processes require ATP, there must be an increase in the rate of ATP utilization.
Researchers have been able to partition the energy used during contraction into its two constituent parts by stretching muscle beyond myofilament overlap. In muscles from diverse species representing a large range of twitch speeds, it was found that a constant proportion (∼25-40 %) of the total energy during contraction is used by SR-Ca2+ pumps and nearly all of the remainder by the crossbridges (reviewed in Homsher & Kean, 1978). This finding is consistent with the notion that, as twitch speed of the muscle increases, the crossbridge kinetics and the Ca2+ pumping rate increase in unison. However, it is unclear if this must necessarily be the case. If the Ca2+ transient duration were the rate limiting step of relaxation, it seems possible that, in muscles adapted for very high frequency contractions, the SR-Ca2+ pumping rate might have increased far more dramatically than the crossbridge cycling rate. This in turn would increase the proportion of energy used by SR-Ca2+ pumps. Also, in these high frequency contraction muscles, the relative myofibrillar volume is known to be reduced (e.g. to 50 % in toadfish swimbladder) as the space is taken up by increasing volume of SR (Rome & Lindstedt, 1998). This should increase further the proportion of the total energy utilization by the SR-Ca2+ pumps.
To test this hypothesis, we determined the ATP utilization rate and partitioned it between SR-Ca2+ pumps and crossbridges in the swimbladder muscle of toadfish. The swimbladder has the fastest isometric relaxation rate of any vertebrate muscle (e.g. at 15°C it takes only ∼10 ms for force to fall from 90 to 10 % in swimbladder compared with ∼85 ms in fast twitch fibres of frogs (G. Lutz & L. C. Rome, unpublished observation) and has been shown to have the fastest Ca2+ transient (Rome et al. 1996). It is therefore of interest to determine if this muscle has the same partitioning of energy utilization as the slower muscles previously tested, and whether the swimbladder utilizes energy at the superfast rate one would predict from its mechanics.
Partitioning of energy usage is also helpful in exploring the kinetic modifications of the processes involved in contraction. Another goal of this study was to determine the rate of pumping per SR-Ca2+ pump (i.e. pump turn-over rate). The swimbladder provides an ideal opportunity to study the turn-over rate of SR-Ca2+ pumps in an intact system because it represents one of the few muscles where the density of SR-Ca2+ pumps has been accurately quantified (Appelt et al. 1991). Furthermore, the number of SR-Ca2+ pumps is extremely large, hence one anticipates a signal that is sufficiently large to be measured in small bundles of skinned muscle fibres. In addition, measuring the turn-over rate of the SR-Ca2+ pumps in swimbladder muscle should enable us to determine if they are specially modified for rapid Ca2+ reuptake in the fastest of all vertebrate muscles.
METHODS
Preparation of skinned muscle fibres
Toadfish were killed with a blow to the head and double-pithed according to guidelines set out by the Institutional Animal Use and Care Committees of the University of Pennsylvania and the Marine Biological Laboratories. The toadfish swimbladder muscle consists of a single fibre type. Therefore we were able to dissect small bundles of fibres, which were pure in fibre type. Bundles of two to three fibres (fibre diameter ∼40-50 μm) were ‘skinned’ with 50 μg ml−1 saponin for 20 min, which permeablizes the cell membrane (Stienen et al. 1995; Launikonis & Stephenson, 1997; Rome et al. 1999b), but does not affect the Ca2+ pumps or SR membrane. The ‘skinned’ muscle fibres were then bathed in solutions of various [Ca2+] and the rate of ATP utilization was measured. All experiments were conducted at a sarcomere length of 2.2-2.3 μm and a temperature of 15°C. The rate of ATP utilization for a total of 35 fibre bundles was measured under various protocols. All data are reported as mean ±s.e.m. Statistical significance was set at the P= 0.05 level.
ATP utilization measurements
As previously described (Syme et al. 1997), the rate of ATP hydrolysis was measured in ‘skinned’ fibres so that the creatine phosphokinase regenerating step could be bypassed and the regeneration of ATP coupled to a glycolysis-like cascade that takes advantage of the fluorescent properties of NADH. In this assay, ADP was rephosphorylated by phosphoenolpyruvate (PEP) catalysed by pyruvate kinase (PK). This reaction is then coupled to the oxidation of NADH (fluorescent) to NAD (non-fluorescent) and the reduction of pyruvate to lactate, catalysed by lactic dehydrogenase (LDH).
Because of the one-to-one stoichiometric relationship between ADP production and NADH oxidation, ATP utilization by the muscle (production of ADP) was measured by the decrease in NADH fluorescence of the solution surrounding the muscle fibre. The system was calibrated by determining the fluorescence level as a function of [NADH]. The system was also calibrated by direct injection of known amounts of ADP (as in Stienen et al. 1995).
