Abstract
The prevalence of bovine viral diarrhea virus (BVDV) infections was determined in 2 groups of stocker calves with acute respiratory disease. Both studies used calves assembled after purchase from auction markets by an order buyer and transported to feedyards, where they were held for approximately 30 d. In 1 study, the calves were mixed with fresh ranch calves from a single ranch. During the studies, at day 0 and at weekly intervals, blood was collected for viral antibody testing and virus isolation from peripheral blood leukocytes (PBLs), and nasal swabs were taken for virus isolation. Samples from sick calves were also collected. Serum was tested for antibodies to bovine herpesvirus-1 (BHV-1), BVDV1a, 1b, and 2, parainfluenza 3 virus (PI3V), and bovine respiratory syncytial virus (BRSV). The lungs from the calves that died during the studies were examined histopathologically, and viral and bacterial isolation was performed on lung homogenates. BVDV was isolated from calves in both studies; the predominant biotype was noncytopathic (NCP). Differential polymerase chain reaction (PCR) and nucleic acid sequencing showed the predominant subtype to be BVDV1b in both studies. In 1999, NCP BVDV1b was detected in numerous samples over time from 1 persistently infected calf; the calf did not seroconvert to BVDV1a or BVDV2. In both studies, BVDV was isolated from the serum, PBLs, and nasal swabs of the calves, and in the 1999 study, it was isolated from lung tissue at necropsy. BVDV was demonstrated serologically and by virus isolation to be a contributing factor in respiratory disease. It was isolated more frequently from sick calves than healthy calves, by both pen and total number of calves. BVDV1a and BVDV2 seroconversions were related to sickness in selected pens and total number of calves. In the 1999 study, BVDV-infected calves were treated longer than noninfected calves (5.643 vs 4.639 d; P = 0.0902). There was a limited number of BVDV1a isolates and, with BVDV1b used in the virus neutralization test for antibodies in seroconverting calves' serum, BVDV1b titers were higher than BVDV1a titers. This study indicates that BVDV1 strains are involved in acute respiratory disease of calves with pneumonic Mannheimia haemolytica and Pasteurella multocida disease. The BVDV2 antibodies may be due to cross-reactions, as typing of the BVDV strains revealed BVDV1b or 1a but not BVDV2. The BVDV1b subtype has considerable implications, as, with 1 exception, all vaccines licensed in the United States contain BVDV1a, a strain with different antigenic properties. BVDV1b potentially could infect BVDV1a-vaccinated calves.
Introduction
Bovine viral diarrhea virus (BVDV) causes infection and disease in cattle, with involvement of 1 or more organ systems (1,2). The conditions range from inapparent infection in postnatal calves to severe, fatal systemic diseases, such as mucosal disease (1). BVDV has been isolated from several clinical forms of disease and from necropsy samples, including from cattle with signs and, or, lesions of bovine respiratory disease (BRD) (2).
BVDV is classified by biotype and genotype (1,3,4). Biotypes, cytopathic (CP) and noncytopathic (NCP), are based on the presence or absence of visible cytopathic effects (CPE) in infected cell cultures. BVDV genotypes (1 and 2) are detected by polymerase chain reaction (PCR) and antigenic differences (3,4). The type 1 genotype has been further subdivided into types 1a and 1b on the basis of PCR and nucleic acid sequencing (5,6). A recent study indicated that BVDV could be clustered into BVDV1a and BVDV1b and also into 11 phylogenetic groups (7).
BVDV has been associated with clinical signs and lesions of BRD (8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28). The involvement of BVDV in BRD has been demonstrated by (1) experimental infections, (2) isolation of virus and, or, identification of BVDV antigen in lesions and, or, other respiratory tract samples from calves with respiratory signs or lesions, and (3) demonstration of active infection through seroconversions in groups of cattle with BRD.
BVDV genotypes have been associated with particular disease forms, PCR being used to differentiate the genotypes. In 1 study, in which clinical conditions were described by veterinarians submitting samples, BVDV NCP biotypes were isolated more frequently than BVDV CP biotypes and BVDV1 genotypes more frequently than BVDV2 genotypes from cattle with respiratory disease (2). Also, BVDV1 genotypes were isolated more frequently than BVDV2 genotypes from necropsy samples from calves with fibrinous pneumonia (2).
Knowledge of the BVDV1 subtypes specific for BRD is limited. However, both of the BVDV strains isolated from Venezuelan dairy calves with BRD were of the 1b subgroup (26). Besides BVDV1a and 1b subtypes, 2 additional clusters have been identified: 1 cluster, 1d, was predominantly associated with field cases of respiratory disease in the southern region of Africa (29). Subsequently, calves experimentally challenged with a BVDV1d subtype developed primary respiratory disease (27).
In this study, postweaning calves were held for approximately 5 wk and observed for natural cases of acute respiratory disease. The purpose was 4-fold: (1) to detect the presence of persistent BVDV infection in calves and determine its role in BVDV transmission; (2) to determine the susceptibility of seronegative calves to BVDV infection; (3) to determine the presence of BVDV in BRD cases by virus isolation and, or, seroconversion; and (4) to determine the BVDV1 subtype involved in BRD cases.
