Abstract
In skeletal muscle, catecholamines and calcitonin gene-related peptide (CGRP) increase the content of cAMP, which mediates stimulation of the Na+–K+ pump. Amylin is structurally very similar to CGRP and also increases cAMP in muscle.
In isolated rat soleus and extensor digitorum longus muscle, amylin produced a rapid and marked decrease in intracellular Na+, which was maintained for several hours. In soleus, amylin was found to induce a 45 % stimulation of Na+ efflux, a 43 % increase in 86Rb influx and a rise in intracellular K+. All these effects were abolished by ouabain, indicating that amylin produces acute stimulation of the Na+–K+ pump.
In contrast, neither the closely related peptides islet amyloid polypeptide (IAPP) and adrenomedullin nor other peptide hormones (C peptide, neuropeptide Y or substance P) produced any detectable change in intracellular Na+ or K+ uptake in soleus.
When contractility in soleus was inhibited by increasing extracellular K+ to 12.5 mm, amylin (10−8m) and insulin (0.7 × 10−8m) both induced partial recovery of force. These effects were additive, and in combination the two hormones elicited 63 and 80 % recovery of tetanic and twitch force, respectively. Higher concentrations produced even larger increases, and all effects were blocked by ouabain.
In buffer containing 12.5 mm K+, dibutyryl cAMP induced 71 % force recovery, which was increased by theophylline. The results indicate that amylin (like catecholamines, cAMP, CGRP and insulin) stimulates the Na+–K+ pump and thereby improves the contractility of depolarized skeletal muscle cells. This adds further support to the concept that the Na+–K+ pump is important for the maintenance of excitability in skeletal muscle.
Amylin is a 37 amino acid peptide present in the pancreatic β-cells (Cooper et al. 1987) and co-secreted with insulin into the bloodstream (Pieber et al. 1993). Its amino acid sequence is 46 % identical to that of CGRP (Cooper et al. 1987), and it has been shown to mimick several of the actions of CGRP (for reviews, see Rink et al. 1993; Pittner et al. 1994). Like CGRP (Stace et al. 1992), amylin was shown to increase the concentration of adenosine 3′,5′-cyclic monophosphate (cAMP) in rat soleus muscle (Pittner et al. 1994, 1995). cAMP mediates the stimulating effect of catecholamines on active Na+–K+ transport in skeletal muscle (Clausen & Flatman, 1977). It was conceivable, therefore, that amylin would exert a similar effect, in particular since CGRP produces a rapid and pronounced stimulation of active Na+–K+ transport in isolated rat skeletal muscle (Andersen & Clausen, 1993). Preliminary experiments showed that amylin increases net Na+ efflux from rat soleus muscle (Clausen, 1996), and a recent study demonstrated that in both soleus and extensor digitorum longus (EDL) muscles, amylin induces a clear-cut decrease in intracellular Na+ (James et al. 1999b).
As shown for a number of other agents that stimulate the Na+–K+ pump (Clausen et al. 1993), CGRP induced a considerable recovery of force in isolated soleus muscles inhibited by exposure to high extracellular K+ (Andersen & Clausen, 1993; Cairns et al. 1995). It was of interest, therefore, to examine the possible effects of amylin on Na+–K+ transport and contractility in rat skeletal muscle.
IAPP is another 37 amino acid peptide co-localized and co-secreted with insulin (Westermark et al. 1986; Novials et al. 1993; O'Brien et al. 1993). Since it has considerable homology with CGRP (Cooper et al. 1987) and amylin (Rink et al. 1993), it could be expected to exert similar actions on Na+–K+ transport. Adrenomedullin is a 52 amino acid peptide showing structural similarity to CGRP and specific binding to rat soleus muscle (Owji et al. 1995). To gain information about the specificity of the effects of amylin, these rather similar peptides were therefore characterized in parallel experiments.
