Abstract
The effects of RGS4 on the voltage-dependent relaxation of G protein-gated K+ (KG) channels were examined by heterologous expression in Xenopus oocytes.
While the relaxation kinetics was unaffected by the acetylcholine concentration ([ACh]) in the absence of RGS4, it became dependent on [ACh] when RGS4 was co-expressed.
Kinetic analyses indicated that RGS4 confers to the KG channel a voltage-independent inhibitory gating mechanism, which was attenuated by ACh in a concentration-dependent fashion.
In vitro biochemical studies showed that RGS4 could bind to the protein complex containing KG channel subunits.
Since the native cardiac KG channel exhibited similar agonist-dependent relaxation kinetics to that mediated by RGS4, it is suggested that KG channel gating is a novel physiological target of RGS protein-mediated regulation.
ACh-induced deceleration of heart beat is mediated by the G protein-gated inwardly rectifying K+ (KG) channel (Yamada et al. 1998). The cardiac KG channel is composed of Kir3.1 and Kir3.4 and its activity can be reconstituted by the expression of both Kir subunits and the m2 muscarinic receptor in Xenopus oocytes (Kubo et al. 1993; Krapivinsky et al. 1995). It was recently shown that the activation, desensitization and deactivation of the reconstituted ACh-induced KG current are accelerated by regulators of G protein signalling (RGS) proteins, thus mimicking the properties of native KG current (Doupnik et al. 1997; Saitoh et al. 1997, 1999; Herlitze et al. 1999). While acceleration of deactivation and short-term desensitization can be accounted for by the enhanced intrinsic GTP hydrolysis of Gα induced by RGS proteins (Doupnik et al. 1997; Saitoh et al. 1997, 1999; Chuang et al. 1998; Herlitze et al. 1999), this cannot explain acceleration of activation without affecting the steady-state current level. It has been shown recently that retinal RGS9 directly inhibits the activity of guanylyl cyclase in addition to its interaction with Gαt (Seno et al. 1998). Thus the RGS proteins might possess functions other than the acceleration of GTPase activity of Gα, which could also be involved in regulation of the KG channel.
Here, we show that co-expression of RGS4 confers an agonist dependence to the voltage-dependent relaxation of the KG channel. The RGS4-induced gating behaviour of the reconstituted KG channel replicated the ACh-induced voltage-dependent relaxation of the native cardiac KG channel. These findings indicate the possibility that KG channel gating is a novel physiological target of RGS protein-mediated regulation.
METHODS
Electrophysiology in Xenopus oocytes and native atrial myocytes
Treatment of Xenopus laevis was in accordance with the guidelines for the use of laboratory animals of Osaka Medical School. The frogs were deeply anaesthetized by immersion in water containing 0.35 % tricaine (Sigma Chemical Co.) and oocytes were surgically removed under clean conditions. Following the operation, the frogs were returned to fresh water to allow them to recover from anaesthesia. Adequate time for healling was allowed between each procedure. Following the last collection, the anaesthetized frogs were killed by decapitation.
Rat Kir3.4, rat RGS4, porcine m2 muscarinic receptor and bovine Gβ1 and Gγ2 cDNAs (kindly provided by Drs D. Clapham, C. Doupnik, T. Kubo and T. Haga, respectively) as well as mouse Kir3.1 cDNA subcloned into pGEMHE vector were transcribed in vitro with T7 RNA polymerase and an mRNA capping kit (Stratagene, La Jolla, CA, USA). A mixture of 160 ng RGS4, 80 ng m2 receptor and 8 ng each of Kir3.1 and Kir3.4 cRNAs was injected into the oocytes which had been defolliculated in 1 mg ml−1 collagenase solution (Wako Pure Chemical, Osaka, Japan). In some oocytes, Gβ1 and Gγ2 cRNAs (10 ng each) were co-injected with RGS4, Kir3.1 and Kir3.4 cRNAs. After injection, the oocytes were maintained in a modified Barth's solution for 72–96 h at 18°C and assayed using a commercially available amplifier (CEZ-1250; Nihon Kohden, Tokyo, Japan). Pipettes were filled with 3 M KCl. The bath solution contained (mM): 90 KCl, 3 MgCl2, 0.15 niflumic acid and 5 Hepes-KOH (pH 7.4).
