Abstract
In cat lumbar motoneurones, disynaptic inhibitory postsynaptic potentials (IPSPs) evoked by stimulation of antagonist motor nerves were depressed for at least 150 ms following conditioning stimulation of flexor (1.7-2 times threshold (T)) and ankle extensor (5T) nerves. The aim of the present study was to investigate the possibility that this depression is caused by presynaptic inhibitory mechanisms acting at the terminals of group I afferent fibres projecting to the Ia inhibitory interneurones and/or the terminals of these interneurones to the target motoneurones.
Conditioning stimulation of flexor, but not ankle extensor, nerves evoked a depression of the monosynaptic Ia excitatory postsynaptic potentials (EPSPs) recorded intracellularly in Ia inhibitory interneurones. This depression lasted between 200 and 700 ms and was not accompanied by a depression of the monosynaptic EPSPs evoked by stimulation of descending pathways. These results suggest that flexor, but not ankle extensor, group I afferent fibres can modulate sensory transmission at the synapse between Ia afferent fibres and Ia inhibitory interneurones.
Conditioning stimulation of flexor muscle nerves, extensor muscle nerves and cutaneous nerves produced a long-lasting increase in excitability of the terminals of the Ia inhibitory interneurones. The increase in the excitability of the terminals was not secondary to an electrotonic spread of synaptic excitation at the soma. Indeed, concomitant with the excitability increase of the terminals there were signs of synaptic inhibition in the soma.
The unitary IPSPs induced in target motoneurones following the spike activity of single Ia inhibitory interneurones were depressed by conditioning stimulation of muscle and cutaneous nerves. Since the conditioning stimulation also evoked compound IPSPs in those motoneurones, a firm conclusion as to whether unitary IPSP depression involved presynaptic inhibitory mechanism of the terminals of the interneurones could not be reached.
The possibility that the changes in excitability of the Ia interneuronal terminals reflect the presence of a presynaptic inhibitory mechanism similar to that operating at the terminals of the afferent fibres (presynaptic inhibition) is discussed.1. In cat lumbar motoneurones, disynaptic inhibitory postsynaptic potentials (IPSPs) evoked by stimulation of antagonist motor nerves were depressed for at least 150 ms following conditioning stimulation of flexor (1.7-2 times threshold (T)) and ankle extensor (5T) nerves. The aim of the present study was to investigate the possibility that this depression is caused by presynaptic inhibitory mechanisms acting at the terminals of group I afferent fibres projecting to the Ia inhibitory interneurones and/or the terminals of these interneurones to the target motoneurones.
The Ia inhibitory interneurones, which mediate disynaptic reciprocal inhibition between motor nuclei innervating antagonist muscle groups, constitute one of the most well characterised and studied populations of interneurones in the spinal cord (for reviews see Baldissera et al. 1981; Jankowska, 1992). Transmission in the pathway of disynaptic reciprocal Ia inhibition has been investigated in the cat by monosynaptic test reflexes (Lloyd, 1946a,b) and, more recently, in human subjects by H-reflex testing (Crone et al. 1987; Crone & Nielsen, 1989). The latter type of study is used extensively to investigate the central control of reflex pathways during voluntary movement in human subjects. It has specifically been proposed that changes in the short-latency inhibition of the soleus H-reflex following stimulation of the antagonist peroneal nerve during voluntary contraction reflects excitability changes in the Ia inhibitory interneurones and that such changes may provide information about the descending supraspinal control of the interneurones (for review see Crone & Nielsen, 1994). However, the non-invasive approach in the human experiments does not permit a distinction between changes in the (postsynaptic) excitability of the interneurones and changes in the synaptic transmission (presynaptic inhibition) from the Ia afferents to the interneurones or from the interneurones to the target motoneurones.
Changes in synaptic transmission in the pathway mediating reciprocal inhibition may occur via presynaptic inhibition acting on the terminals of group Ia afferents and/or the terminals of the Ia interneurones themselves. Already in 1963, Eccles and collaborators suggested that presynaptic mechanisms acting at the terminals of group Ia afferents on the Ia inhibitory interneurones could be responsible for the long-lasting depression of disynaptic reciprocal IPSPs seen after conditioning stimulation of flexor group I afferents in the cat (Eccles et al. 1963). A recent study has also demonstrated that the terminals of interneurones in the cat spinal cord may be depolarised following conditioning stimulation (Aggelopolous et al. 1997). Since depolarisation of the terminals of primary afferents has been associated with presynaptic inhibition (reviewed in Schmidt, 1971; see also Rudomin et al. 1998), the possibility exists that the terminals of interneurones in the cat spinal cord are also subjected to presynaptic inhibition. Presynaptic inhibition of spinal interneurones has also been demonstrated in the lamprey (Alford et al. 1991) and the frog (McDearmid et al. 1997).
The aim of the present study was to investigate whether reciprocal Ia inhibitory interneurones are subjected to presynaptic inhibition in the cat. Preliminary accounts of some of the data have been published in abstract form (Enríquez-Denton et al. 1996, 1997).
METHODS
Dissection
The experiments were approved by the local ethics committee and were performed on twenty male cats weighing between 2.5 and 4.5 kg. The animals were treated in accordance with the National Institutes of Health Guide for the care and use of laboratory animals (NIH publication no. 86-23, revised 1985). Anaesthesia was induced with Saffan (a mixture of 9 mg ml−1 Alphaxolone and 3 mg ml−1 Alphadolone; 1.5 ml kg−1i.m.) or halothane (inhalation of up to 2.5 % in a 50 % mixture of oxygen and NO2). During surgery, a deep level of anaesthesia was maintained with halothane. During the experiment, anaesthesia was maintained with α-chloralose (maximal dose 80 mg kg−1i.v.) supplemented with pentobarbital (Mebumal, 5 mg kg−1i.v.) every 2 h. Testing of paw withdrawal reflexes was used to monitor the depth of the anaesthesia prior to paralysation (see below). At the end of the experiment the animal was killed by an overdose of pentobarbital.
The following nerves were dissected and prepared for stimulation: quadriceps (Q), sartorius (Sar), semimembranosus and anterior biceps (SmAB), posterior biceps and semitendinosus (PBSt), sural (Su), triceps surae (GS), plantaris (Pl), flexor digitorum and hallucis longus and tibialis posterior (FDHL), and tibialis anterior (TA) or deep peroneal nerve (DP; when TA was mounted together with extensor digitorum longus).
The lumbo-sacral spinal cord was exposed for recording by a laminectomy of the L7-L4 vertebrae. The Th12 vertebra was removed for stimulation of the ventro-lateral funiculus (VLF). Ventral roots L5 and L6 were cut and prepared for stimulation. Two cats were spinalised at Th12. In these experiments the recording session started at least 2 h after transection.
The cats were paralysed with pancuronium bromide (Pavulon; initial dose 0.6 ml supplemented with 0.2 ml every 40 min) and artificially ventilated (the expiratory CO2 level was maintained around 4 %). Blood pressure, heart rate and constriction of pupils were routinely monitored as an indication of the depth of anaesthesia. Lack of changes in these parameters following noxious stimulation (paw pincing or high intensity nerve stimulation) was also used to test the adequacy of the anaesthesia. Further mechanical stability was obtained by bilateral pneumothorax.
Data acquisition
Incoming volleys following nerve stimulation were recorded with a silver ball electrode located ventral to the L7 dorsal root and amplified with low noise high gain differential amplifiers (band-pass filters 1 Hz to 10 kHz). The indifferent electrode was located in nearby back muscles. Intracellular recordings were made with potassium acetate (KAc)-filled microelectrodes (2-5 MΩ) coupled to a microelectrode amplifier (Axoclamp-2A). Signals were digitally stored and analysed off-line using custom-built data capture and analysis software (G. R. Detillieux, The University of Manitoba, Winnipeg).
Unit identification
Motoneurones were identified by antidromic invasion after stimulation of peripheral nerves (L7 and S1 ventral roots were left intact) or by the convergence pattern of monosynaptic group I excitation from peripheral muscle nerves (Eccles et al. 1957). Ia inhibitory interneurones were identified by showing: (1) monosynaptic activation by low threshold muscle afferents (see Fig. 1B), (2) ability to discharge without failure following repetitive stimulation of group I afferents at more than 150 Hz (Fig. 1A), (3) depression of excitability after conditioning stimulation of the ventral roots from the same segment (Recurrent inhibition, Fig. 1B), (4) location dorsally and medially to the motor nuclei and (5) by absence of antidromic action potentials following stimulation of the ventral roots (Fig. 1B, see also Hultborn et al. 1971).