The chamber contained a 12.8 μl well (Syme et al. 1997) into which solutions entered at one end and were removed from the opposite end via a suction tube. Instead of mixing the solution with a micromachined stirrer (as in Syme et al. 1997), here the solution was stirred by jets of humidified air directed over the surface of the solution. This had the advantage of avoiding shear from the stirrer blades, which, at the high enzyme concentrations used in this study, appeared to cause protein aggregation and diminished optical clarity of our solutions.
We used solutions (ionic strength ∼200 mm, pH = 7) containing a 50 mm Ca2+-EGTA buffering system. They also contained PK (1000 units ml−1), LDH (200 units ml−1), NADH (0.5-0.75 mm), and PEP (5 mm). P1,P5-di(adenosine-5′)pentaphosphate (0.22 mm) was added to the solutions to inhibit myokinase activity and sodium azide (1 mm) was added to prevent ATP utilization by mitochondria (Syme et al. 1997). As explained later, at various points during the experiment, the SR-Ca2+ pumps were poisoned with a combination of 20 μm 2,5-di-(tert-butyl)-1,4-benzohydroquinone (TBQ) and 20 μm cyclopiazonic acid (CPA).
Because of our expectations for a high fibre ATPase rate, we conducted additional experiments to demonstrate that our measured fibre ATPase values were not limited by reaction rates or diffusion. First, injections of ADP into the chamber demonstrated that the reaction can occur more than 100 times faster than the maximum fibre ATPase rate. This value probably underestimates the true reaction rate because the time response of the system (∼1 s) is limited by the injection speed (∼1 s) and the mixing speed (∼1 s; note that the system fluorescence response for injection of NADH, which involved no reactions, is approximately the same as for the injection of ADP). Second, we measured fibre ATPase with different enzyme and substrate concentrations. The use of solutions that contained 3-fold lower [NADH], 10-fold lower [PK] and 2-fold lower [LDH] in preliminary experiments, did not lead to appreciably lower ATP utilization rates than found in the present experiments. Further, doubling the enzyme and NADH concentrations above their already high concentrations in the present experiments, did not increase the ATP utilization rate either. Hence, we conclude that our assay was sufficiently rapid to accurately track the rate at which the fibre used ATP.
At the end of each experiment, fibres were dried and weighed on a Cahn microbalance (Model C-35). To obtain the equivalent wet weight of intact fibres, the skinned fibre dry weight was multiplied by a conversion factor of 8 (obtained by measuring blotted wet weight of an intact muscle bundle, separating the bundle into small fascicles, skinning the bundle, drying it and weighing). The high conversion factor value reflects the particularly low protein content (9.8 %) of swimbladder muscle. Intact fibre volume was calculated assuming a fibre density of 1.05 kg l−1.
Comparison with other results
It was useful to compare our results with literature values for Xenopus. Because they were presented in terms of skinned fibre volumes or measured at different temperatures, the Xenopus values had to be appropriately scaled. To express skinned fibre measurements in terms of intact fibre volume, we assumed a 2-fold osmotic swelling upon skinning (if data were originally presented in terms of skinned fibre volume) or a myosin concentration of 200 μm in intact fibres (if data were originally presented per myosin head). To convert Vmax values from other temperatures to that at 15°C, we used a Q10 value of 1.8. Conversion factors for other species are presented in the text.
RESULTS
Strategies for partitioning ATP utilization between SR-Ca2+ pumps and crossbridges
The simplest strategy for partitioning ATP utilization, described in pioneering work by Stienen and colleagues (1995), is to measure the ATP utilization rate in fully activated fibres (ATPasetotal; giving ATP utilized by both the crossbridges and SR-Ca2+ pumps), then to poison the SR-Ca2+ pumps and remeasure the ATP utilization rate (ATPasepoisoned; giving the ATP utilized just by the crossbridges). The difference between these values (ATPasetotal-poisoned) gives the ATP utilization rate of the SR-Ca2+ pumps.
There are two potential limitations to this technique which we sought to circumvent. First, the ATP utilization rate by the SR-Ca2+pumps (ATPasetotal-poisoned) represents a relatively small difference between two large numbers (ATPasetotal and ATPasepoisoned), and this could lead to substantial errors in ATPasetotal-poisoned (limitation 1). Second, this procedure depends on the reproducibility of ATP utilization rates from one contraction to the next. Systematic changes in ATP utilization rate between contractions could result in substantial systematic errors in the determination of the ATPasetotal-poisoned (limitation 2).
We were able to circumvent limitation 2 by measuring ATPasetotal and ATPasepoisoned during a single activation. During preliminary experiments it was found that, when a mixture of 20 μm CPA and 20 μm TBQ was administered by either injection or a rapid solution change, it effectively and rapidly (∼30 s) poisoned the SR-Ca2+ pumps well within the normal time of a contraction (1-2 min; see Fig. 2). The effects of CPA and TBQ were not completely reversible; hence they were administered (and their concentrations maintained) to permanently block the Ca2+ pumps.
