Abstract
Interleukin (IL)-6 is an important mediator of the host response to disease and has been proposed, largely based upon circumstantial evidence, as the principal endogenous circulating pyrogen responsible for activating CNS mechanisms in fever during infection and inflammation. In the present investigation, we studied the role of peripheral IL-6 in fever and its relationship with IL-1, itself an important endogenous pyrogen and a potent stimulus of IL-6 production.
Injection of lipopolysaccharide (LPS) into a sterile, subcutaneous air pouch (i.po.) in rats evoked an increase in body temperature which peaked at 3 h, and which was abolished in animals pretreated (intraperitoneally) with IL-6 antiserum.
The increase in body temperature was accompanied by a significant elevation in concentrations of (immunoreactive) IL-1 and IL-6 at the site of inflammation (pouch), but only IL-6 in the circulation and cerebrospinal fluids. We propose that much of the circulating IL-6 originates at the site of inflammation, since injection of human recombinant (hr)IL-6 (i.po.) was detected (10 min after the injection) in the plasma using an ELISA specific for human IL-6.
However, despite the relatively high concentration of IL-6 injected (25 μg kg−1, i.po.), this cytokine had no effect on body temperature when injected alone, but did induce fever when co-injected with a non-pyrogenic dose (when given alone) of IL-1β, and exacerbated the fever to a pyrogenic dose of IL-1β.
The results from the present study demonstrate that IL-6 is a circulating endogenous pyrogen during LPS-induced fever, which acts in concert with IL-1β at the local site of inflammation, before entering the circulation. Circulating IL-6 can then activate CNS mechanisms resulting in the development of the febrile response during disease.
Fever can be induced in experimental animals by administration of lipopolysaccharide (LPS), a cell wall component of Gram-negative bacteria, or by exogenous administration of recombinant cytokines (Kluger, 1991). Considerable evidence indicates that central action of the proinflammatory cytokines, such as interleukin (IL)-1 and IL-6, the production of which are induced by systemic injection of LPS, is a key event in the generation of fever (Kluger, 1991; Klir et al. 1994; Hopkins & Rothwell, 1995; Luheshi et al. 1996).
There remains, however, controversy over the peripheral signal to the brain following injection of exogenous LPS. Much evidence now suggests that brain cytokines are induced in response to a neural afferent signal, for example, the vagus nerve from the peritoneal cavity (Watkins et al. 1995), to activate host defence responses such as fever. Other evidence also exists showing that IL-1 produced in the periphery can signal the brain directly (see Kluger, 1991) by gaining access from the circulation via a number of transport mechanisms, or through regions of the CNS not possessing an effective blood-brain barrier (BBB) (Elmquist et al. 1997). However, failure to detect biologically significant concentrations of IL-1 in the circulation of febrile experimental animals (Luheshi et al. 1997; Miller et al. 1997b) or humans (Damas et al. 1992; Engel et al. 1994) suggests that IL-1 is unlikely to play such a role. This question was addressed in part by our recent studies, showing that in the periphery IL-1 acts locally at the site of inflammation to induce the synthesis and release of a second mediator, IL-6, into the circulation (Luheshi et al. 1997; Miller et al. 1997a,b).
Interleukin-6 is the only inflammatory cytokine that can be measured in significant quantities in the circulation during fever (Nijsten et al. 1987; LeMay et al. 1990a,c; Luheshi et al. 1997; Miller et al. 1997b). The rise in circulating and cerebrospinal fluid (CSF) IL-6 concentrations correlates well with the development of fever in man (Nijsten et al. 1987; Houssiau et al. 1988) and experimental animals (LeMay et al. 1990b,c; Roth et al. 1993). We, and others, have shown that this rise in circulating IL-6 concentrations is directly dependent on IL-1 (LeMay et al. 1990b; Klir et al. 1994; Luheshi et al. 1996; Miller et al. 1997a), which is a potent inducer of IL-6 production, both in vitro (Sironi et al. 1989) and in vivo (Shalaby et al. 1989). Circulating IL-6 can enter the brain via an active transport mechanism (Banks et al. 1994) and injection of IL-6 directly into the brain induces fever (Le May et al. 1990c; Klir et al. 1993; Lenczowski et al. 1999). In addition, mice in which the IL-6 gene has been deleted (IL-6 knockout mice) fail to develop fever in response to systemic injection of LPS or IL-1 (Chai et al. 1996), both potent inducers of IL-6 production in vivo, while a central injection of IL-6 results in a significant rise in body temperature in these same animals (Chai et al. 1996). These data, although circumstantial, strongly suggest that IL-6 is the major circulating pyrogen during inflammation or injury, and that it acts on the brain to induce fever. However, to date, no direct evidence has been provided to confirm the role of peripheral IL-6 in the induction of fever, and systemic administration of a dose of IL-6, equivalent to the circulating concentrations of IL-6 induced by LPS, failed to affect body temperature (LeMay et al. 1990c; Chai et al. 1996; Wang et al. 1997).
Using a model of localised inflammation in rats, the aim of the present study was to clarify the role of circulating IL-6 in fever, by neutralising its action in the periphery using an antiserum raised against rat IL-6. We also investigated the source, and kinetics, of circulating IL-6 and assessed further its relationship with IL-1 in vivo during fever.
METHODS
Male Sprague-Dawley rats (Charles River, Kent, UK) (250-350 g) were used in all experiments. Animals were housed in a controlled environment at an ambient temperature of 21 ± 2°C on a 12 h:12 h light:dark cycle (light on from 08.00 to 20.00). Food (pelleted rat chow, Beekay International, UK) and water were provided ad libitum. All procedures were performed under the UK Animals (Scientific Procedures) Act 1986.
Measurement of body temperature
Core body temperatures of rats were measured by remote biotelemetry (Data Quest IV system, Data Sciences, St Paul, MN, USA), using pre-calibrated radiotransmitters (TA10TA-F40, Data Sciences) implanted intraperitoneally (i.p.) whilst animals were under halothane (Fluorothane, Zeneca, Cheshire, UK) anaesthesia (3 % in oxygen). Animals were housed individually 24 h before the experiments. Transmitter output frequency (Hz) was monitored, at 10 min intervals, by an antenna mounted in a receiver board situated beneath the cage of each animal, and the data were logged into a peripheral processor (BCM 100, Data Sciences) connected to a personal computer. Six days before the start of the experiment, under halothane anaesthesia, a subcutaneous air pouch was formed, as described previously (Edwards et al. 1981). Briefly, animals were anaesthetised (halothane) and 20 ml of sterile air was injected into the subcutaneous tissue of the dorsal midline, caudal to the scapulae. Three days after the initial pouch formation, animals were briefly re-anaesthetised and the air pouches were reinflated with a further 10 ml of sterile air, to maintain open cavities. On day 6, pyrogen or vehicle was injected directly into the air pouches of lightly restrained (hand held), conscious animals.
