Abstract
The effects of Ba2+ on current resulting from the heterologous expression of the human ether-à-go-go related gene (HERG) (IHERG) was studied with two-electrode voltage clamp techniques in Xenopus oocytes.
Ba2+ produced time- and voltage-dependent block of IHERG. Significant inhibition was seen at concentrations as low as 1 μm. Inhibition was greatest at step potentials between -40 and 0 mV; at more positive potentials, inhibition decreased in association with time-dependent unblocking of channels.
An inactivation-attenuated mutant of HERG (S631A) was prepared and expressed in Xenopus oocytes. Ba2+ block of S631A differed from that of HERG in that extensive unblocking was no longer seen at positive potentials and the voltage dependence of step current block was greatly attenuated.
A mathematical model was applied to analyse quantitatively the inhibitory effects of Ba2+ on IHERG. The model suggested similar voltage-dependent affinity of Ba2+ for the open and closed states, along with absence of binding to the inactivated state, and accounted well for Ba2+ effects on both wild-type and S631A channels.
We conclude that Ba2+ potently inhibits IHERG in a characteristic state-dependent fashion, with strong unblocking at positive potentials related to the presence of an intact C-type inactivation mechanism.
The delayed rectifier potassium current (IK) is a key cardiac repolarizing current in a large number of species and tissues (Giles & Shibata, 1985; Colatsky et al. 1990; Balser et al. 1990; Anumonwo et al. 1992; Barry & Nerbonne, 1996). It consists of at least two components, the rapidly activating component, IKr and the slowly activating component, IKs (Noble & Tsien, 1969; Sanguinetti & Jurkiewicz, 1990, 1991).
Our understanding of the properties of IK has been greatly advanced by the identification of important molecular components of both IKr and IKs. The products of HERG (Sanguinetti et al. 1995; Trudeau et al. 1995) and KvLQT1 (Barhanin et al. 1996; Sanguinetti et al. 1996) represent the pore-forming subunits of IKr and IKs, respectively. The products of the MiRP1 (Abbott et al. 1999) and minK (Barhanin et al. 1996; Sanguinetti et al. 1996) genes co-assemble with HERG and KvLQT1 in vivo to form IKr and IKs channels, respectively. Mutations at HERG or KvLQT1 loci are associated with the long QT syndrome, an inherited form of potentially lethal heart disease (Curran et al. 1995; Wang et al. 1996). Recently, double mutations at these loci have been identified in some patients severely affected by the long QT syndrome (Berthet et al. 1999). Due to the clinical importance of these syndromes, substantial effort continues to be dedicated to the detailed study of the characteristics of HERG and KvLQT1, especially with regard to pore structure and block by various compounds and metal ions.
Cations are widely used to probe the structure of K+ channels because of the ease with which they are able to access deeper pore regions, which may not be accessible by more sterically bulky drugs or toxins. One of the cations that is of special interest is Ba2+, which has a similar crystal radius to K+ (0.270 nm for Ba2+vs. 0.266 nm for K+) but is largely impermeable in K+ channel pores. Larger cations such as Tl+ (0.295 nm), Rb+ (0.295 nm) and NH4+ (0.286 nm) exhibit significant pore permeation (see Hille, 1992, for a review), and the non-permeation of Ba2+ is probably due to its divalent charge which results in a tight association with one or more K+ interaction sites in the open pore. Ba2+ interacts strongly with a variety of K+ channels from the intracellular or the extracellular sides of the membrane (Eaton & Brodwick, 1980; Armstrong et al. 1982; Miller et al. 1987; Taglialatela et al. 1993; Zang et al. 1995; Hurst et al. 1995). Ba2+ block is generally voltage dependent, reflecting the effect of the transmembrane voltage field on Ba2+ access to its binding site within the channel. Ba2+ effects on currents carried by HERG expressed in Xenopus oocytes have been reported to be either time and voltage independent (Trudeau et al. 1995) or voltage dependent (Ho et al. 1999). A better understanding of the Ba2+-HERG interaction could provide insight into biophysical properties of the channel. We therefore conducted the present study to determine in detail how Ba2+ affects IHERG. Because Ba2+ effects showed strong voltage and time dependence, we used a mathematical model to test quantitatively a conceptual model of state-dependent Ba2+ interactions with the HERG channel. Results of the present study have been previously presented in abstract form (Weerapura et al. 1998).
METHODS
Oocyte isolation and cRNA injection
Female Xenopus laevis were anaesthetized in 0.13 % w/v tricaine for 30 min at 4°C. Segments of the ovarian lobe were removed through a small abdominal incision. Up to four collections were made from each frog with adequate time allowed for healing between each. After the final collection frogs were killed by exsanguination following a lethal overdose of anaesthetic. The follicular layer was removed by digestion for 1 h with 6 units ml−1 collagenase (Sigma) in Ca2+-free Barth's solution (NaCl, 88 mm; KCl, 1 mm; NaHCO3, 2.4 mm; MgSO4, 0.82 mm; Hepes, 5 mm; pH 7.6; supplemented with 10 mg ml−1 penicillin-streptomycin). The oocytes were incubated at 17°C in L-15 media (50 % v/v Leibowitz's L-15 media, 0.4-g l−1 glutamine, 8 mm Hepes, 40 mg l−1 gentamicin, pH 7.6). For in vitro transcription, HERG cDNA subcloned into pSP64 plasmid vector was linearized with Eco R1 (New England Bio Labs) and then transcribed with SP6 RNA polymerase (Ambion) for 1.5-2 h at 37°C. Twenty four hours after the isolation procedure, stage IV and V oocytes were injected with 25–50 nl of HERG cRNA (∼20-40 ng oocyte−1).
Electrophysiology
Currents were recorded with the two-electrode voltage clamp technique from oocytes 1–3 days after injection of cRNA. Voltage commands were delivered via the GeneClamp 500 amplifier with the use of pCLAMP 6 software (Axon Instruments). Currents were recorded at room temperature in ND96 (ionic components (mM): NaCl, 96; KCl, 2; CaCl2, 1.8; MgCl2, 1; Hepes, 5; pH 7.5). Solutions containing Ba2+ were prepared by adding appropriate volumes of a 0.2 M stock prepared in distilled water. All voltage clamp protocols in the presence of Ba2+ were applied after 3 min of perfusion with Ba2+ at a given concentration to ensure steady state conditions. To examine the possibility of surface charge screening effects of Ba2+, we tested Ba2+ effects on oocytes treated with neuraminidase (Type X from Clostridium perfringens, Sigma). Endogenous currents from oocytes (n= 6) were recorded in the absence and presence of 2 mm Ba2+ 2 days after water injection.
Glass microelectrodes (borosilicate with filament) were pulled using a Flaming/Brown micropipette puller (Sutter Instruments). Pipettes had resistances of 1–3 MΩ for the voltage-sensing electrode and 0.1-0.5 MΩ for the current-injecting electrode when filled with 3 M KCl. The tips of the current-injecting electrodes were back-filled with 1 % agarose in 3 M KCl to prevent KCl leakage (Hebert et al. 1994). ClampFit (Axon) and Origin (version 4, Microcal Software) were used for curve fitting. Data are presented as the means ±s.e.m. Statistical comparisons were made with Student's paired t test.