Figure 2. Method for direct measurement of ATP utilization associated with Ca2+ pumping.
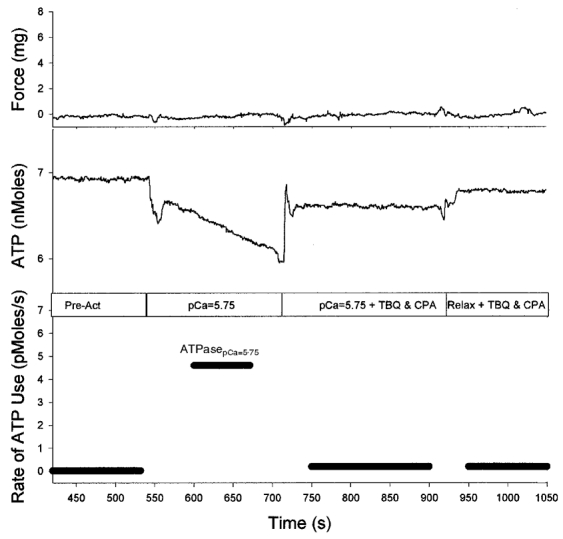
A fibre bundle was first exposed to pre-activating solution with a low [Ca2+] and there was very little utilization of ATP. The solution was then exchanged for one with pCa ≈ 5.75, resulting in the utilization of ATP at a moderate rate (ATPasepCa=5.75; 4.6 pmol s−1) but no force generation. To prove that the ATPasepCa=5.75 does not include ATP utilized by crossbridges, 20 μm TBQ and 20 μm CPA were administered, causing the ATP utilization rate to drop to the baseline. This also shows that TBQ and CPA are effective in poisoning the SR-Ca2+ pumps.
Figure 1 shows an unpoisoned fibre that is maximally activated (pCa = 4.4) and utilizing ATP at a high rate (ATPasetotal) that would normally remain constant for many minutes. In this experiment, however, a potent mixture of Ca2+ pump blockers was administered while keeping the fibre active by changing the solution for an activating solution containing TBQ and CPA. This caused the steady-state ATP utilization rate to drop to a lower level (ATPasepoisoned).
Figure 1. Protocol for determining ATPasetotal and ATPasepoisoned during a single activation.
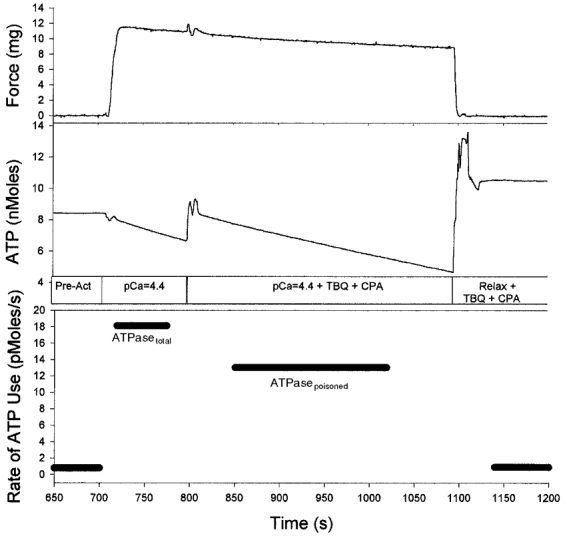
A fibre bundle was first exposed to a pre-activating solution with a low [Ca2+]. The solution was then exchanged for an activating solution with a high [Ca2+] (pCa = 4.4), resulting in maximal force generation and utilization of ATP at a high rate (ATPasetotal; 18.1 pmol s−1). Following administration of 20 μm TBQ and 20 μm CPA to poison the SR-Ca2+ pumps, the ATP utilization rate dropped to a new steady level (ATPasepoisoned; 13.0 pmol s−1). Hence, the difference between ATPasetotal and ATPasepoisoned (ATPasetotal-poisoned) represents the ATP utilization rate associated with Ca2+ pumping (see text for details). Note that for all figures, the middle trace represents the NADH fluorescence, which, based on the calibration of the system, is scaled in terms of quantity of usable ATP in the chamber. In each figure, a small baseline (< 10 % of activated rate) has been subtracted to account for small changes in fluorescence not associated with Ca2-activated ATPase of the crossbridges and Ca2+ pumps. During periods of steady-state changes in fluorescence, the absolute rate of ATP utilization (determined by linear regression) is given in the bottom panel. Note also that for each solution change, there is a change in fluorescence level as well as transients in both the fluorescence and force traces. The fluorescence transients reflect multiple rapid exchanges of solution. The transient in the force trace is a result of these rapid exchanges causing the fibre to vibrate.