Single atrial myocytes were enzymatically isolated from hearts removed from adult male Japanese White rabbits deeply anaesthetized with pentobarbital (Yamada & Kurachi, 1995). Using a Langendorff apparatus, the heart was perfused in a retrograde manner through the coronary arteries with 16 mg of collagenase in 100 ml of nominally Ca2+-free bathing solution at 37°C for 10 min. Whole-cell patch-clamp analysis was performed as described previously (Yamada & Kurachi, 1995). Pipettes were filled with the following solution (mM): KCl 140, KH2PO4 1, MgCl2 1, EGTA-KOH 5, K2ATP 3, Na2GTP 0.1 and Hepes-KOH 5 (pH 7.3). The bath solution contained (mM): NaCl 136.5, KCl 5.4, CaCl2 1.8, MgCl2 0.53, glucose 5.5 and Hepes-NaOH 5 (pH 7.4).
All experiments were performed at room temperature (20-25°C). The ACh-induced K+ currents were obtained by subtracting the control currents from those in the presence of various [ACh] at each potential. In the case of the KG current in the oocytes expressing Gβγ, the Ba2+ (1 mm)-sensitive component was analysed. The data were stored on videotape using a PCM data recording system, and subsequently replayed for computer acquisition and analyses.
In vitro binding assay
GST fusion constructs of RGS4 were prepared by PCR tagging of RGS4 cDNA with Bam HI and Eco RI sites at the 5′ and 3′ end, respectively, and were subcloned into pGEX-2T vector (Amersham Pharmacia Biotech, Uppsala, Sweden). GST-RGS4 and GST were expressed in E. coli and purified from the cell lysates through a glutathione-Sepharose column (Amersham Pharmacia Biotech). Kir3.1 and Kir3.4 cDNAs were subcloned into the pcDNA3 expression vector (Invitrogen, San Diego, CA, USA) and transfected to HEK293T cells with LipofectAMINE and PLUS reagents (Life Technologies Inc., Gaithersburg, MD, USA). Cell lysates containing the expressed Kir subunits were collected with buffer containing 20 mm Hepes-NaOH (pH 7.4), 1 mm Na-EDTA, 0.5 mm Na-EGTA, 1 mm DTT, 145 mm NaCl, 5 mm KCl, 1 % (w/v) CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propane sulphonate), 1 mm PMSF (phenylmethylsulphonyl fluoride), 0.2 mg ml−1 benzamidine HCl, and 5 μg ml−1 each of pepstatin, leupeptin and chymostatin. After centrifugation, the clear supernatants were incubated with GST-RGS4 and GST immobilized on the glutathione-Sepharose beads for 3 h at 4°C. The beads were extensively washed and incubated with Laemmli sample buffer for 5 min at 95°C. The bound proteins were analysed by SDS-PAGE and detected with antibodies specific to Kir3.1 (aG1C-1) and to Kir3.4 (aG4N-10) (Morishige et al. 1999).
RESULTS
Effects of RGS4 on the relaxation of KG channel currents
Xenopus oocytes injected with Kir3.1, Kir3.4 and m2 receptor cRNAs expressed an inwardly rectifying K+ current which responded slowly to ACh (Fig. 1A). On wash-out of ACh, the current disappeared with a time constant of ∼10 s. When RGS4 was co-expressed, the activation and deactivation time courses were accelerated (Fig. 1b; Doupnik et al. 1997; Saitoh et al. 1997, 1999; Herlitze et al. 1999). The steady-state level of ACh-induced current was unaffected by the co-expression of RGS4 (Fig. 1C).
Figure 1. Effect of RGS4 on the relaxation kinetics of the reconstituted KG channel.