Figure 1.
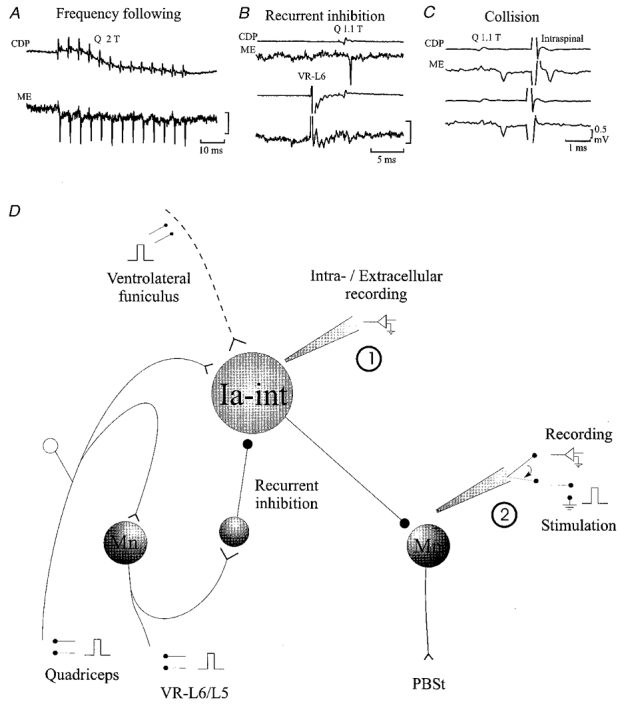
Connectivity of quadriceps-coupled Ia inhibitory interneurones (Ia-int) and recording and stimulation protocols
A-C illustrate the criteria used for identification of quadriceps-coupled Ia inhibitory interneurones. A, ability to follow stimulation frequencies of more than 150 Hz. B, recurrent inhibition by activation of the L6 ventral root (VR-L6). C, antidromic activation from the PBSt motonuclei (Intraspinal) which shows collision with orthodromic action potentials. Voltage calibration is the same in the three panels and refers to microelectrode recordings only. For further details see text. ME, microelectrode recording; CDP, cord dorsum potential. D illustrates the general experimental arrangement. Mn, motoneurone.
Excitability measurement
Extracellular recording from quadriceps-coupled Ia inhibitory interneurones in the caudal L5 or rostral L6 segments was made with KAc-filled and shielded microelectrodes (2-5 MΩ; Fig. 1D, 1). The axon terminals of the interneurones were stimulated at L7 or rostral S1 segments in the PBSt motor nuclei (with 0.1 ms squared pulses) using a shielded glass microelectrode filled with NaCl (2 M) in agar (0.5 %∼1 MΩ; Fig. 1D, 2; see Eide, 1971). The position of the stimulating microelectrode was systematically changed in order to induce antidromic action potentials with the lowest possible intensity (less than 10 μA). Then, the intensity of the intraspinal stimuli was adjusted manually in order to induce an antidromic action potential with a constant probability (usually 50 %; see Jankowska & Roberts, 1972b). An action potential was accepted as being antidromic in origin if it had a latency of less than 0.6 ms with a variability of less than 0.1 ms (Swadlow et al. 1978) and if it collided with an orthodromically induced action potential (see Fig. 1C and Jankowska & Roberts, 1972b). Excitability of the interneuronal terminals was assessed as the ratio between the number of antidromic action potentials elicited by the test stimuli and the total number of test stimuli (firing index; Jankowska & Roberts, 1972b; see also Jankowska et al. 1981).
The excitability of the interneuronal terminals was determined when the intraspinal test stimulus was applied alone and when it was preceded by conditioning stimulation of different nerves at variable intervals. Test alone and test + conditioning stimuli were given in alternation at a repetition frequency of about 1 Hz. Since orthodromic action potentials might affect the excitability of the terminals of the interneurones (see Kocsis et al. 1979; Swadlow et al. 1980), all cases where the interneurones were activated by the conditioning stimulation were discarded from further analysis.
Changes in interneuronal excitability following the conditioning nerve stimulation were determined as significant by comparing the proportion of antidromic spikes in at least 50 sweeps in the presence versus absence of conditioning nerve stimulation (Z test, Jandel SigmaStat for Windows, version 1, Jandel Corporation).
Single unit IPSPs
Unitary IPSPs in PBSt motoneurones were elicited by the discharge of quadriceps-coupled Ia inhibitory interneurones induced by release of sodium glutamate (0.5 M in 0.5 M KAc; Fig. 1D, 1) from the recording electrode with DC currents not larger than 25 nA (anode was the reference). These IPSPs were disclosed using the spike triggered averaging technique (Fig. 7, insets; Jankowska & Roberts, 1972a). Stimulation of extensor muscle nerves, flexor muscle nerves or cutaneous nerves was applied at 1 Hz and the size of the unitary IPSP at different intervals in relation to these conditioning stimuli was measured (see Fig. 7). Changes in the amplitude of the unitary IPSP induced by the conditioning stimulation were evaluated by the use of a Student's t test.
Figure 7.
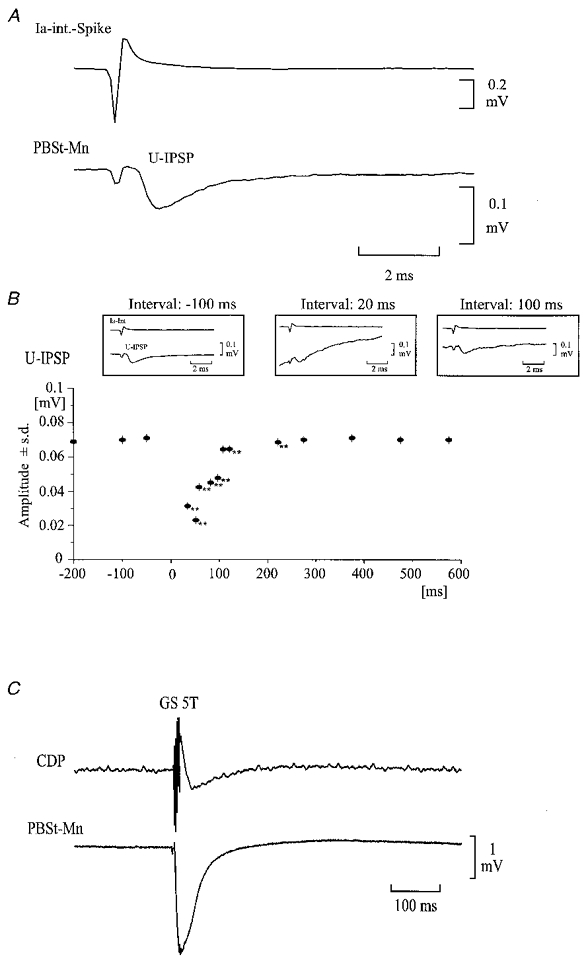
Effect of conditioning GS stimulation on the amplitude of unitary IPSP and membrane potential in a PBSt motoneurone
A, average of the membrane potential in a PBSt motoneurone, triggered by the glutamate-induced activity in a Q-coupled Ia inhibitory interneurone, The upper trace shows the extracellular spike of a single Ia inhibitory interneurone, which was used to trigger the averager. The lower trace shows the unitary IPSP (U-IPSP) evoked by the spike activity. B, time course of the peak amplitude of the U-IPSP following the conditioning GS stimulation (5T; 200 Hz) applied at time 0. Insets illustrate U-IPSPs at -100, 20 and 100 ms from the conditioning stimulation. Each average involved at least 30 spikes. Voltage calibration refers to intracellular recordings only. **P < 0.001 compared with control. C, intracellular recording from the PBSt motoneurone showing that the conditioning GS stimulation induced an IPSP in the motoneurone.
RESULTS
Here we will first provide evidence that stimulation of extensor and flexor nerves depresses the reciprocal inhibition mediated by Ia inhibitory interneurones. This depression may be explained by an inhibitory action at several premotoneuronal sites along the disynaptic reciprocal Ia inhibitory pathway, including: (a) depression of transmitter release from the group Ia afferent terminals to the interneurones, (b) somatic inhibition of the Ia inhibitory interneurones and (c) depression of transmitter release from the terminals of the interneurones to the motoneurones. Each of these possibilities will be addressed in turn.