RESULTS
Ba2+ block of wild-type IHERG
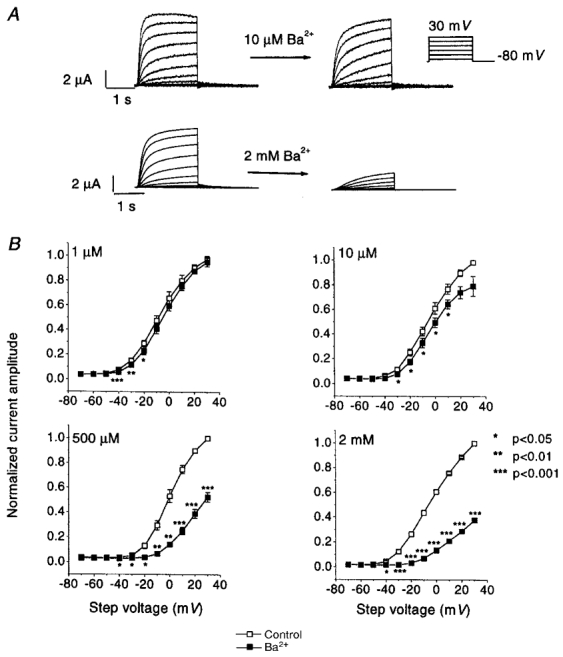
Figure 1A (left panels) shows IHERG upon 2 s depolarizations from a holding potential (Vh) of -80 mV (12 s interpulse interval) 2 days after HERG cRNA injection. Note the increase in time-dependent outward current amplitudes at the lower voltages (-30 and -10 mV) and a subsequent reduction at more positive potentials due to intrinsic voltage-dependent fast C-type inactivation. In contrast, tail currents recorded upon returning to -80 mV increased in amplitude with stronger depolarizing pulses because of rapid recovery from inactivation at the Vh. The middle panels of Fig. 1A illustrate the effects of 10 μm and 2 mm Ba2+. With 10 μm Ba2+, inhibition of step and tail current is evident at voltages of -30 and -10 mV, without apparent effects on currents at more positive potentials nor on tail current kinetics. At a 2 mm concentration, Ba2+ blocked step current at intermediate voltages, whereas at the most positive step potential tested (+30 mV), Ba2+ had little inhibitory effect on step current but inhibited the tail current. In addition to reducing maximum tail current amplitude, 2 mm Ba2+ also accelerated tail current decay. The inhibitory effects of Ba2+ were reversible upon washout (Fig. 1A, right panels). In order to evaluate the potential contaminating role of endogenous currents, currents were recorded from six water-injected oocytes with the same voltage protocol before and after 2 mm Ba2+. As shown in Fig. 1B (left), the endogenous conductance was much smaller than IHERG, with a maximum endogenous step current at +30 mV averaging < 8 % of the IHERG step current at the same voltage. Ba2+ had no effect on endogenous currents (Fig. 1B, right).
Figure 1. Concentration-dependent effects of Ba2+ on IHERG.
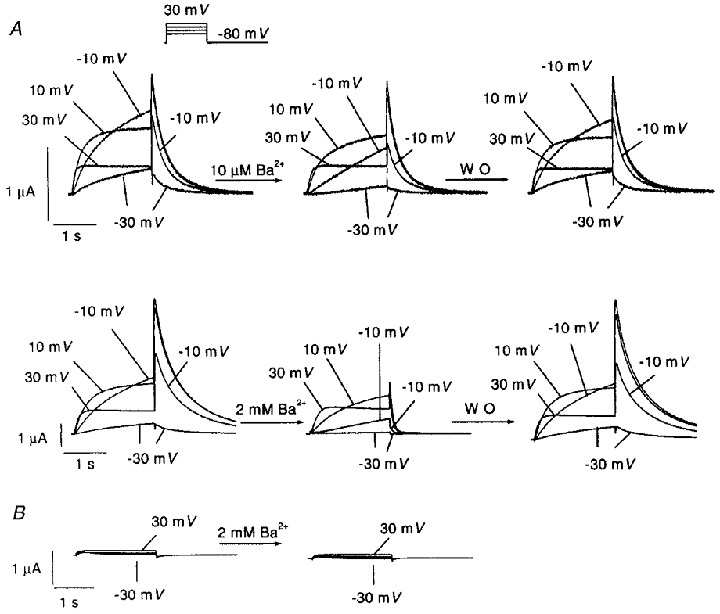
A, original recordings showing effects of 10 μm and 2 mm Ba2+ on IHERG in one oocyte each. IHERG was recorded with 2 s pulses to the voltages indicated (protocol inset). Leftmost panels are control recordings. Inhibitory effects were recorded 3 min after Ba2+superfusion (centre panels). Effects were reversible upon 12 min of washout (WO, right panels). Capacitance transients have been blanked out. B, typical example of currents recorded from water-injected oocytes before (left) and after (right) exposure to 2 mm Ba2+.
Mean data for Ba2+ effects on step currents at the end of 2 s test pulses are shown in Fig. 2. Data are normalized to the maximum step current in each oocyte to control for inter-oocyte variation in current amplitude. Significant inhibition was seen at all voltages between -40 and 0 mV for all concentrations tested. Ba2+ block decreased substantially at voltages on the descending limb of the IHERG current-voltage relation (i.e. > 0 mV), corresponding to voltages with increasingly important inactivation.
Figure 2. Mean data for Ba2+ inhibition of HERG step current.
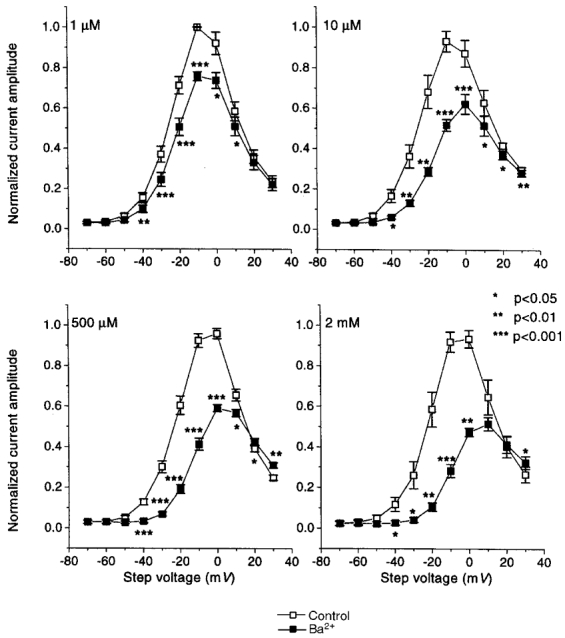
Step current amplitudes at the end of the 2 s pulses were measured before and after 3 min of perfusion with the indicated Ba2+ concentrations. Currents in each oocyte were normalized to the maximum current under control conditions, to control for varying current amplitudes among oocytes. The results shown are from 5, 5, 5 and 4 oocytes at 1, 10, 500 μm and 2 mm Ba2+, respectively. Data are plotted as means ±s.e.m.; * P < 0.05, ** P < 0.01, *** P < 0.001 vs. control).
Mean data for Ba2+ effects on peak tail current amplitude are shown in Fig. 3. A concentration-dependent inhibition of tail current was observed, with minimal effects at 1 μm, inhibition at voltages between -40 and -10 mV noted at 10 μm and inhibition at all potentials positive to -40 mV with 500 μm and 2 mm Ba2+. The half-maximal activation voltages (V½) were estimated by Boltzmann fits to tail current data. V½ averaged -18.9 ± 0.5, -17.7 ± 1, -17.2 ± 0.7 and -16.3 ± 0.7 mV under control conditions and -16 ± 0.5, -10.7 ± 1, -4.4 ± 0.8 and 1.0 ± 1.7 mV after 1 μm, 10 μm, 500 μm and 2 mm Ba2+, respectively.
Figure 3. Inhibition of HERG tail current by various concentrations of Ba2+.
