Abstract
The purpose of the present study was to compare the contractile properties of single motor units in the intrinsic (short) and extrinsic (long) finger muscles in awake human subjects using intraneural motor axon stimulation.
Twitch properties were measured for 17 intrinsic and 11 extrinsic motor units by selective stimulation of a single motor axon in the ulnar or the median nerve. Force was measured from the appropriate digit, just distal to the muscle's point of insertion and single unit EMG was recorded with surface electrodes. Force-frequency relationships (2-100 Hz) were established for 16 of these units (7 intrinsic, 9 extrinsic). Across the 16 motor units for which force-frequency data were obtained, twitch contraction time (63.7 ± 6.0 ms, mean ±s.e.m.) was inversely correlated with the frequency required to generate half-maximum tetanic force (12.0 ± 1.1 Hz).
We found no systematic differences between the contractile properties of intrinsic and extrinsic motor units. There was no evidence of a bimodal distribution into large/small or fast/slow units based on maximum force or contraction times, although both fast and slow motor units were encountered.
The peak slope of the sigmoidal force-frequency relationship for intrinsic motor units (9.1 and 4.4-12.9 mN Hz−1, median and interquartile range) was significantly higher than that for extrinsic motor units (2.9 and 2.3-3.1 mN Hz−1; P= 0.028), i.e. greater force steps were produced by the intrinsic motor units for a given change in stimulation frequency. This difference suggests that motor units in the intrinsic muscles of the hand are more sensitive to modulating force output by changes in motoneurone firing rates than are those in the extrinsic muscles. This reflects the important role of the intrinsic hand muscles in the fine manipulation of objects.
Intraneural motor axon stimulation permits the detailed study of the contractile properties of single motor units by allowing selective stimulation of single motor axons in awake human subjects (Westling et al. 1990). This technique has previously been used to examine both long and short muscles; that is, extrinsic muscles with long extramuscular tendons and intrinsic muscles with short or negligible extramuscular tendons. The electrical and mechanical properties of two groups of long muscles have been studied, the toe extensors (Macefield et al. 1996) and long finger flexors (Fuglevand et al. 1999), and one group of short muscles, the thenar muscles (Thomas et al. 1990b, 1991a). Both studies of long muscles included some short muscles but with insufficient numbers to enable comparisons between groups.
Single motor units in human toe extensors were stronger and slower than those in the thenar muscles – presumably reflecting their role in standing and locomotion – while thenar muscles were more sensitive to a change in stimulation frequency. This implies a greater use of rate coding to modulate force production by the thenar muscles, leading to greater control for fine manipulation of held objects (Macefield et al. 1996). Motor units in the long finger flexors were stronger than those in either the thenar or toe extensor muscles, with one group faster than either toe or thenar muscles and another group slower. Attempts to classify human thenar motor units using intraneural stimulation, following the standard criteria developed for studying motor units in the cat (Burke et al. 1973, 1974; Burke, 1981; Kernell et al. 1983a), revealed physiological differences between human and cat muscle (Thomas et al. 1991b). This study found that while motor units could be labelled ‘fast’ or ‘slow’ using arbitrary criteria, human thenar motor units appear to operate along a continuum of responses, rather than being described by discrete classifications. However, the long finger flexors could be separated into a fast and slow group by the stimulation frequency required to generate half-maximum tetanic force, although there was no bimodal distribution of any other parameter including fatigue index (Fuglevand et al. 1999).
In the present study we compared the contractile properties of long and short muscles in the same experiments. We were also able to extend the range of muscles examined using intraneural motor axon microstimulation and to assess the consistency of the technique by comparing the results obtained in the present study with those reported by two other laboratories. The intrinsic (short) hand muscles studied were first dorsal interosseous muscle (FDI), adductor pollicis (AP) and abductor digiti minimi (ADM); the extrinsic (long) flexors of the digits were flexor pollicis longus (FPL) and flexor digitorum superficialis (FDS). Some of this work has been published in abstract form (Falland & Macefield, 1997; McNulty et al. 1998).
METHODS
General procedures
Data were obtained from 24 experiments performed predominantly on the left hand (n= 19; right hand, n= 5) of healthy human subjects (11 female and 11 male; age range, 18–47 years); two subjects participated in more than one experiment. Experiments were performed according to the Declaration of Helsinki. Ethical approval was given by the Committee on Experimental Procedures Involving Human Subjects of the University of New South Wales and all subjects gave written, informed consent. Subjects reclined in a comfortable chair with the arm slightly abducted and extended with the forearm and hand semi-pronated. The arm rested on a stable platform while the hand was supported by a pillar. Extraneous movements of the arm and hand were prevented by Velcro straps. Mechanical responses to intraneural motor axon stimulation were recorded from the intrinsic hand muscles FDI, AP and ADM; and two extrinsic long finger flexors, FPL and FDS. According to the muscle being studied, the hand and digits were immobilised against the pillar allowing free movement of the proximal (PIP) and distal (DIP) interphalangeal joints. When recording from FDI, the pillar was adjusted to allow abduction at the second metacarpophalangeal (MCP) joint. The thumb support was positioned to allow free movement at either the interphalangeal joint (IP) when recording from FPL or the MCP joint when recording from a unit in AP. A typical experimental set-up is illustrated in Fig. 1.
Figure 1. Experimental set-up.
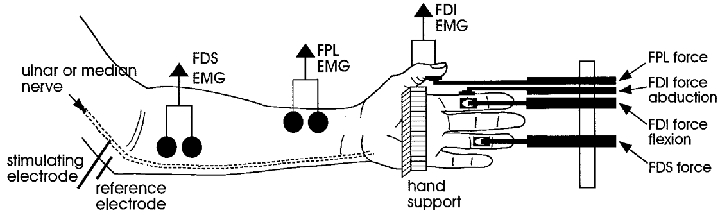
Typical set-up for recording from first dorsal interosseous (FDI) muscle during microstimulation within the ulnar nerve, or from flexor pollicis longus (FPL) or flexor digitorum superficialis (FDS) within the median nerve.
Intraneural stimulation
An insulated tungsten microelectrode (200 μm diameter, 5 μm length uninsulated tip, 1 μm tip diameter; type TM33B20, World Precision Instruments, USA) was inserted through the skin and directed towards either the ulnar or the median nerve in the upper arm approximately 10 cm above the cubital fossa. A reference electrode similar to the microelectrode but with 1 mm of insulation removed from the tip was inserted subdermally approximately 2 cm away. The stimulating electrode was then advanced towards the nerve while delivering low voltage, square wave, negative pulses, 0.01-1 mA of 0.1 ms duration at 1 Hz from a programmable, optically isolated, constant-current stimulator (ADInstruments, Australia). Microstimulation, in conjunction with verbal feedback from subjects, was used to guide electrode positioning to first locate the nerve and then isolate a motor fibre within the nerve bundle. Motor areas were evident from twitching in appropriate muscles time locked to the stimulus pulse while cutaneous fascicles elicited pulse-synchronous sensations of ‘pins and needles’.
