Abstract
Ocular exposure to early morning room light can significantly advance the timing of the human circadian pacemaker. The resetting response to such light has a non-linear relationship to illuminance. The dose-response relationship of the human circadian pacemaker to late evening light of dim to moderate intensity has not been well established.
Twenty-three healthy young male and female volunteers took part in a 9 day protocol in which a single experimental light exposure6.5 h in duration was given in the early biological night. The effects of the light exposure on the endogenous circadian phase of the melatonin rhythm and the acute effects of the light exposure on plasma melatonin concentration were calculated.
We demonstrate that humans are highly responsive to the phase-delaying effects of light during the early biological night and that both the phase resetting response to light and the acute suppressive effects of light on plasma melatonin follow a logistic dose-response curve, as do many circadian responses to light in mammals.
Contrary to expectations, we found that half of the maximal phase-delaying response achieved in response to a single episode of evening bright light (∼9000 lux (lx)) can be obtained with just over 1 % of this light (dim room light of ∼100 lx). The same held true for the acute suppressive effects of light on plasma melatonin concentrations. This indicates that even small changes in ordinary light exposure during the late evening hours can significantly affect both plasma melatonin concentrations and the entrained phase of the human circadian pacemaker.
The light:dark cycle is the pre-eminent synchronizer of mammalian circadian pacemakers (Roenneberg & Foster, 1997; Czeisler & Wright, 1999). The response of mammalian pacemakers varies with both the timing and intensity of the photic stimuli. Both human and non-human mammals have been shown to share the same characteristic responses to variation in the timing of light exposure. Retinal light exposure in the early subjective night will delay the timing of the clock while light exposure in the late subjective night and early subjective morning will advance the timing of the clock (Czeisler et al. 1989; Johnson, 1990). Experimental light exposure at either time will induce a suppression of pineal melatonin production (Honma et al. 1992; Brainard et al. 1997). In non-human mammals, the intensity dependence of both phase shifting of the circadian pacemaker and acute suppression of melatonin have been well characterized (Brainard et al. 1983; Nelson & Takahashi, 1991b; Bauer, 1992; Sharma et al. 1999). In humans, it has been reported recently that three consecutive days of morning room-light exposure (∼180 lx) can significantly phase advance the human circadian pacemaker (Boivin et al. 1996). The magnitude of the resetting response increased with the illuminance in a non-linear manner. This non-linearity was consistent with a cube-root compression of illuminance, one that had been reported previously for visual perception (Stevens, 1961). Though there were limited data below 180 lx, it was recognized that this postulated cube-root relationship could not account for responses observed to light below 180 lx (Jewett & Kronauer, 1998). Furthermore, the use of three consecutive days of light administration, each with a 5 h pulse of light administered at the same clock time, complicates characterization of the dose-response relationship from those data because of the interaction of non-linear intensity-dependent and non-linear phase-dependent effects of light on the circadian pacemaker.
We therefore embarked on a refined assessment of the response of the human circadian pacemaker to a single episode of nocturnal light exposure. We have discovered that the human circadian pacemaker can be phase delayed by dimmer light than previously reported. Not only is the pacemaker responsive to the resetting effects of exposure to ∼100 lx of light in the horizontal angle of gaze, but we found this illuminance to be so effective that it generated half of the maximum circadian phase delay resetting response that was observed at this phase in response to light two orders of magnitude greater in intensity (∼9000 lx).