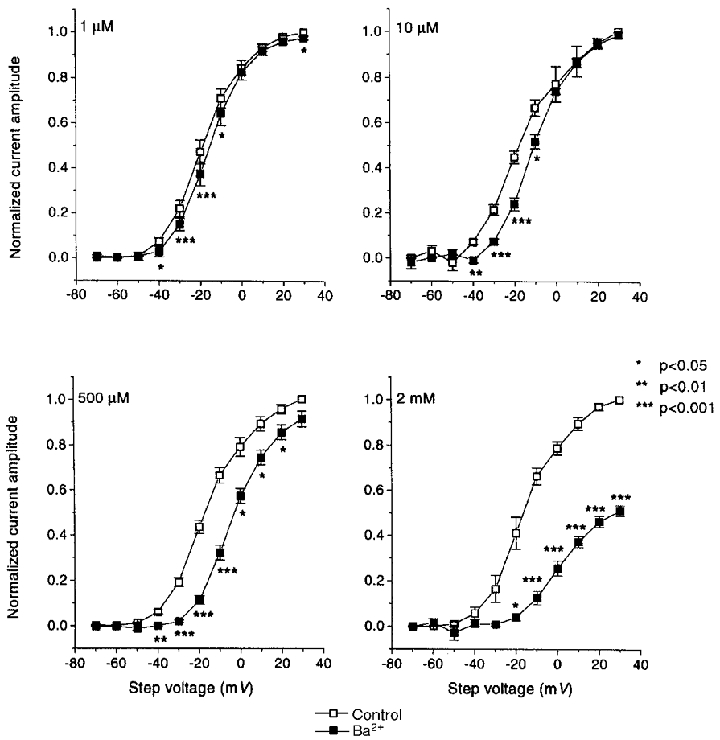
Results are means ±s.e.m. for 5, 5, 5 and 4 oocytes studied before and after 1, 10, 500 μm and 2 mm Ba2+, respectively. Tail currents were recorded returning to the Vh of -80 mV following 2 s steps to the voltages indicated. Current amplitudes were estimated by fitting the deactivation phase of the tails to biexponential functions and extrapolating back to the beginning of the repolarizing step. All currents were normalised to the maximum control current amplitude (* P < 0.05, ** P < 0.01, *** P < 0.001 vs. control). Data were fitted to Boltzman functions (IHERG= (1 + exp [(V½+Vt/k)])−1) where V½ is the half-activation voltage, Vt is the test voltage applied and k is the slope factor. V½ and k (in mV) estimated from the fits were: -18.9 ± 0.5, 9.7 ± 0.5; -17.7 ± 1, 11.1 ± 1.0; -17.2 ± 0.7, 10.5 ± 0.7; -16.3 ± 0.7, 10.1 ± 0.7 under control conditions and -16.0 ± 0.5, 8.7 ± 0.4; -10.7 ± 1, 9.0 ± 1.0; -4.4 ± 0.8, 8.9 ± 0.7; 1.0 ± 1.7, 9.6 ± 1.4 mV for 1, 10, 500 μm and 2 mm Ba2+, respectively.
The reversal of Ba2+-induced IHERG inhibition at positive voltages (Fig. 2) was further studied by analysing the evolution of block over time during depolarizing pulses. Figure 4 shows original recordings of IHERG before and after exposure to 500 μm Ba2+ at 0 and 30 mV (top panel), along with quantitative plots of Ba2+-induced block in the same experiment during 2 s pulses to each of the six voltages indicated (lower panel). Fractional block is maximal towards the beginning of the pulse, and decreases thereafter at a rate that is voltage dependent. Unblocking is slowest at the most negative voltages and becomes increasingly rapid and more complete at positive potentials associated with prominent fast inactivation. Thus Ba2+ appears to unblock from HERG channels upon depolarization to positive voltages. Block is re-established upon repolarization to -80 mV, as indicated by the acceleration of tail current deactivation (Fig. 1). Tail current block is established at a rate that becomes increasingly rapid as Ba2+ concentration increases; e.g. τ= 207 ± 19 and 71 ± 10 ms for 100 μm (n= 6) and 2 mm Ba2+ (n= 4), respectively.
Figure 4. Evolution of Ba2+ block of IHERG during depolarization.
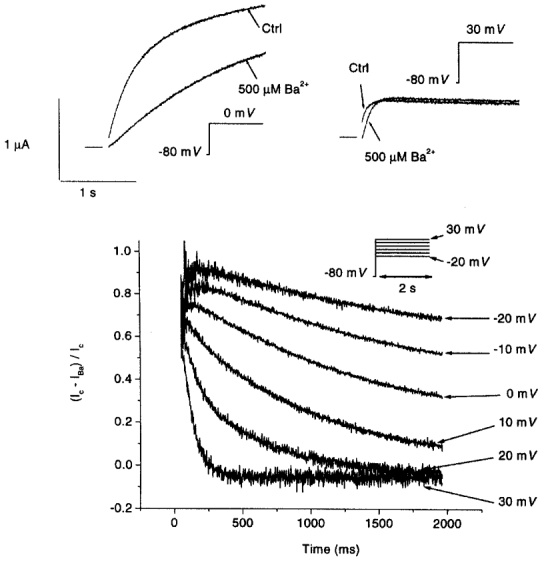
Top, original recordings during 2 s depolarizations to 0 mV (left) and 30 mV (right) before and after superfusion with 500 μm Ba2+. Capacitive transients have been removed. Horizontal bars indicate zero current levels for the adjacent traces. Bottom, evolution of fractional block (Ic–IBa)/Ic, where Ic and IBa are currents under control conditions and in the presence of 500 μm Ba2+, respectively, in the same experiment, upon depolarization to the 6 voltage levels indicated. Similar results were obtained in 4 other oocytes.
Ba2+ block of the inactivation-deficient mutant S631A
Ba2+ unblocking at voltages at which inward rectification due to rapid inactivation becomes prominent, and re-blocking at more negative voltages at which inactivation is removed, suggest that HERG inactivation interferes with Ba2+ block. To evaluate further the potential role of rapid inactivation in governing the effects of Ba2+ on IHERG, we created the inactivation-attenuated mutant S631A (Zou et al. 1998). The single amino acid substitution in the mutant is sufficient to shift the voltage dependence of inactivation by about 100 mV in the depolarizing direction. Thus, within the voltage range used in these studies, the channels are inactivation deficient and show greatly reduced inward rectification (Fig. 5A, left panels) compared with the wild-type (Fig. 1). The effect of Ba2+ was markedly altered in the S631A mutant. The qualitatively different behaviour of Ba2+ block at different test potentials noted for wild-type IHERG (Figs 1 and 2) was replaced by qualitatively similar responses at each test potential in the mutant (Fig. 5A). Figure 5B shows mean (±s.e.m.) IS631A step current under control conditions and in the presence of 1, 10, 500 μm and 2 mm Ba2+ (n= 5, 5, 5 and 7 oocytes, respectively). Significant reductions in current were produced at all voltages positive to -40 mV by Ba2+ concentrations of 10 μm or greater.
Figure 5. Response of the HERG S631A mutant to Ba2+.
A, IS631A (left panels) recorded from two different oocytes during the application of pulse protocol (inset) identical to that used in Fig. 1. Effects of 10 μm and 2 mm Ba2+ on these currents are illustrated on the right. B, mean data for Ba2+ inhibition of step current carried by S631A mutant channels. Step current amplitudes were measured before and after 3 min of perfusion with the indicated Ba2+ concentrations, with the use of the voltage protocol illustrated above. Currents in each oocyte were normalized to the maximum current under control conditions, to control for varying current amplitudes among oocytes. The results shown are from 5, 5, 5 and 7 oocytes at 1, 10, 500 μm and 2 mm Ba2+, respectively. Data are plotted as means ±s.e.m.; * P < 0.05, ** P < 0.01, *** P < 0.001 vs. control.
Figure 6 illustrates the evolution of block of IS631A by 500 μm Ba2+ (compare with Fig. 4). At 0 mV, the response to Ba2+ is qualitatively comparable to that of wild-type channels; however, at 30 mV there is a major difference. The strong time-dependent unblocking noted in wild-type channels is lost. The differences between wild-type and mutant channels are further illustrated by the plot of block as a function of time at step voltages between -20 and +30 mV (Fig. 6, lower panel). In place of the important unblocking at positive voltages observed for the wild-type (Fig. 4) only a modest degree of unblocking occurs.