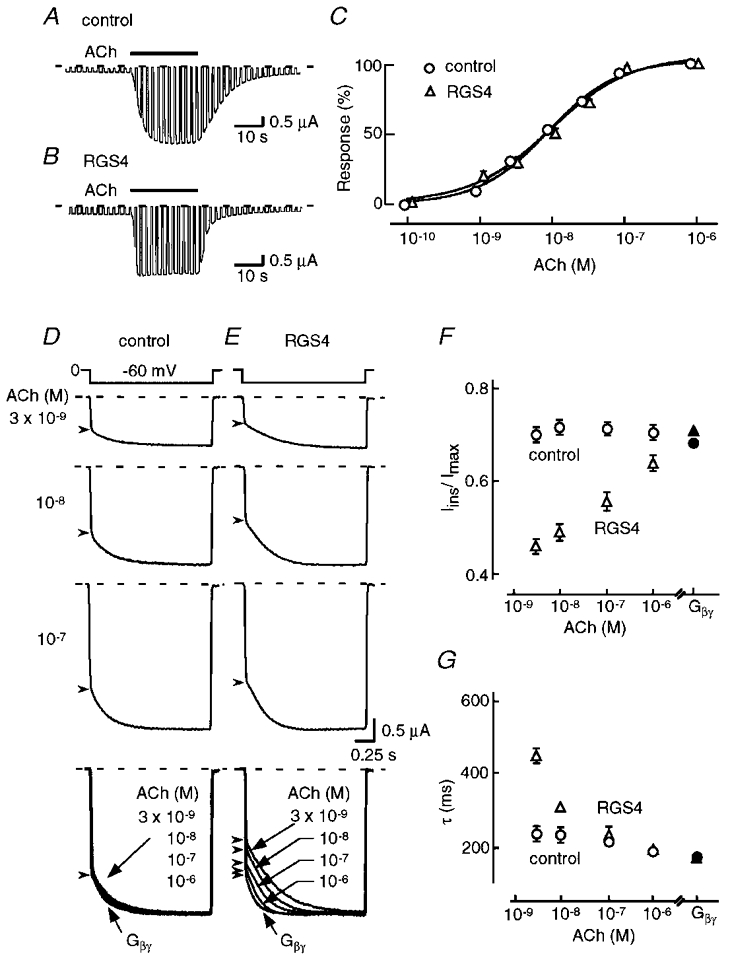
Whole-cell currents were recorded from Xenopus oocytes expressing m2 receptor and heteromeric Kir3.1/Kir3.4 KG channels in the absence or presence of RGS4 (A and B, respectively). Test pulses (2 s) to -60 mV were applied every 3 s from a holding potential of 0 mV. Acetylcholine (ACh, 1 μm) was added to the bath as indicated by the horizontal bar above each trace. C, ACh-induced KG current amplitude was plotted against [ACh]. The mean (±s.e.m.) values of EC50 and Imax of the ACh-induced currents in the presence and absence of RGS4 were 9.1 and 9.9 nM, and 4.3 ± 0.52 and 4.1 ± 0.49 μA, respectively (n= 10). D and E, effect of RGS4 on the gating properties of ACh-induced KG current. Inward current traces in the absence (D) or presence (E) of RGS4 were recorded with a voltage step to -60 mV from 0 mV and basal currents were subtracted. The arrowheads represent the end and start points of the instantaneous and relaxation components of the currents. Traces in the bottom panel represent normalized and superimposed current evoked by the various [ACh] or directly activated by Gβγ. Traces from the same series of oocytes are compared, and similar results were obtained in oocytes from three different frogs. The dashed lines in A-E indicate the zero current level. F and G, the Iins/Imax ratio (F) and the time constant (τ) of the relaxation (G) of KG currents at each [ACh] (open symbols) and with over-expressed Gβγ (filled symbols; n= 10 for each point). Results obtained in the presence and absence of RGS4 are represented by triangles and circles, respectively.
A slowly increasing K+ current during hyperpolarizing voltage pulses, known as relaxation, is one of the features of G protein-gated K+ (KG) channels containing Kir3.1. The ACh-induced K+ current during a voltage step could be divided into instantaneous and relaxing components. In the control (Fig. 1D), at all concentrations of ACh, the ratio of the instantaneous component to the pulse-end current (Iins/Imax) was ∼0.71 (Fig. 1F, ○), and the relaxation time constant (τ) was ∼200 ms (Fig. 1G, ○), this notwithstanding that the current amplitude increased as [ACh] was raised (Fig. 1C). The superimposed normalized KG currents at the bottom of Fig. 1D indicated that Iins/Imax and τ remained constant at various [ACh] and these values were comparable to those of the KG current directly activated by Gβγ in the same series of oocytes.