Although administering a Ca2+ pump blocker during an activation eliminated errors associated with limitation 2, the remaining errors associated with taking the difference between large numbers (limitation 1) compelled us to devise a new method to determine the ATP utilization associated with Ca2+ pumping directly. The swimbladder muscle has an extremely right-shifted force-pCa relationship (Rome et al. 1996). The pCa50 is approximately 5.23, but it is associated with a sharp onset of force (threshold ≈ 5.4). Our goal was to choose a free calcium concentration at which SR-Ca2+ pumps are pumping at their maximum rate, but where crossbridges would not be interacting and splitting ATP. At this [Ca2+], the ATPase would be a direct measure of the rate of ATP utilization by SR-Ca2+ pumps.
When we exposed the swimbladder muscle to pCa ≈ 5.75, it utilized ATP at a significant rate, but did not generate any force (Fig. 2). To demonstrate that the ATP was utilized exclusively by SR-Ca2+ pumps (and not by crossbridges), the fibre was poisoned by administering the Ca2+ pump blockers. This had the effect of rapidly reducing the ATP utilization rate to zero, providing strong evidence that the ATP utilization measured was not associated with crossbridges, only with SR-Ca2+ pumps (Fig. 2). We were unable to obtain a direct measure of ATP utilization by SR-Ca2+ pumps at higher [Ca2+] because of interference by the crossbridges. The 5 mm caffeine required to prevent accumulation of Ca2+ in the SR, is well known to shift the force-pCa relationship to lower [Ca2+] (Wendt & Stephenson, 1983).
This technique provided a direct measure of ATP utilization associated with Ca2+ pumping at pCa ≈ 5.75 (ATPasepCa=5.75), but is this the maximal ATP utilization rate of the SR-Ca2+ pumps? To assess this, we combined this protocol with the one described in Fig. 1. In this combined protocol (illustrated in Fig. 3), we first measured ATPasepCa=5.75. The fibre was then exposed to pCa = 4.4 and we measured ATPasetotal and ATPasepoisoned. In a set of 14 bundles, we found that the rate of ATP utilization associated with Ca2+ pumping at pCa = 5.75 (ATPasepCa=5.75) was slightly higher than, but not statistically different (Student's paired t test, P= 0.08) from, the ATP utilization rate associated with Ca2+ pumping at pCa = 4.4 (ATPasetotal-poisoned) This suggests that the Ca2+ pumping rate was maximal at pCa = 5.75, and our measurements of ATPasepCa=5.75 gives an accurate estimate of the rate of ATP utilization associated with Ca2+ pumping during maximal activation.
Figure 3. Protocol for determining whether ATPasepCa=5.75 represents the maximal ATP utilization rate of the SR-Ca2+ pumps.
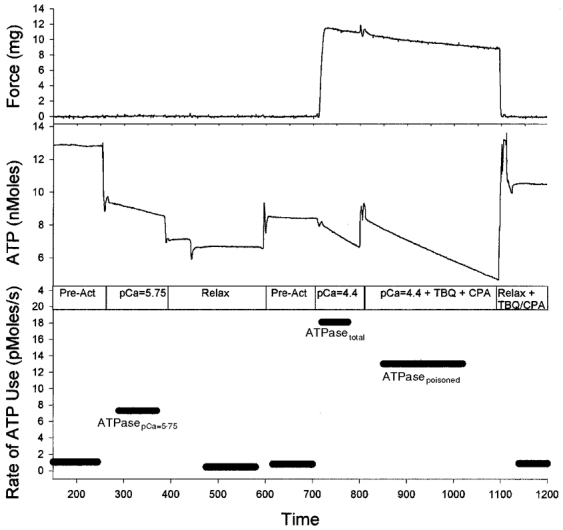
We first measured the ATP utilization rate at pCa = 5.75 (7.3 pmol s−1; similar to Fig. 2, except the fibre was not immediately poisoned by TBQ and CPA), and then changed the solution to pCa = 4.4 and measured ATPasetotal (18.1 pmol s−1) and ATPasepoisoned (13.0 pmol s−1; same fibre as in Fig. 1). We found ATPasepCa=5.75 was similar to the ATP utilization rate associated with Ca2+ pumping at pCa = 4.4 (ATPasetotal-poisoned). This suggests that the Ca2+ pumping rate was nearly maximal at pCa = 5.75.
Our final experimental protocol (illustrated in Fig. 4) consisted of exposing the fibre to pCa = 5.75, measuring ATPasepCa=5.75, poisoning the SR-Ca2+ pumps and remeasuring the rate of ATP utilization to verify the efficacy of the poison. The poisoned fibre was then exposed to pCa = 4.4 and ATPasepoisoned was measured. This protocol had the advantage of eliminating the need for calculating the difference between large values (limitation 1) and, because the crossbridge ATPase was measured on the first force-producing contraction, limitation 2 was also avoided.
Figure 4. Standard protocol for direct measurements of the ATP utilization associated with SR-Ca2+ pumping and crossbridge cycling on a given fibre bundle.