Stimulation of ankle extensor nerves induces a long-lasting depression of reciprocal Ia IPSPs
It was demonstrated by Eccles et al. (1963) that stimulation of flexor nerves induces a long-lasting depression of disynaptic Ia IPSPs. It was suggested that such a depression, which was confirmed in the present investigation (not illustrated), was due to presynaptic inhibition of Ia afferent terminals on the Ia inhibitory interneurones. Figure 2 demonstrates a similar long-lasting depression of disynaptic Ia IPSPs in PBSt motoneurones following stimulation of ankle extensor nerves. Stimulation of ankle extensor nerves has been shown to evoke no or only weak presynaptic inhibition of Ia afferents terminating on motoneurones (reviewed in Schmidt, 1971). In confirmation of this, we found that stimulation of the GS nerve (5 shocks, 5T) failed to depress the monosynaptic Ia EPSPs evoked in a PBSt motoneurone by stimulation of the PBSt nerve at any interval from 20 to 300 ms (compare dashed and continuous lines in upper panel of Fig. 2A; see also open circles in Fig. 2B). However, the GS stimulation effectively depressed the reciprocal Ia IPSP evoked by stimulation of the Q nerve in that motoneurone (compare dashed and continuous lines in lower panel of Fig. 2A). This depression lasted more than 200 ms (Fig. 2B, filled circles). GS stimulation induced a similar long-lasting depression of the disynaptic Ia IPSP in two additional PBSt motoneurones, whereas no depression of either the monosynaptic EPSP or the disynaptic IPSP was seen in a fourth motoneurone. The mean depression of the IPSP was 77 ± 8.7 %, whereas there was no difference between the mean control and conditioned EPSP for the four motoneurones as a group. In contrast, flexor stimulation (DP; 5 shocks, 5T) evoked a clear depression of both IPSPs and EPSPs in the same motoneurones.
Figure 2.

Long-lasting depression of Ia IPSPs in lumbar flexor motoneurones following stimulation of extensor nerves
A, the upper two traces show the cord dorsum potential (CDP) and intracellular recording from a PBSt motoneurone (Mn) of a group Ia EPSP evoked by stimulation of the PBSt motor nerve. The lower two traces show the CDP and disynaptic Ia IPSP evoked by stimulation of the antagonist Q motor nerve in the same PBSt motoneurone. Measurements with and without conditioning stimulation of GS (5T, 5 shocks, 200 Hz, conditioning-test interval 37 ms) are shown as dashed and continuous lines, respectively. Each trace is the average of 32 sweeps. B, time course of the changes in the postsynaptic potentials illustrated in A. The abscissa is the interval between the conditioning GS stimulation and the test stimulation of either the PBSt (EPSP, ○) or Q nerve (IPSP, •). The ordinate indicates the size of the conditioned EPSP or IPSP as a percentage of the control EPSP or IPSP. Records were obtained by averaging 32 individual sweeps. Error bars indicate one standard error of the mean.
One interpretation of these observations is that stimulation of both flexor and extensor nerves induces presynaptic inhibition of the terminals of Ia afferents on the Ia inhibitory interneurones. However, that would require that the terminals of Ia afferents on interneurones and on motoneurones are controlled differently by presynaptic inhibition, since conditioning stimulation of the ankle extensor nerve (GS) depressed disynaptic IPSPs, but not monosynaptic EPSPs in motoneurones. An alternative explanation is that the depression of disynaptic Ia IPSPs by GS stimulation is caused by a direct inhibition of the interneurones at the level of the soma, at the level of their terminals or a combination of both. The following experiments were designed to address these possibilities.
Presynaptic inhibition of Ia afferent terminals on Ia inhibitory interneurones
Figure 3 shows intracellular recordings from two quadriceps-coupled Ia inhibitory interneurones, in which we tested the effect of conditioning stimulation of the flexor (PBSt) or extensor (GS) motor nerves on monosynaptic EPSPs evoked by stimulation of either quadriceps group I afferents (Fig. 3A and C) or descending fibres in the VLF (Fig. 3B and D). As illustrated in Fig. 3A, conditioning stimulation of the PBSt (5 shocks, 2T) caused a clear depression of the monosynaptic EPSP evoked by stimulation of quadriceps group I afferents (compare continuous and dotted lines). In contrast, the EPSP elicited by stimulation of VLF was not depressed (Fig. 3B). This selectivity suggests that the depression of the Ia EPSP was caused by presynaptic inhibition of the terminals of the Ia afferents and not postsynaptic inhibition of the interneurone (i.e. remote dendritic inhibition, see Frank, 1959; Rudomin et al. 1975). A similar depression of Ia EPSPs without depression of VLF-induced EPSPs by conditioning flexor stimulation was obtained from two other quadriceps-coupled Ia inhibitory interneurones and one gracilis-coupled interneurone. The mean depression of the Ia EPSPs was 77 ± 10 % (n = 5).
Figure 3.
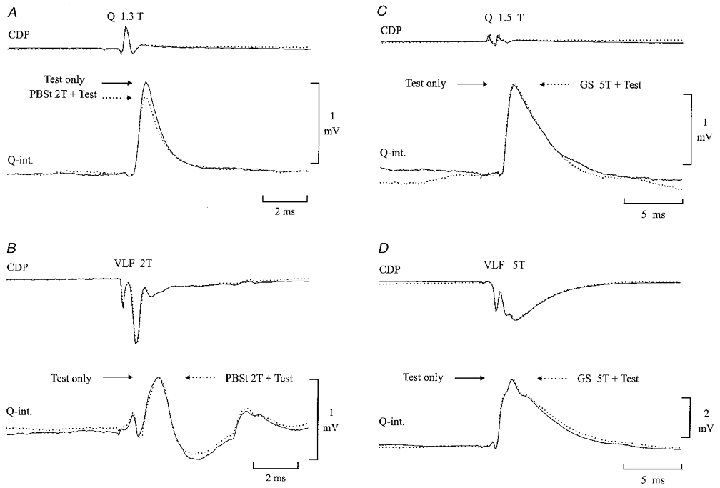
Evidence of presynaptic inhibition of the terminals of group Ia afferents on Ia inhibitory interneurones
Intracellular EPSPs were evoked in a Q-coupled Ia inhibitory interneurone by stimulation of either the Q motor nerve (A and C) or descending tracts in the ventrolateral funiculus (VLF;B and D). The EPSPs were conditioned by stimulation of either the PBSt (A and B; 5 pulses, 375 Hz, 2T, conditioning-test interval 46 ms) or GS motor nerve (C and D; 5 pulses, 275 Hz, 5T, conditioning-test interval 46 ms). EPSPs with (dotted lines) and without (continuous lines) conditioning stimulation were alternated with each other. The CDP is shown above the intracellular recording.
In contrast to flexor nerve stimulation, stimulation of GS had no effect on either the Ia- or the VLF-induced EPSPs (Fig. 3C and D). In the same interneurone, stimulation of the hip extensor nerve (SmAB) also failed to elicit any depression of the monosynaptic EPSPs. It was observed in one more quadriceps-coupled interneurone that GS stimulation had no effect on the Ia-induced EPSP (in the same interneurone PBSt stimulation caused a clear depression of the EPSP).