Skeletal muscle cells are exposed to a number of peptide hormones reaching the sarcolemma by diffusion from the blood plasma (adrenomedullin, amylin, CGRP, C peptide, IAAP, insulin) or by release from blood vessels or nerve endings (adrenomedullin, CGRP, neuropeptide Y, substance P). Few of these have been examined for possible effects on the transport and distribution of Na+ and K+ in skeletal muscle. Rat muscle and a rat muscle cell line (L6) were shown to express specific adrenomedullin binding sites and adrenomedullin activates adenylate cyclase in L6 cells (Coppock et al. 1996). It seemed justifiable, therefore, to supplement the present analysis with studies on other peptide hormones. Moreover, like CGRP, several of these peptides are localized in nerve endings and blood vessels from where they may be released during electrical stimulation. We have recently shown that electrical stimulation produces a marked increase in the rate of active Na+–K+ transport in skeletal muscle which could in part be related to a release of CGRP from nerve endings in the muscle (Nielsen et al. 1998). It was of interest, therefore, to identify other peptide hormones that might possibly mediate the stimulation of the Na+–K+ pump associated with electrical stimulation.
METHODS
Animals
All handling and use of animals complied with Danish animal welfare regulations. All experiments were performed using fed 4-week-old female or male Wistar rats weighing 60–75 g. The animals were of own breed, had free access to food (Altromin pellets no. 1314, Spezialfutterwerke, Lage, Germany) and water and were kept in a thermostatically controlled environment (21°C) with a constant day length (12 h).
Muscle preparation and incubation
Animals were killed by decapitation, and intact soleus and EDL muscles weighing 20–25 mg were dissected out as described previously (Kohn & Clausen, 1971; Chinet et al. 1977).
The standard incubation medium was Krebs-Ringer bicarbonate buffer (pH 7.4 at 30°C) containing (mm): 120.1 NaCl, 25.1 NaHCO3, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1.3 CaCl2 and 5 D-glucose. In addition to the standard buffer, Krebs-Ringer bicarbonate buffers containing 4 or 12.5 mm K+ were used in studies on contractile performance. In these buffers an equivalent amount of Na+ was added or omitted to maintain isosmolarity.
Before incubation or tension recordings, all muscles were equilibrated for at least 30 min in the standard medium. With one exception, all experiments were carried out at 30°C to reduce metabolic requirements and thus ensure sufficient oxygenation of the central muscle fibres. The buffer was continuously gassed with a mixture of 95 % O2 and 5 % CO2. This procedure was shown to allow the maintenance of a high intracellular K+/Na+ ratio and a constant membrane potential for several hours in vitro and to prevent internal anoxia and ensuing muscle contractures (Kohn & Clausen, 1971; Clausen & Kohn, 1977; Clausen & Flatman, 1977).
Na+–K+ content and 86Rb uptake
Na+–K+ content and 86Rb uptake were measured as described in detail elsewhere (Andersen & Clausen, 1993). Brief descriptions are given in the legends to the figures and tables. 86Rb was used as a tracer for K+ and in all experiments only trace amounts of the element were present in the standard Krebs-Ringer buffer containing 5.93 mm K+. 86Rb was assumed to be representative of K+ and for the calculation of K+ uptake, the 86Rb activity taken up by the muscles (μCi (g wet wt)−1) was compared to that of the incubation medium (expressed as μCi (nmol K+)−1). Thus the K+ uptake could be expressed as nmoles per gram wet weight. For the quantification of Na+–K+ pump-mediated K+ uptake, 86Rb was earlier shown to yield the same results as 42K (Dørup & Clausen, 1997), and for the present purpose, therefore, could be expected to be a reliable tracer for K+.
[3H]Ouabain binding
In order to obtain a more specific indication of Na+–K+ pump activation, the rate of [3H]ouabain binding was measured with the use of a previously established procedure (Clausen & Hansen, 1977). In brief, soleus muscles were incubated for 15 min in standard buffer containing [3H]ouabain (1.6 μCi ml−1) and unlabelled ouabain added to a final concentration of 10−7m. Subsequently, the muscles were washed for 4 × 30 min in ice-cold unlabelled buffer to remove unbound [3H]ouabain from the extracellular phase. The muscles were blotted, weighed and taken for counting of 3H activity. After correction for unspecific uptake, the amount of specifically bound [3H]ouabain was expressed in picomoles per gram wet weight per 15 min, and the relative change given as a percentage of that bound to untreated control muscles. At the low concentration of ouabain used, the amount of [3H]ouabain bound corresponds to only a few per cent of the total population of [3H]ouabain binding sites present in the muscles, and it is well documented that this allows for a quantification of the initial rate of ouabain binding (Clausen & Hansen, 1977).