Materials and treatments
Materials
The following injected substances were obtained from the sources indicated. Purified LPS (Escherichia coli, serotype: 0128:B12, Sigma, UK), dissolved in 0.9 % sterile pyrogen-free saline (100 μg ml−1) and injected intrapouch (i.po.) at a dose of 100 μg kg−1. Sheep anti-rat IL-6 serum (1.8 ml, NIBSC, South Mimms, Potters Bar, Herts, UK) or normal (pre-immune) sheep serum (NSS, NIBSC; for control injections), injected i.p. The anti-IL-6 serum recognises both recombinant (E. coli-derived) and natural rat IL-6, but does not cross-react with rat recombinant (rr)IL-1α, IL-1β or TNF-α (Rees et al. 1999). Human recombinant (hr)IL-6 (glycosylated, CHO-derived, specific activity 100000 international units (i.u.) per microgram, was a generous gift from the ARES-SERONO Group). RrIL-6 (E. coli-derived, non-glycosylated, specific activity 250 000 i.u. μg−1, as measured in a B9 mouse hybridoma bioassay, NIBSC). RrIL-1β (E. coli-derived, specific activity 100 000 i.u. μg−1, NIBSC). The cytokines were diluted in pyrogen-free saline containing 0.1 % bovine serum albumin (BSA, fatty acid free, low endotoxin, Sigma, UK) on the day of the experiment, unless otherwise stated, and injected i.po. (see below). The endotoxin content of the recombinant cytokines was <4 ng (40 i.u.) mg−1 of protein, as measured in a Limulus Amoebocyte Lysate test (European Pharmacopaeia, 1999).
Experimental protocol
Experiment 1
Animals were injected i.po. with either LPS (100 μg kg−1) or vehicle (sterile, pyrogen-free saline, 1 ml kg−1) and core body temperature monitored for 5 h after injection. Pouch fluid, plasma and CSF samples were collected from groups of animals (n = 5 per treatment per time point), under terminal anaesthesia (with halothane), before (0 h) or 1, 2, 3 and 5 h after injection of LPS or vehicle. Sampling of the inflammatory exudate within the pouch was achieved by lavaging the pouch with 1 ml of sterile saline. The lavage fluid was quickly aspirated, centrifuged (3000 g, 4°C, 10 min), and the resultant supernatant collected and stored at -70°C until assayed. Blood was collected by cardiac puncture, into sterile tubes containing pyrogen-free heparin (10 U ml−1) and centrifuged (5300 g, 4°C, 10 min). Plasma was stored at -70°C until assayed. CSF (∼80 μl) was drawn from the cisterna magna, through a 25 gauge hypodermic needle connected with plastic PE-10 tubing to a 1 ml syringe, ensuring that no contamination with blood occurred. Samples were centrifuged (3000 g, 4°C, 10 min), supernatants collected and stored at -70°C. Samples containing any visual trace of blood, following centrifugation (3000 g, 4°C, 10 min), were discarded from all subsequent analyses. Samples were analysed for IL-1β and IL-6 using specific ELISAs (NIBSC) (Safieh-Garabedian et al. 1995; Rees et al. 1999).
Experiment 2
Naive animals (n = 5) were injected i.po. with hrIL-6 (25 μg kg−1) and blood samples collected and assayed for human IL-6 using a human IL-6-specific ELISA (Taktak et al. 1991). Blood samples (0.4 ml) were collected at various times after injection of hrIL-6 (10 min to 5 h) via an indwelling jugular catheter, implanted (under halothane anaesthesia) 3 days prior to experimentation, as described previously (Turnbull & Rivier, 1996). Body temperature was monitored via remote radiotelemetry for the duration of the experiment (5 h). The collected volume of blood was replaced with an equal volume of pyrogen-free heparinised saline (10 U heparin (ml saline)−1) immediately following each sampling procedure. Blood was collected into sterile, pyrogen-free tubes containing pyrogen-free heparin (10 U ml−1) and centrifuged 3000 g, 4°C, 10 min). Plasma was stored at -70°C for cytokine analyses. At the end of the experiment (5 h), animals were killed by cervical dislocation and pouch fluid extracted (see above), centrifuged (3000 g, 4°C, 10 min), and the supernatants stored at -70°C until assayed.
In other animals, hrIL-6 (25 μg kg−1) or vehicle (saline, 1 ml kg−1) was injected i.po. and core body temperature monitored by remote radiotelemetry. Groups of animals (n = 5) were killed 1.5, 3 or 5 h after injection (i.po.) of hrIL-6 or vehicle and plasma, and pouch fluid and CSF collected (see above).
Experiment 3
Naive animals, previously implanted with abdominal radiotransmitters, were injected (i.p.) with 1.8 ml sheep anti-IL-6 serum, NSS or saline. Four hours later, animals were injected with LPS (100 μg kg−1, i.po.), or vehicle (saline, 1 ml kg−1, i.po.), and body temperature was monitored for 5 h after injection of LPS or vehicle. At the end of the experiment (5 h), animals were placed under terminal anaesthesia (with halothane) and blood samples collected (see above), centrifuged (5300 g, 4°C, 10 min), and the plasma stored at -70°C until assayed.
Experiment 4
RrIL-6 (0.8-25 μg kg−1) or vehicle (pyrogen-free saline containing 0.1 % BSA, 1 ml kg−1) was injected i.po. and core temperature was monitored, for a minimum of 1 h before and 24 h after injection, by remote radiotelemetry.
Other animals were injected (i.po.) with either rrIL-6 (0.8-25 μg kg−1) and together with rrIL-1β (0.03 or 0.3 μg kg−1), or rrIL-6 (25 μg kg−1, i.po.) 1 h before rrIL-1β (0.3 μg kg−1, i.po.). At the end of the experiments, animals were killed by cervical dislocation.
ELISAs
IL-1β and IL-6 concentrations in the various biological fluids (see above) were quantified by specific ELISAs as described previously (Safieh-Garabedian et al. 1995; Rees et al. 1999). At the concentrations used in this study, human IL-6 did not cross-react with rat IL-6 in the respective ELISAs (data not shown).
Statistical and data analysis
All data are presented as means ±s.e.m. for the number of animals given. The body temperature responses were plotted as abdominal temperature-time curves and data analysed using either analysis of variance (ANOVA) followed by a Tukey-Kramer Multiple Comparisons post hoc test for differences between more than two groups, or Student's t test for differences between two groups. Cytokine concentrations, at the various time points and in the various biological fluids, were compared with the corresponding concentrations measured in control animals, at the same time and in the same biological fluid (please refer to Results for more detail), using Student's t test. Where cytokine levels were undetectable, samples were assigned a value equivalent to the detection limit of the assay. A two-tailed probability P < 0.05 was considered statistically significant.