When RGS4 was co-expressed with KG channels, the relaxation of the ACh-induced currents became dependent on [ACh] (Fig. 1E). At 3 × 10−9 M ACh, Iins/Imax was 0.45 and τ was ∼450 ms. As [ACh] was raised, the Iins/Imax ratio increased and τ became smaller. At 10−6 M ACh, both values were similar to those with over-expressed Gβγ (Fig. 1F and G, ▵ and ▴). The superimposed normalized currents at the bottom of Fig. 1E clearly show the alteration in the relaxation properties of ACh-induced K+ current at various [ACh] in the presence of RGS4. Because Iins/Imax and τ of the K+ current induced by over-expression of Gβγ were the same in the absence and presence of RGS4 (filled symbols in Fig. 1F and G), it would seem that RGS4 may modulate KG channel gating in an inhibitory manner, which appears to be attenuated by the activation pathway followed by Gβγ.
RGS4 inhibits the gating of KG channel
In Fig. 2, we examined the quasi-steady-state relative open probability (Po) of the KG channel relaxation gate at various potentials in the absence and presence of RGS4. A double pulse voltage-clamp protocol was used. In the absence of RGS4, at all [ACh] examined and with over-expressed Gβγ, the Iins/Imax ratio of the tail currents recorded at -100 mV was ∼1 following pre-pulse voltage steps to between -100 and -80 mV and it decreased to ∼0.7 with pre-pulses to positive membrane potentials. Thus, the quasi-steady-state relative Po curves obtained at different [ACh] and with over-expression of Gβγ were very similar (Fig. 2C). On the other hand, when RGS4 was co-expressed, the instantaneous component of the tail currents following the depolarizing pulses was clearly smaller than that in the control (Fig. 2A and B). As [ACh] was increased, the instantaneous component of the tail currents increased and the relative Po curves were shifted upwards at depolarized potentials in a concentration-dependent fashion (Fig. 2B and D). The relative Po curve at 10−6 M ACh even in the presence of RGS4 was similar to that with over-expressed Gβγ (Fig. 2D, ○ and ▾, respectively).
Figure 2. The effect of RGS4 on the quasi-steady-state relative open probability (Po) curve of the KG channel relaxation gate.
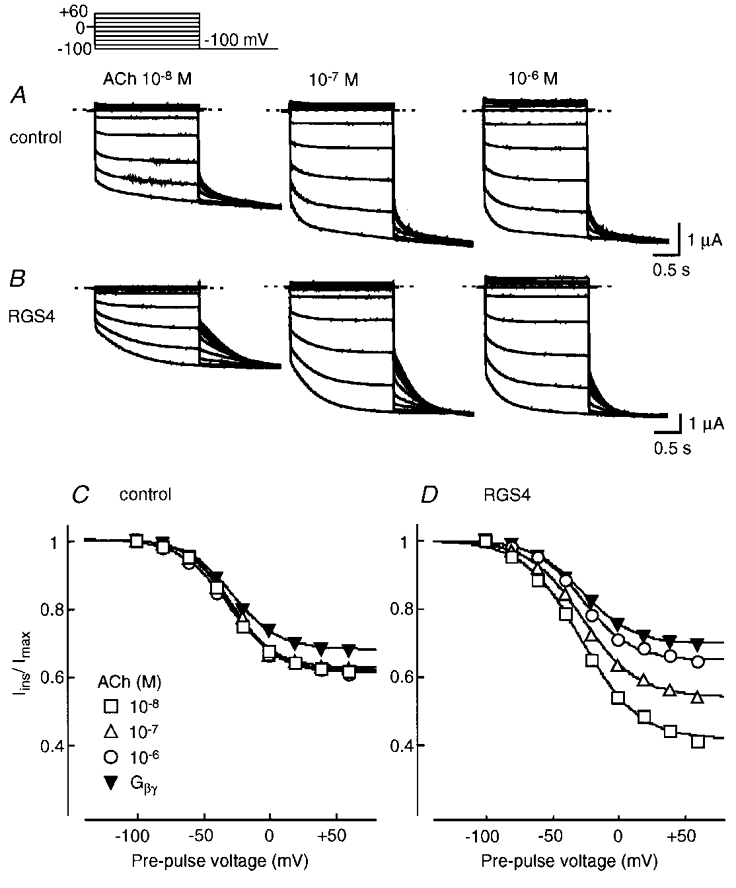
The relative Po curves at various [ACh] and with over-expressed Gβγ were fitted by the modified Boltzmann equation (Fig. 2 legend). RGS4 affected exclusively the A value but neither the V½ nor k values of the relative Po curve. Therefore, RGS4 regulates a voltage-independent parameter of the KG channel relaxation relative Po curve.