Figure 6. Evolution of Ba2+ block of current in S631A mutant channels during depolarizing steps.
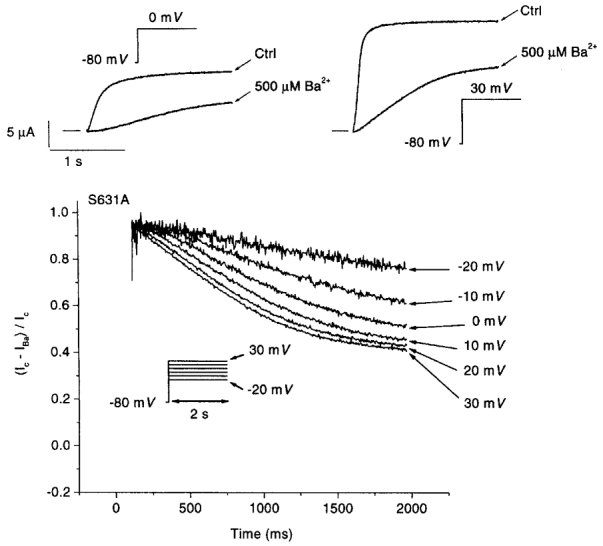
Top panel, original recordings during 2 s depolarizations to 0 mV (left) and 30 mV (right) before and after superfusion with 500 μm Ba2+. Capacitive transients have been removed. Bottom, development of fractional block, (Ic–IBa)/Ic, in the presence of 500 μm Ba2+ in the same experiment, upon depolarization to the 6 voltage levels indicated. Similar results were obtained in 6 other oocytes.
Mathematical model of Ba2+ effects on IHERG
In order to provide a quantitative explanation for the Ba2+-dependent alterations in kinetics, half-activation voltage, and maximal conductance of HERG, we applied a mathematical model of the channel and its interaction with Ba2+. Our model is based on the work of (Wang et al. 1997a), who developed a mathematical model of wild-type HERG channels expressed in Xenopus oocytes, based on two-electrode and cut-open oocyte clamp current recordings. They showed that the best fit to their data was obtained using a model with three closed states (C2, C1, C0), one open state (O), and one inactivated state (I) (Scheme 1; Wang et al. 1997a).
Scheme 1.
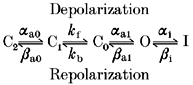
We implemented their formulation exactly, except for one modification to the inactivation kinetics of HERG that prevented the large initial current spikes observed at positive step potentials (+30 mV) in the original model. Faster inactivation kinetics reduced the amplitude of the spike, consistent with experimental recordings (Fig. 1).
The resulting modified functions for the rate constants of the O 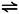 I transition are:
I transition are:
and
After establishing the basic model for wild-type IHERG, we investigated the consequences of voltage-dependent or independent binding of Ba2+ to one or more channel states, aiming to reproduce the altered kinetics, half-activation voltage and maximal conductance of IHERG in the presence of Ba2+. The strong block immediately after depolarization and the more pronounced sigmoid activation profile of IHERG in the presence of Ba2+ at negative potentials suggest closed state block. The absence of significant steady-state block at positive potentials suggests voltage-dependent action. Acceleration of tail current decay in the presence of Ba2+ requires shunting of open channels through open-blocked and closed-blocked states.
Based on the available data, we selected a simple model of voltage-dependent Ba2+ binding. This is in general terms similar to the Ba2+ blocking scheme suggested by Ho et al. (1999); however, these investigators did not study a full HERG model incorporating inactivation, which prevented them from drawing conclusions about the role of Ba2+ in decreasing maximal HERG tail currents and from fully understanding effects at voltages with substantial inactivation. We assumed that Ba2+ blocked only non-inactivated HERG channels, since we had no direct experimental evidence to suggest a role for binding of Ba2+ to the inactivated state. In addition, the low level of IHERG block at positive potentials makes high affinity for inactivated channels unlikely. The O ⇌ I transition was maintained in the model so as to simulate all the gating properties in the absence and presence of Ba2+. Four new states are included in the model, C0B, C1B, C2B and OB, corresponding to barium-bound closed and open states. Based on the step current values in the presence of Ba2+, its binding affinity for the closed and open states is high at negative potentials and low at positive potentials. A unique steady-state relation for both the C0,1,2 ⇌ C0,1,2B and O ⇌ OB transitions in the presence of Ba2+ at a 100 μm concentration was formulated as:
Rapid unblocking of step currents at +30 mV suggests rapid kinetics of Ba2+ interaction at positive potentials. Acceleration of tail currents suggests rapid blocking kinetics for high Ba2+ concentrations at negative potentials. The resulting fitted functions of the rate constants for the C0,1,2 ⇌ C0,1,2B and O ⇌ OB transitions are:
and
The model parameters describing the transitions between states C0,1,2B and OB are identical to those describing the transitions between states C0,1,2 and O, suggesting similar voltage-dependent effects on Ba2+ interaction with the closed and open states.
The voltage protocol used in Fig. 1A was applied to the model to produce model-generated step and tail currents in the presence of 10 μm and 2 mm Ba2+ (Fig. 7). An IHERG reversal potential (Vrev) of -90 mV was used, and the maximum IHERG conductance (gmax) was 0.72542 mS. Instantaneous IHERG was then computed as IHERG=gmax O (V – Vrev). The model reproduces the experimentally observed voltage-dependent step current block, delayed current activation and acceleration of tails caused by Ba2+ in a concentration-dependent manner (compare Fig. 7 with Fig. 1A). The quantitative results of the model simulations are summarized in the form of step (left) and tail (right) current-voltage relations at different Ba2+ concentrations in Fig. 8. The model accurately reproduces the main features of the corresponding experimental observations shown in Figs 2 and 3.
Figure 7. Model-simulated IHERG in the absence and presence of low (10 μm) and high (2 mm) Ba2+ concentrations.
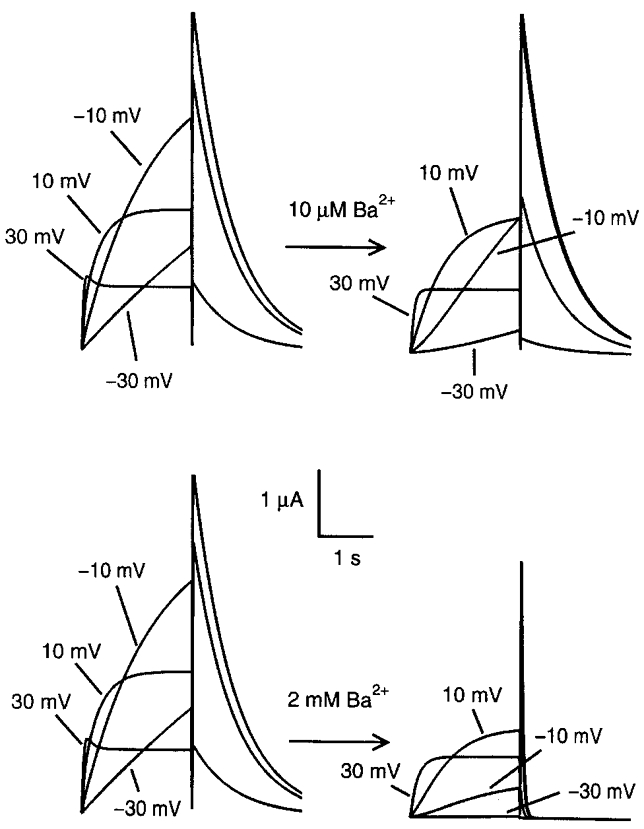
Applied pulse protocols were identical to those used experimentally, i.e. -80 mV holding potential and 2 s depolarizations to the test voltages followed by return to the holding potential. Note the similarity in responses to experimental recordings (Fig. 1A).
Figure 8. Current-voltage relations from model simulations of wild-type step and tail currents in the absence and presence of Ba2+.