Materials and methods
Cattle
The cattle were involved in 2 studies to determine factors associated with acute respiratory disease in young (postweaning) beef cattle. In both the 1999 study and the 2000 study, cattle were purchased from local auctions in eastern Tennessee and transported to a nearby order buyer's (OB) pens. There they received their initial treatments and vaccinations, and samples were collected, including nasal swabs for virus isolation, EDTA blood for isolation of virus from peripheral blood leukocytes (PBLs), and clotted blood samples for viral serologic testing (20). On day 0 of both studies, the calves received anthelmintic, monovalent BHV-1 modified-live virus (MLV) vaccine, and clostridial vaccine, as previously described (20). The calves were then shipped via semi-trailer truck to research feedyards: in Clayton, New Mexico, for the 1999 study and in Bushland, Texas, for the 2000 study. Samples were collected as described above at weekly intervals until the final day of each study: in the 1999 study, day 33; and in the 2000 study, day 32. The isolation and transmission of Mannheimia haemolytica and Pasteurella multocida, primary causes of bacterial pneumonias in BRD, are the subjects of other investigations.
Lung samples were collected at necropsy from each calf dying during the studies. These samples were tested for viruses and bacteria at the Oklahoma Animal Disease Diagnostic Laboratory (OADDL), Oklahoma State University, Stillwater, Oklahoma. Samples were also collected for histopathological study.
Serologic tests
A virus neutralization test (VNT) in Madin–Darby bovine kidney (MDBK) cells in 96-well microtiter plates was used to quantitate virus-neutralizing antibodies to BVDV types 1a, 1b, and 2, PI3V, and BRSV. The viruses used in this test were CP BVDV type 1a (Singer strain), CP BVDV1b (TGAC 8HB), CP BVDV type 2 (125-C strain), PI3V (SF-4 strain), and a BRSV vaccine strain (20,30,31). The 1:4 final dilution was the lowest dilution tested. A plaque reduction assay in MDBK cells in 24-well plates was used to detect virus-neutralizing antibodies to the Cooper strain of BHV-1 (20,30,31). The 1:10 final dilution was the lowest dilution tested. In this study, 0 or negative titers represented < 1:4 for BVDV types 1 and 2, PI3V, and BRSV, and < 1:10 for BHV-1. Titers expressed represent reciprocals of the endpoint titers.
Microbiologic studies
The virus isolations were performed at the OADDL. A monolayer enzyme-linked immunosorbent assay was used to detect BVDV in serum (20,32). To isolate viruses, nasal swabs, PBLs, and lung samples were inoculated onto BT monolayers in 24-well plates, as previously described (20,32). Cytopathic agents other than BVDV (BRSV, PI3V, and BHV-1) were tested for by direct fluorescent antibody tests. The BVDV isolates were typed with the use of differential PCR and sequencing of the 5́ untranslated region, as previously described (5,6). Bacterial isolation from lung tissues was also performed by the OADDL.
Pathological studies
Lung samples collected at necropsy were fixed in 10% buffered formalin and processed for histopathological study. Sections were stained with hematoxylin and eosin, and, in a blinded study, were examined by 1 of the authors (A.W.C.), who rendered a morphologic diagnosis.
Subtypes in vaccines
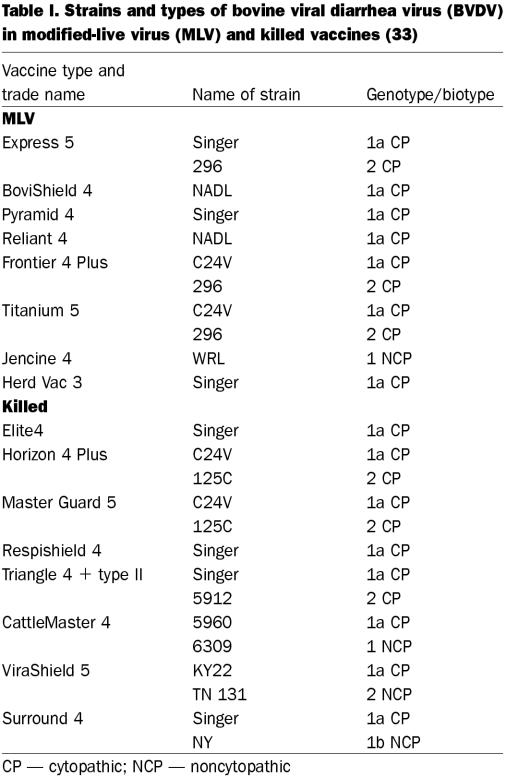
Vaccine manufacturers in the United States were contacted to determine the name(s) of the BVDV strain(s) in both killed and MLV vaccines licensed and marketed in the United States. The genotype and biotype for each vaccine strain was provided by the vaccine manufacturer. In addition, product descriptions were available to the public in a reference text containing bovine-vaccine product information (33); these descriptions are presented in Table I.
Table I.
Statistical analysis
The percentages of isolations from sick vs healthy calves and of seroconversions were compared with Fisher's exact 2-sided test (34). The effect of BVDV infection on the duration of treatment was analyzed with a 2-population t-test (34). All tests were performed at the 0.05 level of significance.