Biochemical study of a link between RGS4 and KG channel
The possible association between RGS4 and the KG channel was examined using GST-RGS4 and CHAPS extract from HEK293T cells expressing Kir3.1 and/or Kir3.4 (Fig. 3). Kir3.1 and Kir3.4 immunoreactivity was retained in the complex of GST-RSG4 and glutathione-Sepharose beads when incubated with the lysate from cells co-transfected with Kir3.1 and Kir3.4, but neither signal was observed in the control (GST alone; Fig. 3A and B). Next we prepared lysates from cells separately expressing Kir3.1 or Kir3.4 and mixed them with GST-RGS4 or GST (see Fig. 3C). Although the lysates from cells transfected with the separate Kir subunits contained equal amounts of protein to those co-transfected with both subunits (right panels in Fig. 3A and B), only a small amount of Kir3.1 and little of Kir3.4 were recovered using the GST-RGS4-glutathione-Sepharose beads (Fig. 3A and B). These results suggest that heteromeric Kir3.1/Kir3.4 channel proteins are more effective than homomeric Kir subunits in forming protein complexes containing RGS4.
Figure 3. In vitro association between RGS4 and the Kir3.1/Kir 3.4 KG channel.
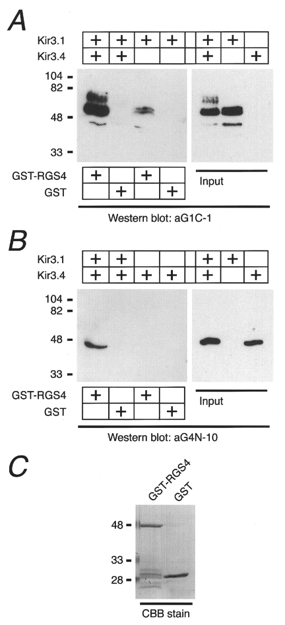
RGS4 fused with GST (GST-RGS4) and GST (1 μg each) were immobilized on glutathione-Sepharose beads and incubated with the lysates from HEK293T cells transfected with various combinations of Kir3.1 and Kir3.4 as indicated above each panel. The proteins recovered on the beads were analysed with anti-Kir3.1 (aG1C-1; A) or anti-Kir3.4 antibodies (aG4N-10; B). Both subunits in the input lysates were detected by this method (right panels). C, protein bands were visualized by staining the gel with Coomassie brilliant blue R-250 (CBB) to show GST-RGS4 and GST. Numbers on the left side of each panel represent the molecular mass of the standard markers in kilodaltons.
[ACh]-dependent alteration of relaxation in native KG channels
In Fig. 4, we examined the effects of various [ACh] on the relaxation of native KG channels in rabbit atrial myocytes. The amplitude of ACh-induced K+ current increased as [ACh] was raised (Fig. 4A). The ACh-induced K+ current increased slowly during hyperpolarizing pulses and upon returning to the holding potential the outward current gradually decreased. As [ACh] was raised, the Iins/Imax ratio increased (Fig. 4B) and τ became smaller (Fig. 4C). The superimposed normalized currents in Fig. 4A were very similar to those of the reconstituted Kir3.1/Kir3.4 KG channel with RGS4. This strongly suggests that RGS proteins may participate in the physiological regulation of KG channel relaxation gating in atrial myocytes.