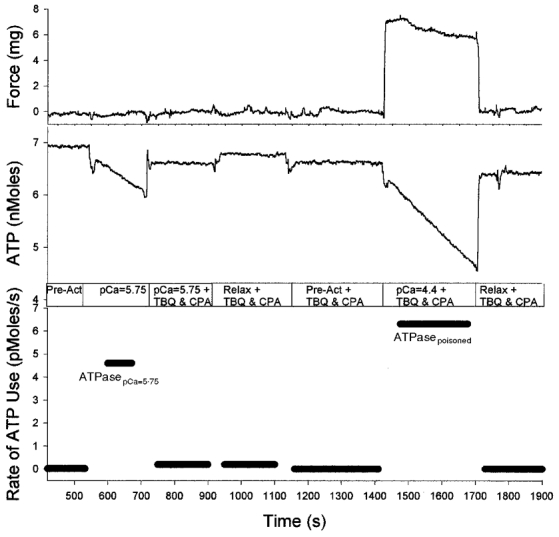
First, ATPasepCa=5.75 was measured (4.6 pmol s−1). The SR-Ca2+ pumps were then poisoned, and ATP utilization was remeasured at pCa = 5.75 to verify complete blockage of the pumps (same fibre as Fig. 2.). The poisoned fibre was then placed at a pCa of 4.4 and ATPasepoisoned was measured (6.3 pmol s−1).
ATPase of Ca2+ pumps and crossbridges in swimbladder fibres
For a set of seven fibres, the mean rate of ATP utilization by SR-Ca2+ pumps was 0.45 ± 0.04 mmol ATP s−1 per litre of intact muscle ((l muscle)−1) and the mean rate of ATP utilization by the crossbridges was 0.77 ± 0.04 mmol ATP s−1 (l muscle)−1. Thus, on average, the SR-Ca2+ pumps utilized 37 ± 2 % of the ATP used in isometric contraction compared with 63 ± 2 % by the crossbridges. In another set of bundles (N= 14) run under a slightly different protocol (control experiments illustrated in Fig. 3 to test whether SR-Ca2+ pumps were operating at their maximum rate), we obtained approximately 20 % lower ATP utilization rates for both the SR-Ca2+ pumps and crossbridges. However, the proportions (38 ± 2 and 62 ± 2 % for the SR-Ca2+ pumps and the crossbridges, respectively) were nearly identical to those reported above.
DISCUSSION
High twitch speeds at low steady-state costs
Despite the extraordinary twitch speed of the swimbladder muscle, the steady-state rate of ATP utilization in skinned fibres is not exceptionally fast. At 15°C the swimbladder muscle uses ATP at a somewhat slower rate (1.22 mmol s−1 per litre of intact muscle) than fast-twitch amphibian fibres (e.g. ∼1.6 mmol s−1 per litre of intact muscle) measured in Xenopus laevis skinned fibres (Stienen et al. 1995) and ∼2 mmol s−1 per litre of intact muscle measured in Rana pipiens intact muscle (DeFuria & Kushmerick, 1977; Rome & Kushmerick, 1983)). Marked modifications in the functioning of the crossbridges and probable reliance on intracellular Ca2+ buffers are necessary to permit the much higher twitch speeds in the swimbladder without high steady-state rates of ATP utilization.
First, although the ATP turn-over rate per crossbridge (11.2 s−1) in swimbladder is about twice that found in Xenopus (∼6 s−1; note that the Vmax was also about twice as large: 12 vs.∼5.5 muscle lengths s−1 (Lannergren & Hoh, 1984)), on the whole the swimbladder crossbridges had a somewhat smaller total ATPase rate than those in Xenopus. The swimbladder contains only about 50 % myofibrils (to make room for the large SR volume; Appelt et al. 1991) and thus a low myosin crossbridge concentration (69 μm) (Rome et al. 1999a). Therefore, for a given ATP turn-over per crossbridge, having fewer crossbridges reduces the amount of ATP that the fibre uses. More importantly, the swimbladder has evolved a mechanism that achieves superfast crossbridge kinetics with a modest ATP utilization per head. Compared with toadfish fast-twitch fibres, swimbladder fibres have a similar crossbridge attachment rate constant (f), but they have a 10-fold faster crossbridge detachment rate constant (g) (Rome et al. 1999a). It is the very high g that permits their very fast relaxation rate necessary for high frequency contractions. In most fibres, the rate of ATP utilization increases in proportion to g because the rate of ATP utilization is proportional to fg/(f+g) (Huxley, 1957) and f and g generally increase in unison. However, in swimbladder, f does not increase and thus ATP utilization rate is low. A rapid detachment rate constant with a modest ATP utilization rate comes with a price: the number of attached crossbridges (=f/(f+g)), and hence force, is only about 1/5 that of normal locomotory fibres (Rome et al. 1999a).