METHODS
Protocol
We exposed 23 healthy young male and female volunteers, aged 18-44 years (27.8 ± 8.91 years (mean ±s.d.)), each to a single illuminance of light, ranging from 3 to 9100 lx in the horizontal angle of gaze (Fig. 1), during a 9 day protocol (for screening procedures and more extensive protocol details, refer to Boivin et al. 1994 and Duffy et al. 1996). Prior to beginning the protocol, all subjects gave informed, written consent; the Human Research Committee at the Brigham and Women's Hospital monitored the protocol and all procedures. All experimental procedures were carried out in accordance with the principles of the Declaration of Helsinki. Following at least 2 weeks of a regular, at home sleep:wake schedule and three in-patient baseline days in a room free of time cues, we estimated the initial timing (phase) of the circadian system from the fitted minimum of the endogenous core temperature (Tmin) rhythm recorded continuously via a rectal thermistor (YSI, Yellow Springs, OH, USA). Core temperature was estimated during a constant routine (Mills et al. 1978; Czeisler et al. 1989, 1990) of ∼50 h, using a two harmonic regression model (Czeisler et al. 1989; Brown & Czeisler, 1992). Following an 8 h scheduled sleep episode, subjects awoke to 16 h of wakefulness centred within which was a 6.5 h pulse of experimental light exposure. The experimental light exposure was scheduled to start 6.75 h before Tmin and to end 0.25 h before Tmin. It was thus centred 3.5 h before Tmin, a time during which bright light is known to induce phase delays of the circadian system (Jewett et al. 1994). Subjects were required to remain seated for the duration of the 6.5 h exposure, alternating their gaze between a fixed spot on the wall and free gaze every 6 min. No photophobic behaviour (e.g. closing one's eyes, reading) was allowed during any portion of the experimental light exposure. Light was generated using overhead cool white fluorescent lamps (North American Philips Lighting, Bloomfield, NJ, USA) filtered with a Lexan 9030 UV-restricting lens (General Electric Plastics, Pittsfield, MA, USA) and designed to provide uniform illuminance to the whole experimental room (Philips Lighting, The Netherlands). To quantify the illuminance of light to which the subject was exposed during the experimental light exposure, light measurements were taken from the corneal level in the horizontal angle of gaze during the fixed portions of exposure, using a research photometer (IL1400, International Light, Newburyport, MA, USA). The illuminance values reported are the mean of the illuminances recorded during these fixed periods of gaze. During the period of free gaze, illuminance values were typically ∼70 % of those during the fixed gaze. The next day, subjects awoke into a second constant routine of ∼30 h duration in order to assess the effects of the experimental light exposure on endogenous circadian phase. Subjects were allotted a final 8 h sleep episode and were discharged upon awakening on Day 9. During the baseline days, illuminance in the horizontal angle of gaze was < 150 lx during wake episodes and < 0.03 lx during sleep episodes. For all other days (except for during the experimental light exposure) subjects were exposed to no more than 10 lx during wake episodes and < 0.03 lx during sleep episodes.
Figure 1. Phase shift of the human circadian pacemaker and acute suppression of plasma melatonin.
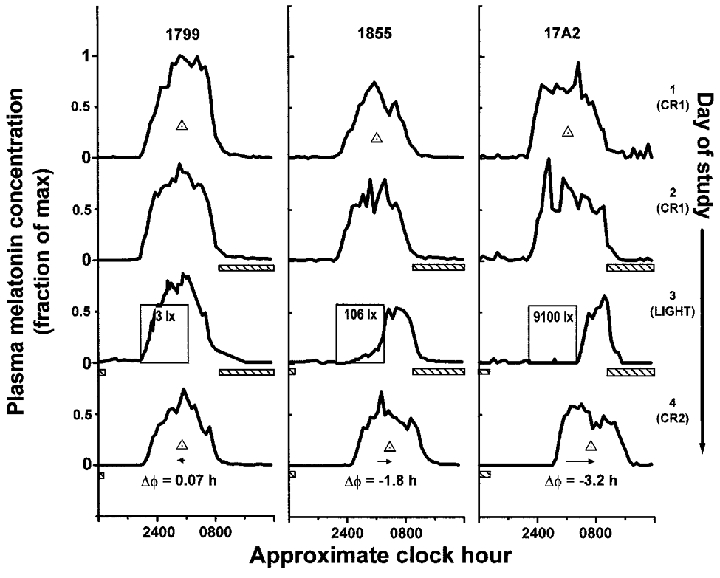
Melatonin profiles during days on which the first constant routine (CR) (1 and 2), the single experimental light exposure 6.5 h in duration (3), and the second CR (4) occurred are shown for three representative subjects (1799, 1855, 17A2). In the dimmest light condition, exposure to the dim light stimulus (∼3 lx) had little effect on either the phase of the melatonin rhythm (phase shift (ΔΦ) 0.07 h) or concentration of plasma melatonin (suppression 11 %). In the brightest light condition (∼9100 lx), light both shifted the rhythm (ΔΦ -3.2 h) and completely suppressed plasma melatonin (98 %). Exposure to dim room light (∼106 lx) evoked more than half of the shift observed in the brightest light condition (ΔΦ -1.8 h compared with -3.2 h) and a nearly equal amount of suppression (88 %). During the CRs and day of experimental light exposure, subjects were exposed to no more than 5 lx in the horizontal angle of gaze at any time except during the scheduled sleep episodes (hatched bars < 0.03 lx) and the experimental light exposure (labelled open boxes, see Fig. 2). Individual subject data were plotted on a time scale in which their habitual wake time was assigned a reference value of 08.00 h. Phase of the melatonin maximum (midpoint of the upward and downward mean crossings) during each CR is noted as the ▵. For graphical purposes, ordinate values were normalized to each subject's absolute peak plasma melatonin concentration.