Once a potential single motor unit was located, the optimal stimulation site was identified and monitored based on the appearance of, and changes in, EMG and twitch force signals. The criteria for identifying a single motor unit were developed by Westling and colleagues (Westling et al. 1990) and Macefield and co-workers (Macefield et al. 1996) and are illustrated in Fig. 2A. Once the initial stimulus threshold for the unit is identified, the following criteria must be met: (a) parallel changes in EMG and force production with an all-or-none initial response; (b) parallel quantal increments in both force and EMG as second and subsequent motor units are recruited with increasing stimulation intensity; (c) EMG and force disappear simultaneously as additional motor units are derecruited in reverse order with decreasing stimulus intensity; (d) a difference of two or more minimal stimulus increments (2 μA in the present study) in the recruitment thresholds between the unit being recorded and subsequently recruited units (this difference constitutes the safety margin of the unit being studied). If a site became unstable or EMG or force drop-outs occurred, the electrode was repositioned and the search and identification procedures repeated.
Figure 2. Intraneural motor axon stimulation.
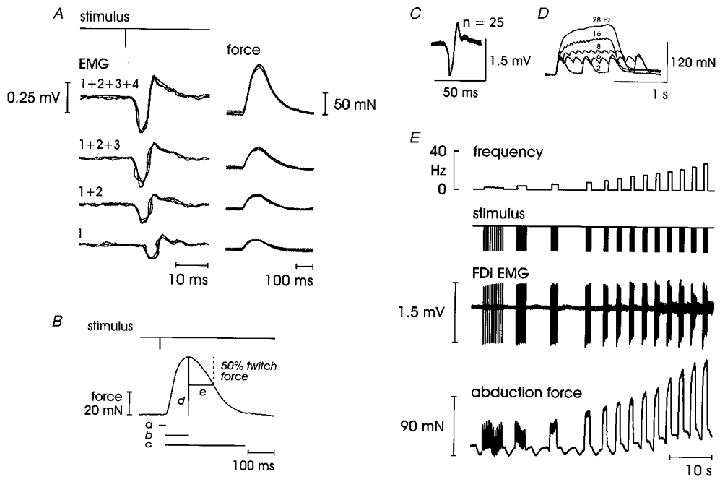
A and B, twitches. A, simultaneous appearance of both force and EMG as a single motor unit is recruited; parallel and quantal increases in both force and EMG indicate the recruitment of additional units with increasing stimulation intensity. B, measurement of twitch parameters: a, onset latency to force; b, contraction time; c, twitch duration; d, twitch force; e, half-relaxation time. C-E, force-frequency relationships in a single motor unit in FDI. C, superimposed EMG potentials confirming a unitary recording. D, expanded force traces showing the development of full fusion from unfused twitches at 2 Hz, 62 % fusion at 6 Hz, 85 % at 8 Hz, 92 % at 16 Hz and 100 % at 28 Hz stimulation. E, raw data to 28 Hz (at which point maximum force and 100 % fusion had been reached); peak slope of 4.4 mN Hz−1 was developed between 6 and 12 Hz; note, there is some EMG signal cancellation at higher frequencies and a respiratory modulation accompanied by a slight baseline drift is evident in the force trace.
EMG recording
EMG signals were recorded using disposable 6 mm Ag-AgCl surface electrodes placed over the belly of the muscle being studied. Electrodes were positioned either before the experiment for more inaccessible muscles, or during the experiment once the nerve entered by the microelectrode had been identified or the muscle being stimulated was determined. EMG was recorded from FDS and FPL if the electrode was positioned in the median nerve, or AP, ADM and FDI if it was positioned in the ulnar nerve. Unitary EMG was readily identifiable using surface electrodes. EMG activity was amplified (bandwidth, 10 Hz to 1 kHz; gain, 2 × 103), digitised at 3.2 kHz (MacLab BioAmp, ADInstruments) and stored on computer with all other signals for off-line analysis using SC/ZOOM data acquisition and analysis software (Department of Physiology, University of Umeå, Sweden).
Force recording
Semi-isometric, semi-conductor force transducers (UFI, USA) were used to record the force production of single motor units and were taped to the palmar surface of the digits to ensure secure mechanical transmission of the signal. Force was amplified (gain, 103; bandwidth, DC to 2 kHz; ADInstruments) and digitised at 800 Hz. When recording from FDI, transducers were placed over both the anterior and radial surfaces of the PIP joint of the second digit to simultaneously measure flexion and abduction components of force production. Twitch parameters were measured from the derived resultant force where flexion was present (8 out of 14 motor units in FDI produced no flexion force), but only abduction force was used in measuring force-frequency relationships. To record from FDS, the transducers were placed just proximal to the DIP joints of digits II, III and IV. When single motor units in the long finger flexors produced force in more than one finger (Kilbreath et al. 1998; Fuglevand et al. 1999) data were recorded from the digit with the greatest force production. The volar surface of the distal phalanx of the thumb rested on a force transducer (see Fig. 1) to record force from FPL so that the thumb assumed a natural position. An additional transducer could be attached to the radial side of the IP joint of the thumb when recording from AP or to the ulnar side of the PIP of digit V for ADM. To minimise distortion from arterial pulse pressure when recording force output, the test stimuli were triggered from the cardiac cycle R wave of the ECG recorded with Ag-AgCl surface electrodes on the chest wall (Westling et al. 1990).
Stimulation procedures
Once a stable recording site was found, the recruitment threshold, safety margin and twitch properties for each unit were determined by ascending and descending series of five pulses (at approximately 1 Hz, depending on the subject's heart rate) delivered to the motor axon with an increase of 1 μA for each series. Each motor unit was studied using a stimulus intensity set in the middle of the unit's safety margin. A series of ascending constant-frequency trains of stimuli were then injected into the nerve to examine the force-frequency response of the motor unit. These stimulus volleys employed 20 frequencies: 2, 4, 6, 8, 10, 12.5, 14, 16, 18, 20, 22, 25, 28, 30, 33.3, 40, 50, 66.6, 80 and 100 Hz. Frequency trains consisted of 10 pulses per frequency between 2 and 10 Hz, 1 s of stimulation from 12 to 50 Hz and 50 pulses per train from 66.6 to 100 Hz. Twitch stimuli and pulse trains were triggered from the R wave with the latter separated by 0.5-1 s depending on subject's heart rate.
Data analysis
The unitary nature of each motor unit was verified by a tight alignment of both the morphology and amplitude of single superimposed twitches (Fig. 2C). Superimposition also demonstrated that force and EMG remained constant within the safety margin and thus eliminated the need to electronically reset the force baseline prior to each stimulus for both single twitches and force-frequency stimulus trains.
Twitch properties (Fig. 2B), measured from an average of five single twitches, included: twitch force (baseline to peak), latency of force production and EMG (time from stimulus to onset of force increase and change in the EMG baseline, respectively), contraction time (time to peak), twitch duration (time from force (F) onset to where dF/dt returns to zero) and half-relaxation time (time for decay from peak force to half-maximum force). Maximum contraction and relaxation rates were calculated from the first time derivative of the twitch force and were normalised to the twitch amplitude to enable comparison of units with greatly differing amplitudes (Bigland-Ritchie et al. 1983).