Figure 4. Relaxation of the ACh-induced K+ current in rabbit atrial myocytes.
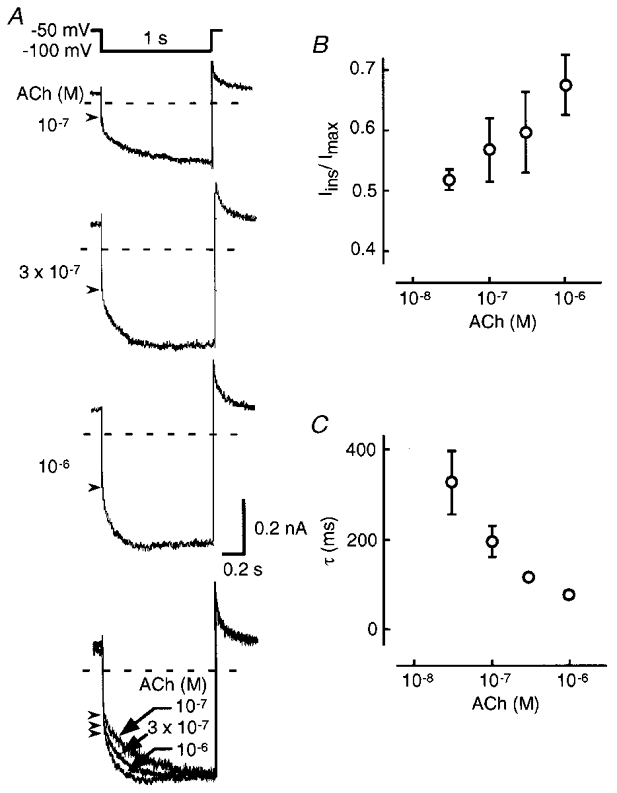
A, whole-cell currents in response to various [ACh] were obtained by application of hyperpolarizing pulses to -100 mV for 1 s every 7 s from the holding potential of -50 mV. In the bottom panel, superimposed normalized ACh-induced currents are shown. Dashed lines and arrowheads indicate the zero current level and the point separating the instantaneous and relaxation components, respectively. B and C, the Iins/Imax ratio (B) and the time constant (τ) of relaxation (C; means ±s.e.m., n= 3).
DISCUSSION
This study shows that RGS4 confers ACh dependence on the relaxation gating of Kir3.1/Kir3.4 KG channels. Since the native cardiac KG channel shows similar ACh-dependent relaxation kinetics, regulation of KG channel gating by RGS-mediated signalling may occur physiologically.
The analyses of quasi-steady-state relative Po curves of KG channels in the absence and presence of RGS4 clearly indicated that RGS4 decreased a voltage-independent parameter (A). The voltage-independent decrease of A was attenuated by ACh in a concentration-dependent manner. Furthermore, over-expression of Gβγ completely cancelled the inhibitory effect of RGS4 on the relative Po curve. Therefore, a RGS4-mediated regulatory mechanism may act on the non-activated KG channels and free Gβγ may hinder the effect of RGS4 on channel gating. The in vitro biochemical experiments indicated that RGS4 and KG channels composed of Kir3.1 and Kir3.4 were closely associated and also suggested that different Kir3.0 subunits and their combinations may have different affinities for RGS-mediated regulatory mechanisms. However, we could not determine in this study whether the interaction between KG channel subunits and RGS4 is direct or involves other intermediate proteins. Further studies are needed to clarify the molecular mechanisms for the linkage and interaction between the KG channel subunits, RGS proteins and Gβγ.
The fast deactivation of the ACh-induced KG current after wash-out of the agonist and the short-term desensitization have been attributed to the acceleration of the GTPase activity of Gα by RGS proteins (Doupnik et al. 1997; Saitoh et al. 1997, 1999; Chuang et al. 1998; Herlitze et al. 1999). Acceleration of activation was not simply explained by the GTPase-activating protein (GAP) action of RGS proteins (Doupnik et al. 1997). Saitoh et al. (1997) proposed that RGS proteins may function as an on-rate accelerator. They further showed that RGS7 accelerates activation with only a weak effect on deactivation (Saitoh et al. 1999). Herlitze et al. (1999), however, suggested using mutational analysis of RGS2 that the underlying mechanism for acceleration of activation and that of deactivation may share the same pathway. Bünemann & Hosey (1998) showed that RGS proteins, in addition to their GAP action, increase the availability of Gβγ in HEK293T cells. Therefore, it seems likely that RGS proteins possess multiple functional arms to the G protein activation of KG channel system, which have not yet been fully clarified. The effect of RGS proteins on KG channel gating reported here has not been recognized at all in previous studies, and may provide a novel insight into the physiological regulation of KG channel current.