This set of observations confirms that stimulation of flexor nerves evokes presynaptic inhibition of the terminals of Ia afferents on Ia inhibitory interneurones. However, this does not seem to be the case for extensor nerve stimulation. Although, the observations are limited, they are in line with the observations regarding Ia EPSPs in motoneurones (see previous section). Accordingly, the depression of reciprocal inhibition by GS stimulation is more likely to be due to postsynaptic inhibition of the interneurones or an inhibitory effect on the terminals of the interneurones
Postsynaptic inhibition of Ia inhibitory interneurones
Stimulation of GS has been shown to evoke postsynaptic inhibition in the soma of quadriceps-coupled Ia inhibitory interneurones (Fig. 4; Hultborn et al. 1976). This inhibition could certainly contribute to the depression of the disynaptic reciprocal IPSP by GS stimulation. In order to determine the extent of this contribution, we constructed peristimulus time histograms (PSTHs) of the glutamate-induced activity in Ia inhibitory interneurones (n = 13). In Fig. 4, such PSTHs are shown for three Ia inhibitory interneurones. In the interneurone of Fig. 4A, a short-lasting facilitation followed by a long-lasting depression was observed. In the interneurone illustrated in Fig. 4B, only the short-lasting facilitation was seen, while in the unit illustrated in Fig. 4C only the depression was observed. In eight interneurones a mixed pattern of facilitation followed by inhibition was seen. Facilitation only or inhibition only was observed in each case in two interneurones. In the remaining interneurone neither facilitation nor inhibition was observed. The mean latency and duration of the facilitation (10 interneurones) was 4.2 ± 3.1 ms (range 2-11 ms) and 8.4 ± 5.4 ms (range 5.5-17 ms), respectively. The depression (10 interneurones) had, on average, a longer latency of 12.4 ± 8.2 ms (range 7.7-24 ms) and duration of 29.4 ± 30.1 ms (range 8-131 ms).
Figure 4.

The effect of GS stimulation on the activity of Ia inhibitory interneurones
A-C, peristimulus time histograms of the activity of Ia inhibitory interneurones following stimulation of the GS motor nerve (5T, 5 shocks, 300 Hz). The background activity of the interneurones was facilitated by application of glutamate through the recording electrode. Time zero corresponds to the arrival in the spinal cord of the incoming volley from the GS stimulation. The conditioning stimulation was applied every second. Bin width, 1 ms. D, intracellular recording from a Q-coupled Ia inhibitory interneurone following stimulation of the GS motor nerve (5T, 5 shocks, 300 Hz). The CDP is shown above the intracellular recording.
The effect of conditioning stimulation of the flexor nerves to PBSt or DP (5T, 5 shocks, 300 Hz) and cutaneous nerves (2T) on the glutamate-induced activity of Ia inhibitory interneurones was also investigated. Stimulation of PBSt (2 interneurones) evoked a depression at a latency of 2 ms with a duration of 2 ms. This depression was followed by an increase in the activity at about 5.5 ± 4.9 ms from the conditioning stimulation. Stimulation of DP (3 interneurones from 3 different preparations) caused an increase in the activity at a latency of 3.9 ± 2.25 ms followed by a depression at a latency of 19.9 ± 4.7 ms and lasting 34.6 ± 25.6 ms. Stimulation of cutaneous nerves (5 units from 5 different preparations) evoked a mixed pattern of excitation and inhibition. The first excitation was seen at a latency of 3.8 ± 4 ms and lasted 13 ± 10.7 ms. It was followed by a depression at a latency of 18.4 ± 10.7 ms which lasted 21.2 ± 30.8 ms. In three units this last phase was followed by an excitation at a latency of 24 ± 14.5 ms and a duration of 9.6 ± 11.5 ms. In one unit these changes were followed by an inhibition at a latency of 36 ms and a duration of 53 ms.
The depression of glutamate-induced firing of Ia interneurones following stimulation of GS was characterised further by intracellular recording from two Q-coupled interneurones. In both interneurones stimulation of GS induced an EPSP followed by an IPSP of short duration (40 ms). An example from one of the interneurones is illustrated in Fig. 4D.
These findings demonstrate that changes in the somatic excitability of Ia inhibitory interneurones induced by GS stimulation (5 shocks, 300 Hz, 5T) are not of sufficient duration to explain the long-lasting depression of the disynaptic Ia IPSP following GS stimulation as illustrated in Fig. 2. The long-lasting depression of the Ia IPSPs following GS stimulation could then be explained by a long-lasting depression in synaptic transmission from the terminals of the interneurones to the target motoneurones.
Excitability changes in the terminals of Ia inhibitory interneurones
As a possible indicator of presynaptic inhibition of the terminals of Ia inhibitory interneurones we investigated whether it was possible by conditioning nerve stimuli to induce excitability changes in these terminals. Antidromic spikes elicited in quadriceps-coupled Ia inhibitory interneurones (21 neurones from 14 preparations) by stimulation of their terminals, located in the PBSt motor nucleus, were recorded with and without prior conditioning stimulation of various muscle and cutaneous nerves.
Figure 5 illustrates a raster display of 52 extracellular recordings of the response to intraspinal stimulation from one of these interneurones. A constant intraspinal stimulus of 8 μA induced antidromic action potentials in 16 of 100 trials in the absence of conditioning nerve stimulation. When the intraspinal stimulation was preceded by a train of 5 stimuli to the GS nerve (5T, 5 stimuli at 400 Hz, conditioning-test interval 30 ms) an increase in the firing probability of the interneurone to 47 spikes in 100 trials was observed. This increase in the firing index of the unit was significant (P < 0.001). It should be noted that since the conditioning stimulation alone did not discharge the interneurone, the observed changes in the firing index cannot be explained by an increase in the excitability following the propagation of the orthodromic action potential (supranormal period, Swadlow et al. 1980) in the terminals of the interneurones.
Figure 5.
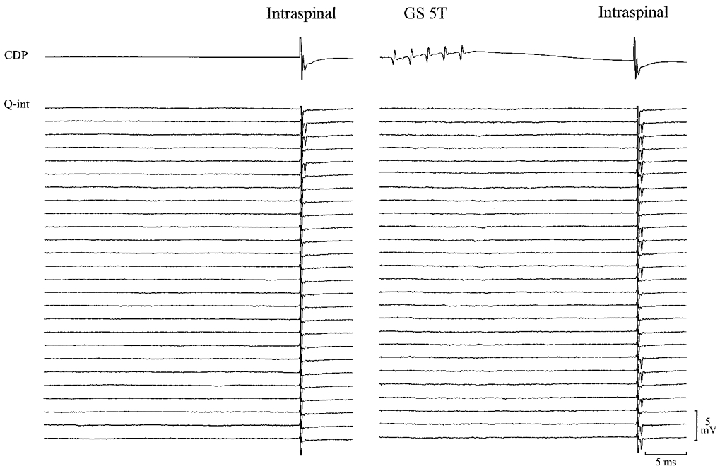
Increase in excitability of the terminals of Ia inhibitory interneurones following GS stimulation
Raster display showing the occurrence of antidromic action potentials recorded extracellularly from a Ia inhibitory interneurone with (right panel) and without (left panel) conditioning GS stimulation (5 pulses, 30 ms before; 375 Hz). The action potentials were evoked by single intraspinal pulses of constant amplitude in the PBSt motor nucleus. Conditioned and unconditioned trials were alternated at 1 Hz.
Source, threshold and time course of the excitability changes
The data from all recorded interneurones are summarised in Table 1. Conditioning stimulation of the GS at stimulation intensities of 2-5T induced a significant increase of the firing index in 12 of 14 interneurones (conditioning-test interval 25-100 ms). Weaker stimuli were tested in the same interneurone in four cases, in two of them a significant increase in the firing index was still observed at stimulation intensities between 1.6 and 2T.
Table 1.
Changes in the excitability of the terminals of Ia inhibitory interneurones.