Force-frequency data were obtained from single trials (Fig. 2E). The properties measured for each frequency train were: maximum force production, measured as both peak and average force (the former a single point of maximum force, the latter calculated over the final 250 ms of stimulation, which provides a more conservative measure reducing the influence of respiratory modulation); twitch fusion – the difference between the peak and the trough of subsequent tetanic twitches in the middle of each stimulus train, normalised to the amplitude of the unfused twitch at 2 Hz and expressed as a percentage, where 0 % indicates no fusion and 100 % indicates complete fusion (Fig. 2D); maximum contraction and relaxation rates (both normalised to twitch force); stimulation frequency required to generate half-maximum tetanic force and half-maximum fusion; and peak slope measured from the steepest portion of the force-frequency curve (determined by line fitting).
Normally distributed data have been summarised as means ±s.e.m. and were analysed using Pearson product moment correlations and independent Student's paired t tests (with post hoc Bonferroni corrections). Spearman rank order correlations and Mann-Whitney U tests (with post hoc Bonferroni corrections) were used for non-parametric samples which are expressed as median and interquartile range (IQR). Differences were considered significant at P < 0.05.
RESULTS
Stimulated motor units
Twenty-eight single motor axons supplying muscles acting on the fingers and thumb were successfully stimulated. Seventeen motor units were located in short, intrinsic muscles: 14 in first dorsal interosseous (FDI), two in adductor pollicis (AP) and one in abductor digiti minimi (ADM); and 11 in long, extrinsic muscles: eight in flexor digitorum superficialis (FDS) and three in flexor pollicis longus (FPL). Force-frequency data were recorded in 16 of these motor units (4 in FDI, 2 in AP, 1 in ADM, 7 in FDS and 2 in FPL).
Twitch parameters
We found no systematic differences between the twitch properties of intrinsic and extrinsic motor units (Table 1). However, differences did exist between the individual parameters of latency to force production and the normalised maximum contraction rate. Extrinsic motor units had a shorter onset latency to EMG than intrinsic units (6.3 ± 0.3 and 12.3 ± 0.5 ms, respectively; P < 0.001; unpaired t test) reflecting the shorter length of the motor axon, and consequently a shorter latency to force production (extrinsic units, 15.4 ± 1.1 ms; and intrinsic units, 25.1 ± 2.1 ms; P= 0.015; unpaired t test). However, the electro-mechanical delay, the time difference between EMG and the onset of force, showed no significant difference (extrinsic units, 8.0 and 6.6-11.5 ms, median and IQR; intrinsic units, 10.4 and 7.5-16.3 ms, median and IQR). Interestingly, extrinsic units showed a significantly faster normalised maximum contraction rate than did intrinsic units (P= 0.017; Mann-Whitney U test).
Table 1.
Twitch properties of single motor units in human hand muscles
| Intrinsic(n= 17) | Extrinsic(n= 11) | Pooled(n= 28) | |
|---|---|---|---|
| Twitch force(mN) | 14.7[7.9–21.3] | 15.7[8.0–26.9] | 15.2[8.0–24.3] |
| 2.2–72.8 | 2.8–80.7 | 2.2–80.7 | |
| Contraction time(ms) | 70.3 ± 5.0 | 57.9 ± 5.0 | 65.0 ± 3.7 |
| 32.0–111.3 | 31.2–82.6 | 31.2–111.3 | |
| Twitch duration(ms) | 183.8[158.1–261.3] | 186.3[153.0–257.5] | 185[157.0–259.4] |
| 101.3–468.8 | 136.4–285.6 | 101.3–468.8 | |
| Half-relaxation time(ms) | 70.2 ± 6.5 | 69.5 ± 9.1 | 70.0 ± 5.2 |
| 20.0–115.9 | 43.1–150.6 | 20.0–150.6 | |
| Normalised maximum contraction rate(s−1)* | 20.9 ± 1.3 | 25.4 ± 1.0 | 22.6 ± 1.0 |
| 13.3–37.0 | 19.9–30.8 | 13.3–37.0 | |
| Normalised maximum relaxation rate(s−1) | 11.2 ± 1.0 | 11.5 ± 0.9 | 11.4 ± 0.7 |
| 6.1–20.1 | 7.0–17.2 | 6.1–20.1 |
Data are expressed as means ± S.E.M. and range; non-parametric data (in italics) are expressed as the median, interquartile range (in brackets) and range.
Significant difference between intrinsic and extrinsic motor units at P < 0.05.
As illustrated in Fig. 3A and C, the twitch forces recorded in both intrinsic and extrinsic motor units were skewed towards lower values. The contraction time histograms (Fig. 3B and D) reveal a unimodal distribution of human motor unit contraction speeds as reported in previous studies (Milner-Brown et al. 1973b; Stephens & Usherwood, 1977; Thomas et al. 1990b; Elek et al. 1992; Kossev et al. 1994; Macefield et al. 1996; Fuglevand et al. 1999).
Figure 3. Twitch force and contraction time histograms for intrinsic and extrinsic motor units.
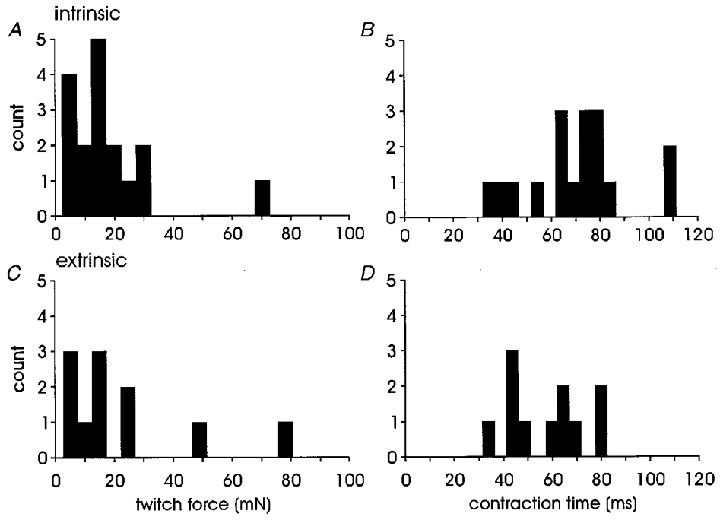
A and B, intrinsic motor units, n= 17; C and D, extrinsic motor units, n= 11. Bin width, 5 mN twitch force (A and C) or 5 ms contraction time (B and D). There were no statistical differences between intrinsic and extrinsic motor units for either parameter.
Twitch duration and half-relaxation times were distributed over a wide range and normalised maximum relaxation rates were half the normalised maximum contraction rates. Twitch force was unrelated to contraction time (ρ= 0.095, P= 0.63; Spearman rank order correlation) and half-relaxation time (ρ= 0.28, P= 0.15), but was significantly correlated to twitch duration (ρ= 0.47, P= 0.01), whereas contraction time was correlated to twitch duration (ρ= 0.61, P < 0.001), half-relaxation time (ρ= 0.47, P= 0.01), normalised maximum contraction rate (ρ= -0.47, P= 0.01) and normalised maximum relaxation rate (ρ= -0.41, P= 0.03). Twitch duration was correlated to half-relaxation time (ρ= 0.79, P < 0.001) and normalised maximum relaxation rate (ρ= -0.55, P= 0.003).