Results
Serologic findings at arrival
The blood collected on day 0 in both the 1999 and 2000 studies was tested for BHV-1, BVDV1a, BVDV2, PI3V, and BRSV antibodies. Of the OB calves in the 1999 and 2000 studies, respectively, 92.6% and 95.0% were seronegative for BHV-1, 76.9% and 65.8% for BVDV1a, 82.6% and 80.0% for BVDV2, 55.4% and 45.0% for PI3V, and 86.0% and 87.5% for BRSV. Of the ranch calves in the 1999 study, 70.2% were seronegative for BHV-1, 71.4% for BVDV1a, 81.0% for BVDV2, 30.0% for PI3V, and 41.7% for BRSV. The titers of antibody to BVDV1a, BVDV2, PI3V, and BRSV could be measured for evidence of active infection (4-fold or greater rise) or maternal antibody decay (a drop of 1 dilution or more). BHV-1 maternal antibody determinations could not be made, as all the calves in each study had received the MLV BHV-1 vaccine. Based on the above-defined evidence of maternal antibody decay, there were animals in both studies with maternal antibodies at entry. It was subsequently learned that the cow herd from which the ranch calves were obtained for the 1999 study had been receiving annual vaccinations with viral vaccine containing BHV-1, BVDV1, PI3V, and BRSV (CattleMaster 4, Pfizer Animal Health, Exton, Pennsylvania, USA).
Respiratory disease morbidity and mortality
During the 1999 study, the morbidity rate was 124/205 (60.5%) among the calves, which included the 118 surviving calves treated after clinical signs of BRD were observed and the calves that died with BRD lesions (6). The morbidity rate was 81/121 (66.9%) for the OB calves and 43/84 (51.2%) for the ranch calves. Of the 6 calves (2.9%) that died during the 1999 study, 5/121 (4.1%) were OB calves and 1/84 (1.2%) ranch calves. In the 2000 study, the morbidity rate was 70/120 (58.3%) for the OB calves, and 6/120 (5%) died during the study. Respiratory disease signs included fever [temperature ≥ 104°F (40°C)], nasal and ocular discharge, coughing, and labored breathing. Treatment included antimicrobial therapy. The duration of treatment ranged from 1 to 7 d in the 1999 study and 3 to 13 d in the 2000 study.
Calves that died: lung lesions and isolates
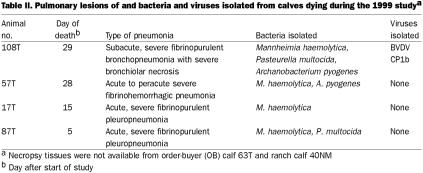
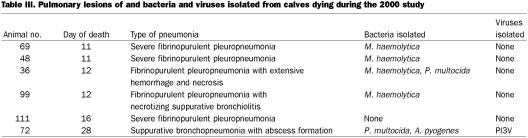
Necropsy tissue was not available from 1 OB calf and 1 ranch calf. The lesions in the lungs of the other 10 calves that died during the studies were consistent with those of pneumonia caused by M. haemolytica and, or, P. multocida — primarily fibrinopurulent pneumonia in 9 cases and bronchopneumonia in 1 case (Tables II and III). M. haemolytica and, or, P. multocida was isolated from the lungs in both studies. Viruses were isolated from the lung homogenates in each study: 1 lung in the 1999 study was positive for CP BVDV (subtype 1b), and 1 in the 2000 study was positive for PI3V.
Table II.
Table III.
Virus isolates from survivors
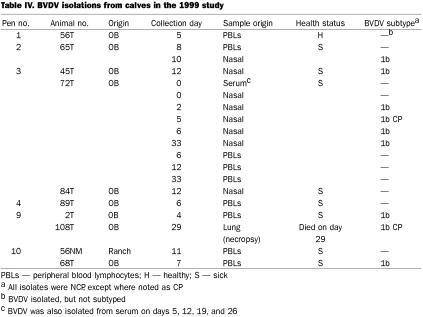
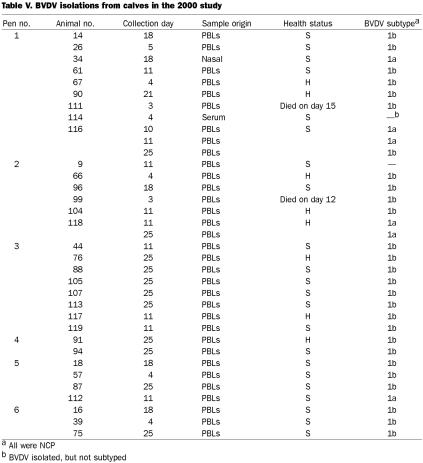
In 1999, there was only 1 calf positive for BVDV on day 0 (Table IV). This calf, 72T, an OB calf, proved to be persistently infected, as samples were positive through day 33; in addition, BHV-1 was isolated on day 26 and PI3V on day 19. Of the remaining 9 BVDV-positive calves, all but 2 had NCP BVDV; 1 isolate from a nasal swab from calf 72T was CP, as was an isolate from necropsy lung tissue. Six of the 10 pens (nos. 1 to 4, 9, and 10) had calves from which BVDV was isolated. The only subtype was 1b. Of the live calves from which BVDV was isolated, 8 were sick, and 1 was healthy. The probability of BVDV being isolated from sick calves more frequently than healthy calves approached significance (P = 0.0887) with Fisher's exact 2-sided test.