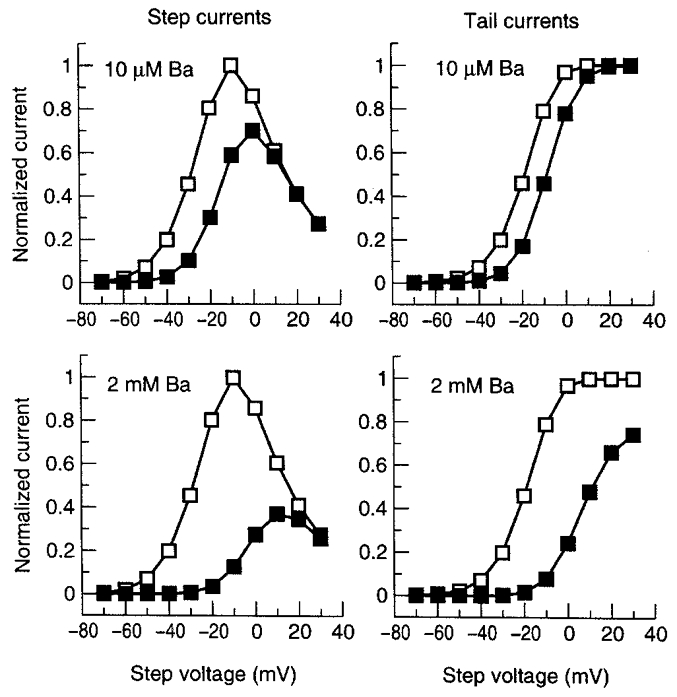
The model was subjected to the pulse protocol studied in Fig. 7. Step current amplitudes (left panels) were calculated from simulated current at the end of 2 s depolarizing pulses. Tail currents (right panels) were obtained from current amplitudes upon returning to -80 mV.
We then turned to the assessment of model predictions about Ba2+ interaction with the S631A mutant. The IHERG model based on wild-type data could be modified to reproduce currents carried by S631A mutant channels simply by incorporating a +120 mV shift in inactivation voltage dependence (Fig. 9A). The shift required is close to the +102 mV experimentally measured shift reported by Zou et al. (1998). As was noted in experimental recordings (Fig. 5A), 2 mm Ba2+ substantially reduced step currents in the model at all voltages (Fig. 9A, right). The results of voltage steps were then analysed in the model for both drug-free IS631A and IS631A in the presence of various Ba2+ concentrations. Substantial concentration-dependent step current block by Ba2+ was observed at positive potentials (Fig. 9B), in contrast to the lack of block at positive potentials in the wild-type model (Fig. 8, left panels) and in agreement with the experimental results with the mutant (Fig. 5B). Figure 10 illustrates the evolution of Ba2+ block as a function of time during depolarizing voltage steps in the wild-type (upper panel) and the mutant (lower panel) model. The wild-type model reproduces the experimentally observed profiles (Fig. 4, bottom panel), with rapid unblocking at more positive potentials and slower, incomplete unblocking at lesser potentials. The model for the mutant (Fig. 10B) reproduces the reduced degree of Ba2+ unblocking observed for IS631A (Fig. 6, bottom panel), although unblocking in the model remains faster than that observed experimentally.
Figure 9. Model-simulated S631A currents in the absence and presence of low (10 μm) and high (2 mm) Ba2+ concentrations (A) and their representative current-voltage relationships under control conditions and in the presence of 10, 500 μm and 2 mm Ba2+ (B).
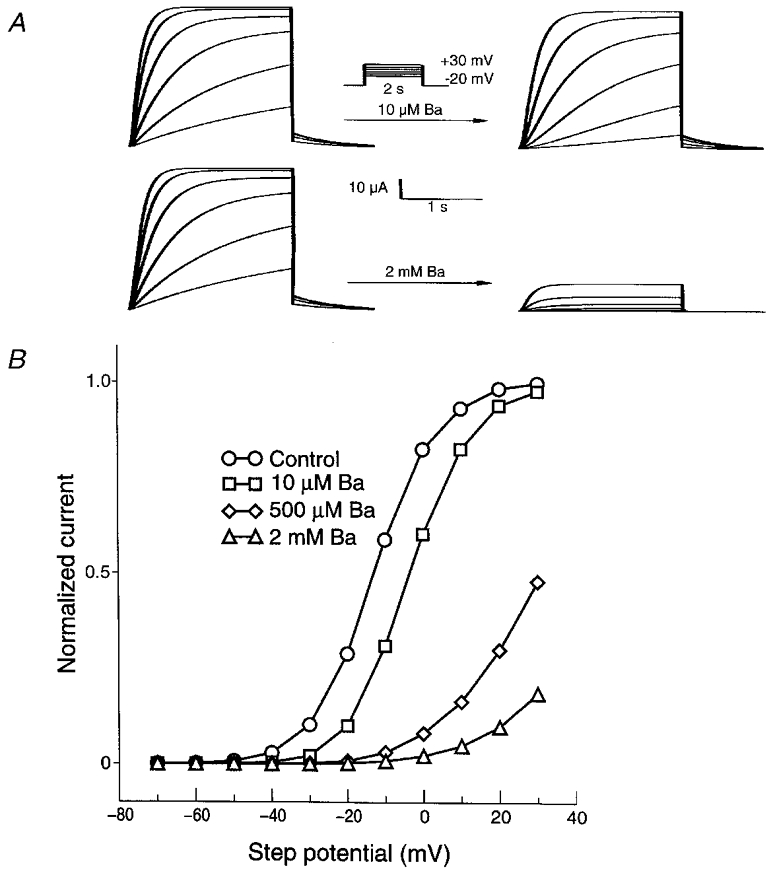
Note the similarity of the simulated current profiles to experimental results (Fig. 5).
Figure 10. Model-simulated fractional Ba2+ block of wild-type (top panel) and S631A mutant (lower panel) currents during 2 s voltage steps.
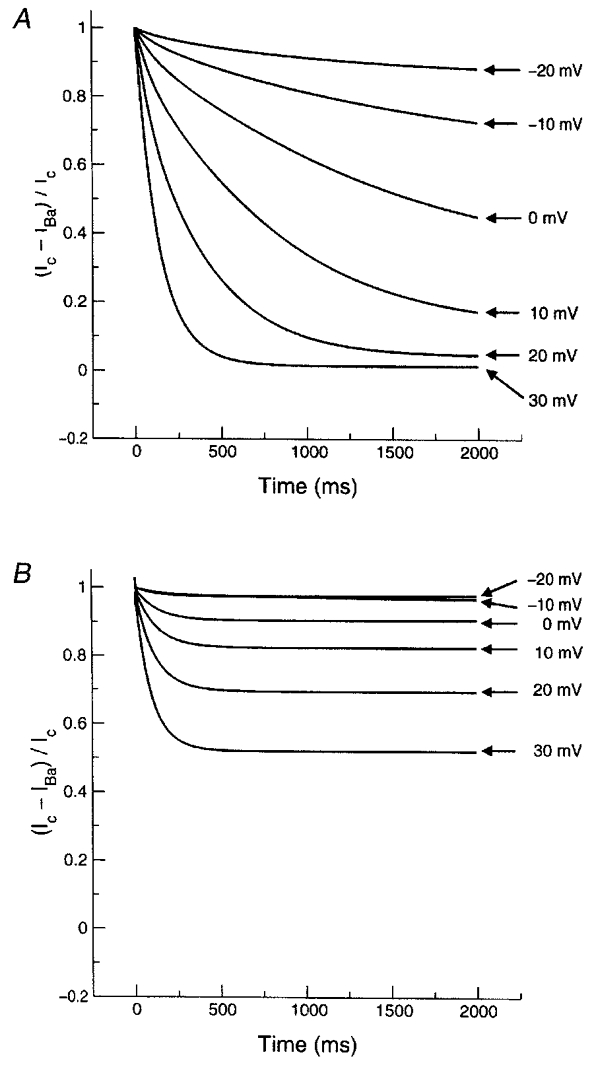
Note the prominent unblocking of Ba2+ from wild-type channels at positive potentials, similar to experimental observations (Fig. 4, lower panel). Note also the attenuation of unblocking in the simulations for the mutant (compare with experimental observations in Fig. 6, lower panel).