RESULTS
Experiment 1
LPS injected into a subcutaneous air pouch (100 μg kg−1, i.po.) induced an increase in body temperature which peaked at 3 h (38.7 ± 0.1°C compared with 37.1 ± 0.1°C for vehicle-injected controls, P < 0.001, Student's t test) and remained elevated at 5 h (38.0 ± 0.2°C compared with 37.1 ± 0.1°C for controls, P < 0.001, Student's t test). IL-1β was undetectable in pouch fluid (< 19 pg ml−1), plasma (< 38 pg ml−1) and CSF (< 38 pg ml−1) of animals injected with vehicle (saline control), at all time points (data not shown). In contrast, IL-1β was produced in the pouch (3467 ± 1190 pg ml−1), within 1 h of injection (i.po.) of LPS, and concentrations peaked (41 900 ± 7935 pg ml−1) at the 5 h time point (Fig. 1A). IL-1β was undetectable in both the plasma (Fig. 1B) and CSF (< 38 pg ml−1) of animals injected intrapouch with LPS. IL-6 was detected locally (pouch) and in the plasma of animals injected with LPS (Fig. 1A and B). Pouch IL-6 was detected (1 h) before plasma IL-6 (2 h), both of which preceded any significant increase in body temperature. Pouch IL-6 concentrations peaked 2 h after LPS (20 880 ± 4 157 pg ml−1, Fig. 1A) and were 35-fold greater than peak plasma concentrations after 2 h (411 ± 87 pg ml−1, Fig. 1B). IL-6 increased significantly in the CSF in response to LPS administration at 3 h (150 ± 19 pg ml−1, P < 0.001, Student's t test, Fig. 1C).
Figure 1. Concentrations of immunoreactive interleukin (IL)-1β and IL-6 at the site of inflammation, in the plasma, and in the CSF after intrapouch (i.po.) injection of lipopolysaccharide (LPS).
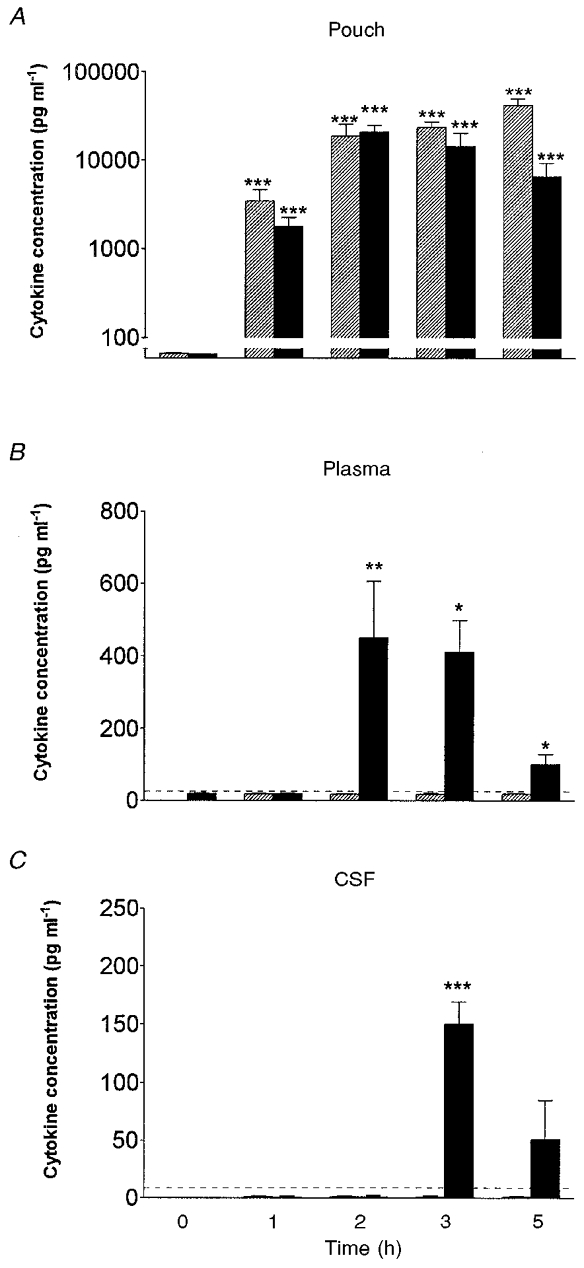
Kinetics of the appearance of immunoreactive IL-1β (hatched columns) and IL-6 (filled columns) at: the site of injection (air pouch lavage fluid, A), in plasma (B), and in the CSF of rats (C) (n = 5) at various time points after injection of LPS (100 μg kg−1, i.po.). Data are expressed as means ±s.e.m. IL-1β and IL-6 were undetectable in pouch fluid, plasma and CSF of vehicle-injected (saline control) animals (data not shown). *P < 0.05, **P < 0.01, ***P < 0.001 (compared with control animals, Student's t test); horizontal dashed line indicates assay detection limit.
Experiment 2
Injection (i.po.) of hrIL-6 (25 μg kg−1) had no effect on body temperature for the duration of the experiment (3 h, 37.1 ± 0.1°C compared with 37.1 ± 0.1°C for vehicle-injected controls, P > 0.05, Student's t test). Injection of hrIL-6 i.po. resulted in a significant increase in hrIL-6 in the plasma at 10 min (3225 ± 57 pg ml−1) which peaked at 40 min (5716 ± 550 pg ml−1) and decreased thereafter, but was still present at the 5 h time point (181 ± 132 pg ml−1; Fig. 2). Comparison of the kinetics of plasma IL-6 in relation to local IL-6 revealed that 1.5 h after hrIL-6 injection (i.po.), 107-fold more hrIL-6 was present in the pouch than in the plasma. This relationship was maintained throughout the duration of the experiment (data not shown). At all time points investigated, hrIL-6 was undetectable (< 38 pg ml−1) in the CSF of animals injected i.po. with hrIL-6 alone and was undetectable in the pouch fluid, plasma and CSF of vehicle-injected controls.
Figure 2. Circulating IL-6 derives from the site of inflammation.
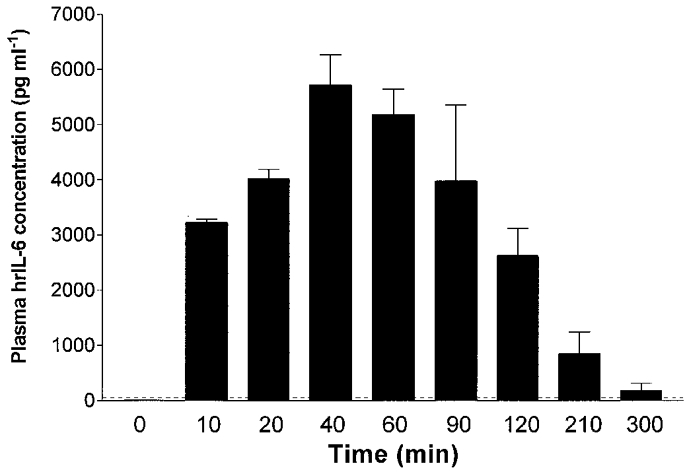
Kinetics of immunoreactive human IL-6 in the plasma of rats (n = 5) injected with human recombinant IL-6 (25 μg kg−1, i.po). Data are expressed as means ±s.e.m. Human IL-6 was undetectable in the plasma of vehicle-injected animals (n = 3, data not shown). IL-6 was measured using an immunoassay specific for human IL-6 that did not cross-react with rat IL-6; horizontal dashed line indicates assay detection limit.