Table IV.
In 2000, no calves were positive for BVDV on day 0, and none were persistently infected during the study. There were 35 BVDV isolates, from 32 calves; all isolates were NCP (Table V). All 6 pens had cattle from which BVDV was isolated, the numbers ranging from 9 calves in pens 1 and 8 calves in pen 3 to 2 calves in pen 4. BVDV was isolated from 24 sick calves and 8 healthy calves; the probability that BVDV was isolated more frequently from sick calves than from healthy calves was significant (P = 0.0323). Calves 116 and 118 had multiple isolations of BVDV. Of the 33 isolates available for subtyping, 27 were 1b and 6 were 1a. Three calves (nos. 34, 118, and 112) had subtype 1a alone, whereas calf 116 was BVDV1a-positive on days 10 and 11, yet BVDV1b-positive on day 25.
Table V.
PI3V was isolated from nasal swabs of both healthy and sick calves, in all pens, in both studies. BHV-1 was isolated from nasal swabs of 15 OB calves in the 1999 study (1 in each of pens 1 and 4, 2 in pen 2, 3 in pen 3, and 4 in each of pens 8 and 9) but from no calves in the 2000 study. Generally, BHV-1 was isolated from calves only once; 2 calves were positive on days 19 and 26, and 1 calf was positive on days 5, 11, and 19. BHV-1 was isolated from 4 healthy and 11 sick calves. Eight calves had both PI3V and BHV-1 isolations, at the same or different collections. BVDV was isolated from either the nasal swabs or the PBLs of 2 BHV-1-positive calves.
Seroconversions to BVDV types 1a and 2, PI3V, and BRSV in the surviving calves
In 1999, 199 of the 205 calves survived the study duration; in 2000, 114 of the 120 calves survived. Seroconversion, or active infection with BVDV1a, BVDV2, PI3V, or BRSV, was defined by a 4-fold or greater rise in the antibody titer from day 0 to the final day of blood collection. The attempt to determine pen effect on viral infection and disease is illustrated in Tables VI-IX. For BVDV1a and BVDV2, the origin of the calf (OB or ranch) and the disease state (sick or healthy) is shown for each of pens. Owing to the low numbers by origin in the 1999 study, the 2 origins were pooled for comparisons by pen of the total sick to healthy ratios.
Table VI.
Table VII.
Table VIII.
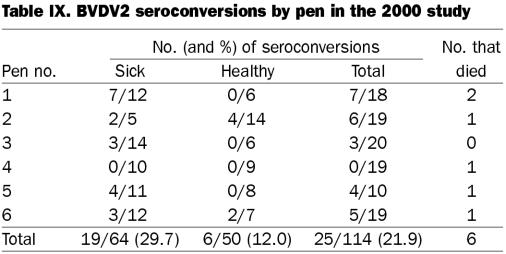
Table IX.
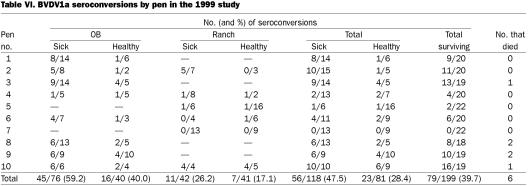
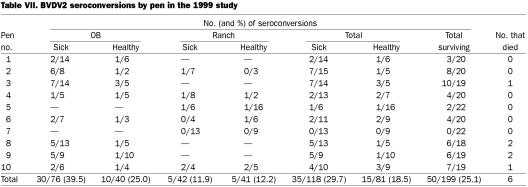
There were significantly more BVDV1a seroconversions in sick (47.5%) than in healthy (28.4%) calves (P = 0.008 by Fisher's exact 2-sided test) in the 1999 study. There was no significant difference by pen; however, for some pens, the difference approached significance: pen 1, P = 0.1571; pen 2, P = 0.1273; and pen 10, P = 0.0867. Pen 10 had the highest seropositivity rate among the pens for BVDV1a, 16/19 (84.2%), and all 10 sick calves in this pen seroconverted. Pen 3 contained the persistently infected calf, 72T, and had a BVDV1a seropositivity rate of 68.4% (13/19); of the seroconverting calves, 9 were sick and 4 healthy, which indicated transmission to both sick and healthy calves. It appears that the major source of the BVDV in the study was the OB calves, as only 2 of the 44 ranch calves in the 2 pens containing only ranch calves seroconverted to BVDV. Of the 116 OB calves, 61 (52.6%) seroconverted to BVDV1a, and of the 83 ranch calves, 18 (21.7%) did so (considering only surviving calves). However, when, as in pen 10, ranch calves were mixed with OB calves that had a high rate of seroconversion to BVDV1a among the sick calves (6/6), then most (8/9) of the ranch calves became infected with BVDV1a. For BVDV2 in that study, the P value was 0.0960 for the relation of BVDV infection to sickness; seroconversion occurred in 29.7% of the sick and 18.5% of the healthy calves.