Assays
Core temperature was used to estimate circadian phase during the experiment in order to schedule the timing of the experimental light exposure. However, comparative analysis has shown melatonin phase, as defined by the midpoint between the upward and downward crossings of the 24 h mean (Shanahan, 1995; Zeitzer et al. 1999), to be a more reliable and accurate measure of circadian phase (Gershengorn et al. 1998). Therefore, we collected blood samples twice an hour throughout the protocol and later assayed them for plasma melatonin concentrations (assay sensitivity of 2.5 pg ml−1; intra-assay and interassay percentage coefficients of variation, were 8 and 13 %, respectively; DiagnosTech, Osceola, WI, USA). Post hoc analysis of initial melatonin phases revealed that the experimental light exposure was mistimed in two subjects. In these two subjects, the midpoint of the experimental light exposure was at or after the melatonin maximum, a time at which light will begin to advance the phase of the circadian pacemaker. In the remaining 21 subjects, the light on average (±s.d.) occurred 2.0 ± 0.95 h before the melatonin maximum.
Statistics
Phase shifts were calculated as the difference between the melatonin phase during the first constant routine and the melatonin phase during the second constant routine. Melatonin suppression was calculated using the following equation:
in which AUC is the area under the plasma melatonin profile as calculated by the trapezoidal method (Microcal Origin 5.0; Zeitzer, 1999). Baseline AUC was calculated during the 4 h before the melatonin maximum during the second melatonin cycle of the first constant routine, during which time the subject was continuously semirecumbent and exposed to < 10 lx of light. The light AUC was calculated during the same four clock hours of the next melatonin cycle, during which time all subjects were seated and exposed to the experimental light.
The following models were fitted to the data (Tables 1 and 2): power model, cube root model (power model with the power term fixed at 0.33) (Boivin et al. 1996), log model, three parameter logistic model (from which the Naka-Rushton and Michaelis-Menten equations are derived) (Nelson & Takahashi, 1991b), and a four parameter logistic model (a version of the three parameter logistic model with an added power term that has been shown to be useful in modelling some biological responses to light (Boynton & Whitten, 1970; Brainard et al. 1983; Nelson & Takahashi, 1991b)). The three and four parameter logistic models estimate well responses that have a sigmoidal relationship with increasing stimulus strength. In the logistic models, a is the estimated response of the system to 0 lx of light, b is the lux value at which 50 % of the maximal shift (or suppression, as appropriate) is observed, c is the asymptotic maximal responsiveness of the system, and d, in the four parameter logistic model, is a measure of the steepness of the rising portion of the curve. Data were fitted with a non-linear least squares fitting analysis based upon the Levenberg-Marquardt method (CurveExpert v.1.34; Microcal Origin 5.0). The goodness-of-fit of each model was assessed by calculating the adjusted correlation coefficient (R2), Akaike's Information Criterion (AIC) (Priestley, 1981), and the residual squared error (RSE, square root of the residual sum of squares divided by its degrees of freedom). When fitting the phase shift data in both the logistic models, we constrained a from -0.96 to -0.24, as this is the mean estimated drift (i.e. movement of the clock, unaltered by the environment) between the two phase estimations (Duffy et al. 1996; Czeisler et al. 1999). We also examined the logistic models leaving a unconstrained, and found that the goodness of fit measures changed little (data not shown). When fitting the acute suppressive effects of light on plasma melatonin secretion, we constrained c of the logistic models to be less than, or equal to one, as the maximal response of the system is 1 (i.e. 100 % suppression).
Table 1.
Statistical comparison of models fitted to the illuminance-response circadian phase shift data
| Model | Formula | Parameter estimates | Adjusted R2 | AIC | RSE |
|---|---|---|---|---|---|
| log | y = a + blnx | a = 0·387 ± 0·379 | 0·67 | 11·19 | 0·379 |
| b = 0·407 ± 0·0629 | |||||
| 3 Parameter logistic | 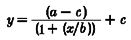 |
a = 0·240 ± 0·389 | 0·75 | 11·16 | 0·287 |
| b = 120 ± 61·2 | |||||
| c =−3·00 ± 0·235 | |||||
| 4 Parameter logistic | 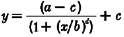 |
a =−0·240 ± 0·409 | 0·75 | 12·81 | 0·283 |
| b = 119 ± 43·1 | |||||
| c =−2·90 ± 0·238 | |||||
| d = 1·42 ± 0·661 | |||||
| Power | y = axb | a =−0674 ± 0·200 | 0·55 | 13·81 | 0·516 |
| b = 0·175 ± 0·0401 | |||||
| Cube root | y = ax0·33 | a =−2·01 ± 0·0204 | 0·25 | 19·20 | 0·860 |
Parameters are shown with the standard deviation of the fit. Adjusted R2 is the correlation coefficient, AIC Akaike's Information Criterion, RSE residual squared error.