Experiment 3
Animals injected (i.p.) with NSS had body temperature responses no different from animals injected (i.p.) with saline (data not shown; P > 0.05, ANOVA) for the duration of the study (9 h in total). Injection (i.p.) of 1.8 ml sheep-anti-rat IL-6 serum slightly decreased body temperature for the first 60 min after injection (sheep anti-IL-6 serum, 37.3 ± 0.2°C compared with NSS, 37.7 ± 0.2°C; saline, 37.6 ± 0.1°C, data not shown). Thereafter, body temperature returned to pre-injection levels, and was similar to that of control animals, for the remaining 2 h prior to injection (i.po.) of LPS (Fig. 3). Injection of LPS (100 μg kg−1, i.po.) in rats pretreated with NSS (i.p.) or saline (i.p.) resulted in a fever which was maximal 3 h later (NSS + LPS, 38.4 ± 0.1°C, Fig. 3; saline + LPS, 38.3 ± 0.2°C, data not shown; compared with NSS + saline, 37.2 ± 0.1°C, Fig. 3; P < 0.001, ANOVA). Pretreatment with sheep anti-IL-6 serum (i.p.) had no effect on the body temperature response to local (i.po.) vehicle (saline 3 h, sheep anti-IL-6 serum + saline, 37.3 ± 0.1°C, Fig. 3), yet abolished the increase in body temperature to local (i.po.) LPS for the duration of the 5 h period (3 h, sheep anti-IL-6 serum + LPS, 37.3 ± 0.1°C). IL-6 was detected in the plasma of animals injected with saline (i.p.) + LPS (i.po.) (98 ± 20 pg ml−1, n = 5), or NSS (i.p.) + LPS (i.po.) (158 ± 26 pg ml−1, n = 4) at the 5 h time point. In contrast, IL-6 was undetectable in the plasma (5 h, < 38 pg ml−1) of animals injected with NSS (i.p.) + saline (i.po.) (n = 4), or sheep anti-IL-6 serum (i.p.) + LPS (i.po.) (n = 5).
Figure 3. Intraperitoneal (i.p.) treatment of rats with sheep anti-IL-6 serum abolishes the febrile response to localised (i.po.) injection of LPS.
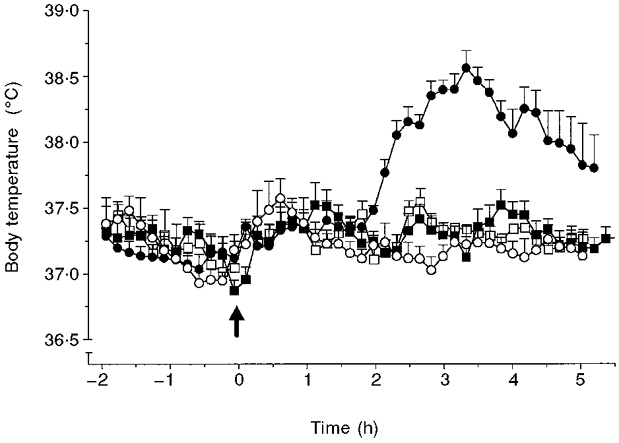
Injection (at time -4 h) of sheep anti-IL-6 serum (1.8 ml, i.p., □, ▪) or pre-immune serum (NSS, ○, •), followed by injection of LPS (100 μg kg−1, filled symbols) or vehicle (saline, 1 ml kg−1, open symbols) into a subcutaneous air pouch, at time 0 h (arrow). Baseline temperatures of all experimental groups were not significantly different from each other at time 0 h (P > 0.05, ANOVA). The results are presented as time curves (means ±s.e.m.) of core body temperature (°C) for: NSS i.p., vehicle i.po. (○, n = 8); NSS i.p., LPS i.po. (•, n = 9); anti-IL-6 serum i.p., vehicle i.po. (□, n = 5); anti-IL-6 serum i.p., LPS i.po. (▪, n = 10).
Experiment 4
None of the doses of rrIL-6 tested (0.8-25 μg kg−1, i.po.) elicited significant fever up to 5 h post-injection (0.8-8 μg kg−1, data not shown; P > 0.05, ANOVA; 25 μg kg−1 i.po., Fig. 4A–C). Injection of rrIL-1β (0.03 μg kg−1, i.po.) also had no effect on body temperature when compared with vehicle-injected animals (Fig. 4A). A separate group of animals injected with rrIL-1β (0.3 μg kg−1, i.po.) developed fever 1-2 h after injection, and this peaked at 2.5 h (38.3 ± 0.1°C, compared with 37.1 ± 0.1°C in vehicle-injected controls, P < 0.001, Fig. 4B), and returned to baseline levels by 6 h after injection (data not shown). Co-administration of IL-6 (25 μg kg−1) with a non-pyrogenic dose (when given alone) of IL-1β (0.03 μg kg−1, i.po.) evoked a significant rise in body temperature which commenced 0.5-1 h post-injection and peaked at 2 h (38.0 ± 0.1°C compared with 37.0 ± 0.1°C in vehicle-injected controls; Fig. 4A). Doses of IL-6 less than 2.5 μg kg−1, co-injected (i.po.) with a non-pyrogenic dose of IL-1β, did not evoke fever (data not shown). Co-injection of IL-6 (25 μg kg−1, i.po.) and a pyrogenic dose of IL-1β (0.3 μg kg−1, i.po.) increased the magnitude of the febrile response compared with injection of IL-1β (0.3 μg kg−1, i.po.) alone (Fig. 4B). Co-injection of IL-6 at doses below 2.5 μg kg−1 (i.po.) with a pyrogenic dose of IL-1β (0.3 μg kg−1, i.po.) did not potentiate the response to IL-1β (data not shown). Injection of IL-6 (25 μg kg−1, i.po.) 1 h before injection of a pyrogenic dose of IL-1β (0.3 μg kg−1, i.po.) increased the magnitude, but not the duration, of the febrile response to IL-1β (Fig. 4C).
Figure 4. IL-6 potentiates IL-1β-evoked fever.