Force-frequency relationships of single motor units
Data from 16 single motor units studied with constant-frequency trains of increasing rates of stimulation are summarised in Table 2. No systematic differences between intrinsic and extrinsic motor units were evident. However, significant differences were found between long and short muscles in the peak slope of the force-frequency curve. Despite the divergence in the mean maximum force values for intrinsic and extrinsic motor units, there was no significant difference between the two groups. Extrinsic motor unit maximum force values were normally distributed, while intrinsic units generated maximum force within a narrow range (152.7-168.9 mN) with the exception of a single outlier (28.9 mN). If this outlying unit is excluded from the analysis, the two groups become significantly different. As has been found previously in human motor units (Thomas et al. 1990b; Macefield et al. 1996; Fuglevand et al. 1999) there was a significant inverse correlation between twitch contraction time and the stimulus frequency necessary to generate half-maximum tetanic force (r= -0.55, P= 0.028; Pearson product moment correlation).
Table 2.
Tetanic properties of single motor units in human hand muscles
| Intrinsic(n= 7) | Extrinsic(n= 9) | Pooled(n= 16) | |
|---|---|---|---|
| Maximum force(mN) | 141.0 ± 18.8 | 71.5 ± 12.6 | 101.9 ± 13.7 |
| 28.9–168.9 | 23.8–139.0 | 23.8–168.9 | |
| Frequency for maximum force(Hz) | 34.7 ± 6.3 | 40.6 ± 4.6 | 38.0 ± 3.7 |
| 14.0–66.0 | 18.0–66.0 | 14.0–66.0 | |
| Frequency for 50% tetanic force(Hz) | 11.0 ± 1.8 | 12.8 ± 1.5 | 12.0 ± 1.1 |
| 5.2–16.9 | 8.8–20.8 | 5.2–20.8 | |
| Force at 8 Hz(% maximum force) | 34.0[25.6–75.5] | 34.7[20.68–38.9] | 34.3[23.8–41.7] |
| 22.1–75.8 | 13.9–41.7 | 13.9–75.8 | |
| Frequency of peak fusion(Hz) | 17.3 ± 3.1 | 19.6 ± 1.9 | 18.6 ± 1.7 |
| 8.0–33.0 | 12.0–30.0 | 8.0–33.0 | |
| Frequency for 50% fusion(Hz) | 6.1 ± 0.8 | 8.0 ± 0.8 | 7.2 ± 0.6 |
| 3.2–9.5 | 3.3–10.7 | 3.2–10.7 | |
| Twitch:tetanic ratio | 0.27 ± 0.05 | 0.24 ± 0.03 | 0.25 ± 0.03 |
| 0.11–0.45 | 0.14–0.45 | 0.11–0.45 | |
| Peak slope(mN Hz−1)* | 9.1[4.4–12.9] | 2.9[2.3–3.1] | 3.3[2.5–8.3] |
| 1.0–13.0 | 1.2–4.4 | 1.0–13.0 | |
| Frequency range for peak slope(Hz) | 10.0[6.0–12.0] | 8.0[6.0–16.0] | 9.0[6.0–12.0] |
| 6.0–12.0 | 6.0–27.0 | 6.0–27.0 |
Data are expressed as means ± S.E.M. and range; non-parametric data (in italics) are expressed as the median, interquartile range (in brackets) and range.
Significant difference between intrinsic and extrinsic motor units at P < 0.05.
Examples of the force-frequency curves used to derive the peak slope of each unit are illustrated in Fig. 4 for typical intrinsic and extrinsic motor units with arrows identifying the steepest portion of the curve or peak slope. The intrinsic unit (Fig. 4A) reached a peak force of 155.9 mN at 28 Hz and peak fusion at 12 Hz; its twitch:tetanic ratio was 0.11. The peak slope of 9.1 mN Hz−1 was developed between 6 and 18 Hz and represents the sensitivity of muscle fibres to increases in motoneurone firing rate: the change in force for a given change in frequency. The extrinsic unit (Fig. 4C) reached a peak force of 116.3 mN at 50 Hz, with a twitch:tetanic ratio of 0.21. Peak fusion was developed by 22 Hz, and the peak slope of 3.1 mN Hz−1 occurred between 6 and 33 Hz. Maximum force was measured at the highest point once a plateau had been reached. Occasionally some subsequent points were marginally higher, but due to the influence of the respiratory modulation, these were not thought to be true increases in force output (it is also possible that this increase resulted from potentiation of the motor unit). In some units it was obvious by a sharp upwards inflection of the force-frequency curve that a second unit had been recruited between 66 and 100 Hz and these data points were removed from the analysis.
Figure 4. Peak slope of the force-frequency curve.
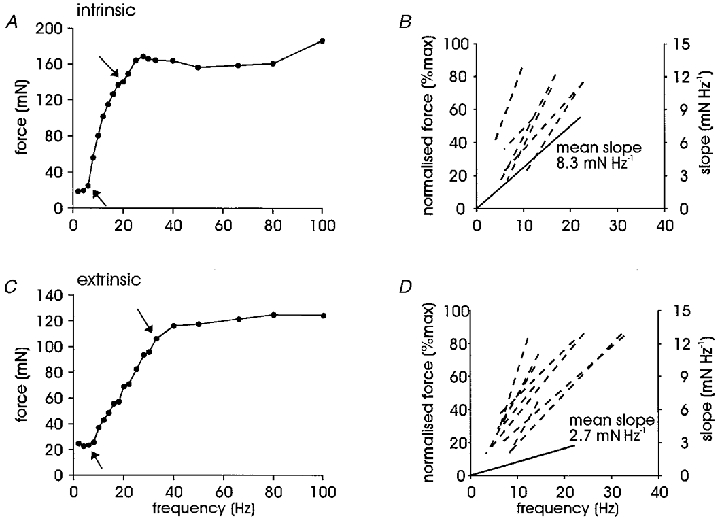
A, an intrinsic motor unit recorded in FDI; arrows indicate the steepest portion of the force-frequency curve for this unit (9.1 mN Hz−1). B, dashed lines, peak slopes for all intrinsic motor units measured from the normalised force-frequency curve indicating the range of frequencies required to generate peak slope (n= 7); note that there is some superimposition of traces. Continuous line, mean peak slope for intrinsic motor units normalised to the origin (without reference to the abscissa and not as a percentage of maximum force, right-hand scale), without reference to the range of frequencies required for development. C, force-frequency curve for an extrinsic motor unit recorded in FPL with arrows indicating the peak slope (3.1 mN Hz−1). D, dashed lines, extrinsic unit peak slopes (n= 9) measured as in B; continuous line, mean extrinsic peak slope.