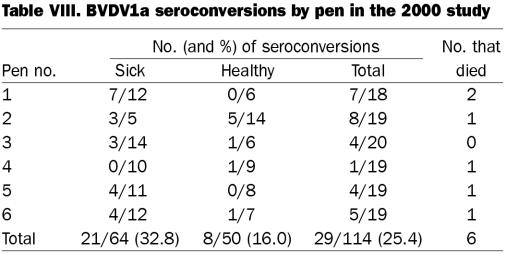
In the 2000 study, BVDV1a seroconversion, based on the total from the 6 pens, was positively related to sickness (P = 0.0517), with 32.8% of the sick and 16.0% of the healthy calves seroconverting. Pen 1 had a P value of 0.0377 (7/12 sick calves and 0/6 healthy calves seroconverted), and pen 5 had a P value of 0.1032 (7/11 sick calves and 0/18 healthy calves seroconverted), which demonstrated that seroconversions occurred at a greater rate in the sick calves than in the healthy calves. Similarly for BVDV2, among the total group of survivors, seroconversion was positively related to sickness (P = 0.0387), with 29.7% of the sick and 12.0% of the healthy calves seroconverting. Pens 1 and 5 had the same P values in demonstrating a relationship between seroconversion to BVDV1a and sickness.
In 1999, 64.3% of the calves seroconverted to BRSV and 37.7% to PI3V. There was no relationship between seroconversion and sickness for either virus, for either the total group of survivors or by pen. In 2000, 86.8% of the calves seroconverted to BRSV and 65.8% to PI3V. Again, there was no relationship between seroconversion and health status for either virus, for either the total group or by pen.
Effect of BVDV infection on duration of treatment
For this evaluation, a calf was classified as having BVDV infection if either the virus was isolated from PBLs or nasal swabs or there was seroconversion (a 4-fold or greater rise in titer of antibody to either BVDV1a or BVDV2). In the 1999 study, duration of treatment was 5.643 d for BVDV-infected calves and 4.639 d for calves without BVDV. The probability that BVDV caused the greater duration of treatment approached significance (P = 0.0902). In the 2000 study, the duration of treatment was 2.15 d for BVDV-infected calves and 1.90 d for calves without BVDV; the probability that BVDV caused the greater duration of treatment did not approach significance (P = 0.3013).
BVDV infection in calves that died
During the 1999 study, 6 calves died with BRD, and 4 were necropsied. Calf 108T died on day 29; BVDV1b CP was isolated from the lung tissues. In the 2000 study, 6 calves died with BRD, and 4 were necropsied. Calf 99 died on day 12 of the study; BVDV1b had been isolated from PBLs on day 4. Calf 111 died on day 16; BVDV1b had been isolated from PBLs on day 4.
Comparison of BVDV1a and BVDV1b titers
The BVDV1a (Singer) and BVDV2 (125-C) viruses were used initially in the VNT to detect BVDV antibodies. This serology was done prior to subtyping, which showed that BVDV1b subtypes predominated in both studies. In both 1999 and 2000, the serum from calves seroconverting to BVDV1a was then tested for BVDV1b antibodies, in both acute and convalescent phases. This test was performed to determine if the BVDV1b antibody titers were higher than the BVDV1a titers using the Singer strain.
There were 75 serum samples available for BVDV1b serologic testing in the 1999 study. Of the 58 from OB calves, 34 had higher BVDV1b than BVDV1a titers (by 1 dilution or more), 14 had the same titer, and 10 had lower BVDV1b than BVDV1a titers (by 1 dilution or more). Of the 17 samples from ranch calves, 7 had higher and 10 had lower BVDV1b than BVDV1a titers. The only ranch-calf BVDV isolate was not available for typing. In the 2000 study, there were 29 serum samples available for BVDV1b serologic testing: 12 had higher BVDV1b than BVDV1a titers, 7 had the same titer, and 10 had lower BVDV1b than BVDV1a titers.
BVDV subtypes in vaccines
At the time of the studies, the vaccines in the United States primarily contained BVDV1a CP strains, although some killed and MLV vaccines contained BVDV2 CP strains (Table I). In all but 2 vaccines, the biotypes were CP; 1 killed and 1 MLV vaccine contained NCP strains. One MLV vaccine contained an NCP strain, WRL, referred to as BVDV1; however, the subtype was not identified. One killed vaccine contained strain 6309, a BVDV1 NCP strain whose subtype was not specified. Only 1 vaccine contained BVDV1b.
Discussion
BVDV was identified in cases of BRD in 2 separate studies of calves of mixed source (OB procured) that were commingled, transported to an experiment feedyard, and observed for 4 to 5 wk. In 1 study, fresh ranch calves were mixed with the OB calves. These 2 studies, in 1999 and 2000, are continuing studies of BRD and follow, in general, our study from 1998 (20). In contrast to the 1998 study, in which blood was collected only at days 0 and 33 for virus isolation and serologic testing, additional collections were made in the 1999 and 2000 studies: nasal swabs and additional blood samples were collected for virus isolation from PBLs on a weekly basis and from sick calves. The addition of nasal swabs was designed to detect viruses in upper respiratory tract secretions that might not be present in serum or PBLs. PBLs were used to detect systemic viruses and, especially, those causing acute BVDV infection. Weekly collection permitted virus detection over time. Samples of lung tissue from calves dying during the studies were assayed for viruses and bacteria, and viral serologic testing was performed, with an additional BVDV subtype, BVDV1b. The plan was to identify additional viruses in calves held over this period. The number of viruses, both BVDV and others, in both the 1999 and 2000 studies was increased, as expected. Also, viruses were isolated from both healthy and sick calves.