Table 2.
Statistical comparison of models fitted to the illuminance-response melatonin suppression data
| Model | Formula | Parameter estimates | Adjusted R2 | AIC | RSE |
|---|---|---|---|---|---|
| log | y = a + binx | a =−0·242 ± 0·157 | 0·63 | 5·23 | 0·065 |
| b = 0·154 ± 0·0260 | |||||
| 3 Parameter logistic | 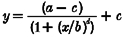 |
a =−0·161 ± 0·160 | 0·78 | 4·73 | 0·038 |
| b = 88·0 ± 41·3 | |||||
| c = 1·0 | |||||
| 4 Parameter logistic | 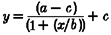 |
a =−0·0156 ± 0·0832 | 0·86 | 8·42 | 0·024 |
| b = 106 ± 13·3 | |||||
| c = 0·936 ± 0·504 | |||||
| d = 3·55 ± 1·58 | |||||
| Power | y = axb | a = 0·188 ± 0·0707 | 0·52 | 5·59 | 0·084 |
| b = 0·201 ± 0·0500 | |||||
| Cube root | y = ax0·33 | a = 0·0683 ± 0·00725 | 0·39 | 4·15 | 0·107 |
Parameters are shown with the standard deviation of the fit, and adjusted R2 is the correlation coefficient; AIC and RSE as Table 1.
RESULTS
Plasma melatonin concentration was suppressed in a dose-dependent manner during the single 6.5 h light stimulus that was administered from approximately 23.00-05.30 h during this 9 day protocol. Low illuminances evoked little change in plasma melatonin concentrations, whereas bright room light and higher illuminances completely suppressed plasma melatonin (Figs 1 and 2B). The acute response of melatonin to increasing illuminance occurred in a nearly step-wise manner, with suppression of melatonin occurring at illuminances greater than ∼200 lx, with minimal suppression below 80 lux, and variable responses between the two illuminances. Endogenous circadian phase assessments conducted under carefully controlled conditions before and after the stimulus revealed that, like the acute suppressive effects of light, the circadian phase-resetting response to light varied with illuminance in a dose-dependent manner (Figs 1 and 2A). Low illuminances (below 15 lux) evoked little phase shift, while bright room light (above 500 lux) caused an apparent saturating phase shift of the endogenous circadian melatonin rhythm. Between these illuminances (normal range of room light), the circadian phase-shifting response rose rapidly with increasing illuminance.
Figure 2. Illuminance-response curve of the human circadian pacemaker.
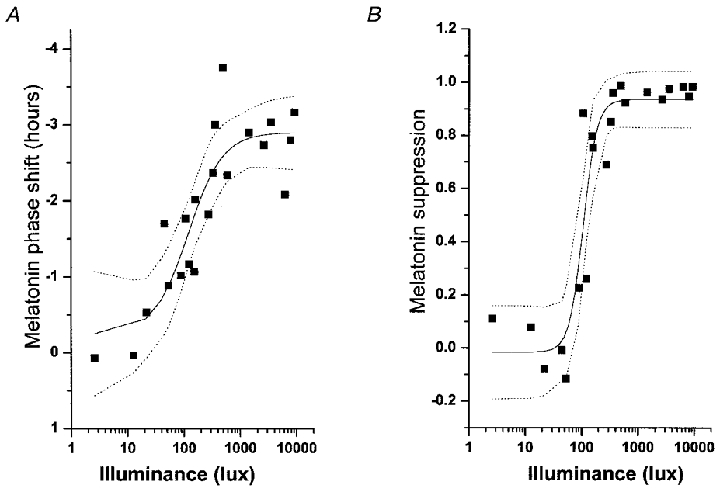
The shift in the phase of the melatonin rhythm (A), as assessed on the day following exposure to a 6.5 h experimental light stimulus, has been fitted with a four parameter logistic model (see Table 1) using a non-linear least squares analysis. Acute suppression of plasma melatonin (B) during the light exposure also has been fitted with a four parameter logistic model (see Table 2) using a non-linear least squares analysis. The logistic models predict an inflection point of the curve (i.e. the sensitivity of the system) at ∼120 lx. Saturation of the phase-shift response is predicted to occur with ∼550 lx and saturation of the melatonin-suppression response is predicted to occur with ∼200 lx. Individual subjects are represented by ▪, the model by the continuous line, and the 95 % confidence intervals by the dotted lines.