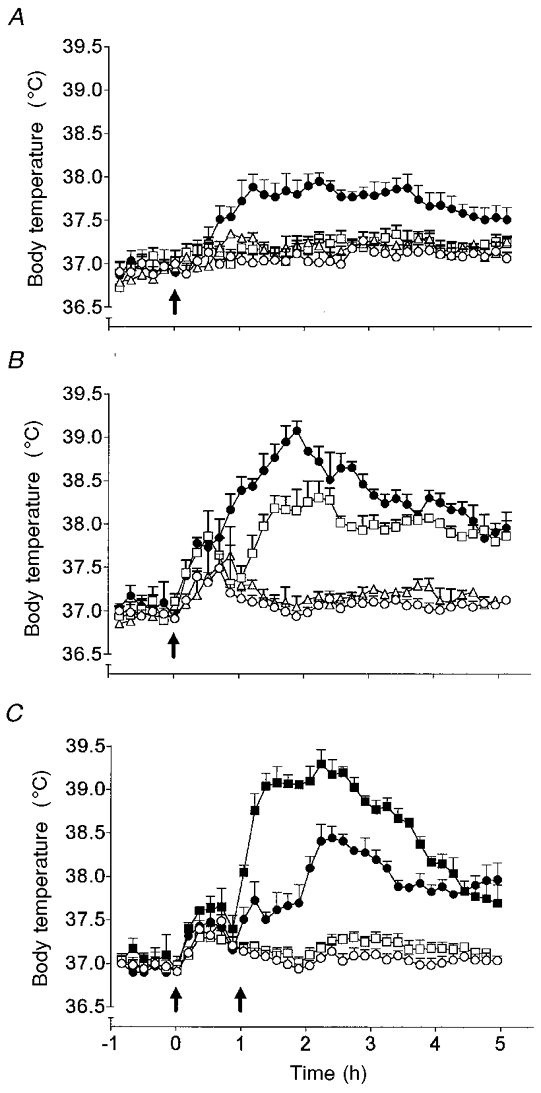
Body temperature responses of rats injected (i.po.) with: A, vehicle (○, n = 4), rat recombinant (rr)IL-1β (0.03 μg kg−1, □, n = 4) or rrIL-6 (25 μg kg−1, Δ, n = 4) alone, or in combination (•, n = 5); B, vehicle (○, n = 4), rrIL-1β (0.3 μg kg−1, □, n = 4) or rrIL-6 (25 μg kg−1, Δ, n = 4) alone, or in combination (•, n = 5); and C, vehicle at time 0 h (arrow), followed by vehicle (○, n = 6) or a pyrogenic dose of rrIL-1β (0.3 μg kg−1, •, n = 8) at time 1 h (arrow), and rrIL-6 (25 μg kg−1) at time 0 h (arrow) followed by vehicle (□, n = 6) or a pyrogenic dose of rrIL-1β (0.3 μg kg−1, ▪, n = 8) at time 1 h (arrow).
DISCUSSION
Circulating IL-6 has been implicated previously as an important mediator of fever, and can be induced in response to diverse stimuli not necessarily restricted to inflammatory insults (Zhou et al. 1993). Despite the strong association between this cytokine and inflammation, it has been difficult to establish a specific role for circulating IL-6 during infection, probably because of complex interactions between cytokines with overlapping biological activity (see Kluger, 1991). The present study reports for the first time that pretreatment (i.p.) of rats with an anti-IL-6 serum abolished the febrile response to localised (i.po.) injection of LPS. This finding extends earlier observations indicating the involvement of circulating endogenous IL-6 in fever (LeMay et al. 1990a,b,c; Engel et al. 1994; Luheshi et al. 1997; Miller et al. 1997a) and sepsis (Damas et al. 1992), and provides the first direct evidence that endogenous IL-6 can act as the afferent circulating signal to the brain to evoke fever in response to localised injection of LPS in rats. Our findings also suggest that while IL-6 is essential for LPS-induced fever, alone it does not cause fever when injected systemically, but appears to act in concert with IL-1.
We have shown previously that injection of LPS into a subcutaneous air pouch in rats elicited a marked fever, which was accompanied by a dramatic increase in local (pouch) concentrations of (bioactive) IL-1 and IL-6, and increased concentrations of circulating IL-6, but not IL-1 (Miller et al. 1997b). The results of the present study confirm these data and accord with earlier results reported for related models of systemic infection or inflammation (LeMay et al. 1990a,b; Roth et al. 1993; Klir et al. 1994; Luheshi et al. 1997). The marked elevation in concentrations of (immunoreactive) IL-1β and IL-6 in the pouch fluid, sampled after LPS, preceded the rise in body temperature suggesting that these cytokines are involved in the generation of the febrile response. In addition, we showed that circulating IL-6 concentrations increased dramatically (22-fold above basal concentrations), within 2 h of LPS administration, unlike IL-1β concentrations, which remained undetectable in the plasma. These data support our earlier hypothesis that IL-1 most likely acts locally in infected and inflamed tissues to induce the synthesis and release of IL-6 into the circulation (Miller et al. 1997a).
That the source of circulating IL-6 (in our experiments) is the air pouch was confirmed by injecting human IL-6 (i.po.) and measuring its appearance in the circulation, using an immunoassay specific for human IL-6 that does not cross-react with rat IL-6. These experiments revealed the rapid appearance in significant quantities of human IL-6 in the circulation (but not in the CSF) which at 40 min after the injection was (14-fold) higher than peak endogenous (i.e. rat) IL-6 concentrations detected after intrapouch injection of LPS. Despite this, none of the animals injected (i.po.) with hrIL-6 showed any significant change in body temperature. A robust fever (1.5°C increase in body temperature at 4 h) was, however, evoked when this same preparation was injected directly into the brain, even at a lower dose (100 ng hrIL-6 in 1 μl saline, intracerebroventricularly (i.c.v.), authors’ unpublished observations), highlighting the importance of brain IL-6 in the induction of fever. Although the expected initial plasma half-life of IL-6 is short (3 min) (Castell et al. 1988), plasma concentrations of human IL-6 were elevated for up to 5 h after injection (i.po.) suggesting a slow distribution from the pouch into the blood. The failure of i.po. hrIL-6 to induce fever cannot, therefore, be ascribed to a short half-life or, for that matter, to the use of a heterologous preparation since injection (i.po.), into rats, of rat IL-6 (at similar or higher doses than those detected in the circulation after LPS injection) also failed to induce fever.
Despite the failure to induce fever by administration of IL-6, it is nevertheless clear from the present study, that the role played by endogenous circulating IL-6 is pivotal to the generation of fever. This was dramatically demonstrated by neutralising its action in the circulation (using an antiserum raised against rat IL-6) and subsequently abolishing the LPS-induced fever. It is unclear, however, how circulating IL-6 activates the brain mechanisms that are essential for triggering the febrile response (Chai et al. 1996). One possibility is that circulating IL-6 gains entry into the brain via an active transport system (Banks et al. 1994) or via sites of the brain lacking a BBB, such as the circumventricular organs (CVOs; Blatteis, 1992). These CVOs not only respond to, but can also produce IL-6 (Vallières & Rivest, 1997) and could, therefore, be routes of entry for IL-6. Once in the brain, IL-6 can act directly on the hypothalamus to induce fever (Klir et al. 1993). This hypothesis is supported partly by our finding that rat IL-6 concentrations were significantly increased in the CSF of animals injected (i.po.) with LPS, and were maximum at the time when body temperature peaked (3 h). The importance of IL-6 in the CSF, however, remains unclear since body temperature started to rise, despite undetectable levels of IL-6 in the CSF at 2 h (present study). Furthermore, we have observed previously a fever of greater magnitude in the presence of much lower CSF IL-6 concentrations after injection (i.po.) of turpentine compared with injection (i.po) of LPS (authors’ unpublished observations). It is likely, therefore, that the concentration of IL-6 detected in the CSF does not accurately reflect that within specific brain tissue. In vivo studies using push-pull perfusion as performed previously (Klir et al. 1993; Roth et al. 1993) would more clearly determine the level of IL-6 production and release in discrete areas of the brain, such as the anterior hypothalamus.