BVDV infection occurred in both studies and in numerous instances appeared to contribute to BRD. This was demonstrated by both virus isolation from affected animals and seroconversion in calves with BRD signs. Subtyping of the BVDV isolates was important, in that the predominant subtype in these studies was BVDV1b. The expectation that BVDV1 would be more common than BVDV2 was based on our prior study (2), in which we found that BVDV1 and NCP strains were more common in cattle with a history of BRD than were CP and BVDV2 strains (2). Also, BVDV1 was isolated more frequently than BVDV2 from necropsied cattle with fibrinous pneumonia. The results in this study are similar to the Venezuelan study, in which BVDV1b was isolated from dairy calves with respiratory disease (26).
There are implications for BVDV vaccines to control these infections. BVDV has been isolated from cattle receiving BVDV1a vaccines (2,35). The current BVDV vaccines, both MLV and killed, contain BVDV1a, with 1 exception: a killed BVDV containing the NCP BVDV New York strain, of subtype 1b (reference 33, plus individual product descriptions in the text, and correspondence from the company listing the vaccine components). There are antigenic differences between BVDV1a and BVDV1b, as demonstrated by a recent study (36). Calves were either vaccinated with a BVDV1a MLV vaccine or inoculated intranasally with either an NCP BVDV1b strain or an NCP BVDV2 strain. Postexposure serum samples were tested for antibodies in the VNT with the CP BVDV1a NADL strain, the CP BVDV1b TGAC strain, or the CP BVDV2 125-C strain. Cross-reactive and type-specific antibodies to all 3 viruses were found in the individual animals. Usually the titers were higher to the same genotype (subtype) as the challenge or vaccine strain. Knowledge of the ability of the BVDV1a vaccines to protect against BVDV1b strains is limited. It is known, however, that BVDV1a vaccines, both killed and MLV, induce a wide range of antibodies to several BVDV1a strains (37). The presence of BVDV1 strains in calves subsequent to BVDV1a vaccination may represent field strains for which there was little protection afforded by the BVDV1a vaccine strain. Both experimental and field studies should be performed to demonstrate the efficacy of BVDV1a vaccines against BVDV1b strains. Also, it is likely that BVDV1b strains may be incorporated into the BVDV1a vaccines, as have the BVDV2 strains (33).
Our studies have also demonstrated concurrent infections with BHV-1, PI3V, and BRSV. The PI3V and BRSV seroconversions were expected, as these viruses appear to circulate in commingled cattle, as had been observed in our prior study (20). From seroconversions in the 1999 and 2000 studies in both healthy and sick calves, we could not demonstrate a significant relationship between the presence of either virus and BRD status. The calves had not received any PI3V, BRSV, or BVDV vaccines at entry to the study; thus, it is most likely these infections represent field infections. However, the detection of PI3V in nasal secretions, late in the holding period in both studies, supports widespread infection. Perhaps, if the cattle had been held 2 to 3 wk longer, there would have been time for an increase in antibody titers, indicating active infection.
The isolation of BHV-1 from calves in the 1999 study was also a repeat of the finding in our prior study (20). The calves in all 3 studies had received an MLV monovalent BHV-1 vaccine at day 0. In the 1999 study, several calves were shedding BHV-1 nasally. Since all calves had received the BHV-1 vaccine, there were no unvaccinated calves to serve as sentinels for field BHV-1 strains. It is likely that the BHV-1 isolates represented natural infections brought by the OB calves during commingling and transportation, because BHV-1 was not isolated from any of the ranch calves. BHV-1 was also found in outbreaks of BHV-1 disease in calves that had been vaccinated with an MLV BHV-1 vaccine after arrival at a feedlot (38). It is not unexpected to find multiple viruses in both sick and healthy calves involved in BRD, as BHV-1, BVDV, PI3V, BRSV, bovine adenoviruses, and bovine coronaviruses are listed as etiologic agents for BRD (39).
In summary, BVDV1b was the predominant BVDV subtype identified in 2 studies of acute BRD in commingled calves that had received no BVDV vaccines. In 1 study, a calf persistently infected with BVDV1b likely exposed other calves during the commingling and transportation. The transmission of virus from calves with acute BVDV infection cannot be ruled out in either study, particularly since the 2000 study had no persistently infected calves. The implications for effective vaccination against BVDV1b are obvious: (1) there must be demonstration of the efficacy of current BVDV1a vaccines against BVDV1b; (2) new BVDV1b components to add to current BVDV1a vaccines should be developed; or (3) current BVDV1b vaccine should be used. From a diagnostic standpoint, 2 issues should be addressed: (1) the epidemiologic features of the BVDV subtypes should be determined from isolates from clinical cases by differential PCR and sequencing; and (2) diagnostic laboratories should consider incorporating serologic testing for BVDV1b in addition to the tests for BVDV1a and BVDV2 that most laboratories currently use to detect active infection by seroconversion.
Footnotes
Acknowledgments
This research was supported by a grant from The Noble Foundation, Ardmore, Oklahoma, and Oklahoma Agricultural Experiment Station Projects OKLO 2276, OKLO 1438, and OKLO 2386. We appreciate the assistance of Ms. Diana Moffeit in the preparation of this manuscript.