Acknowledgments
The authors thank Dr Ian Findlay (Tours University, Tours, France) for his critical reading of this manuscript. This work was supported by grants from the Ministry of Education, Culture, Sports and Science of Japan, from the Research for the Future Program of the Japan Society for the Promotion of Science (96L00302), and from the Human Frontier Science Program (RG0158/1997-B).
References
- Bünemann M, Hosey MM. Regulators of G protein signaling (RGS) proteins constitutively activate Gβγ-gated potassium channels. Journal of Biological Chemistry. 1998;273:31186–31190. doi: 10.1074/jbc.273.47.31186. [DOI] [PubMed] [Google Scholar]
- Chuang H-H, Yu M, Jan YN, Jan LY. Evidence that the nucleotide exchange and hydrolysis cycle of G proteins causes acute desensitization of G-protein gated inward rectifier K+ channels. Proceedings of the National Academy of Sciences of the USA. 1998;95:11727–11732. doi: 10.1073/pnas.95.20.11727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupnik CA, Davidson N, Lester HA, Kofuji P. RGS proteins reconstitute the rapid gating kinetics of Gβγ-activated inwardly rectifying K+ channels. Proceedings of the National Academy of Sciences of the USA. 1997;94:10461–10466. doi: 10.1073/pnas.94.19.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze S, Ruppersberg JP, Mark MD. New roles for RGS2, 5 and 8 on the ratio-dependent modulation of recombinant GIRK channels expressed in Xenopus oocytes. The Journal of Physiology. 1999;517:341–352. doi: 10.1111/j.1469-7793.1999.0341t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature. 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Reuveny E, Slesinger PA, Jan YN, Jan LY. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 1993;364:802–806. doi: 10.1038/364802a0. [DOI] [PubMed] [Google Scholar]
- Morishige K, Inanobe A, Yoshimoto Y, Kurachi H, Murata Y, Tokunaga Y, Maeda T, Maruyama Y, Kurachi Y. Secretagogue-induced exocytosis recruits G protein-gated K channels to plasma membrane in endocrine cells. Journal of Biological Chemistry. 1999;274:7969–7974. doi: 10.1074/jbc.274.12.7969. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Kubo Y, Miyatani Y, Asano T, Nakata H. RGS8 accelerates G-protein-mediated modulation of K+ currents. Nature. 1997;390:525–529. doi: 10.1038/37385. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Kubo Y, Odagiri M, Ichikawa M, Yamagata K, Sekine T. RGS7 and RGS8 differentially accelerate G protein-mediated modulation of K+ currents. Journal of Biological Chemistry. 1999;274:9899–9904. doi: 10.1074/jbc.274.14.9899. [DOI] [PubMed] [Google Scholar]
- Seno K, Kishigami A, Ihara S, Maeda T, Bondarenko VA, Nishizawa Y, Usukura J, Yamazaki A, Hayashi F. A possible role of RGS9 in phototransduction. Journal of Biological Chemistry. 1998;273:22169–22172. doi: 10.1074/jbc.273.35.22169. [DOI] [PubMed] [Google Scholar]
- Yamada M, Inanobe A, Kurachi Y. G protein regulation of potassium ion channels. Pharmacological Reviews. 1998;50:723–757. [PubMed] [Google Scholar]
- Yamada M, Kurachi Y. Spermine gates inward-rectifying muscarinic but not ATP-sensitive K+ channels in rabbit atrial myocytes. Journal of Biological Chemistry. 1995;270:9289–9294. doi: 10.1074/jbc.270.16.9289. [DOI] [PubMed] [Google Scholar]