Peak slope was the only force-frequency parameter that differed significantly between intrinsic and extrinsic motor units (P= 0.028; unpaired t test). This region of the curve lay between 6 Hz (6.0 ± 0.8 Hz) and 16 Hz (15.4 ± 1.8 Hz) for intrinsic units and between 6 Hz (5.8 ± 0.4 Hz) and 20 Hz (20.7 ± 2.9 Hz) for extrinsic units. There was no difference in the range of frequencies over which peak slope was developed (Table 2). We plotted peak slope values in two ways. Firstly, with reference to the start and end points as measured on the normalised force-frequency curve (dashed lines in Fig. 4B and D), which revealed that the peak slopes for intrinsic units tended to be steeper than for extrinsic units. Secondly, as the slope of the absolute force-frequency curve (i.e. not normalised to maximum force) without reference to start and end points and normalised to the origin, by which the difference between mean values for intrinsic and extrinsic motor units became apparent (continuous lines, Fig. 4B and D). There was no bimodal distribution of peak slopes. A continuum exists with intrinsic motor units towards the higher end and extrinsic motor units at the lower end of the spectrum.
The absence of a fast/slow modality of motor units is evident when the force-frequency curves of all units recorded in the present series are plotted together (Fig. 5A). Unlike Fuglevand and colleagues (Fuglevand et al. 1999), we did not see a segregation into fast and slow units based on the stimulation frequency required to reach half-maximum tetanic force. Neither did we see any extremely fast and large motor units (Fuglevand et al. 1999) or any difference between intrinsic and extrinsic motor units (P > 0.05; Mann-Whitney U test).
Figure 5. Force-frequency relationships.
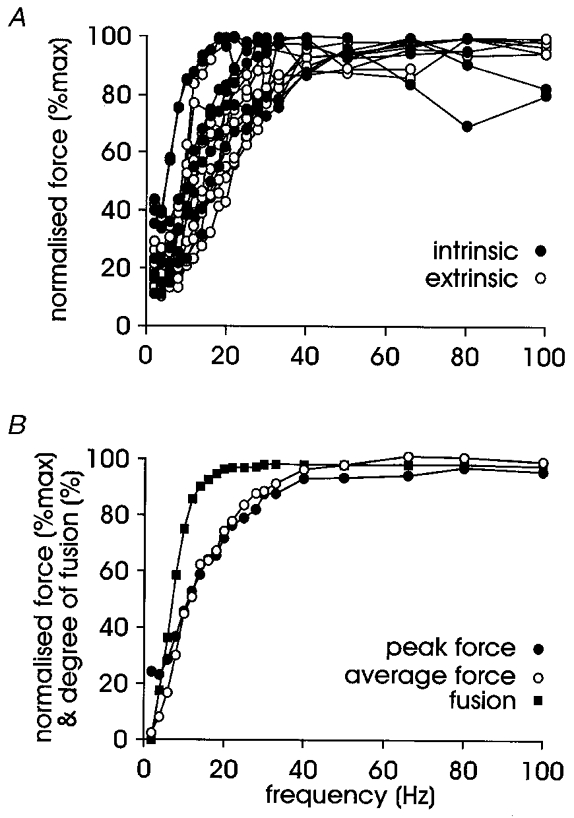
A, force-frequency curve plotted for all units (7 intrinsic and 9 extrinsic units); note the absence of a bimodal distribution into fast and slow motor units (nor was there a difference between the mean values for intrinsic and extrinsic units). B, the relationship between force and fusion for pooled data, indicating that the development of full fusion cannot explain all of the increase in force production; there was no difference between maximum force whether measured as a single point of peak force or over the last 250 ms of stimulation (average force).
All motor units, except one, were fully fused before maximum force output was reached as illustrated in Fig. 5B, where full fusion occurs at a lower frequency than maximum force. The relationship between fusion and force indicates that the generation of maximum force cannot be fully explained by the development of fusion. Unfused twitches were always seen at 2 Hz stimulation; partial fusion usually began at 4 or 6 Hz but in some units did not begin until 8 Hz. However, all motor units were partially fused at 8 Hz and several units were approximately 90 % fused by this frequency. We also considered the percentage of maximum force developed at 8 Hz – the rate at which motor units are commonly recruited during voluntary contractions in human muscles (Milner-Brown et al. 1973b; Monster & Chan, 1977; Petajan, 1981; De Luca et al. 1982). There was no difference in the percentage of force produced at this frequency between intrinsic and extrinsic motor units, with a pooled median of 34.3 % maximum force (mean, 36.7 %). This fits well with the mean value of 39.3 % for the slow motor units (Group I) in the long finger flexors of the human hand, despite these units reaching a much higher level of maximum force production (Fuglevand et al. 1999).
Maximum contraction and relaxation rates were normalised to twitch force to allow an ensemble response to be considered. There were no differences between intrinsic and extrinsic motor units. Normalised maximum contraction rates increased monotonically from 10 to 100 Hz, with the increase being linear from 10 to 50 Hz (r= 0.98, P < 0.001) and then slowing between 50 and 100 Hz. The relationship between stimulation frequency and normalised maximum relaxation rate was sigmoidal with a linearly increasing portion between 4 and 28 Hz.
DISCUSSION
In this study we have used intraneural motor axon stimulation to examine the contractile and mechanical properties of 28 single motor units in both intrinsic and extrinsic muscles acting on the human hand. We found no systematic differences between intrinsic and extrinsic muscles, nor a bimodal distribution of fast and slow motor units. Single motor units in FDI and ADM have been stimulated and recorded for the first time using this technique.
Methodological considerations
Intraneural motor axon stimulation overcomes many of the problems associated with earlier techniques for studying single human motor units (Westling et al. 1990; Thomas et al. 1990a; Elek & Dengler, 1995; Thomas, 1995). These techniques include spike-triggered averaging (Buchthal & Schmalbruch, 1970; Sica & McComas, 1971; Milner-Brown et al. 1973a; Monster & Chan, 1977; Calancie & Bawa, 1986; Nordstrom et al. 1989; Stein & Yang, 1990; Elek et al. 1992; Kossev et al. 1994; Elek & Dengler, 1995; Thomas, 1995) and intramuscular microstimulation (Stein et al. 1972; Milner-Brown et al. 1973a; Taylor & Stephens, 1976; Garnett et al. 1979; Stein & Yang, 1990; Elek et al. 1992; Kossev et al. 1994; Mateika et al. 1998). However, a disadvantage of intraneural motor axon stimulation is its low experiment yield, providing small numbers of motor units from any one subject and for any one muscle. The low sample number in this and previous studies limits the ability to generalise from these results. Nevertheless, the results of three different laboratories with three different set-ups and muscles acting on both fingers and toes, reveal a consistent pattern of responses. These responses cover the expected spectrum of motor units found in human muscles from the smallest units (2.2 mN twitch force, present study) to very large, presumably fast fatiguable (FF)-type, units (135.3 mN twitch force; Fuglevand et al. 1999). The stability of the electrode stimulation site, critical for experiment outcomes, is confirmed by matching pre- and post-experiment motor unit stimulation safety margins. Only recordings with identified single units were considered suitable for analysis as no motor unit discrimination techniques were used.