DISCUSSION
We have demonstrated that Ba2+ inhibits IHERG in a concentration-, voltage- and time-dependent fashion. An unusual aspect of Ba2+ block of IHERG is its complete relief at positive voltages, which was no longer present in an inactivation-deficient mutant, suggesting that the phenomenon is related to rapid C-type inactivation. A mathematical model of HERG-Ba2+ interaction incorporating voltage-dependent Ba2+ block of open and closed states and no binding to the inactivated state provided simulations that were in broad agreement with experimental observations.
Comparison with previous observations of Ba2+ block of IHERG
Studies of Ba2+ interaction with IHERG have been limited. Trudeau et al. (1995) showed that Ba2+ inhibits inward currents carried by IHERG, with an ∼50 % reduction at 0.5 mm concentration. They did not observe time- or voltage-dependent block under a limited range of conditions. Subsequent to the completion and initial submission of the present study, Ho et al. (1999) reported a detailed analysis of the effects of divalent cations, including Ba2+, on IHERG. They noted a variety of effects of Ba2+ on IHERG similar to those we observed, including voltage-dependent effects on step current, a slowing in the onset of step currents and an acceleration of tail currents. They were able to simulate effects on IHERG activation voltage dependence and tail current kinetics on the basis of a voltage-dependent interaction of Ba2+ with open channels qualitatively similar to that incorporated in our model. Their model did not incorporate IHERG inactivation and they only studied Ba2+ effects on currents carried by wild-type HERG channels.
Ho et al. (1999) justified the omission of channel inactivation in their model because the inactivation process seemed little affected by Ba2+. Our observations also suggest little direct interaction between Ba2+ and IHERG inactivation; however, inactivation appears to play a potentially important role in determining blocking behaviour at positive potentials, as shown by our results with the S631A mutant and supported by our modelling work. The loss of Ba2+ block of IHERG step currents at positive potentials (Fig. 2) was no longer evident in the mutant (Fig. 5B), and the strong time-dependent unblocking during steps to positive potentials (Fig. 4, bottom) seen with wild-type channels was greatly attenuated in the mutant (Fig. 6, bottom). Furthermore, simply shifting inactivation by +120 mV in the mathematical model of HERG was sufficient to reproduce the major experimentally observed alterations in Ba2+ action caused by the S631A mutation. These results suggest that Ba2+ has negligible affinity for HERG channels in the inactivated state. Thus, at positive potentials there is a functional competition between Ba2+ block and the inactivation process for removal of channels from the open state. At such potentials, the steady-state ratio of inactivated to open channels is much greater than that between Ba2+ blocked and unblocked open channels, resulting in negligible effects of Ba2+ on the number of conducting, open channels. In the mutant, inactivation is greatly attenuated or lost over the voltage range examined in the present study, and the blocking of open channels is apparent at positive voltages.
Comparison with Ba2+ effects on other K+ channels
Ba2+ has long been known to interact with a variety of native K+ channels (Werman & Grundfest, 1961). Eaton & Brodwick (1980) showed that Ba2+ inhibits K+ currents in squid giant axon upon either external or internal application. Ba2+ block is voltage and time dependent, with features that suggest a blocking site in the middle of the membrane electrical field. Increasing external [K+] decreases block by external Ba2+, suggesting that Ba2+ and K+ compete for a common binding site. Armstrong et al. (1982) further evaluated Ba2+ interactions with the squid K+ conductance, noting that internal Ba2+ enters the channel only when activation gates are open, producing time-dependent block and that the entry of external Ba2+ is particularly remarkable when the activation gates are closed. The blocking site appears to be the same for both external and internal Ba2+, and is located towards the internal mouth of the channel, two thirds of the way from the external side. Ba2+ is a strong blocker of inward rectifier cardiac K+ channels, blocking both the background conductance (IK1) and the acetylcholine-activated conductance (IKACh) with 50 %-inhibitory concentrations in the range of 10 to 20 μm (Carmeliet & Mubagwa, 1986). Ba2+ block of IKACh is subject to the same type of ‘knock-off’ interaction with external K+ as seen for the K+ conductance in squid giant axon, and shows time dependence.
Over the past several years, substantial work has been performed to evaluate the interaction between Ba2+ and various cloned K+ channels, primarily to assess structural determinants of Ba2+ block. Hurst et al. (1995) showed that exposure of Shaker channels in Xenopus oocytes to external Ba2+ is followed by progressive channel block with two kinetic phases, apparently due to distinct blocking sites. The rapid-component site has relatively low affinity (Kd at 0 mV of about 19.1 mm) and its Ba2+ affinity is less voltage dependent than the slower site. The same group subsequently showed that mutations in the pore region of Shaker could reduce Ba2+ block by decreasing the rate of block (Hurst et al. 1996). Harris et al. (1998) studied the properties of Ba2+ block of Shaker further and identified three potential interaction sites. All three sites are accessible to the external solution when the activation gates are closed, and the deep site lies between the activation gate and the structure responsible for C-type inactivation. They also identified mutations in the pore region that disrupt two of the binding sites. More recently Basso et al. (1998) determined that Ba2+ binds tightly to C-type inactivated Shaker channels, with C-type inactivation creating high-energy barriers that hinder Ba2+ egress. Ba2+ also interacts with Kv2.1 channels, with block occurring at both internal and external sites, and showing strong voltage and state dependence (Taglialatela et al. 1993).
Some of the properties of Ba2+ block of IHERG resemble those previously reported for other K+ channels as discussed above. These include the voltage dependence of block, with inhibition more marked at negative voltages, and the time dependence of blocking behaviour. The feature which appears to differentiate Ba2+ block of IHERG and that of other channels studied to date (which have generally lacked inactivation) is the strong attenuation of IHERG block at positive potentials related to the presence of an intact rapid C-type inactivation mechanism.
Potential limitations
It would have been desirable to isolate Ba2+ block of individual channel states with selective voltage protocols, in order to test state-dependent block of the model both directly and independently. Unfortunately, because Ba2+ interacts with closed and open channel states, it is impossible to design voltage protocols that evaluate Ba2+ block of single states. For example, for HERG channels in the absence of Ba2+, the inactivated state can be studied by pulsing briefly from a positive (e.g. 30 mV) to a negative voltage (e.g. -120 mV) to remove inactivation and then observing fast inactivation during a pulse to a positive potential. However, Ba2+ blocks closed channels in a time-dependent way on pulsing to the negative potential and will unblock in a time-dependent way on pulsing back to the more positive potential. Thus, the peak current during the final depolarizing pulse will reflect both intrinsic voltage-dependent conductance and Ba2+ block of open and closed channels. The kinetics of current decay will reflect intrinsic inactivation, but also the kinetic changes in Ba2+ block as channels cycle through closed, open and inactivated states. The closed state cannot be studied in isolation because channels have to be opened to record current and evaluate block. The open state cannot be studied in isolation because some degree of inactivation occurs in wild-type channels over most of the voltage range over which there is significant channel opening. Therefore, we relied on the use of an inactivation-deficient mutant and mathematical modelling to understand the Ba2+ interaction with the channel.
At some test potentials (particularly negative to 0 mV), steady-state current was not achieved at the end of a 2 s voltage pulse. This could have affected the derived half-activation voltages. In addition, a direct effect of Ba2+ to slow activation could have contributed to apparent unblocking by reducing current at the beginning of a pulse. A slowing in activation cannot be clearly separated experimentally from time-dependent unblocking of inactivated channels. The more marked slowing of current development during a pulse in wild-type (e.g. Fig. 1, -10 mV) compared with inactivation-deficient (Fig. 5, top) channels argues in favour of unblocking during inactivation as an important mechanism for apparent slowing of wild-type activation in the presence of Ba2+. Nonetheless, a possible contribution from Ba2+-induced activation slowing cannot be excluded.