| PBSt | GS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unit | 1−1.2T | 1.21−1.6T | 1.61−2T | 2.1−5T | >5T | 1−1.2T | 1.21−1.6T | 1.61−2T | 2.1−5T | >5T |
| i2-010995 | — | — | — | — | — | — | — | — | — | — |
| i4-230296 | — | 12* | 59** | — | — | — | — | — | — | — |
| i5-230296 | 13**(2) | — | 38** | 34** | — | — | — | 17** | — | — |
| i3-230296 | — | — | 30**(2) | — | — | — | — | — | — | — |
| i1-040396 | — | 12*(2) | — | 29**(3) | — | — | — | — | — | — |
| i2-040396 | — | 6 | 16 | 34**(2) | — | — | — | — | 17** | — |
| i2-080396 | — | — | 30**(3) | — | — | — | — | — | — | — |
| i3-270496 | — | — | — | 14(2) | — | –10 | –12 | — | 13 | 21** |
| i4-270496 | — | — | — | — | — | — | — | 31** | 31**(2) | — |
| i2-030596 | −7 | 18*(3) | 38**(2) | 48**(4) | — | — | — | — | 24** | — |
| i6-030596 | — | — | — | 28** | — | — | — | — | 14 | — |
| i1-310796 | — | — | — | — | — | — | — | — | 8*(2) | — |
| i2-070896 | — | — | — | — | — | — | — | — | 32** | — |
| i6-070896 | — | — | — | — | — | — | — | — | 5 | 8 |
| i7-070896 | — | — | — | — | — | — | — | — | — | 4 |
| i4-301296 | — | — | — | — | — | — | — | 1(2) | 22* | — |
| i1-220897 | — | — | — | — | — | — | — | 5(2) | 16*(3) | — |
| i2-220897 | — | — | — | 1 | — | — | — | — | 23*(2) | — |
| i1-260897 | — | — | — | — | — | −8 | −6 | 12*(2) | 15*(3) | — |
| i1-280897 | 12 | — | 4 | 4(2) | 5 | — | — | — | 17*(2) | — |
| i3-200298 | 7(4) | 16* | 21** | 25** | — | — | — | — | 24** | — |
| DP | SU | Sp | ||||||
|---|---|---|---|---|---|---|---|---|
| Unit | 1.2–1.6T | 1.61–2T | 2.1–5T | <5T | 1–1.6T | 1.61–2T | <2T | 5T |
| i2-010995 | — | — | — | — | — | — | — | 25** |
| i4-230296 | — | — | — | — | — | 14*(2) | — | — |
| i5-230296 | — | 24** | — | — | — | 65**(2) | — | — |
| i3-230296 | — | — | — | — | — | — | — | — |
| i1-040396 | — | — | — | — | — | — | — | — |
| i2-040396 | — | — | 1 | — | — | 21**(2) | — | — |
| i2-080396 | — | — | — | — | 5 | 16(*) | — | — |
| i3-270496 | 5 | 10(2) | 21* | 4 | — | 2 | 3 | — |
| i4-270496 | −7 | −4(3) | 34**(2) | — | — | — | 18* | — |
| i2-030596 | — | — | 22** | — | — | — | 2 | — |
| i6-030596 | — | — | — | — | — | — | — | — |
| i1-310796 | — | — | — | — | — | — | — | — |
| i2-070896 | — | — | — | — | — | — | — | — |
| i6-707896 | — | — | — | — | — | — | — | — |
| i7-070896 | — | — | — | — | — | — | — | — |
| i4-301296 | — | 2(2) | 15* | — | — | — | — | — |
| i1-220897 | — | — | — | — | — | — | — | — |
| i2-220897 | — | — | −2 | — | — | — | −2 | — |
| i1-260897 | — | — | −5 | — | — | — | 11 | — |
| i1-280897 | — | — | — | — | — | — | — | — |
| i3-200298 | — | — | — | — | — | — | — | — |
Changes in the proportion (expressed as percentage change in relation to the control firing probability) of antidromic action potentials are grouped to the nerves stimulated and range of stimulation. The interval from the conditioning stimulation was 100 ms in all cases. The number of similar observations in the same unit is presented in parenthesis. Statistical significance:
P < 0.01
P < 0.001.
Excitability changes in the terminals of the Ia inhibitory interneurones were not only produced by stimulation of GS, but also by stimulation of other extensor motor nerves, flexor motor nerves and cutaneous nerves (see Table 1). With PBSt stimulation, 9 of 12 interneurones significantly increased their firing index. The effect was largest when the intensity of stimulation was above 1.6T, but a significant increase in the firing index was often observed at stimulation intensities below 1.6T (5 of 7 interneurones). In 1 of 4 interneurones a significant increase in the firing index was even observed at stimulation intensities between 1.0 and 1.2T. This interneurone was used for the illustration in Fig. 6A. The amount of change and its significance increased steeply with higher stimulation intensities and reached a maximum around 3T. The increased excitability was well correlated to the size of the incoming volley from the conditioning nerve stimulation for stimulation intensities below 2T.
Figure 6.
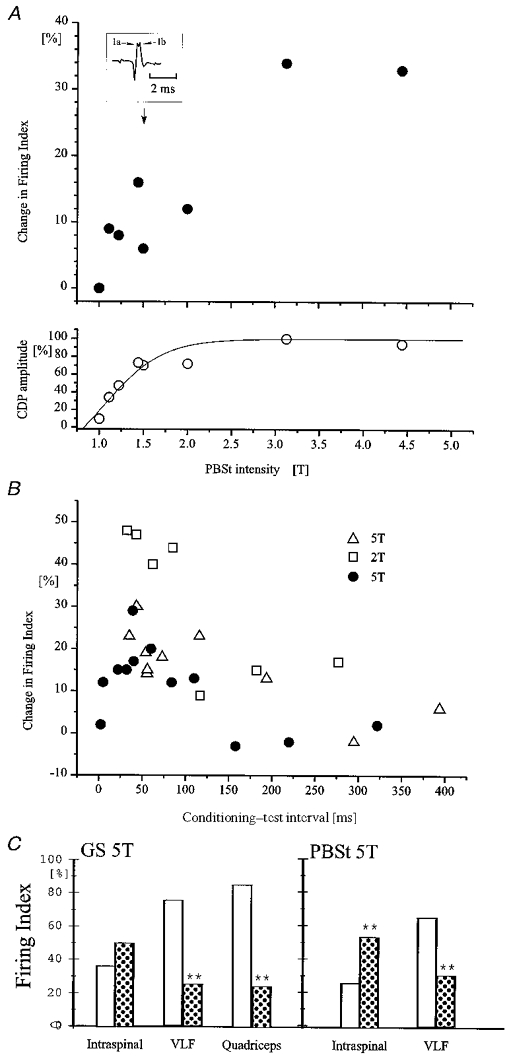
Threshold and time course of excitability changes in the terminals of Ia interneurones evoked by flexor and extensor nerve stimulation
The excitability of single Q-coupled Ia inhibitory interneurones was tested by intraspinal stimulation as in Fig. 5. A, data from a single interneurone. The conditioning stimulation was applied to the PBSt motor nerve at an interval of 45 ms (5 shocks, 200 Hz). The upper graph shows the increase of terminal excitability (expressed as the percentage change in firing index (number of action potentials evoked per number of stimuli)) as a function of the intensity of the PBSt stimulation. The lower graph shows the size of the incoming volley (expressed as a percentage of the maximal volley) recorded from the cord dorsum as a function of the PBSt stimulation intensity. The inset illustrates an example of the incoming volley at an intensity of 1.5T. Notice that a clear separation of Ia and Ib components of the incoming volley can be seen. B, data from three different interneurones (indicated by different symbols). The graph shows excitability changes (expressed as in A) in the terminals of the interneurones induced by conditioning PBSt stimulation (5T, 2T and 5T; 5 pulses, 175 Hz in all cases) as a function of the conditioning-test interval. C, data from a single interneurone in which a comparison was made of the effect of conditioning GS (left-hand columns; 5T; 5 pulses, 200 Hz, conditioning-test interval 45 ms) and PBSt stimulation (right-hand columns; 5T, 5 pulses, 200 Hz, conditioning-test interval 45 ms) on the responsiveness of Ia inhibitory interneurones to (1) intraspinal stimulation of the terminals of the interneurones (Intraspinal), (2) stimulation of descending pathways in VLF (VLF) and (3) stimulation of the Q motor nerve (Quadriceps). The open bars show the firing index obtained in the absence of the conditioning stimulation. Dotted bars show the firing index when the conditioning stimuli were also applied. Asterisks indicate the level of statistical significance in relation to the control: **P < 0.001.
Stimulation of DP similarly produced a significant increase in the firing index in 4 of 7 interneurones when the stimulation intensity was above 2T. Stimulation intensities between 1.6 and 2.0T were effective in only one of these interneurones. Stimulation of the cutaneous nerves (Su or SP) produced a significant increase in the firing index in 2 of 6 interneurones at stimulation intensities above 2T. Weaker stimulation intensities were less effective, but stimuli as weak as 1-1.6T still produced significant increases in the firing index in 4 of 5 interneurones. Stimulation of the extensor nerves to plantaris and to SmAB were also tested in one interneurone where a significant increase in the firing index of more than 10 % was seen at stimulation intensities of 5T (weaker stimuli were not investigated).
These findings demonstrate that stimulation of hindlimb nerves can increase excitability of the terminals of the Ia inhibitory interneurones. Although the changes in excitability may be produced by rather weak intensities of stimulation, (group I afferents), the major contribution seems to come from fibres recruited at intensities above 2T (group II afferents).