Whether the increased IL-6 in the CSF (in response to localised LPS administration) was derived from the periphery or the brain is yet to be determined, as systemic LPS has been shown previously to induce brain IL-6 mRNA directly (Vallières & Rivest, 1997). An alternative and attractive hypothesis is that IL-6 may act on the blood side of the BBB by inducing cyclo-oxygenase (COX)-2 in cerebroendothelial cells (CECs) with the subsequent production of arachidonic metabolites (most probably prostaglandins), which are critical mediators of fever on the brain side of the BBB (Dinarello et al. 1991, 1999). Indeed it has been shown that local (turpentine; Lacroix & Rivest, 1998) or systemic (LPS) inflammation (Matsumura et al. 1998) induces COX-2 mRNA in CECs, that fever due to either stimulus is attenuated or prevented by COX inhibitors, and that IL-6 stimulates prostaglandin production from rat CECs in cell culture (De Vries et al. 1995). It is possible that other cytokines (or co-factors) cause or allow circulating IL-6 to enter the brain during LPS-induced fever, especially since LPS, at high doses, is known to alter the permeability of the BBB (see Kluger, 1991).
Our failure to induce fever by systemic administration of recombinant IL-6 is in agreement with earlier studies (LeMay et al. 1990b; Wang et al. 1997), where IL-6 injected alone failed to increase body temperature, even at very high doses (up to 40 μg kg−1, authors’ unpublished observations). Given the importance of endogenous circulating IL-6 in the development of localised LPS-induced fever (present study), the lack of response to exogenous IL-6 administration suggests that endogenous IL-6 is working together with another factor, to trigger the febrile response. Several of the non-pyrogenic actions of IL-6 are known to be augmented by IL-1 in vitro (Tritarelli et al. 1994). In vivo, IL-1 is an important stimulator of IL-6 production in peripheral immune cells (Akira et al. 1993) as well as in the central nervous system and endogenous IL-1 production has been shown to play a key role in the production of IL-6 within the hypothalamus of rats treated with bacterial LPS (Klir et al. 1994). Consequently, we tested the hypothesis that in vivo IL-6 acts in synergy with IL-1 in the periphery, to induce a febrile response. The results show that IL-6 can induce fever, but only in the presence of IL-1. The threshold dose of IL-6 required to evoke this effect was 2.5 μg kg−1. In agreement with the above data, injection (i.p.) of either IL-6 or LPS, alone, did not induce fever in IL-6 knockout mice, yet in combination, evoked fever (Chai et al. 1996). Further, it has been proposed that the likely mechanism for the loss of a febrile response to turpentine in IL-1β knockout mice is the reduction of IL-6 production (Zheng et al. 1995). Interestingly, preclinical safety studies with a daily injection of hrIL-6 in primates (1000 μg kg−1 day−1, subcutaneously (s.c.), over 9 weeks) and rats (500 μg kg−1 day−1s.c. for 4 weeks) did not report fever (Ryffel & Weber, 1995), whereas a phase I and/or II trial of a daily injection of hrIL-6 (0.5-5 μg kg−1 day−1s.c. for 28 days) in patients with aplastic anaemia (which may be associated with increased production of other cytokines, including IL-1), was discontinued prematurely on account of the observed toxicity of hrIL-6 in the patients, the adverse events of which included fever (Schrezenmeier et al. 1995).
The mechanism through which a non-pyrogenic dose of IL-1 and IL-6 induces fever, warrants further investigation. In vitro the synergism between IL-1 and IL-6 is mostly on prostanoid synthesis and probably involves the capacity of IL-6 to release arachidonate, and of IL-1 to stimulate COX-2 synthesis (Lacroix & Rivest, 1998; Dinarello et al. 1999). The failure to observe a rise in body temperature, in response to peripheral injection of IL-6 alone may be due to the availability of the soluble IL-6 receptor (sIL-6R). Soluble p80 IL-6R is abundant in biological fluids in various diseases in man (Gaillard et al. 1993; Keul et al. 1998), and ‘chaperones’ IL-6 and confers IL-6 responsiveness on a variety of cells that are unresponsive to ‘free’ IL-6 (Hirota et al. 1996; Romano et al. 1997) or that do not express endogenous IL-6R mRNA. Studies in rats show i.c.v. injection of sIL-6R, which lacks detectable biological activity, enhances and prolongs the central effects of IL-6 (Schobitz et al. 1995). Moreover, in the presence of human sIL-6R, mice are sensitised to human IL-6 and the detected acute phase response persists for a longer period (Peters et al. 1996). In the present study, the binding of IL-6 to its sIL-6R may be enhanced in animals treated with LPS or IL-1 and may enable endothelial cells (which lack the IL-6R (Romano et al. 1997)) to become responsive to circulating IL-6.
In conclusion, the present data confirm that (immunoreactive) IL-1β and IL-6 are induced locally at the site of inflammation in response to LPS administration. The locally produced IL-6 can be released into the circulatory system and appears to be an essential mediator of the febrile response to local LPS-induced inflammation. Although IL-6 itself had no effect on body temperature when injected into a subcutaneous air pouch, co-administration of IL-6 with a non-pyrogenic dose of IL-1β induced a significant increase in body temperature, and given together with a pyrogenic dose of IL-1β exacerbated the febrile response to the IL-1β. Thus, IL-6 appears to act in concert with IL-1 and perhaps other soluble mediators to induce or exacerbate fever.
Acknowledgments
This work was supported by a Wellcome Trust International Travelling Postdoctoral Fellowship (T.C.) and the MRC (G.N.L. and N.J.R.). Recombinant cytokines were generously provided by the centralised facility of the European Community BIOMED I program ‘Cytokines in the Brain’ (PL96, NIBSC).