To quantify the responsiveness of the human circadian timing system to light, we fitted the phase shift data with a variety of models that had been previously suggested for describing the response of mammalian circadian systems to light (Table 1). While each model significantly fitted the phase shift data (P < 0.01, approximate F tests), the three and four parameter logistic models fitted the data best. Both the logistic models estimate that the maximal response of the human circadian system to a light pulse of this duration and timing is approximately 3 h, while the half-maximal response occurs with only 80-160 lx of light, a typical range of ambient indoor room illumination (Fig. 2A). AIC measures indicate that, though the three parameter logistic model is more parsimonious, the four parameter logistic model has a smaller RSE and larger adjusted R2.
Given that suppression of plasma melatonin is often used as a proxy for the effects of light on the human circadian pacemaker, we also quantified the dose-dependent acute suppression of plasma melatonin during the 6.5 h light stimulus. As with the phase shift data, we examined the same five models on this data set (Table 2). Though all models significantly fitted the data (P < 0.01, approximate F tests), the three and four parameter logistic models fitted the data best (Fig. 2B). The four parameter logistic model may fit the data better than the three parameter logistic model as the power term (d), which models the steepness of the rising portion of the curve, accounts for the extremely rapid rise in the suppression of melatonin in response to increasing light intensity. Both models predict a half-maximal response at ∼50-130 lx, again within the range of normal, ambient room illumination.
DISCUSSION
Our data demonstrate that the human circadian pacemaker is much more sensitive than previously recognized to low intensity room light during the first 6.5 h of the biological night. In mammals, various models, including the power, log, cube root and logistic, have been used to describe circadian responses to photic stimuli (Stevens, 1961; Brainard et al. 1983; Nelson & Takahashi, 1991b; Boivin et al. 1996). While each of these models predicted a monotonic, non-linear increase in the response of the human circadian pacemaker to an increasing light stimulus strength, and each model was statistically consistent with the experimental data, we found that the two logistic models were particularly effective in capturing the rate of change in response to increasing illuminances. This type of relationship between light intensity and a circadian response has been previously observed in studies of non-human mammals in which light pulses of shorter duration were administered against a background of darkness (Brainard et al. 1983; Nelson & Takahashi, 1991b; Bauer, 1992; Sharma et al. 1999) as well as electrophysiological studies of the responsiveness of the suprachiasmatic nucleus of the hypothalamus (SCN) to light (Meijer, 1991). Estimates derived from the logistic model indicate that, at this phase and duration, the response of the human circadian timing system to a single episode of light exposure saturates (i.e.90 % of the asymptotic maximum response) at ∼550 lx for phase-shifting responses and ∼200 lx for melatonin suppression. Such a difference in the sensitivity of two responses that are both mediated by the circadian pacemaker has been previously observed in non-human mammals (Nelson & Takahashi, 1991a; Kanematsu et al. 1991). The greatest rate of change (i.e. the largest derivative), as predicted by the logistic models, occurs between ∼50 and 600 lx (typical range of indoor room light). The occurrence of the inflection point of this curve between ∼50 and 160 lx indicates further that the human circadian pacemaker is highly sensitive to ordinary room light and that minor changes in room light intensity could have a major impact on entrainment of the human circadian pacemaker. However, whether the illuminance at which the inflection point and saturating response occur holds for different durations of light exposure remains to be seen. Further examination of the mechanism of integration of light over time by the human circadian pacemaker will be necessary to more fully answer this question.
The pathway from photoreception to the melatonin responses that are influenced by the human circadian pacemaker involves a complex neural pathway that is postulated to lead from the SCN to the hypothalamic paraventricular nucleus (PVN), through the median forebrain bundle into the spinal cord and down the intermediolateral cell column, and then from the spinal cord to the superior cervical ganglion, which in turn innervates the pineal gland (Klein, 1993). The observed illuminance-response relationship that we have described is likely to reflect signal transduction mechanisms operative at these different stages of regulation, including gene regulation in the SCN (Akiyama et al. 1999), of the circadian rhythm of melatonin. The first step in the pathway is the transduction of radiant energy into a neurochemical signal. Although data from one human study have suggested otherwise (Campbell & Murphy, 1998), which has recently been refuted (Foster, 1998; Lockley et al. 1998), mammalian circadian photoreception occurs only in the eyes, as bilateral enucleation results in a loss of photic responsiveness (Groos & van der Kooy, 1981; Czeisler et al. 1995). Our current and previous data are consistent with the hypothesis that the same long-, medium- and short-wavelength-sensitive cones that are used by humans for image formation are sufficient to transduce photic information to the SCN (Zeitzer et al. 1997). However, our data do not preclude the existence of a novel circadian photoreceptor, utilized either in conjunction with the known cones or independently, as has been suggested in human and non-human mammals (Foster et al. 1993; Provencio & Foster, 1995; Thresher et al. 1998; Lucas et al. 1999; Provencio et al. 2000). However, the demonstration that the human circadian system is differentially sensitive to a large dynamic range of illuminances implies that the photoreceptor(s) involved in circadian responses must have cone-like saturation properties. From both anatomical and physiological evidence, it has been hypothesized that mammals use type III ganglion cells to transmit photic information to the SCN (Groos & Mason, 1980; Moore et al. 1995; Provencio et al. 1998). The high threshold response (in comparison with that of image formation) that is implied by our data and the model fitted is consistent with the use of these ganglion cells in humans, as these cells have an elevated activation threshold. However, such a threshold could also reflect a threshold sensitivity of the SCN to the glutamatergic input from the retina or by a threshold response of downstream effector mechanisms at the PVN or pineal gland (Ding et al. 1994; Jiao et al. 1999). Likewise, the asymptotic upper threshold may be due to the kinetics of response in the retina, SCN or downstream.