Dr. Duff's current address is Department of Animal Sciences, University of Arizona, Tucson, Arizona 85721, USA.
Address correspondence and reprint requests to Dr. Robert W. Fulton, Department of Veterinary Pathobiology, Room 250, McElroy Hall, College of Veterinary Medicine, Oklahoma State University, Stillwater, Oklahoma 74078, USA, tel: 405-744-8170, fax: 405-744-5275, e-mail:rfulton@okstate.edu
Received January 4, 2002. Accepted April 29, 2002.
References
- 1.Baker JC. The clinical manifestations of bovine viral diarrhea infection. Vet Clin North Am Food Anim 1995;11:425–445. [DOI] [PubMed]
- 2.Fulton RW, Saliki JT, Confer AW, et al. Bovine viral diarrhea virus cytopathic and noncytopathic biotypes and type 1 and 2 genotypes in diagnostic laboratory accessions: clinical and necropsy samples from cattle. J Vet Diagn Invest 2000;12: 33–38. [DOI] [PubMed]
- 3.Pellerin CJ, van den Hurk J, Lecomte J, Tussen P. Identification of a new group of bovine viral diarrhea virus strains associated with severe outbreaks and high mortalities. Virology 1994;203: 260–268. [DOI] [PubMed]
- 4.Ridpath JF, Bolin SR, Dubovi EJ. Segregation of bovine viral diarrhea virus into genotypes. Virology 1994;205:66–74. [DOI] [PubMed]
- 5.Ridpath JF, Bolin SR. Differentiation of types 1a, 1b, and 2 bovine viral diarrhoea virus (BVDV) by PCR. Molec Cell Probes 1998;12:101–106. [DOI] [PubMed]
- 6.Ridpath JF, Neill JD, Frey M, et al. Phylogenetic, antigenic and clinical characterization of type 2 BVDV from North America. Vet Microbiol 2000;77:145–155. [DOI] [PubMed]
- 7.Vilcek S, Paton DJ, Durkovic B, et al. Bovine viral diarrhoea virus genotype 1 can be separated into at least eleven genetic groups. Arch Virol 2001;146:99–115. [DOI] [PubMed]
- 8.Potgeiter LND, McCracken MD, Hopkins FM, Walker RD, Guy JS. Experimental production of bovine respiratory tract disease with bovine viral diarrhea virus. Am J Vet Res 1984;45: 1582–1585. [PubMed]
- 9.Potgeiter LND, McCracken MD, Hopkins FM, Guy JS. Comparison of the pneumopathogenicity of two strains of bovine viral diarrhea virus. Am J Vet Res 1985;46:151–153. [PubMed]
- 10.Lehmkuhl HD, Gough PM. Investigation of causative agents of bovine respiratory tract disease in a beef cow-calf herd with an early weaning program. Am J Vet Res 1977;38:1717–1720. [PubMed]
- 11.Martin SW, Bohac JG. The association between serological titers in infectious bovine rhinotracheitis virus, bovine virus diarrhea virus, parainfluenza-3 virus, respiratory syncytial virus and treatment for respiratory disease in Ontario feedlot calves. Can J Vet Res 1986;50:351–358. [PMC free article] [PubMed]
- 12.Richer L, Marois P, Lamontagne L. Association of bovine viral diarrhea virus with multiple viral infections in bovine respiratory disease outbreaks. Can Vet J 1988;29:713–717. [PMC free article] [PubMed]
- 13.Martin SW, Bateman KG, Shewen PE, et al. A group level analysis of the associations between antibodies to seven putative pathogens and respiratory disease and weight gain in Ontario feedlot calves. Can J Vet Res 1990;54:337–342. [PMC free article] [PubMed]
- 14.Allen JW, Viel L, Bateman KG, et al. Serological titers to bovine herpesvirus 1, bovine viral diarrhea virus, parainfluenza 3 virus, bovine respiratory syncytial virus and Pasteurella haemolytica in feedlot calves with respiratory disease: association with bacteriological and pulmonary cytological values. Can J Vet Res 1992;56:281–288. [PMC free article] [PubMed]
- 15.Caldow GL, Edwards S, Peters AR, et al. Association between viral infections and respiratory disease in artificially reared calves. Vet Rec 1993;133:85–89. [DOI] [PubMed]
- 16.Dinter Z, Bakos K. Viruses associated with acute respiratory and enteric disease in Swedish cattle. Bull Off Int Epizoot 1961;56: 29–34.
- 17.Ganaba R, Belanger D, Dea S, Bigras-Poulin M. A seroepidemiological study of the importance of respiratory and enteric viruses in beef operations from northwest Quebec. Can J Vet Res 1995;59:26–33. [PMC free article] [PubMed]
- 18.Stott EJ, Thomas LH, Collins AP, et al. A survey of virus infections of the respiratory tract of cattle and their association with disease. J Hyg 1980;85:257–269. [DOI] [PMC free article] [PubMed]
- 19.Reggiardo C. Role of BVD virus in shipping fever of feedlot cattle. Case studies and diagnostic considerations. Proc 22nd Annu Meet Am Assoc Lab Diagnosticians, San Diego, California, 1979:315–320.