Our measurement of ATPase rate associated with Ca2+ pumping (0.45 mmol s−1 (l muscle)−1) is also not extraordinarily fast considering the swimbladder muscle has the fastest Ca2+ transient ever measured (Rome et al. 1996). Assuming a stoichiometry of 2 Ca2+ per ATP, the Ca2+ pumping rate is equivalent to 0.9 mmol s−1 (l muscle)−1. Recent measurements of Ca2+ uptake by homogenized swimbladder SR vesicles in the presence of oxalate, gave a similar value (∼0.5 mmol s−1 (l muscle)−1 (original measurements at 23°C are corrected to 15°C values using a Q10 of 3); Feher et al. 1998). The results from the vesicle measurements, which were made at high [Ca2+] (200 μm) and in the presence of a CPK-regenerating system, suggest that our measurements of the rate of ATP utilization by the SR-Ca2+ pumps were not limited by a lower [Ca2+].
It is striking that the Ca2+ pumping rate measured in the skinned fibre is far slower than what appears to be needed for the swimbladder muscle to twitch continuously at the high frequencies used for calling. The swimbladder muscle contracts at 80–100 Hz during a call at 15°C. If one assumes that the amount of Ca2+ released during every contraction is equal to the amount needed to saturate troponin C (TnC, 140 μm; TnC concentration is taken to be equal to myosin heavy chain concentration (Yates & Greaser, 1983a, b)), then continuous calling would require a steady-state Ca2+ pumping rate of 11–14 mmol s−1 (l muscle)−1 (12-15 times the Ca2+ pumping rate measured in this study). However, toadfish do not call continuously, rather they make short calls (∼0.2 s) followed by relatively long pauses (6-60 s) (Fine, 1978). The intermittent nature of their calling may ultimately reflect the inability of the SR-Ca2+ pumps to keep up with Ca2+ cycling in a steady state.
As previously hypothesized by Feher et al. (1998), our skinned fibre results and analysis suggest that, during the actual call, most of the released Ca2+ may have to bind to parvalbumin (Hou et al. 1991; Jiang et al. 1996) or to the SR-Ca2+ pumps. This Ca2+ would be subsequently pumped back into the SR during the relatively long inter-call pauses. The high concentration of Ca2+ binding sites associated with parvalbumin (3 mm) and SR-Ca2+ pumps (0.76 mm) (Appelt et al. 1991) seems to be sufficient to sequester the Ca2+ released during a call (∼2.5 mmol (l muscle)−1). Further, the off-rate of Ca2+ from parvalbumin (0.7 s−1 at 15°C in frog muscle; Hou et al. 1992) and the Ca2+ pumping rate measured here are both sufficiently fast to ensure that all the Ca2+ bound to parvalbumin can be unloaded and pumped back into the SR during the inter-call pause. Finally, preliminary evidence using a technique for measuring Ca2+ sequestration in skinned fibres (Rome et al. 1999b) suggests that the swimbladder SR is capable of sequestering ∼30 mmol Ca2+ per litre of muscle. This is 12-fold more Ca2+ than would be released during a call, and hence, the SR should not run out.
Although this is a quantitatively appealing hypothesis, we cannot exclude the possibility that either far less Ca2+ is released per contraction (i.e. calling does not require the alternate complete saturation and complete removal of Ca2+ from TnC) or that the Ca2+ pumping rates in intact cells are substantially higher than we measure in the steady state in skinned fibres. Measurements in intact cells are necessary to address these possibilities
In conclusion, by employing two mechanisms (having a small number of attached crossbridges and probably by employing intracellular Ca2+ buffers to spread out the time over which Ca2+ pumping can occur (i.e. a ‘use now, pay later’ strategy)), the swimbladder muscle is able to achieve tremendous twitch speeds with a modest rate of steady-state ATP utilization. Thus the swimbladder muscle appears to have circumvented the apparent rule linking muscle speed and the rate of ATP utilization.
Partitioning energy utilization during contraction (SR-Ca2+ pumps vs. crossbridges)
Although the total rate of ATP utilization does not appear to be nearly as high as expected, the relative proportions of energy usage by the crossbridges and SR-Ca2+ pumps under these steady-state conditions are similar to that of other muscles. In our experiments the SR-Ca2+ pumps account for about 37 % of the steady-state ATP utilization. Previously it has been shown that in muscles ranging about 60-fold in speed, between 25 and 40 % of the energy for contraction is used by the SR-Ca2+ pumps (Homsher & Kean, 1978; Crow & Kushmerick, 1983; Woledge et al. 1985; Burchfield & Rall, 1986; Stienen et al. 1995). The agreement between our measurements from the fastest vertebrate muscle (Rome et al. 1996, 1999a) and these previous observations make this relationship particularly compelling in view of the reduction of myosin crossbridge concentration in swimbladder. Overall, these data show that crossbridge kinetics and the kinetics of Ca2+ pumping change in unison, maintaining a nearly constant proportion of energy usage by these processes over a remarkably large range of muscle speeds. Hence it appears that, during the evolution of muscular systems, the overall kinetics of crossbridges and SR-Ca2+ pumps are linked, but the mechanism for this linkage is unknown. It should be noted that this apparent empirical rule holds only for steady-state measurements. It is possible that, when examining in vivo calling behaviour, the proportion of the energy that is used for Ca2+ pumping (which would include the pumping of Ca2+ between calls while the crossbridges are quiescent) will become a larger proportion of the total energy utilized.