Responsivity of the human circadian pacemaker to phase resetting by room light has been questioned in both past and recent studies (Wever, 1970; Aschoff et al. 1971; Wever, 1989; Van Cauter et al. 1998). Our data, however, strongly indicate that dim room light is not a neutral circadian stimulus and must be considered in the interpretation of human circadian experimentation. This finding is supported by considerable evidence from other studies (Czeisler et al. 1981; Boivin et al. 1996; Waterhouse et al. 1998; Boivin & Czeisler, 1998). It should be noted that in the present study, our light stimulus was administered on a background of very dim light. The contrast and timing of both the experimental stimulus and the background light (i.e. the temporal organization and contrast of all light received by the pacemaker) may provide information to the pacemaker and determine the magnitude of induced phase changes, though this postulate requires further testing.
The present data indicate that the human circadian timing system is approximately a log unit more sensitive to light than was previously thought and that at the tested phase, exposure to a single 6.5 h episode of ∼100 lx of light will generate half of the response observed for a stimulus that is nearly 100-fold brighter (∼9000 lx). However, it must be emphasized that the sensitivity of the system to light administered at different phases, especially in the region of critical resetting, will be necessary to understand more fully the physiology of human photic resetting. Nonetheless, the logistic kinetics that we observe imply that minor changes in indoor lighting condition may have a major impact on human circadian entrainment and its dysregulation, as observed in certain sleep disturbances associated with ageing, shift work and rapid time-zone changes.
Acknowledgments
We are indebted to the study subjects, to the research technicians, and to Mr Edward F. Hall for protocol assistance. This work was supported by the National Aeronautics and Space Administration, the National Institute of Mental Health, National Heart, Lung, and Blood Institute, the National Institute on Aging, National Space Biomedical Research Institute, the National Institute of General Medical Sciences and the General Clinical Research Center Program of the National Center for Research Resources. D.-J.D. was supported by a Philips Fellowship.
References
- Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, Watanabe S, Tei H, Sakaki Y, Shibata S. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. Journal of Neuroscience. 1999;19:1115–1121. doi: 10.1523/JNEUROSCI.19-03-01115.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschoff J, Fatranská M, Giedke H, Doerr P, Stamm D, Wisser H. Human circadian rhythms in continuous darkness: Entrainment by social cues. Science. 1971;171:213–215. doi: 10.1126/science.171.3967.213. [DOI] [PubMed] [Google Scholar]
- Bauer MS. Irradiance responsivity and unequivocal type-1 phase responsivity of rat circadian activity rhythms. American Journal of Physiology. 1992;263:R1110–1114. doi: 10.1152/ajpregu.1992.263.5.R1110. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Czeisler CA. Resetting of the circadian melatonin and cortisol rhythms in humans by ordinary room light. NeuroReport. 1998;9:779–782. doi: 10.1097/00001756-199803300-00002. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Sensitivity of the human circadian pacemaker to moderately bright light. Journal of Biological Rhythms. 1994;9:315–331. doi: 10.1177/074873049400900311. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379:540–542. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- Boynton RM, Whitten DN. Visual adaptation in monkey cones: recordings of late receptor potentials. Science. 1970;170:1423–1426. doi: 10.1126/science.170.3965.1423. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Richardson BA, King TS, Matthews SA, Reiter RJ. The suppression of pineal melatonin content and N-acetyltransferase activity by different light irradiances in the Syrian hamster: a dose-response relationship. Endocrinology. 1983;113:293–296. doi: 10.1210/endo-113-1-293. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Rollag MD, Hanifin JP. Photic regulation of melatonin in humans: ocular and neural signal transduction. Journal of Biological Rhythms. 1997;12:537–546. doi: 10.1177/074873049701200608. [DOI] [PubMed] [Google Scholar]