In other experiments, the saturable capacity for [3H]ouabain binding was measured by incubating soleus muscles for 120 min in K+-free Krebs-Ringer bicarbonate buffer containing 5 × 10−6m[3H]ouabain. Following washout at 0°C as described above, the concentration of [3H]ouabain binding sites was determined, and after correction for unspecific uptake, the concentration of [3H]ouabain binding sites was expressed as picomoles per gram wet weight.
Force development
Force development was recorded as described in detail elsewhere (Clausen & Everts, 1991). Soleus muscles with intact tendons were mounted in a thermostatically controlled (30°C) chamber (volume, 35 ml) containing Krebs-Ringer bicarbonate buffer with 4 mm K+ and stimulated directly via two platinum electrodes in contact with the muscle surface. Supramaximal pulses (10–15 V and 1 ms duration) were obtained from a pulse generator, and force development was measured with a force displacement transducer (Grass FTO3) using a chart recorder calibrated with standard weights. After adjustment to optimal length, active force of single twitches and tetanic contractions (30 Hz for 2 s) was tested. Changes in contractile performance were followed by applying single pulses or 2 s pulse trains of 30 Hz every 5 min.
Chemicals and isotopes
All chemicals were of analytical grade. 86Rb (400 mCi mol−1) was from the Danish Atomic Energy Commission (Isotope Laboratory, Risø, Denmark). [3H]Ouabain (15 Ci mmol−1) and 22NaCl (736 Ci mol−1) were from the Radiochemical Centre (Amersham). Peninsula Inc. supplied the following peptides (catalogue no. given in parentheses): rat adrenomedullin (9508), rat amylin (an amide, 7323), IAPP (an acid, 7321), rat CGRP (an amide, 6006), neuropeptide Y (an amide, 7180) and substance P (an amide, 7451). Dibutyryl cyclic AMP (dbcAMP), ouabain and theophylline were obtained from Sigma. Insulin was a gift from Novo Nordisk A/S (Copenhagen, Denmark) and C peptide was a gift from Professor Jon Wahren (Stockholm, Sweden).
Statistics
All results are given as mean values ±s.e.m., with n indicating the number of muscles used in each experiment. When only two groups were compared, the difference between their means was tested for statistical significance using Student's two-tailed t test for groups of non-paired observations. In one instance where the contractile force of the same muscle was compared before and after the addition of a compound, the analysis was based on paired comparison. For the comparison of multiple groups, a one-way ANOVA was performed. Group means were tested for significant differences using the Tukey test and a significance level of 0.05.
RESULTS
Na+–K+ content
Figure 1 shows the time course of the changes in intracellular Na+ and K+ in soleus muscles exposed to amylin (10−7m) for 60 min. Within 5 min after the onset of exposure to the hormone, there was a highly significant (P < 0.001) decrease in Na+ content. This decrease continued and was associated with a consistent increase in K+ content of approximately the same magnitude. Since the increase in K+ content was small compared to the scatter, however, this effect did not reach statistical significance except in one instance (10 min incubation, P < 0.025).
Figure 1. Time course of amylin action on intracellular Na+ and K+ in rat soleus muscle.