Figure 6B shows the time course of the change in the proportion of antidromic action potentials induced by conditioning stimulation of the PBSt motor nerve at intensities of 2T (open squares) and 5T (filled circles and open triangles) from three interneurones recorded in three different experiments. As can be seen, the conditioning stimulation of PBSt increased the excitability of these interneurones by more than 10 % at intervals between 7 and 300 ms with a maximum around 50 ms. Overall, excitability changes induced by GS stimulation were shown to last at least 100 ms in 13 interneurones. A similar long duration of the excitability changes was observed for 10 interneurones following PBSt stimulation, in five following DP stimulation, in six following sural stimulation, in one following SP stimulation and in one unit following stimulation of SmAB.
Electrotonic spread from the soma?
As pointed out originally by Jankowska & Roberts (1972b) it is possible that the observed changes in excitability of the terminals of the Ia interneurones are due to a depolarisation spreading electrotonically from the soma to the terminals. However, in Fig. 4 we provided evidence that, apart from an occasional short-lasting facilitation, the dominating effect of GS stimulation on the soma of the Q-coupled interneurones is an inhibition. In Fig. 6C it is further shown that the increased terminal excitability following GS stimulation could be seen concurrent with this inhibition at the soma. As can be seen, conditioning GS stimulation (5T, 5 shocks, 300 Hz) increased the probability of evoking an antidromic action potential by intraspinal stimulation from 36 to 50 % (P = 0.148). In contrast to this increase in terminal excitability, GS stimulation depressed the probability of orthodromic spikes induced by stimulation of the VLF (from 74 of 99 to 14 of 56 trials; P < 0.01) or by stimulation of the Q motor nerve (from 84 of 99 to 16 of 64 selected trials; P < 0.001). In the same unit, conditioning PBSt stimulation increased the probability of antidromic spikes (from 26 of 100 trials to 54 of 100 trials; P < 0.001), but decreased the response of the unit to stimulation of the VLF (from 65 of 100 to 30 of 100 trials; P < 0.001). This pattern of an excitability increase in the terminals of the interneurones versus an excitability decrease at somatic level by GS stimulation was documented in one more interneurone. In two interneurones similar findings were also obtained following stimulation of DP (5T; 5 shocks, 300 Hz).
Taken together, these data thus suggest that the excitability increase in the terminals of the Ia inhibitory interneurones following conditioning nerve stimulation is not due to spread of depolarisation from the soma. The increase in excitability of the terminals was seen even with a concurrent inhibition at the soma by the conditioning stimulation. Thus, the increase in the excitability of the terminals of the Ia inhibitory interneurones was not secondary to an electrotonic spread of excitation at the soma of the interneurones.
Depression of unitary IPSPs from single Ia inhibitory interneurones
In order to examine whether the observed changes in the excitability of the terminals of the Ia inhibitory interneurones are related to presynaptic inhibition, we recorded unitary IPSPs in PBSt motoneurones induced by the spontaneous or glutamate-induced discharge of individual Q Ia inhibitory interneurones. For this purpose, recordings from 21 quadriceps-coupled Ia inhibitory interneurones were performed simultaneously with intracellular recording from 47 PBSt motoneurones (8 preparations). Functional connections, as disclosed by spike triggered averaging, were only found for eight interneurones. On average, the eight interneurones had functional connections with only 21 % of the recorded motoneurones. Because of this limited connectivity we were able to analyse only 10 functional connections. Figure 7 demonstrates data from one of these connections. It is shown in Fig. 7B that conditioning GS stimulation (5T, 5 shocks, 250 Hz) significantly depressed the peak amplitude of the unitary IPSP (Fig. 7A and insets in Fig. 7B) in the motoneurone (averages of at least 30 traces) at an interval between 20 and 200 ms. A similar long-lasting depression of the unitary IPSP was observed in another combination, while an increase was seen in two cases and no effect in the remaining five combinations. When the conditioning stimulation was applied to the ankle flexors (DP or TA 5T) a depression of the unitary IPSP was seen in only 1 of 4 cases. When the stimulation was applied to SU (2T), a depression was observed in 3 of the 4 tested combinations.
Figure 7C demonstrates that the conditioning GS stimulation produced an IPSP in the recorded PBSt motoneurone. This was the case in all the motoneurones in which functional connections were investigated regardless of whether a depression of the unitary IPSP was seen or not. The duration of the motoneuronal IPSP was perhaps somewhat shorter than the duration of the depression of the unitary IPSP, but we cannot exclude that the depression of the unitary IPSP was caused by the hyperpolarisation or the shunting effect of the motoneuronal IPSP.
DISCUSSION
The experiments in the present study have confirmed the previous findings by Eccles et al. (1963) that conditioning stimulation of flexor group I afferents induces a long-lasting depression of the IPSPs mediated by Ia inhibitory interneurones. In addition, we have found that stimulation of the ankle extensor nerve, GS, can also induce similar long-lasting depression of the reciprocal disynaptic IPSPs. While the initial part of the depression evoked by flexors or ankle extensor nerves could be due to postsynaptic inhibition of the Ia inhibitory interneurones, such a mechanism is less likely to explain the later parts of the depression. As for the depression evoked by stimulation of flexor nerves, it is likely that presynaptic inhibition of the terminals of the Ia afferents on the Ia inhibitory interneurones has a significant contribution, since long-lasting depression of monosynaptic Ia EPSPs measured intracellularly in Ia inhibitory interneurones was observed. Stimulation of the GS motor nerve, however, had no effect on the monosynaptic EPSPs in the interneurones and presynaptic inhibition of the Ia afferents therefore cannot explain the long duration of the depression of the disynaptic IPSPs following stimulation of this extensor nerve. Stimulation of GS, DP and PBSt, as well as cutaneous nerves, however, caused a significant, long-lasting increase in the excitability of the terminals of the Ia inhibitory interneurones. However, the mechanisms responsible for these changes in excitability and the functional relation to presynaptic inhibition remain unclear.
Evidence for presynaptic inhibition of Ia afferent terminals on Ia inhibitory interneurones
Eccles et al. (1963) were of the opinion that the depression of the disynaptic Ia IPSP following flexor nerve stimulation was caused by presynaptic inhibition of the terminals of the Ia afferents on the interneurones although they did not exclude the possibility of presynaptic inhibition acting on the terminals of the interneurones. The observation in the present study that the monosynaptic Ia EPSP in the Ia inhibitory interneurones was depressed following flexor nerve stimulation with a time course similar to that seen for presynaptic inhibition of Ia EPSPs in motoneurones provides evidence in favour of this interpretation. The presynaptic origin of this depression was confirmed by the observation that the monosynaptic EPSPs evoked by stimulation of descending pathways in the VLF were not depressed by the conditioning flexor nerve stimulation. Descending axons have been demonstrated not to be depressed by presynaptic inhibition from group I afferents (Rudomin et al. 1975, 1991). There are several reasons why it could not be taken for granted that the terminals of the Ia afferents on the Ia inhibitory interneurones should be subjected to presynaptic inhibition. The interneurones, which mediate presynaptic inhibition, are divided into several different groups and subgroups, which ensures that different classes of afferents and even different branches of the same afferents may be differentially regulated. Presynaptic inhibition of the terminals of Ia afferents on motoneurones and on DSCT cells is thus mediated by different populations of interneurones (Jankowska & Padel, 1984). Recent experiments have also demonstrated that branches of the same afferent fibre may show a different magnitude of changes in excitability and even different patterns of depolarisation following peripheral nerve stimulation (Eguibar et al. 1997; Quevedo et al. 1997; Lomelíet al. 1998). Differential presynaptic inhibition of the terminals of the Ia afferents on interneurones and motoneurones would therefore not be impossible. It should also be pointed out that our data do not provide any evidence of whether similar or different interneurones are responsible for the presynaptic inhibition of the Ia afferent terminals on the two types of neurones. We therefore have to remain open to the possibility that presynaptic inhibition of Ia afferents on corresponding interneurones and motoneurones are not controlled in parallel by the brain (see further considerations below).
Possible mechanisms for the excitability changes in the terminals of the Ia interneurones
One possibility is that the observed changes in the excitability of the terminals of Ia inhibitory interneurones are caused by a somatic depolarisation spreading electrotonically to the terminals of the interneurones (Jankowska & Roberts, 1972b). This possibility is not unlikely taking the short distance between the soma and the terminals of the interneurones into consideration (11 mm). However, the present material shows that increased terminal excitability can occur at the same time as an IPSP is induced in the soma of the Ia inhibitory interneurones. We therefore feel rather confident that the observed excitability changes originate from the terminals of the interneurones.