Divalent cations can cause voltage shifts of activation and inactivation, which can complicate analysis of their direct actions. In a separate set of experiments, we applied a three-step protocol to examine the voltage dependence of inactivation in oocytes expressing HERG. Only a modest shift (∼10 mV) of the voltage dependence was observed with 500 μm or 2 mm Ba2+ (data not shown), in agreement with the observations of Ho et al. (1999). This moderate shift would not explain phenomenon at very positive voltages, at which inactivation is saturated. We also studied the effects of Ba2+ in neuraminidase-treated oocytes. Neuraminidase is known to selectively hydrolyse negatively charged sialic acid groups on the cell surface, eliminating their potential influence on voltage-dependent gating processes (Fermini & Nathan, 1991). Effects of neither 500 μm nor 2 mm Ba2+ (n= 5 each) were quantitatively different in neuraminidase treated vs. untreated oocytes (data not shown). Nonetheless, we cannot exclude a possible contribution of surface charge screening effects to the voltage-dependent actions of Ba2+, nor can we be positive that the S631A mutant had no effect on affinity for Ba2+ independent of effects on C-type inactivation.
Although our mathematical model reproduced many of the features of currents carried by wild-type and S631A HERG in the absence and presence of Ba2+, not all experimental findings were noted in the model. Most notably, the rate of apparent Ba2+ unblocking during step pulses in mutant channels was slower in experimental recordings (Fig. 6) than predicted by the model (Fig. 10B). Some of the discrepancies may be due to our use of the model of Wang et al. (1997a) with minimal modification. However, it is quite possible that our relatively simple model does not take into consideration additional complexities of the Ba2+-HERG interaction. In addition, the agreement between model predictions and experimental behaviour does not prove the validity of the assumptions underlying the model. It simply indicates that the conceptual notions incorporated in the model are sufficient to explain most of the observed behaviour. We cannot eliminate the possibility that an alternative model based on a different set of assumptions could account for experimental behaviour just as well.
Novel aspects and potential importance
The HERG gene sprang into popular attention upon the demonstration of the role of HERG mutations in the type 2 congenital long QT syndrome (Curran et al. 1995). HERG was found to encode a channel with macroscopic current properties corresponding to IKr (Sanguinetti et al. 1995; Trudeau et al. 1995). The single-channel properties of IHERG and its pharmacologic responses were also found to resemble those of IKr (Kiehn et al. 1996; Zou et al. 1997). One of the distinctive properties of IKr is its strong inward rectification, caused by very rapid C-type inactivation of the HERG channel (Smith et al. 1996; Spector et al. 1996). This unusual form of inactivation is probably central to the physiological role of the channel, allowing it to contribute importantly to phase 3 repolarization without interfering with the long plateau phase which is typical of cardiac action potentials and essential to normal mechanical and electrical function. Rapid C-type inactivation is associated with the specific pharmacological sensitivity of IKr to methanesulfonanilides like dofetilide, and mutations that remove fast inactivation (like the S631A mutation which we studied) greatly reduce sensitivity to methanesulfonanilide block (Wang et al. 1997b; Ficker et al. 1998). We found that the C-type inactivation mechanism of IHERG was associated with unusual behaviour of Ba2+ block, with strong unblocking of wild-type channels at positive voltages that disappeared when fast inactivation was removed. It is perhaps because of this behaviour that Ba2+ block of IKr has not previously been noted – the rapid inactivation mechanism of the channel minimizes block by Ba2+ at voltages positive to 0 mV. It is presently unknown, and would be interesting to determine, whether Ba2+ unblocking in the presence of inactivation is limited to IHERG, or also occurs with other K+ channels demonstrating inactivation. A variety of other divalent cations, including Mn2+, Zn2+, Ca2+ and Mg2+, also show reduced ability to block IHERG at positive potentials (Ho et al. 1998, 1999). It would be interesting to establish whether the removal of inactivation alters their interactions with IHERG, and to determine the molecular mechanism of this phenomenon. Futhermore, it would be interesting to determine how co-expression with MiRP alters HERG block by Ba2+ and other cations, since native IKr is believed to be carried by channel complexes involving both HERG and MiRP (Abbott et al. 1999).
Ba2+ has been used extensively to eliminate potential contaminating effects of IK1 channels in the recording of other K+ currents (DiFrancesco et al. 1984; Shimoni et al. 1992; Brochu et al. 1992; Li et al. 1996). Based on similar reasoning with respect to the specificity of Ba2+ for IK1 inhibition, Ba2+ has also been used to examine the potential role of IK1 as a repolarizing current in intact heart studies (Gillis et al. 1998). Paquette et al. (1998) studied the effects of a variety of divalent cations on IKr in rabbit ventricular myocytes, but time-dependent IK1 block precluded evaluation of the effects of Ba2+ on IKr. Since IK1 is time dependent at some voltages and Ba2+ block is itself time and voltage dependent, studies of Ba2+ effects on IKr in native myocytes are difficult to perform and interpret. Nonetheless, our observations urge caution in the use of Ba2+ as a tool to remove or isolate effects on IK1, since IHERG (and presumably the native equivalent IKr) can be suppressed by relatively low Ba2+ concentrations.
Acknowledgments
HERG cDNA was a generous gift of Dr M. Keating and Dr M. Sanguinetti, University of Utah. The authors thank Luce Bégin for expert secretarial help. This work was supported by the Medical Research Council of Canada, the Quebec Heart Foundation, the Natural Science and Engineering Research Council and the Fonds de Recherche de l'Institut de Cardiologie de Montréal. T.E.H. and M.C. are research scholars of the Fonds de la Recherche en Santé du Québec, and M.W. is supported by a Medical Research Council studentship.