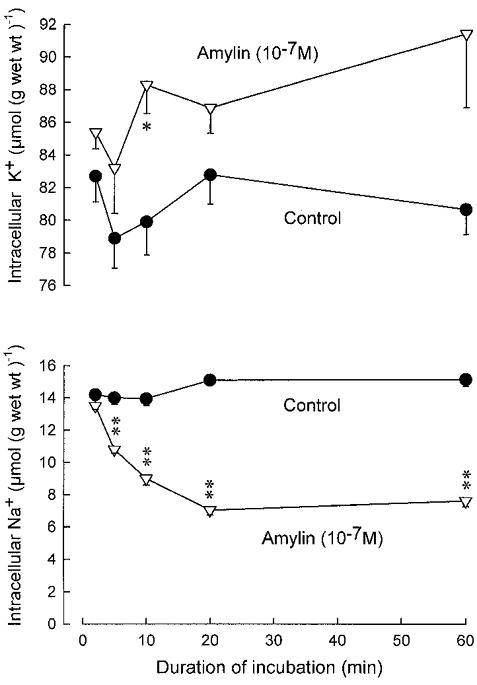
Groups of four muscles were incubated for between 2 and 60 min in the absence or presence of rat amylin (10−7m), followed by washout for 4 × 15 min in ice-cold Na+-free Tris-sucrose buffer. The amount of Na+ and K+ retained after the cold wash was determined as described previously (Andersen & Clausen, 1993). Each point represents the mean of observations on four to eight muscles with vertical bars denoting s.e.m. The statistical significance of the differences between control muscles and muscles incubated with amylin is indicated by asterisks: *P < 0.025, **P < 0.001.
When fully developed (after 60 min), the decline in Na+ content amounted to 49.7 % (7.5 μmol (g wet wt)−1) and the rise in K+ content to 13 % (10.8 μmol (g wet wt)−1). Other experiments showed that the amylin-induced decrease in intracellular Na+ was maintained for up to 240 min. In the presence of ouabain (10−3m), the effects of amylin on Na+ and K+ content were abolished.
At 37°C, incubation for 10 min with amylin (10−7m) also induced a significant decrease in intracellular Na+ content (from 12.7 ± 0.4 to 8.0 ± 0.4 μmol (g wet wt)−1; n = 4vs. 4, P < 0.001). All of the following experiments were performed at 30°C.
The dose–response relationship for the effect of amylin on intracellular Na+ in soleus and EDL muscles was explored in incubations of 120 min duration. As shown in Fig. 2, the Na+ content of untreated EDL was clearly lower than that of soleus. When exposed to amylin, the Na+ content of both muscles was reduced, reaching the same level of around 6 μmol (g wet wt)−1. In soleus, the relative decrease in Na+ content was more pronounced than in EDL and a significant effect was seen at a much lower concentration of amylin (10−8m). In these experiments, amylin (10−7 and 10−6m) was found to produce a small (4 %), but significant (P < 0.05) increase in the K+ content of soleus. In EDL, amylin only produced a significant increase (5 %; P < 0.05) in K+ content when present at a concentration of 10−6m.
Figure 2. Effect of varying concentrations of rat amylin on intracellular Na+ in soleus and EDL muscles.
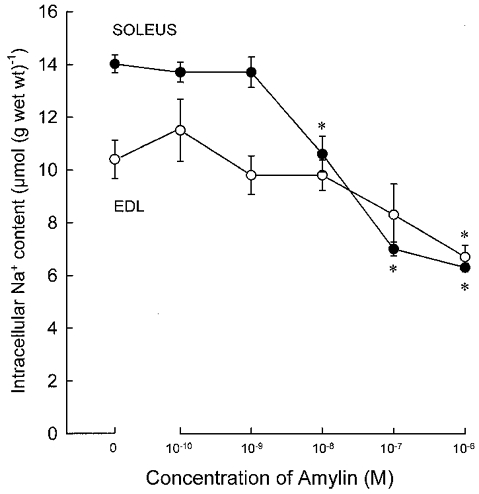
Experimental conditions as described in the legend to Fig. 1, except that all incubations lasted for 120 min. Each point represents the mean of observations on 8–12 muscles with bars denoting 2 s.e.m.* Significant difference from control muscles (P < 0.001).