Differences between extrinsic and intrinsic motor units
Previous studies using intraneural motor axon stimulation have considered either intrinsic (short) muscles (Thomas et al. 1990b, 1991a) or extrinsic (long) muscles (Macefield et al. 1996; Fuglevand et al. 1999). The last study included a small number (3/13) of intrinsic motor units but not enough to enable comparisons between these groups (Fuglevand et al. 1999). In the present study we found intrinsic hand muscles had a higher peak slope of the force-frequency curve than did extrinsic muscles.
Force-frequency relationships have been widely used to study the contribution of rate coding to increasing force production of single motor units (Adrian & Bronk, 1929; Bigland & Lippold, 1954; Kernell et al. 1983b; Botterman et al. 1986; Thomas et al. 1991b; Fuglevand et al. 1999). The differences in peak slope of long and short muscles highlighted in the force-frequency relationship of this study suggest that intrinsic motor units are better suited to modulating force (output) for a given change in stimulus frequency (input); that is, controlling force by changes in firing rate. Interestingly, the peak slope for the extrinsic motor units recorded in the current study (2.9 mN Hz−1) is very similar to that reported for the long toe extensor muscles (3.7 mN Hz−1; Macefield et al. 1996).
It has been proposed (Macefield et al. 1996) that different tendon lengths may partially explain twitch force and twitch contraction time differences between intrinsic thenar motor units (Thomas et al. 1990b) and extrinsic toe extensor muscles (Macefield et al. 1996). In the present study there was no difference between intrinsic and extrinsic motor units in the electro-mechanical delay. This suggests a high fidelity of transmission between the electrical activity in extrinsic muscles and the force event in the finger, with no disadvantage in the muscle being more remote from the site of force application.
Comparison with data recorded in other laboratories
Single motor units have now been studied with intraneural microstimulation in three separate laboratories, including the present one. The similarities between findings are striking given the small sample sizes involved. Major differences, such as the maximum force, can be explained by the size of the units recorded rather than by fundamental differences resulting from the same technique.
The maximum tetanic force of 80.0 ± 6.9 mN (mean ±s.e.m.) reported for the thenar muscles (Thomas et al. 1991a), was similar to that of the toe extensors, 89.0 ± 16.5 mN (Macefield et al. 1996), and hand muscles, 101.9 ± 13.7 mN (present study). On average, the maximum force produced by the long finger flexors was larger: 200.2 ± 20.8 and 222.6 ± 98.4 mN for Group I (slow) and Group II (fast) motor units, respectively (Fuglevand et al. 1999). Twitch parameters also showed similarities between laboratories. For example, contraction time for thenar motor units (49.9 ± 1.3 ms) was similar to that of the Group II long finger flexors (45.9 ± 2.0 ms), while contraction time for Group I long finger flexors (65.0 ± 4.7 ms) was similar to that of the toe extensors (74.8 ± 3.9 ms) and to our pooled data in the hand (65.0 ± 3.7 ms). In all studies the twitch normalised maximum relaxation rate (NMRR) was approximately half the normalised maximum contraction rate (NMCR). Again, the thenar data (14.7 ± 0.8 s−1, NMRR and 32.5 ± 1.0 s−1, NMCR) were similar to those of Group II long finger flexors (16.0 ± 4.4 s−1, NMRR and 34.5 ± 5.1 s−1, NMCR), whereas the Group I long finger flexor (9.0 ± 0.5 s−1, NMRR and 23.3 ± 0.8 s−1, NMCR), toe extensor (11.7 ± 0.7 s−1, NMRR and 21.6 ± 0.6 s−1, NMCR) and hand data (11.4 ± 0.7 s−1, NMRR and 22.6 ± 1.0 s−1, NMCR) were all similar.
A unimodal distribution of motor unit contraction time and force production is apparent in all studies with the exception of that of the long finger flexors (Fuglevand et al. 1999). The stimulation frequency necessary to generate half-maximum tetanic force was used to classify these motor units as fast or slow (Fuglevand et al. 1999). Interestingly, this study included several large units not reported previously using this technique which we believe are FF-type motor units. Furthermore, while a significant inverse correlation between the frequency necessary to generate half-maximum tetanic force and contraction time existed, the latter showed no such bimodal distribution.
When data from the two fastest and two slowest motor units from the present study were compared (Fig. 6), a significant difference became evident between these units based on speed-related force-frequency properties. The mean frequency required to generate half-maximum tetanic force for the two slow units in Fig. 6 is 5.3 ± 0.05 Hz (mean ±s.e.m.), compared to Fuglevand and colleagues’ Group I data, 9.1 ± 0.18 Hz, and for the fast motor units is 19.6 ± 1.25 Hz, compared with Fuglevand and colleagues’ Group 2 data, 15.5 ± 0.49 Hz (Fuglevand et al. 1999). Thus we have recorded both faster and slower motor units in the present study. However, when all our data are included, as in Fig. 5A, there is no evidence of this separation. Therefore, the clustering seen in the data of Fuglevand et al. (1999) suggests they have not recorded the intermediate units seen in the current study.
Figure 6. Extremes in force-frequency relationship.
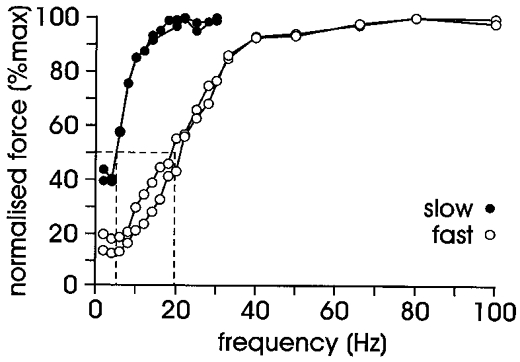
Force-frequency curves of the two slowest and two fastest motor units recorded in the current series (i.e. data from Fig. 5A with all intermediate units removed). Dashed lines indicate the mean frequency required to generate half-maximum tetanic force: fastest group, 19.6 Hz; and slowest group, 5.3 Hz. Note that the units in the slow group were both intrinsic motor units and those in the fast group were both extrinsic motor units, and that both slow motor units had reached maximum force by 33.3 Hz.
Some methodological differences exist between the studies, such as more stimulation frequencies in the force-frequency curve in the present study (n= 20) which gives a clearer understanding of force development in single motor units up to 100 Hz. Given that, on average, motor units were maximally fused at 19 Hz, maximum tetanic force was not produced until 38 Hz, suggesting stimulation frequencies beyond that required for full fusion will produce potentiation of the motor unit. For most units, force increased sharply before reaching a plateau which in some units drifted upwards, indicating continuing slight potentiation. We judged maximum force to have been reached at the beginning of such plateaus. While undoubtedly some units, most noticeably FF-type motor units, can produce additional force at stimulation frequencies up to 100 Hz, or by firing short bursts or doublets (short interspike intervals < 12 ms), it seems unlikely that most human motor units should continue to generate extra force at such high, prolonged and unphysiological firing rates.