SR-Ca2+ pump turn-over rate and muscle design
From our measurements of ATP utilization by the SR-Ca2+ pumps, and knowing the quantity of pumps from quantitative electron microscopy (0.38 mm; the value is corrected by 20 % for possible volume shrinkage during fixation; Appelt et al. 1991), we calculated the pump turn-over rate as a modest ∼1.2 ATP molecules s−1. In contrast, a value of ∼2.5 s−1 has been calculated for rabbit SR vesicles (Inesi & Scarpa, 1972) (original measurements at 25°C are corrected to 15°C values using a Q10 of 4 (Inesi et al. 1973)). If any muscle should have SR-Ca2+ pumps with a fast turn-over rate, the swimbladder would be a good candidate. The Ca2+ transient is the fastest known, the pump density on the SR is maximized and the overall SR surface density is very large (Appelt et al. 1991). As explained above, the fast calcium transient requires such a high SR volume that this causes a significant reduction in myofibril volume. Yet, our results show that an increased pump turn-over rate does not occur, suggesting that Ca2+ turn-over rate cannot be increased.
Therefore fast Ca2+ pumping is not due to an increase in pump turn-over rate, but rather to a high concentration of SR-Ca2+ pumps. Thus, unlike for crossbridges where the number is fixed and the kinetics vary dramatically (for instance the detachment rate constant is 50 times faster in swimbladder than in toadfish red muscle (Rome et al. 1999a)), for SR-Ca2+ pumps the kinetics appears nearly fixed, and faster pumping rates can only be achieved by increasing pump number.
Acknowledgments
This work was supported by grants NIH AR38404, NSF IBN-9514383 and NIH AR46125. We thank Dr I. S. Young for carefully reviewing the manuscript. We dedicate this manuscript to the memory of our friend Professor J. David Johnson whose insights on Ca2+-parvalbumin interactions inspired much of this work.
References
- Appelt D, Shen V, Franzini-Armstrong C. Quantitation of Ca ATPase, feet and mitochondria in super fast muscle fibers from the toadfish, Opsanus tau. Journal of Muscle Research and Cell Motility. 1991;12:543–552. doi: 10.1007/BF01738442. [DOI] [PubMed] [Google Scholar]
- Burchfield D, Rall JA. Temperature dependence of the crossbridge cycle during unloaded shortening and maximum isometric tetanus in frog skeletal muscle. Journal of Muscle Research and Cell Motility. 1986;7:320–326. doi: 10.1007/BF01753652. [DOI] [PubMed] [Google Scholar]
- Crow MT, Kushmerick MJ. Correlated reduction of velocity of shortening and the rate of energy utilization in mouse fast-twitch muscle during a continuous tetanus. Journal of General Physiology. 1983;82:703–720. doi: 10.1085/jgp.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defuria RF, Kushmerick MJ. ATP utilization associated with recovery metabolism in anaerobic frog muscle. American Journal of Physiology. 1977;232:C30–36. doi: 10.1152/ajpcell.1977.232.1.C30. [DOI] [PubMed] [Google Scholar]
- Feher JJ, Waybright TD, Fine ML. Comparison of sarcoplasmic reticulum capabilities in toadfish (Opsanus tau) sonic muscle and rat fast twitch muscle. Journal of Muscle Research and Cell Motility. 1998;19:661–674. doi: 10.1023/a:1005333215172. [DOI] [PubMed] [Google Scholar]
- Fine ML. Seasonal and geographical variation of the mating call of the oyster toadfish Opsanus tau L. Oecologia. 1978;36:45–57. doi: 10.1007/BF00344570. [DOI] [PubMed] [Google Scholar]
- Hill AV. The heat of shortening and the dynamic constants of muscle. Proceedings of the Royal Society. 1938;B 126:136–195. [Google Scholar]
- Hill AV. The dimensions of animals and their muscular dynamics. Science Progress. 1950;38:209–229. [Google Scholar]
- Homsher E, Kean CJ. Skeletal muscle energetics and metabolism. Annual Review of Physiology. 1978;40:93–131. doi: 10.1146/annurev.ph.40.030178.000521. [DOI] [PubMed] [Google Scholar]