A second possibility is that the increased excitability of the Ia inhibitory interneurones is due to preceding activity in the axon (Waxman & Swadlow, 1976; Swadlow et al. 1980). However, we may disregard this possibility, since we excluded from the present material all the cases in which the conditioning stimulation elicited orthodromic action potentials.
A third possibility is accumulation of potassium in the extracellular space following the conditioning stimuli. Although, we cannot exclude this possibility entirely, the contribution of this mechanism is probably negligible. First, the potassium accumulation mainly occurs in the dorsal areas of the spinal cord (Jiménez et al. 1987), whereas the accumulation in the motor nucleus, where we stimulated the terminals of the Ia inhibitory interneurones, is only minor (Kríz et al. 1974; Jiménez et al. 1984). Second, the time course of the potassium accumulation is much longer than the time course of the excitability changes that we have observed here (see Jiménez et al. 1984).
One remaining possibility is that the excitability changes are due to specific synaptic interactions with the terminals of the Ia inhibitory interneurones, similar to the ones on terminals of primary afferents. In that case the excitability increase of the interneuronal terminals may also be linked to presynaptic inhibition Three observations support this possibility. (1) The duration of the depression by GS stimulation of Ia IPSPs in flexor motoneurones always outlasted the duration of the IPSP in the Ia inhibitory interneurone. (2) Pentobarbital is known to facilitate GABA actions (Schmidt, 1964; Evans, 1979; Higashi & Nishi, 1982). During the present investigation (authors’ unpublished observations) we occasionally observed that application of pentobarbital seemed to increase the effect of the conditioning stimulation on the excitability of the Ia interneuronal terminals. One possible explanation of this observation is that the excitability changes are caused by activation of GABA-ergic interneurones in the same way as presynaptic inhibition of primary afferents. Primary afferent depolarisation has similarly been observed to be increased by application of barbiturates (Schmidt, 1964; Mokha et al. 1983). (3) For 6 of 10 functional connections between Ia inhibitory interneurones and PBSt motoneurones, we observed that conditioning nerve stimulation reduced the size of Ia unitary IPSPs in motoneurones. Although this may indicate the presence of presynaptic inhibition at the terminals of the interneurones, a definite conclusion cannot be reached because the conditioning stimuli also caused IPSPs in the target motoneurones.
So far there is no positive anatomical evidence for axo-axonic projections or the presence of GABA-ergic receptors on the terminals of the Ia interneurones (Fyffe, 1991 and personal communication). Recent confocal inmunohistochemical studies from Bannatyne et al. (1998) also failed to demonstrate GABA-ergic-containing neuronal elements on terminals of group II interneurones, although these terminals have been demonstrated to show changes in excitability following activation of group II pathways (Aggelopoulos et al. 1997). These observations thus raise the possibility that the increase in the excitability of the terminals of interneurones is mediated by a different mechanism than the classical GABAergic axo-axonal presynaptic inhibition.
It should also be noted that the pattern and distribution of the observed excitability changes in the interneuronal terminals differ from that of depolarisation of Ia afferent terminals. We found, firstly, that the excitability changes were equally well evoked by stimulation of flexor and extensor muscle afferents as well as cutaneous afferents. Secondly, the low threshold of the excitability changes in several cases indicated that group I afferents are capable of evoking the excitability changes, but the increase of the occurrence and size of the excitability changes at stimulation intensities above 2.0T indicates that group II afferents make a contribution.
Regardless of whether the excitability changes of the terminals of the Ia inhibitory interneurones are related to presynaptic inhibition or not, this indicates that the excitability changes are not mediated by the same neuronal system, which is responsible for the excitability changes in primary afferents. The characteristics of the excitability changes also differ from those reported for excitability changes in group II interneurones by Aggelopoulos et al. (1997). Future research will have to elucidate the exact nature of the mechanism.
Functional considerations
The demonstration of presynaptic inhibition of the terminals of Ia afferents on Ia inhibitory interneurones indicates that modulation of the transmitter release from these terminals has to be taken into account when interpreting the modulation of reciprocal Ia inhibition in non-invasive human experiments (Crone & Nielsen, 1994). Although our experiments have not elucidated whether presynaptic inhibition of Ia afferent terminals on motoneurones and Ia inhibitory interneurones are controlled in parallel, they do suggest that this is a likely possibility. If so, many of the observations of modulation of transmission in the disynaptic reciprocal Ia inhibitory pathway during extension-flexion movements (Tanaka, 1974; Iles, 1986; Crone et al. 1987, 1989), co-contraction of antagonistic muscles (Nielsen & Kagamihara, 1992) and walking (Capaday et al. 1990; Petersen et al. 1999) may be explained by modulation of presynaptic inhibition of the terminals of the Ia afferents or (possibly) even the terminals of the interneurones themselves.
Since it is unclear whether our observations of excitability changes in the terminals of the Ia inhibitory interneurones are associated to presynaptic control of their transmission, it is not possible to make any convincing functional interpretation of those data. However, our data suggest the possible existence of an interneuronal system involved in the control of the efficacy of synapses of interneurones in the spinal cord. If so, this interneuronal system would appear to be separate from the system involved in presynaptic inhibition of primary afferents (cf. the different source and distribution characteristics of PAD and the excitability changes of the terminals of the interneurones) and the brain would thus be provided with a means of modulating transmitter release from primary afferents and interneurones separately. It may even be speculated that such modulatory effects could be involved not only in on-going sensory-motor control, but also in long-term changes in synaptic efficacy which may be associated with motor learning.
Acknowledgments
We wish to express our gratitude to Gilles R. Detillieux from the SCRC, University of Manitoba for his assistance with the use of the SCRC analysis system. We would also like to thank Lillian Grøndahl, Conni Temdrup, Egil Gudbrandsen and the late Jan Nielsen for technical support. We are grateful to Allan Djorup for computational support. The study was supported by the Danish Health Research Council, The NOVO Nordisk Foundation and the Danish Society for Multiple Sclerosis. M.E.-D. received a Postdoctoral Scholarship from CONACyT (Mexico).