Another phenomenon apparent in the force-frequency curve, due to the inclusion of more stimulation frequencies, was the behaviour of motor units when stimulated at 4 Hz. This frequency was not used in the earlier studies (Thomas et al. 1991a; Macefield et al. 1996; Fuglevand et al. 1999). Thirteen of the sixteen motor units studied produced less force at 4 Hz than at 2 Hz. By 8 Hz all these units produced more force than at 2 Hz, but in four units, stimulation at 6 Hz produced more force than at 4 Hz, but still less than at 2 Hz. We are at a loss to explain this result, which was observed in both intrinsic and extrinsic motor units.
As in previous studies of single human motor units using intraneural motor axon stimulation, we found a significant inverse correlation between twitch contraction time and the stimulation frequency required to generate half-maximum tetanic force. Thus twitch properties can be predicted from the force-frequency curve and vice versa. The stimulation frequency required to generate half-maximum tetanic force falls almost exactly half-way along the peak slope of the force-frequency curve. Twitch:tetanic ratio, like peak slope, indicates the sensitivity of the unit to modulating force production as a function of rate coding. This has been measured slightly differently in the various studies to date. In the current series and that of Fuglevand and co-workers (Fuglevand et al. 1999), tetanic force was taken from the maximum force produced, regardless of the frequency at which it occurred. Earlier studies (Thomas et al. 1990b; Macefield et al. 1996) used the force generated at 100 Hz. Given the discussion above regarding force production at 100 Hz we feel that using the maximum force generated, irrespective of frequency, is a better measure.
Comparisons with other studies of the FDI muscle
The electrical and mechanical twitch properties of single motor units in human FDI have been examined using spike-triggered averaging (Milner-Brown et al. 1973a,b; Stephens & Usherwood, 1977; Kossev et al. 1994) and intramuscular microstimulation (Milner-Brown et al. 1973a; Elek et al. 1992; Kossev et al. 1994). We have studied this muscle for the first time using intraneural motor axon stimulation (4 of the 7 intrinsic units were FDI) as well as reporting the first force-frequency relationships for FDI (examples of which can be seen in Figs 2C-E and 4A).
The data recorded in this study fit well with those reported using spike-triggered averaging and intramuscular microstimulation (Table 3). The similarity of these results adds strength to the findings reported here despite the relatively small number of single units examined. All techniques found a unimodal distribution of motor units for both twitch force and contraction time.
Table 3.
Comparison of FDI twitch properties recorded using three different techniques
| n | Recording method | Twitch force(mN) | Twitch contraction time(mS) | Half-relaxation time(ms) | |
|---|---|---|---|---|---|
| Present study | 14 | IMS | 13.8[2.2–27.8] | 70.8 ± 5.0 | 68.8 ± 7.3 |
| (2.2–72.8) | (32.0–111.3) | (20.0–115.9) | |||
| Milner-Brown et al.(1973b) | 137 | STA | – | – | – |
| (1.8–300) | (30–100) | – | |||
| Stephens & Usherwood (1977) | 67 | STA | – | – | – |
| (1.0–100) | (32–122) | – | |||
| Kossev et al. (1994) | 236 | STA | 17.7 ± 1.3 | 47.3 ± 0.8 | 33.9 ± 0.7 |
| (0.2–105) | (20.0–90.0) | (14.0–75.0) | |||
| 200 | IMMS | 14.9 ± 1.2 | 63.1 ± 1.0 | 60.4 ± 1.2 | |
| (1.0–140) | (30–135) | (24.0–130.0) | |||
| Elek et al. (1992) | 209 | IMMS | 16.0 ± 1.3 | 63 ± 1.0 | 61.0 ± 1.2 |
| (1–137) | (30–135) | (20–125) |
Data are expressed as means ± S.E.M. with the range given in parentheses; non-parametric data (in italics) are expressed as the median, interquartile range (in brackets) and range (in parentheses). Recording methods: IMS, intraneural motor-axon stimulation; STA, spike-triggered averaging; IMMS, intramuscular microstimulation.
Conclusions
We have used intraneural motor axon stimulation to selectively stimulate single motor units in muscles acting on the human hand, namely FDI, AP, ADM, FDS and FPL. Our results have shown consistency with the data recorded in previous experiments using this technique. Most of the differences between studies can be explained by the select population of motor units recorded, given the small sample sizes reported in all studies. The distribution of motor units in this study showed a continuum of responses with respect to force output and contraction speed, as opposed to a bimodal separation into fast/slow and large/small motor units. We also found no systematic differences between single motor units in extrinsic and intrinsic motor units with one major exception. Single motor units of intrinsic hand muscles display a much higher peak slope of the force-frequency curve, suggesting a better adaptation to modulating force output by changes in firing rates than motor units of the extrinsic hand muscles. This reflects the important role of the intrinsic hand muscles in the fine manipulation of held objects.
Acknowledgments
We are grateful to Professor Simon Gandevia for his comments on the manuscript. This work was supported by the National Health and Medical Research Council of Australia (programme grant 963206).
References
- Adrian ED, Bronk DW. Discharge of impulses in motor nerve fibres, part II. The Journal of Physiology. 1929;67:119–151. [PMC free article] [PubMed] [Google Scholar]
- Bigland B, Lippold OCJ. Motor unit activity in the voluntary contraction of human muscle. The Journal of Physiology. 1954;125:322–335. doi: 10.1113/jphysiol.1954.sp005161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Johansson R, Lippold OCJ, Woods JJ. Contractile speed and EMG changes during fatigue of sustained maximal voluntary contractions. Journal of Neurophysiology. 1983;50:313–324. doi: 10.1152/jn.1983.50.1.313. [DOI] [PubMed] [Google Scholar]
- Botterman BR, Iwamoto GA, Gonyea WJ. Gradation of isometric tension by different activation rates in motor units of cat flexor carpi radialis muscle. Journal of Neurophysiology. 1986;56:494–506. doi: 10.1152/jn.1986.56.2.494. [DOI] [PubMed] [Google Scholar]
- Buchthal F, Schmalbruch H. Contraction times and fibre types in intact human muscle. Acta Physiologica Scandinavica. 1970;79:435–452. doi: 10.1111/j.1748-1716.1970.tb04744.x. [DOI] [PubMed] [Google Scholar]
- Burke RE. Motor units: anatomy, physiology, and functional organization. In: Brookhart JM, Mountcastle VB, editors. Handbook of Physiology, section I, The Nervous System, Motor Control. part 1. II. Bethesda, MD, USA: American Physiological Society; 1981. pp. 345–422. [Google Scholar]