- Brown EN, Czeisler CA. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. Journal of Biological Rhythms. 1992;7:177–202. doi: 10.1177/074873049200700301. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Murphy PJ. Extraocular circadian phototransduction in humans. Science. 1998;279:396–399. doi: 10.1126/science.279.5349.396. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk D-J, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Johnson MP, Duffy JF, Brown EN, Ronda JM, Kronauer RE. Exposure to bright light and darkness to treat physiologic maladaptation to night work. New England Journal of Medicine. 1990;322:1253–1259. doi: 10.1056/NEJM199005033221801. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Richardson GS, Zimmerman JC, Moore-Ede MC, Weitzman ED. Entrainment of human circadian rhythms by light-dark cycles: a reassessment. Photochemistry and Photobiology. 1981;34:239–247. [PubMed] [Google Scholar]
- Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, Klein T, Rizzo JF., III Suppression of melatonin secretion in some blind patients by exposure to bright light. New England Journal of Medicine. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Wright KP., Jr . Influence of light on circadian rhythmicity in humans. In: Turek FW, Zee PC, editors. Regulation of Sleep and Circadian Rhythms. New York: Marcel Dekker, Inc; 1999. pp. 149–180. [Google Scholar]
- Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Kronauer RE, Czeisler CA. Phase-shifting human circadian rhythms: influence of sleep timing, social contact and light exposure. The Journal of Physiology. 1996;495:289–297. doi: 10.1113/jphysiol.1996.sp021593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RG. Shedding light on the biological clock. Neuron. 1998;20:829–832. doi: 10.1016/s0896-6273(00)80464-x. [DOI] [PubMed] [Google Scholar]
- Foster RG, Argamaso S, Coleman S, Colwell CS, Lederman A, Provencio I. Photoreceptors regulating circadian behavior: A mouse model. Journal of Biological Rhythms. 1993;8S:S17–23. [PubMed] [Google Scholar]
- Gershengorn H, Klerman EB, Kronauer RE. Circadian phase assessment from plasma melatonin – a comparison of measures. Society for Research on Biological Rhythms. 1998;6:120. [Google Scholar]
- Groos GA, Mason R. The visual properties of rat and cat suprachiasmatic neurones. Journal of Comparative Physiology. 1980;135:349–356. [Google Scholar]
- Groos GA, van der Kooy D. Functional absence of brain photoreceptors mediating entrainment of circadian rhythms in the adult rat. Experientia. 1981;37:71–72. doi: 10.1007/BF01965576. [DOI] [PubMed] [Google Scholar]
- Honma S, Kanematsu N, Katsuno Y, Honma K. Light suppression of nocturnal pineal and plasma melatonin in rats depends on wavelength and time of day. Neuroscience Letters. 1992;147:201–204. doi: 10.1016/0304-3940(92)90595-x. [DOI] [PubMed] [Google Scholar]
- Jewett ME, Kronauer RE. Refinement of a limit cycle oscillator model of the effects of light on the human circadian pacemaker. Journal of Theoretical Biology. 1998;192:455–465. doi: 10.1006/jtbi.1998.0667. [DOI] [PubMed] [Google Scholar]
- Jewett ME, Kronauer RE, Czeisler CA. Phase/amplitude resetting of the human circadian pacemaker via bright light: A further analysis. Journal of Biological Rhythms. 1994;9:295–314. doi: 10.1177/074873049400900310. [DOI] [PubMed] [Google Scholar]
- Jiao Y-Y, Lee TM, Rusak B. Photic responses of suprachiasmatic area neurons in diurnal degus (Octodon degus) and nocturnal rats (Rattus norvegicus) Brain Research. 1999;817:93–103. doi: 10.1016/s0006-8993(98)01218-9. [DOI] [PubMed] [Google Scholar]
- Johnson CH. An Atlas of Phase Response Curves for Circadian and Circatidal Rhythms. Nashville, TN, USA: Department of Biology, Vanderbilt University; 1990. [Google Scholar]
- Kanematsu N, Honma S, Honma K-i, Hiroshige T. Red dim light suppresses the pineal melatonin without affecting the circadian pacemaker. Sapporo Symposium on Biological Rhythms. 1991:53. [Google Scholar]
- Klein DC. The mammalian melatonin rhythm generating system. In: Wetterberg L, editor. Light and Biological Rhythms in Man. New York: Pergamon Press; 1993. pp. 55–70. [Google Scholar]
- Lockley SW, Skene DJ, Thapan K, English J, Ribeiro D, Haimov I, Hampton S, Middleton B, von Schantz M, Arendt J. Extraocular light exposure does not suppress plasma melatonin in humans. Journal of Clinical Endocrinology and Metabolism. 1998;83:3369–3372. doi: 10.1210/jcem.83.9.5244. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Freedman MS, Muñoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- Meijer JH. Integration of visual information by the suprachiasmatic nucleus. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus: The Mind's Clock. New York: Oxford University Press; 1991. pp. 107–119. [Google Scholar]