References
- Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Advances in Immunology. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neuroscience Letters. 1994;179:53–56. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- Blatteis CM. Role of the OVLT in the febrile response to circulating pyrogens. Progressive Brain Research. 1992;91:409–412. doi: 10.1016/s0079-6123(08)62360-2. [DOI] [PubMed] [Google Scholar]
- Castell JV, Geiger T, Gross V, Andus T, Walter E, Hirano T, Kishimoto T, Heinrich PC. Plasma clearance, organ distribution and target cells on interleukin-6/ hepatocyte-stimulating factor in the rat. European Journal of Biochemistry. 1988;177:357–361. doi: 10.1111/j.1432-1033.1988.tb14384.x. [DOI] [PubMed] [Google Scholar]
- Chai Z, Gatti S, Toniatti C, Poli V, Bartfai T. Interleukin (IL)-6 gene expression in the central nervous system is necessary for fever response to lipopolysaccharide or IL-1β: a study on IL-6-deficient mice. Journal of Experimental Medicine. 1996;183:311–316. doi: 10.1084/jem.183.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas P, Ledoux D, Nys M, Vrindts Y, De Groot D, Franchimont P, Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Annals of Surgery. 1992;215:356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries HE, Hoogendoorn KH, van Dijk J, Zijlstra FJ, van Dam AM, Breimer DD, van Berkel TJ, de Boer AG, Kuiper J. Eicosanoid production by rat cerebral endothelial cells: stimulation by lipopolysaccharide, interleukin-1 and interleukin-6. Journal of Neuroimmunology. 1995;59:1–8. doi: 10.1016/0165-5728(95)00009-q. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Cannon JG, Mancilla J, Bishai I, Lees J, Coceani F. Interleukin-6 as an endogenous pyrogen: induction of prostaglandin E2 in brain but not in peripheral blood mononuclear cells. Brain Research. 1991;562:199–206. doi: 10.1016/0006-8993(91)90622-3. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Gatti S, Bartfai T. Fever: links with an ancient receptor. Current Biology. 1999;9:R147–150. doi: 10.1016/s0960-9822(99)80085-2. [DOI] [PubMed] [Google Scholar]
- Edwards JCW, Sedgwick AD, Willoughby DA. The formation of a structure with the features of synovial lining by subcutaneous injection of air: An in vivo tissue culture system. Journal of Pathology. 1981;134:147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Scammell TE, Saper CB. Mechanisms of CNS response to systemic immune challenge: the febrile response. Trends in Neurosciences. 1997;20:565–570. doi: 10.1016/s0166-2236(97)01138-7. [DOI] [PubMed] [Google Scholar]
- Engel A, Kern WV, Mürdter G, Kern P. Kinetics and correlation with body temperature of circulating interleukin-6, interleukin-8, tumor necrosis factor alpha and interleukin-1 beta in patients with fever and neutropenia. Infection. 1994;22:160–164. doi: 10.1007/BF01716695. [DOI] [PubMed] [Google Scholar]
- European Pharmacopaeia. Bacterial Endotoxins. 3. Strasbourg: European Pharmacopaeia; 1999. pp. 41–46. 2.6.14. [Google Scholar]
- Gaillard JP, Bataille R, Brailly H, Zuber C, Yasukawa K, Attal M, Maruo N, Taga T, Kishimoto T, Klein B. Increased and highly stable levels of functional soluble interleukin-6 receptor in sera of patients with monoclonal gammopathy. European Journal of Immunology. 1993;23:820–824. doi: 10.1002/eji.1830230408. [DOI] [PubMed] [Google Scholar]
- Hirota H, Kiyama H, Kishimoto T, Taga T. Accelerated nerve regeneration in mice by upregulated expression of interleukin (IL)-6 and IL-6 receptor after trauma. Journal of Experimental Medicine. 1996;183:2627–2634. doi: 10.1084/jem.183.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins SJ, Rothwell NJ. Cytokines and the nervous system I: expression and recognition. Trends in Neurosciences. 1995;18:83–88. [PubMed] [Google Scholar]
- Houssiau FA, Bukasa K, Sindic CJ, Van-Damme J, Van Snick J. Elevated levels of the 26K human hybridoma growth factor (interleukin 6) in cerebrospinal fluid of patients with acute infection of the central nervous system. Clinical and Experimental Immunology. 1988;71:320–323. [PMC free article] [PubMed] [Google Scholar]
- Keul R, Heinrich PC, Muller-Neuwen G, Muller K, Woo P. A possible role for soluble IL-6 receptor in the pathogenesis of systemic onset juvenile chronic arthritis. Cytokine. 1998;10:729–734. doi: 10.1006/cyto.1997.0343. [DOI] [PubMed] [Google Scholar]
- Klir JJ, McClellan JL, Kluger MJ. Interleukin 1β causes the increase in anterior hypothalamic interleukin-6 during LPS-induced fever in rats. American Journal of Physiology. 1994;266:R1845–1848. doi: 10.1152/ajpregu.1994.266.6.R1845. [DOI] [PubMed] [Google Scholar]
- Klir JJ, Roth J, Szelényi Z, McClellan JL, Kluger MJ. Role of hypothalamic interleukin-6 and tumor necrosis factor-α in LPS fever in rat. American Journal of Physiology. 1993;265:R512–517. doi: 10.1152/ajpregu.1993.265.3.R512. [DOI] [PubMed] [Google Scholar]
- Kluger MJ. Fever: role of pyrogens and cryogens. Physiological Reviews. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix S, Rivest S. Effect of systemic inflammatory response and cytokines on the transcription of the genes encoding cyclooxygenase enzymes (COX-1 and COX-2) in the rat brain. Journal of Neurochemistry. 1998;70:452–466. doi: 10.1046/j.1471-4159.1998.70020452.x. [DOI] [PubMed] [Google Scholar]
- Le May DR, LeMay LG, Kluger MJ, D'Alecy LG. Plasma profiles of IL-6 and TNF with fever-inducing doses of lipopolysaccharide in dogs. American Journal of Physiology. 1990a;259:R126–132. doi: 10.1152/ajpregu.1990.259.1.R126. [DOI] [PubMed] [Google Scholar]
- Le May LG, Otterness IG, Vander AJ, Kluger MJ. In vivo evidence that the rise in plasma IL-6 following injection of a fever-inducing dose of LPS is mediated by IL-1β. Cytokine. 1990b;2:99–204. doi: 10.1016/1043-4666(90)90016-m. [DOI] [PubMed] [Google Scholar]
- Le May LG, Vander AJ, Kluger MJ. Role of interleukin 6 in fever in rats. American Journal of Physiology. 1990c;258:R798–803. doi: 10.1152/ajpregu.1990.258.3.R798. [DOI] [PubMed] [Google Scholar]
- Lenczowski MJ, Bluthe R-M, Roth J, Rees GS, Rushforth DA, van Dam AM, Tilders FJ, Dantzer R, Rothwell NJ, Luheshi GN. Central administration of rat IL-6 induces HPA activation and fever but not sickness behaviour in rats. American Journal of Physiology. 1999;276:R652–658. doi: 10.1152/ajpregu.1999.276.3.R652. [DOI] [PubMed] [Google Scholar]
- Luheshi GN, Miller AN, Brouwer S, Dascombe MJ, Rothwell NJ, Hopkins SJ. Interleukin-1 receptor antagonist inhibits endotoxin fever and systemic interleukin-6 induction in the rat. American Journal of Physiology. 1996;270:E91–95. doi: 10.1152/ajpendo.1996.270.1.E91. [DOI] [PubMed] [Google Scholar]