- Hou T-T, Johnson JD, Rall JA. Parvalbumin content and Ca2+ and Mg2+ dissociation rates correlated with changes in relaxation rate of frog muscle fibres. The Journal of Physiology. 1991;441:285–304. doi: 10.1113/jphysiol.1991.sp018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T-T, Johnson JD, Rall JA. Effect of temperature on relaxation rate and Ca2+, Mg2+ dissociation rates from parvalbumin of frog fibres. The Journal of Physiology. 1992;449:399–410. doi: 10.1113/jphysiol.1992.sp019092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley AF. Muscle structure and theories of contraction. Progress in Biophysics and Biophysical Chemistry. 1957;7:255–318. [PubMed] [Google Scholar]
- Inesi G, Millman M, Eletr S. Temperature-induced transitions of function and structure in sarcoplasmic reticulum membranes. Journal of Molecular Biology. 1973;81:483–504. doi: 10.1016/0022-2836(73)90518-4. [DOI] [PubMed] [Google Scholar]
- Inesi G, Scarpa A. Fast kinetics of adenosine triphosphate dependent Ca2+ uptake by fragmented sarcoplasmic reticulum. Biochemistry. 1972;11:356–359. doi: 10.1021/bi00753a008. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Johnson JD, Rall JA. Parvalbumin relaxes frog skeletal muscle when the sarcoplasmic reticulum Ca-ATPase is inhibited. American Journal of Physiology. 1996;270:C411–417. doi: 10.1152/ajpcell.1996.270.2.C411. [DOI] [PubMed] [Google Scholar]
- Lannergren J, Hoh JFY. Myosin isoenzymes in single muscle fibers from Xenopus laevis: analysis of five different functional types. Proceedings of the Royal Society. 1984;B 222:401–408. doi: 10.1098/rspb.1984.0072. [DOI] [PubMed] [Google Scholar]
- Launikonis BS, Stephenson DG. Effect of saponin treatment on the sarcoplasmic reticulum of rat, cane toad and crustacean (yabby) skeletal muscle. The Journal of Physiology. 1997;504:425–437. doi: 10.1111/j.1469-7793.1997.425be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome LC, Cook C, Syme DA, Connaughton MA, Ashley-Ross M, Klimov AA, Tikunov BA, Goldman YE. Trading force for speed: Why superfast crossbridge kinetics leads to superlow forces. Proceedings of the National Academy of Sciences of the USA. 1999a;96:5826–5831. doi: 10.1073/pnas.96.10.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome LC, Klimov AA, Young IS. A new approach for measuring real-time calcium pumping and SR function in muscle fibers. Biological Bulletin. 1999b;197:227–228. doi: 10.2307/1542618. [DOI] [PubMed] [Google Scholar]
- Rome LC, Kushmerick MJ. The energetic cost of generating isometric force as a function of temperature in isolated frog muscle. American Journal of Physiology. 1983;244:C100–109. doi: 10.1152/ajpcell.1983.244.1.C100. [DOI] [PubMed] [Google Scholar]
- Rome LC, Lindstedt SL. The quest for speed: muscles built for high frequency contractions. News in Physiological Sciences. 1998;13:261–268. doi: 10.1152/physiologyonline.1998.13.6.261. [DOI] [PubMed] [Google Scholar]
- Rome LC, Syme DA, Hollingworth S, Lindstedt SL, Baylor SM. The whistle and the rattle: the design of sound producing muscles. Proceedings of the National Academy of Sciences of the USA. 1996;93:8095–8100. doi: 10.1073/pnas.93.15.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienen GJM, Zaremba R, Elzinga G. ATP utilization of calcium uptake and force production in skinned muscle fibres of Xenopus laevis. Journal of Physiology. 1995;482:109–122. doi: 10.1113/jphysiol.1995.sp020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syme DA, Connaughton MA, Rome LC. A device for measuring steady-state ATP utilization in single skinned muscle fibers. Biological Bulletin. 1997;193:251–252. doi: 10.1086/BBLv193n2p251. [DOI] [PubMed] [Google Scholar]
- Wendt IR, Stephenson DG. Effects of caffeine on Ca-activated force production in skinned cardiac and skeletal muscle fibres of the rat. Pflügers Archiv. 1983;398:210–216. doi: 10.1007/BF00657153. [DOI] [PubMed] [Google Scholar]
- Woledge RC, Curtin NA, Homsher E. Energetic Aspects of Muscle Contraction. New York: Academic Press; 1985. [PubMed] [Google Scholar]
- Yates LD, Greaser ML. Quantitative determination of myosin and actin in rabbit skeletal muscle. Journal of Molecular Biology. 1983a;168:123–141. doi: 10.1016/s0022-2836(83)80326-x. [DOI] [PubMed] [Google Scholar]
- Yates LD, Greaser ML. Troponin subunit stoichiometry and content in rabbit skeletal muscle and myofibrils. Journal of Biological Chemistry. 1983b;258:5770–5774. [PubMed] [Google Scholar]