- Burke RE, Levine DN, Salcman M, Tsairis P. Motor units in cat soleus muscle: physiological, histochemical and morphological characteristics. The Journal of Physiology. 1974;234:723–748. doi: 10.1113/jphysiol.1974.sp010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. The Journal of Physiology. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calancie B, Bawa P. Limitations of the spike-triggered averaging technique. Muscle and Nerve. 1986;9:78–83. doi: 10.1002/mus.880090113. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Behaviour of human motor units in different muscles during linearly varying contractions. The Journal of Physiology. 1982;329:113–128. doi: 10.1113/jphysiol.1982.sp014293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elek JM, Dengler R. Human motor units studied by intramuscular microstimulation. Advances in Experimental Medicine and Biology. 1995;384:161–171. doi: 10.1007/978-1-4899-1016-5_13. [DOI] [PubMed] [Google Scholar]
- Elek JM, Kossev A, Dengler R, Schubert M, Wohlfahrt K, Wolf W. Parameters of human motor unit twitches obtained by intramuscular microstimulation. Neuromuscular Disorders. 1992;2:261–267. doi: 10.1016/0960-8966(92)90058-e. [DOI] [PubMed] [Google Scholar]
- Falland KJ, Macefield VG. Contractile properties of single human FDI motor units assessed by intraneural motor axon stimulation. Proceedings of the Australian Neuroscience Society. 1997;8:77. [Google Scholar]
- Fuglevand AJ, Macefield VG, Bigland-Ritchie B. Force-frequency and fatigue properties of motor units in muscles that control digits of the human hand. Journal of Neurophysiology. 1999;81:1718–1729. doi: 10.1152/jn.1999.81.4.1718. [DOI] [PubMed] [Google Scholar]
- Garnett RAF, O'Donovan MJ, Stephens JA, Taylor A. Motor unit organization of human medial gastrocnemius. The Journal of Physiology. 1979;287:33–43. doi: 10.1113/jphysiol.1979.sp012643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D, Eerbeek O, Verhey BA. Motor unit categorization on basis of contractile properties: an experimental analysis of the composition of the cat's m. peroneus longus. Experimental Brain Research. 1983a;50:211–219. doi: 10.1007/BF00239185. [DOI] [PubMed] [Google Scholar]
- Kernell D, Eerbeek O, Verhey BA. Relation between isometric force and stimulus rate in cat's hindlimb motor units of different twitch contraction time. Experimental Brain Research. 1983b;50:220–227. doi: 10.1007/BF00239186. [DOI] [PubMed] [Google Scholar]
- Kilbreath SL, Raymond J, Gorman RB, Gandevia SC. Distribution of forces produced by single motor units in human flexor digitorum profundus. Society for Neuroscience Abstracts. 1998;24:672. [Google Scholar]
- Kossev A, Elek JM, Wohlfarth K, Schubert M, Dengler R, Wolf W. Assessment of human motor unit twitches – a comparison of spike-triggered averaging and intramuscular microstimulation. Electroencephalography and Clinical Neurophysiology. 1994;93:100–105. doi: 10.1016/0168-5597(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Fuglevand AJ, Bigland-Ritchie B. Contractile properties of single motor units in human toe extensors assessed by intraneural motor-axon stimulation. Journal of Neurophysiology. 1996;75:2509–2519. doi: 10.1152/jn.1996.75.6.2509. [DOI] [PubMed] [Google Scholar]
- McNulty PA, Falland K, Macefield VG. Differences in contractile properties of single human motor units assessed by intraneural motor-axon stimulation. Peripheral and Spinal Mechanisms in the Neural Control of Movement, Tucson Arizona, Symposium Proceedings. 1998;47 [Google Scholar]
- Mateika JH, Essif EG, Dellorusso C, Fregosi RF. Contractile properties of human nasal dilator motor units. Journal of Neurophysiology. 1998;56:494–506. doi: 10.1152/jn.1998.79.1.371. [DOI] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Yemm R. The contractile properties of human motor units during voluntary isometric contractions. The Journal of Physiology. 1973a;228:285–306. doi: 10.1113/jphysiol.1973.sp010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. The Journal of Physiology. 1973b;230:359–370. doi: 10.1113/jphysiol.1973.sp010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monster AW, Chan H. Isometric force production by motor units of extensor digitorum communis muscle in man. Journal of Neurophysiology. 1977;40:1432–1443. doi: 10.1152/jn.1977.40.6.1432. [DOI] [PubMed] [Google Scholar]
- Nordstrom MA, Miles TS, Veale JL. Effect of motor unit firing pattern on twitches obtained by spike-triggered averaging. Muscle and Nerve. 1989;12:556–567. doi: 10.1002/mus.880120706. [DOI] [PubMed] [Google Scholar]
- Petajan JH. Motor unit frequency control in normal man. Progress in Clinical Neurophysiology. 1981;9:184–200. [Google Scholar]
- Sica REP, McComas AJ. Fast and slow twitch units in a human muscle. Journal of Neurology, Neurosurgery and Psychiatry. 1971;34:113–120. doi: 10.1136/jnnp.34.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB, French AS, Mannard A, Yemm R. New methods for analysing motor function in man and animals. Brain Research. 1972;40:187–192. doi: 10.1016/0006-8993(72)90126-6. [DOI] [PubMed] [Google Scholar]
- Stein RB, Yang JF. Methods for estimating the number of motor units in human muscles. Annals of Neurology. 1990;28:487–495. doi: 10.1002/ana.410280404. [DOI] [PubMed] [Google Scholar]
- Stephens JA, Usherwood TP. The mechanical properties of human motor units with special reference to their fatiguability and recruitment threshold. Brain Research. 1977;125:91–97. doi: 10.1016/0006-8993(77)90361-4. [DOI] [PubMed] [Google Scholar]
- Taylor A, Stephens JA. Study of human motor unit contractions by controlled intramuscular microstimulation. Brain Research. 1976;117:331–335. doi: 10.1016/0006-8993(76)90742-3. [DOI] [PubMed] [Google Scholar]
- Thomas CK. Human motor units studied by spike-triggered averaging and intraneural motor axon stimulation. Advances in Experimental Medicine and Biology. 1995;384:147–160. doi: 10.1007/978-1-4899-1016-5_12. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Bigland-Ritchie B, Johansson RS. Force-frequency relationships of human thenar motor units. Journal of Neurophysiology. 1991a;65:1509–1516. doi: 10.1152/jn.1991.65.6.1509. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Bigland-Ritchie B, Westling G, Johansson RS. A comparison of human thenar motor-unit properties studied by intraneural motor-axon stimulation and spike-triggered averaging. Journal of Neurophysiology. 1990a;64:1347–1351. doi: 10.1152/jn.1990.64.4.1347. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Johansson RS, Bigland-Ritchie B. Attempts to physiologically classify human thenar motor units. Journal of Neurophysiology. 1991b;65:1501–1508. doi: 10.1152/jn.1991.65.6.1501. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Johansson RS, Westling G, Bigland-Ritchie B. Twitch properties of human thenar motor units measured in response to intraneural motor-axon stimulation. Journal of Neurophysiology. 1990b;64:1339–1346. doi: 10.1152/jn.1990.64.4.1339. [DOI] [PubMed] [Google Scholar]
- Westling G, Johansson RS, Thomas CK, Bigland-Ritchie B. Measurement of contractile and electrical properties of single human thenar motor units in response to intraneural motor-axon stimulation. Journal of Neurophysiology. 1990;64:1331–1338. doi: 10.1152/jn.1990.64.4.1331. [DOI] [PubMed] [Google Scholar]