- Mills JN, Minors DS, Waterhouse JM. Adaptation to abrupt time shifts of the oscillator(s) controlling human circadian rhythms. The Journal of Physiology. 1978;285:455–470. doi: 10.1113/jphysiol.1978.sp012582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Speh JC, Card JP. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. Journal of Comparative Neurology. 1995;352:351–366. doi: 10.1002/cne.903520304. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS. Comparison of visual sensitivity for suppression of pineal melatonin and circadian phase-shifting in the golden hamster. Brain Research. 1991a;554:272–277. doi: 10.1016/0006-8993(91)90200-f. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus) The Journal of Physiology. 1991b;439:115–145. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestley MB. Spectral Analysis and Time Series. New York: Academic Press; 1981. [Google Scholar]
- Provencio I, Cooper HM, Foster RG. Retinal projections in mice with inherited retinal degeneration: implications for circadian photoentrainment. Journal of Comparative Neurology. 1998;395:417–439. doi: 10.1002/(sici)1096-9861(19980615)395:4<417::aid-cne1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Provencio I, Foster RG. Circadian rhythms in mice can be regulated by photoreceptors with cone-like characteristics. Brain Research. 1995;694:183–190. doi: 10.1016/0006-8993(95)00694-l. [DOI] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. Journal of Neuroscience. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Foster RG. Twilight times: light and the circadian system. Photochemistry and Photobiology. 1997;66:549–561. doi: 10.1111/j.1751-1097.1997.tb03188.x. [DOI] [PubMed] [Google Scholar]
- Shanahan TL. MD Dissertation. USA: Harvard Medical School; 1995. Circadian Physiology and the Plasma Melatonin Rhythm in Humans. [Google Scholar]
- Sharma VK, Chandrashekaran MK, Singaravel M, Subbaraj R. Relationship between light intensity and phase resetting in a mammalian circadian system. Journal of Experimental Zoology. 1999;283:181–185. doi: 10.1002/(sici)1097-010x(19990201)283:2<181::aid-jez8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Stevens SS. To honor Fechner and repeal his law. Science. 1961;133:80–86. doi: 10.1126/science.133.3446.80. [DOI] [PubMed] [Google Scholar]
- Thresher RJ, Vitaterna MH, Miyamoto Y, Kazantsev A, Hsu DS, Petit C, Selby CP, Dawut L, Smithies O, Takahashi JS, Sancar A. Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science. 1998;282:1490–1494. doi: 10.1126/science.282.5393.1490. [DOI] [PubMed] [Google Scholar]
- van Cauter E, Moreno-Reyes R, Akseki E, L'Hermite-Balériaux M, Hirschfeld U, Leproult R, Copinschi G. Rapid phase advance of the 24-h melatonin profile in response to afternoon dark exposure. American Journal of Physiology. 1998;275:E48–54. doi: 10.1152/ajpendo.1998.275.1.E48. [DOI] [PubMed] [Google Scholar]
- Waterhouse J, Minors D, Folkard S, Owens D, Atkinson G, MacDonald I, Reilly T, Sytnik N, Tucker P. Light of domestic intensity produces phase shifts of the circadian oscillator in humans. Neuroscience Letters. 1998;245:97–100. doi: 10.1016/s0304-3940(98)00174-8. [DOI] [PubMed] [Google Scholar]
- Wever R. Zur Zeitgeber-Stärke eines Licht-Dunkel-Wechsels für die circadiane Periodik des Menschen. Pflügers Archiv. 1970;321:133–142. doi: 10.1007/BF00586368. [DOI] [PubMed] [Google Scholar]
- Wever RA. Light effects on human circadian rhythms: A review of recent Andechs experiments. Journal of Biological Rhythms. 1989;4:161–185. [PubMed] [Google Scholar]
- Zeitzer JM. PhD Dissertation. USA: Harvard University; 1999. Physiology and Anatomy of Human Circadian Photoreception and Melatonin Regulation. [Google Scholar]
- Zeitzer JM, Daniels JE, Duffy JF, Klerman EB, Shanahan TL, Dijk D-J, Czeisler CA. Do plasma melatonin concentrations decline with age? American Journal of Medicine. 1999;107:432–436. doi: 10.1016/s0002-9343(99)00266-1. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Kronauer RE, Czeisler CA. Photopic transduction implicated in human circadian entrainment. Neuroscience Letters. 1997;232:135–138. doi: 10.1016/s0304-3940(97)00599-5. [DOI] [PubMed] [Google Scholar]