- 20.Fulton RW, Purdy CW, Confer AW, et al. Bovine viral diarrhea viral infections in feeder calves with respiratory disease: interactions with Pasteurella spp., parainfluenza-3 virus, and bovine respiratory syncytial virus. Can J Vet Res 2000;64:151–159. [PMC free article] [PubMed]
- 21.Martin SW, Bateman KG, Shewen PE, et al. The frequency, distribution and effects of antibodies, to 7 putative respiratory pathogens, on respiratory disease and weight gain in feedlot calves in Ontario. Can J Vet Res 1989;53:355–362. [PMC free article] [PubMed]
- 22.Martin SW, Nagy E, Armstrong D, Rosendal S. The associations of viral and mycoplasmal antibody titers with respiratory disease and weight gain in feedlot calves. Can Vet J 1999;40:560–567. [PMC free article] [PubMed]
- 23.Booker CW, Guichon PT, Jim GK, et al. Seroepidemiology of undifferentiated fever in feedlot calves in western Canada. Can Vet J 1999;40:40–48. [PMC free article] [PubMed]
- 24.O'Connor A, Martin SW, Nagy E, et al. The relationship between the occurrence of undifferentiated bovine respiratory disease and titer changes to bovine coronavirus and bovine viral diarrhea virus in 3 Ontario feedlots. Can J Vet Res 2001;65:137–142. [PMC free article] [PubMed]
- 25.Alkan F, Ozkul A, Bilge-Dagalp S, et al. Virological and serological studies on the role of PI-3 virus, BRSV, BVDV, and BHV-1 on respiratory infections of cattle. DTW 2000;107:193–195. [PubMed]
- 26.Obando C, Baule C, Pedrique C, et al. Serological and molecular diagnosis of bovine viral diarrhoea virus and evidence of other viral infections in dairy calves with respiratory disease in Venezuela. Acta Vet Scand 1999;40:253–262. [DOI] [PMC free article] [PubMed]
- 27.Baule C, Kulcsar G, Belak K, et al. Pathogenesis of primary respiratory disease induced by isolates from a new genetic cluster of bovine viral diarrhea virus type 1. J Clin Microbiol 2001;39:146–153. [DOI] [PMC free article] [PubMed]
- 28.Taylor LF, Janzen ED, Ellis JA, et al. Performance, survival, necropsy, and virological findings from calves persistently infected with the bovine viral diarrhea virus originating from a single Saskatchewan beef herd. Can Vet J 1997;38:29–37. [PMC free article] [PubMed]
- 29.Baule C, van Vewren M, Lowings JR, et al. Genetic heterogeneity of bovine viral diarrhoea viruses isolated in South Africa. Virus Res 1997;52:205–220. [DOI] [PubMed]
- 30.Fulton RW, Confer AW, Burge LJ, et al. Antibody responses by cattle after vaccination with commercial viral vaccines containing bovine herpesvirus-1, bovine viral diarrhea virus, parainfluenza-3 virus, and bovine respiratory syncytial virus immunogens and subsequent revaccination at day 140. Vaccine 1995;13:725–733. [DOI] [PubMed]
- 31.Fulton RW, Saliki JT, Burge LJ, et al. Neutralizing antibodies to type 1 and 2 bovine viral diarrhea viruses: detection by inhibition of viral cytopathology and infectivity by immunoperoxidase assay. Clin Diagn Immunol 1997;4:380–383. [DOI] [PMC free article] [PubMed]
- 32.Saliki JT, Fulton RW, Hull SR, et al. Microtiter virus isolation and enzyme immunoassays for detection of bovine viral diarrhea virus in cattle serum. J Clin Microbiol 1997;35:803–807. [DOI] [PMC free article] [PubMed]
- 33.Compendium of Veterinary Products. 6th ed. Port Huron, Michigan: North American Compendiums, 2001:302–309.
- 34.Agresti A. Categorical Data Analysis. New York, New York: Wiley and Sons, 1990.
- 35.Van Campen H, Vorpahl P, Huzurbazar S, Edwards J, Cavender J. Evidence for bovine viral diarrhea virus type 2 (BVDV)-associated disease in beef herds vaccinated with a modified-live type 1 BVDV vaccine. J Vet Diagn Invest 2000;12:263–265. [DOI] [PubMed]
- 36.Jones L, Van Campen H, Xu ZC, et al. Comparison of neutralizing antibodies to type 1a, 1b, and 2 bovine viral diarrhea virus from experimentally infected and vaccinated cattle. Bovine Practitioner 2001;35:137–140.
- 37.Fulton RW, Burge LJ. Bovine viral diarrhea virus types 1 and 2 antibody response in calves receiving modified live virus or inactivated vaccines. Vaccine 2000;19:267–274. [DOI] [PubMed]
- 38.van Drunen Littel-van den Hurk S, Myers D, Doig PA, et al. Identification of a mutant bovine herpesvirus-1 (BHV-1) in post-arrival outbreaks of IBR in feedlot calves and protection with conventional vaccination. Can J Vet Res 2001;65:81–88. [PMC free article] [PubMed]
- 39.Kahrs RF. Viral Diseases of Cattle. 2nd ed. Ames, Iowa: Iowa State Univ Pr, 1981:53.