- Luheshi GN, Stefferl A, Turnbull AV, Dascombe MJ, Brouwer S, Hopkins SJ, Rothwell NJ. Febrile response to tissue inflammation involves both peripheral and brain IL-1 and TNF-α in the rat. American Journal of Physiology. 1997;272:R862–868. doi: 10.1152/ajpregu.1997.272.3.R862. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Cao C, Ozaki M, Morii H, Nakadate K, Watanabe Y. Brain endothelial cells express cyclooxygenase-2 during lipopolysaccharide-induced fever: light and electron microscopic immunocytochemical studies. Journal of Neuroscience. 1998;18:6729–6789. doi: 10.1523/JNEUROSCI.18-16-06279.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ, Hopkins SJ, Luheshi GN. Sites of action of IL-1 in the development of fever and cytokine responses to tissue inflammation in the rat. British Journal of Pharmacology. 1997a;120:1274–1279. doi: 10.1038/sj.bjp.0701049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ, Luheshi GN, Rothwell NJ, Hopkins SJ. Local cytokine induction by LPS in the rat air pouch and its relationship to the febrile response. American Journal of Physiology. 1997b;272:R857–861. doi: 10.1152/ajpregu.1997.272.3.R857. [DOI] [PubMed] [Google Scholar]
- Nijsten MWN, De Groot ER, Ten Duis HJ, Klasen HJ, Hack CE, Aarden LA. Serum levels of interleukin-6 and acute phase responses. Lancet. 1987;2:921. doi: 10.1016/s0140-6736(87)91413-9. [DOI] [PubMed] [Google Scholar]
- Peters M, Jacobs S, Ehlers M, Vollmer P, Mullberg J, Wolf E, Brem G, Meyer zum Buschenfelde KH, Rose-John S. The function of soluble interleukin 6 (IL-6) receptor in vivo: sensitization of human soluble IL-6 receptor transgenic mice towards IL-6 and prolongation of the plasma half life of IL-6. Journal of Experimental Medicine. 1996;183:1399–1406. doi: 10.1084/jem.183.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G, Ball C, Ward HL, Gee CK, Tarrant G, Mistry Y, Poole S, Bristow AF. Rat interleukin 6: expression in recombinant Escherichia coli. Cytokine. 1999;11:95–103. doi: 10.1006/cyto.1998.0408. [DOI] [PubMed] [Google Scholar]
- Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, van Hinsbergh V, Sozzani S, Bussolino F, Poli V, Ciliberto G, Mantovani A. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–325. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- Roth J, Conn CA, Kluger MJ, Zeisberger E. Kinetics of systemic and intrahypothalamic IL-6 and tumor necrosis factor during endotoxin fever in guinea pigs. American Journal of Physiology. 1993;265:R653–658. doi: 10.1152/ajpregu.1993.265.3.R653. [DOI] [PubMed] [Google Scholar]
- Ryffel B, Weber M. Preclinical safety studies with recombinant human interleukin 6 (rhIL-6) in the primate Callithrix jacchus (marmoset): comparison with studies in rats. Journal of Applied Toxicology. 1995;15:19–26. doi: 10.1002/jat.2550150106. [DOI] [PubMed] [Google Scholar]
- Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. British Journal of Pharmacology. 1995;115:1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrezenmeier H, March JC, Stromeyer P, Muller H, Heimpel H, Gordon-Smith EC, Raghavachar A. A phase I/II trial of recombinant human interleukin-6 in patients with aplastic anaemia. British Journal of Haematology. 1995;90:283–292. doi: 10.1111/j.1365-2141.1995.tb05148.x. [DOI] [PubMed] [Google Scholar]
- Shalaby MR, Waage A, Aarden L, Espevik T. Endotoxin, tumor necrosis factor α and interleukin 1 induce interleukin 6 productionin vivo. Clinical Immunology and Immunopathology. 1989;53:488–498. doi: 10.1016/0090-1229(89)90010-x. [DOI] [PubMed] [Google Scholar]
- Shobitz B, Pezeshki G, Pohl T, Hemmann U, Heinrich PC, Holsboer F, Reul JM. Soluble interleukin-6 (IL-6) receptor augments central effects of IL-6in vivo. FASEB Journal. 1995;9:659–664. doi: 10.1096/fasebj.9.8.7768358. [DOI] [PubMed] [Google Scholar]
- Sironi M, Brevario F, Prosenpio P, Biondi A, Vecchi A, van Damme J, Dejana EM, Mantovani A. IL-1 stimulates IL-6 production in endothelial cells. Journal of Immunology. 1989;142:549–553. [PubMed] [Google Scholar]
- Sundgren-Andersson AK, Ouml;stlund P, Bartfai T. IL-6 is essential in TNF-α-induced fever. American Journal of Physiology. 1998;275:R2028–2034. doi: 10.1152/ajpregu.1998.275.6.R2028. [DOI] [PubMed] [Google Scholar]
- Taktak YS, Selkirk S, Bristow AF, Carpenter A, Ball C, Rafferty B, Poole S. Assay of pyrogens by interleukin-6 release from monocytic cell lines. Journal of Pharmacy and Pharmacology. 1991;43:578–582. doi: 10.1111/j.2042-7158.1991.tb03540.x. [DOI] [PubMed] [Google Scholar]
- Tritarelli E, Greco G, Testa U, Bellardelli F, Peschle C, Proietti E. Combined interleukin-1beta/interleukin-6 treatment in mice: synergistic myelostimulatory activity and myelorestorative effect after cyclosphamide-induced myelosuppression. Cancer Research. 1994;54:6469–6476. [PubMed] [Google Scholar]
- Turnbull AV, Rivier C. Corticotrophin-releasing factor, vasopressin and prostaglandins mediate, and nitric oxide restrains, the HPA axis response to acute local inflammation in the rat. Endocrinology. 1996;137:455–463. doi: 10.1210/endo.137.2.8593789. [DOI] [PubMed] [Google Scholar]
- Vallières L, Rivest S. Regulation of the genes encoding interleukin-6, its receptor, and gp130 in the rat brain in response to the immune activator lipopolysaccharide and the proinflammatory cytokine interleukin-1β. Journal of Neurochemistry. 1997;69:1668–1683. doi: 10.1046/j.1471-4159.1997.69041668.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Ando T, Dunn AJ. Effect of homologous interleukin-1, interleukin-6 and tumor necrosis factor-α on the core body temperature of mice. Neuroimmunomodulation. 1997;4:230–236. doi: 10.1159/000097341. [DOI] [PubMed] [Google Scholar]
- Watkins L, Maier SF, Goehler LE. Cytokine-to-brain communication: a review and analysis of alternative mechanisms. Life Sciences. 1995;57:1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]
- Zheng H, Fletcher D, Kozak W, Jiang M, Hoffman KJ, Conn CA, Soszynski D, Grabiec C, Trumbauer ME, Shaw A, Kostura MJ, Stevens K, Rosen H, North RJ, Chen HY, Tocci MJ, Kluger MJ, Van der Ploeg LHT. Resistance to fever induction and impaired acute-phase response in interleukin-1β-deficient mice. Immunity. 1995;3:9–19. doi: 10.1016/1074-7613(95)90154-x. [DOI] [PubMed] [Google Scholar]
- Zhou D, Kusnekov AW, Shurin MR, Depaoli M, Rabin BS. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic pituitary-adrenal axis. Endocrinology. 1993;133:2523–2530. doi: 10.1210/endo.133.6.8243274. [DOI] [PubMed] [Google Scholar]