References
- Aggelopoulos NC, Chakrabarty S, Edgley SA. Evoked excitability changes at the terminals of midlumbar premotor interneurons in the cat spinal cord. Journal of Neuroscience. 1997;17:1512–1518. doi: 10.1523/JNEUROSCI.17-04-01512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford S, Christensen J, Grillner S. Presynaptic GABAa and GABAb receptor-mediated phasic modulation in axons of spinal motor interneurons. European Journal of Neuroscience. 1991;3:107–117. doi: 10.1111/j.1460-9568.1991.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Brooks VB, editor. Handbook of Physiology, section 1, The Nervous System, Motor Control. Vol. 2. Bethesda, MD, USA: American Physiological Society; 1981. pp. 509–595. [Google Scholar]
- Bannatyne B, Riddell J, Maxwell D, Scott D. A confocal and electron microscopic examination of the terminals of midlumbar premotor interneurones in the feline spinal cord. The Journal of Physiology. 1998;509.P:171.P. [Google Scholar]
- Capaday C, Cody FW, Stein RB. Reciprocal inhibition of soleus motor output in humans during walking and voluntary tonic activity. Journal of Neurophysiology. 1990;64:607–616. doi: 10.1152/jn.1990.64.2.607. [DOI] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Jespersen B, Nielsen J. Reciprocal Ia inhibition between ankle flexors and extensors in man. The Journal of Physiology. 1987;389:163–185. doi: 10.1113/jphysiol.1987.sp016652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Spinal mechanisms in man contributing to reciprocal inhibition during voluntary dorsiflexion of the foot. The Journal of Physiology. 1989;416:255–272. doi: 10.1113/jphysiol.1989.sp017759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Central control of disynaptic reciprocal inhibition in humans. Acta Physiologica Scandinavica. 1994;152:351–363. doi: 10.1111/j.1748-1716.1994.tb09817.x. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. The Journal of Physiology. 1957;137:22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Schmidt RF, Willis W. The location and mode of action of presynaptic inhibitory pathways on to group I afferent fibres from muscle. Journal of Neurophysiology. 1963;26:506–522. [Google Scholar]
- Eguibar JR, Quevedo J, Rudomin P. Selective cortical and segmental control of primary afferent depolarization of single muscle afferents in the cat spinal cord. Experimental Brain Research. 1997;113:411–430. doi: 10.1007/pl00005595. [DOI] [PubMed] [Google Scholar]
- Eide E. Stimulation and recording with closely spaced microelectrodes. Acta Physiologica Scandinavica. 1971;82:4–5A. doi: 10.1111/j.1748-1716.1971.tb04986.x. [DOI] [PubMed] [Google Scholar]
- Enríquez-Denton M, Nielsen JB, Perrault M-C, Morita H, Petersen N, Hultborn H. Changes in the excitability of the terminals of Ia inhibitory interneurones in the cat spinal cord. The Journal of Physiology. 1997;501.P:40.P. [Google Scholar]
- Enríquez-Denton M, Perrault M-C, Nielsen J, Morita H, Petersen N, Hultborn H. Presynaptic inhibition along the disynaptic reciprocal Ia inhibitory pathway in the cat. Society of Neuroscience Abstracts. 1996;340.4 (Abstract) [Google Scholar]
- Evans RH. Potentiation of the effects of GABA by pentobarbitone. Brain Research. 1979;171:113–120. doi: 10.1016/0006-8993(79)90736-4. [DOI] [PubMed] [Google Scholar]
- Frank K. Basic mechanisms of synaptic transmission in the central nervous system. Institute of Radio Engineers Transactions on Medical Electronics. 1959;6:85–98. [Google Scholar]
- Fyffe RE. Glycine-like immunoreactivity in synaptic boutons of identified inhibitory interneurons in the mammalian spinal cord. Brain Research. 1991;547:175–179. doi: 10.1016/0006-8993(91)90590-r. [DOI] [PubMed] [Google Scholar]
- Higashi H, Nishi S. Effect of barbiturates on the GABA receptor of cat primary afferent neurones. The Journal of Physiology. 1982;332:299–314. doi: 10.1113/jphysiol.1982.sp014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Santini M. Convergence on interneurones mediating the reciprocal Ia inhibition of motoneurones. II. Effects from segmental flexor reflex pathways. Acta Physiologica Scandinavica. 1976;96:351–367. doi: 10.1111/j.1748-1716.1976.tb10205.x. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Jankowska E, Lindstrom S. Recurrent inhibition of interneurones monosynaptically activated from group Ia afferents. The Journal of Physiology. 1971;215:613–636. doi: 10.1113/jphysiol.1971.sp009488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF. Reciprocal inhibition during agonist and antagonist contraction. Experimental Brain Research. 1986;62:212–214. doi: 10.1007/BF00237419. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Progress in Neurobiology. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, McCrea D, Rudomin P, Sykova E. Observations on neuronal pathways subserving primary afferent depolarization. Journal of Neurophysiology. 1981;46:506–516. doi: 10.1152/jn.1981.46.3.506. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Padel Y. On the origin of presynaptic depolarization of group I muscle afferents in Clarke's column in the cat. Brain Research. 1984;295:195–201. doi: 10.1016/0006-8993(84)90967-3. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Roberts WJ. An electrophysiological demonstration of the axonal projections of single spinal interneurones in the cat. The Journal of Physiology. 1972a;222:597–622. doi: 10.1113/jphysiol.1972.sp009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Roberts WJ. Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. The Journal of Physiology. 1972b;222:623–642. doi: 10.1113/jphysiol.1972.sp009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez I, Rudomin P, Solodkin M. Mechanisms involved in the depolarization of cutaneous afferents produced by segmental and descending inputs in the cat spinal cord. Experimental Brain Research. 1987;69:195–207. doi: 10.1007/BF00247042. [DOI] [PubMed] [Google Scholar]
- Jimenez I, Rudomin P, Solodkin M, Vyklicky L. Specific and nonspecific mechanisms involved in generation of PAD of group Ia afferents in cat spinal cord. Journal of Neurophysiology. 1984;52:921–940. doi: 10.1152/jn.1984.52.5.921. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Swadlow HA, Waxman SG, Brill MH. Variation in conduction velocity during the relative refractory and supernormal periods: a mechanism for impulse entrainment in central axons. Experimental Neurology. 1979;65:230–236. doi: 10.1016/0014-4886(79)90263-2. [DOI] [PubMed] [Google Scholar]
- Kríz N, Sykova E, Ujec E, Vyklický L. Changes in extracellular potassium concentration induced by neuronal activity in the spinal cord of the cat. The Journal of Physiology. 1974;238:1–15. doi: 10.1113/jphysiol.1974.sp010507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DPC. Facilitation and inhibition of spinal motoneurons. Journal of Neurophysiology. 1946a;9:421–438. doi: 10.1152/jn.1946.9.6.421. [DOI] [PubMed] [Google Scholar]
- Lloyd DPC. Integrative pattern of excitation and inhibition in two-neuron reflex arcs. Journal of Neurophysiology. 1946b;9:439–444. doi: 10.1152/jn.1946.9.6.439. [DOI] [PubMed] [Google Scholar]
- Lomeli J, Quevedo J, Linares P, Rudomin P. Local control of information flow in segmental and ascending collaterals of single afferents. Nature. 1998;395:600–604. doi: 10.1038/26975. [DOI] [PubMed] [Google Scholar]
- McDearmid JR, Scrymgeour-Wedderburn JF, Sillar KT. Aminergic modulation of glycine release in a spinal network controlling swimming in Xenopus laevis. The Journal of Physiology. 1997;503:111–117. doi: 10.1111/j.1469-7793.1997.111bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokha SS, McMillan JA, Iggo A. Dorsal root potentials in the cat: effects of bicuculline. Brain Research. 1983;259:313–318. doi: 10.1016/0006-8993(83)91265-9. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. The regulation of disynaptic reciprocal Ia inhibition during co-contraction of antagonistic muscles in man. The Journal of Physiology. 1992;456:373–391. doi: 10.1113/jphysiol.1992.sp019341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Morita H, Nielsen J. Modulation of reciprocal inhibition between ankle extensors and flexors during walking in man. The Journal of Physiology. 1999;520:605–61. doi: 10.1111/j.1469-7793.1999.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo J, Eguibar JR, Lomeli J, Rudomin P. Patterns of connectivity of spinal interneurons with single muscle afferents. Experimental Brain Research. 1997;115:387–402. doi: 10.1007/pl00005709. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Jimenez I, Enriques M. Effects of group I flexors on heterosynaptic facilitation of monosynaptic reflexes produced by Ia and descending inputs. Experimental Brain Research. 1991;85:93–102. doi: 10.1007/BF00229990. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Nunez R, Madrid J. Modulation of synaptic effectiveness of Ia and descending fibres in the cat spinal cord. Journal of Neurophysiology. 1975;38:1181–1195. doi: 10.1152/jn.1975.38.5.1181. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Romo R, Mendell L. Presynaptic Inhibition and Neural Control. New York: Oxford University Press; 1998. [Google Scholar]
- Schmidt RF. The pharmacology of presynaptic inhibition. Progress in Brain Research. 1964;12:119–134. doi: 10.1016/s0079-6123(08)60620-2. [DOI] [PubMed] [Google Scholar]
- Schmidt RF. Presynaptic inhibition in the vertebrate central nervous system. Ergebnisse die Physiologie. 1971;63:20–101. doi: 10.1007/BFb0047741. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Kocsis JD, Waxman SG. Modulation of impulse conduction along the axonal tree. Annual Review of Biophysics and Bioengineering. 1980;9:143–179. doi: 10.1146/annurev.bb.09.060180.001043. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Waxman SG, Rosene DL. Latency variability and the identification of antidromically activated neurons in mammalian brain. Experimental Brain Research. 1978;32:439–443. doi: 10.1007/BF00238715. [DOI] [PubMed] [Google Scholar]
- Tanaka R. Reciprocal Ia inhibition during voluntary movements in man. Experimental Brain Research. 1974;21:529–540. doi: 10.1007/BF00237171. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Swadlow HA. Morphology and physiology of visual callosal axons: evidence for a supernormal period in central myelinated axons. Brain Research. 1976;113:179–187. doi: 10.1016/0006-8993(76)90017-2. [DOI] [PubMed] [Google Scholar]