References
- Abbott GW, Sesti FS, Splawski I, Buck ME, Lehmann MH, Timothy KW, Keating MT, Goldstein SA. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- Anumonwo JMB, Freeman LC, Kwok WM, Kass RS. Delayed rectification in single cells isolated from guinea pig sinoatrial node. American Journal of Physiology. 1992;262:H921–925. doi: 10.1152/ajpheart.1992.262.3.H921. [DOI] [PubMed] [Google Scholar]
- Armstrong CM, Swenson RP, Taylor SR. Block of squid axon K channels by internally and externally applied barium ions. Journal of General Physiology. 1982;80:663–682. doi: 10.1085/jgp.80.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balser JR, Bennett PB, Roden DM. Time-dependent outward current in guinea pig ventricular myocytes, gating kinetics of the delayed rectifier. Journal of General Physiology. 1990;96:835–863. doi: 10.1085/jgp.96.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K (V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- Barry DM, Nerbonne JM. Myocardial potassium channels: electrophysiological and molecular diversity. Annual Review of Physiology. 1996;58:363–394. doi: 10.1146/annurev.ph.58.030196.002051. [DOI] [PubMed] [Google Scholar]
- Basso C, Labarca P, Stefani E, Alvarez O, Latorre R. Pore accessibility during C-type inactivation in Shaker K+ channels. FEBS Letters. 1998;429:375–380. doi: 10.1016/s0014-5793(98)00635-8. [DOI] [PubMed] [Google Scholar]
- Berthet M, Denjoy I, Donger C, Demay L, Hammoude H, Klug D, Schulze-Bahr E, Richard P, Funke H, Schwartz K, Coumel P, Hainque B, Guicheney P. C-terminal HERG mutations: the role of hypokalemia and a KCNQ1-associated mutation in cardiac event occurrence. Circulation. 1999;99:1464–1470. doi: 10.1161/01.cir.99.11.1464. [DOI] [PubMed] [Google Scholar]
- Brochu RM, Clay JR, Shrier A. Pacemaker current in single cells and in aggregates of cells dissociated from the embryonic chick heart. The Journal of Physiology. 1992;454:503–515. doi: 10.1113/jphysiol.1992.sp019276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E, Mubagwa K. Characterization of the acetylcholine-induced potassium current in rabbit cardiac Purkinje fibres. The Journal of Physiology. 1986;371:219–237. doi: 10.1113/jphysiol.1986.sp015970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colatsky TJ, Follmer CH, Starmer CF. Channel specificity in antiarrhythmic drug action. Mechanism of potassium channel block and its role in suppressing and aggravating cardiac arrhythmias. Circulation. 1990;82:2235–2242. doi: 10.1161/01.cir.82.6.2235. [DOI] [PubMed] [Google Scholar]
- Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Ferroni A, Visentin S. Barium-induced blockade of the inward rectifier in calf Purkinje fibres. Pflügers Archiv. 1984;402:446–453. doi: 10.1007/BF00583946. [DOI] [PubMed] [Google Scholar]
- Eaton DC, Brodwick MS. Effects of barium on the potassium conductance of squid axon. Journal of General Physiology. 1980;75:727–750. doi: 10.1085/jgp.75.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermini B, Nathan RD. Removal of sialic acid alters both T- and L-type calcium currents in cardiac myocytes. American Journal of Physiology. 1991;260:H735–743. doi: 10.1152/ajpheart.1991.260.3.H735. [DOI] [PubMed] [Google Scholar]
- Ficker E, Jaroliemek W, Kiehn J, Baumann A, Brown AM. Molecular determinants of dofetilide block of HERG K+ channels. Circulation Research. 1998;82:386–395. doi: 10.1161/01.res.82.3.386. [DOI] [PubMed] [Google Scholar]
- Giles WR, Shibata EF. Voltage clamp of bull-frog cardiac pace-maker cells: a quantitative analysis of potassium currents. The Journal of Physiology. 1985;368:265–292. doi: 10.1113/jphysiol.1985.sp015857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis AM, Geonzon RA, Mathison HJ, Kulisz E, Lester WM, Duff HJ. The effects of barium, dofetilide and 4-aminopyridine (4-AP) on ventricular repolarization in normal and hypertrophied rabbit heart. Journal of Pharmacology and Experimental Therapeutics. 1998;285:262–270. [PubMed] [Google Scholar]
- Harris RE, Larsson HP, Isacoff EY. A permeant ion binding site located between two gates of the Shaker K+ channel. Biophysical Journal. 1998;74:1808–1820. doi: 10.1016/s0006-3495(98)77891-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert TE, Monette R, Drapeau P, Dunn RJ. Voltage dependencies of the fast and slow gating modes of RIIA sodium channels. Proceedings of the Royal Society. 1994;B 256:253–261. doi: 10.1098/rspb.1994.0078. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer Associates Inc. Publishers; 1992. [Google Scholar]
- Ho W-K, Kim I, Lee CO, Earm YE. Voltage-dependent blockade of HERG channels expressed in Xenopus oocytes by external Ca2+ and Mg2+ The Journal of Physiology. 1998;507:631–638. doi: 10.1111/j.1469-7793.1998.631bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho W-K, Kim I, Lee CO, Youm JB, Lee SH, Earm YE. Blockade of HERG expresssed in Xenopus laevis oocytes by external divalent cations. Biophysical Journal. 1999;76:1959–1971. doi: 10.1016/S0006-3495(99)77355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Latorre R, Toro L, Stefani E. External barium block of Shaker potassium channels: evidence for two binding sites. Journal of General Physiology. 1995;106:1069–1087. doi: 10.1085/jgp.106.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Toro L, Stefani E. Molecular determinants of external barium block in Shaker potassium channels. FEBS Letters. 1996;388:59–65. doi: 10.1016/0014-5793(96)00516-9. [DOI] [PubMed] [Google Scholar]
- Kiehn J, Lacerda AE, Wible B, Brown AM. Molecular physiology and pharmacology of HERG. Single-channel currents and block by dofetilide. Circulation. 1996;94:2572–2579. doi: 10.1161/01.cir.94.10.2572. [DOI] [PubMed] [Google Scholar]
- Li GR, Feng J, Yue L, Carrier M, Nattel S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circulation Research. 1996;78:689–696. doi: 10.1161/01.res.78.4.689. [DOI] [PubMed] [Google Scholar]
- Miller C, Latorre R, Reisin I. Coupling of voltage-dependent gating and Ba2+ block in the high-conductance, Ca2+-activated K+ channel. Journal of General Physiology. 1987;90:427–449. doi: 10.1085/jgp.90.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D, Tsien RW. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. The Journal of Physiology. 1969;200:205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette T, Clay JR, Ogbaghebriel A, Shrier A. Effects of divalent cations on the E-4031-sensitive repolarization current, IKr, in rabbit ventricular myocytes. Biophysical Journal. 1998;74:1278–1285. doi: 10.1016/S0006-3495(98)77841-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:1–20. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. Journal of General Physiology. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK. Delayed rectifier outward K+ current is composed of two currents in guinea pig atrial cells. American Journal of Physiology. 1991;260:H393–399. doi: 10.1152/ajpheart.1991.260.2.H393. [DOI] [PubMed] [Google Scholar]
- Shimoni Y, Clark RB, Giles WR. Role of an inwardly rectifying potassium current in rabbit ventricular action potential. The Journal of Physiology. 1992;448:709–727. doi: 10.1113/jphysiol.1992.sp019066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PL, Baukrowitz T, Yellen G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 1996;379:833–836. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- Spector PS, Curran ME, Zou A, Keating MT, Sanguinetti MC. Fast inactivation causes rectification of the IKr channel. Journal of General Physiology. 1996;107:611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela M, Drewe JA, Brown AM. Barium blockade of a clonal potassium channel and its regulation by a critical pore residue. Molecular Pharmacology. 1993;44:180–190. [PubMed] [Google Scholar]
- Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, De Jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nature Genetics. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- Wang S, Liu S, Morales MJ, Strauss HC, Rasmusson RL. A quantitative analysis of the activation and inactivation kinetics of HERG expressed in Xenopus oocytes. The Journal of Physiology. 1997a;502:45–60. doi: 10.1111/j.1469-7793.1997.045bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Morales MJ, Liu S, Strauss HC, Rasmusson RL. Modulation of HERG affinity for E-4031 by [K+]o and C-type inactivation. FEBS Letters. 1997b;417:43–47. doi: 10.1016/s0014-5793(97)01245-3. [DOI] [PubMed] [Google Scholar]
- Weerapura M, Hebert T, Nattel S. State-dependent barium block of HERG channels: mutual antagonism with inactivation. Circulation. 1998;98(suppl I):1–126. [Google Scholar]
- Werman R, Grundfest H. Graded all-or-none electrogenesis in arthropod muscle. II. The effects of alkalai earth and onium ions on lobster muscle fibers. Journal of General Physiology. 1961;44:997–1027. doi: 10.1085/jgp.44.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang WJ, Yu XJ, Boyett MR. Barium block of the muscarinic potassium current in guinea-pig atrial cells. Pflügers Archiv. 1995;430:348–357. doi: 10.1007/BF00373909. [DOI] [PubMed] [Google Scholar]
- Zou A, Curran ME, Keating MT, Sanguinetti MC. Single HERG delayed rectifier K+ channels expressed in Xenopus oocytes. American Journal of Physiology. 1997;272:H1309–1314. doi: 10.1152/ajpheart.1997.272.3.H1309. [DOI] [PubMed] [Google Scholar]
- Zou A, Xu QP, Sanguinetti MC. A mutation in the pore region of HERG K+ channels reduces rectification by shifting the voltage dependence of inactivation. The Journal of Physiology. 1998;509:129–137. doi: 10.1111/j.1469-7793.1998.129bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