The effect of amylin on Na+ content in soleus muscle was compared to that of other peptide hormones. As shown in Table 1, rat CGRP (10−7m) produced the same decrease in intracellular Na+ as amylin, whereas insulin (7 × 10−7m) produced a somewhat smaller decrease. In contrast, adrenomedullin, IAPP, neuropeptide Y, C peptide and substance P produced no significant change in intracellular Na+. C peptide (10−8 to 10−6m) also did not produce any detectable change in intracellular Na+ or K+ content in incubations of longer duration (90 min; data not shown). Also, in another series of experiments with incubations of 15 min duration, amylin and insulin both produced a highly significant decrease in intracellular Na+ (n = 8vs. 8; P < 0.05). When combined, amylin and insulin produced a significantly larger decrease in intracellular Na+ than insulin alone (n = 8vs. 8; P < 0.05).
Table 1. Effects of peptide hormones on intracellularNa+ in rat soleus muscle.
| Additions | Intracellular Na+ (μmol (g wet wt)−1) | P |
|---|---|---|
| Control | 13.6 ± 0.3(16) | — |
| Amylin (10−7m) | 7.0 ± 0.1(4) | <0.05 |
| CGRP (10−7m) | 5.8 ± 0.2(4) | <0.05 |
| Insulin(7×10−7m) | 9.0 ± 0.2(4) | <0.05 |
| Control | 14.9 ± 0.1(4) | — |
| Adrenomedullin(10−7m) | 13.0 ± 0.6(4) | n.s. |
| IAPP(10−7m) | 14.5 ± 1.1(4) | n.s. |
| Control | 15.1 ± 0.7(4) | — |
| Neuropeptide Y(10−7m) | 15.9 ± 0.6(4) | n.s. |
| Control | 14.0 ± 0.6(4) | — |
| C peptide(10−7m) | 13.3 ± 0.6(4) | n.s. |
| Control | 14.0 ± 0.2(8) | — |
| Substance P (10−7m) | 13.6 ± 0.4(8) | n.s. |
Experimental conditions as described in the legend to Fig. 2, but using only 20 min incubation periods. Each value represents the mean ±s.e.m. with the number of observations (muscles) indicated in parentheses. The significance of the difference between control and experimental values was assessed by multiple comparison procedures (Tukey test).
22Na fluxes
In order to determine whether the decrease in intracellular Na+ was due to reduced Na+ influx, the effect of 10−7m amylin on 22Na influx was tested in the presence of ouabain (10−3m). 22Na influx amounted to 1192 ± 40 and 1150 ± 23 nmol (g wet wt)−1 min−1 in the absence and presence of amylin, respectively (n = 4vs. 4, P > 0.30). In contrast, as shown in Fig. 3, in the absence of ouabain 10−7m amylin induced a 45 % increase in the fractional loss of 22Na within the first 10 min of exposure. This effect was highly significant (P < 0.001) and was completely blocked by ouabain (10−3m). Measurements of Na+ and K+ content following washout at the end of the experiment showed that amylin had produced a significant decrease in total Na+ (6.4 μmol (g wet wt)−1, P < 0.01), and an equivalent increase in total K+ (6.3 μmol (g wet wt)−1, P < 0.05). These results indicate that the effect of amylin on intracellular Na+ is due to stimulation of Na+–K+ pump-mediated Na+ efflux.
Figure 3. Effect of rat amylin (10−7m) on 22Na extrusion from soleus muscle.

Muscles were loaded for 60 min at 30 °C in buffer containing 22Na (10 μCi ml−1), and then washed for 8 × 10 min at 30 °C in eight tubes containing unlabelled buffer without or with the indicated additions. Ouabain (10−3m), when added, was present from the onset of washout. Horizontal bar indicates the addition of rat amylin. By successive addition of the 22Na activity in the washout tubes to that in the muscle at the end of experiment, activity in the muscle before each transfer was calculated. On the basis of these values, the fractional loss of 22Na activity could be calculated for each 10 min washout period. Each point represents the mean of observations on six muscles with bars denoting s.e.m.* Significant difference from control muscles (P < 0.01).
86Rb uptake
Figure 4 shows the effect of amylin (10−7m) on the time course of 86Rb uptake in rat soleus. It appears that within the first 2 min of exposure to the hormone, 86Rb uptake was already significantly increased (P < 0.01). The stimulating effect seemed to reach its maximum (+43 %) after 10 min.
Figure 4. Effect of rat amylin on the time course of 86Rb uptake in rat soleus muscle.
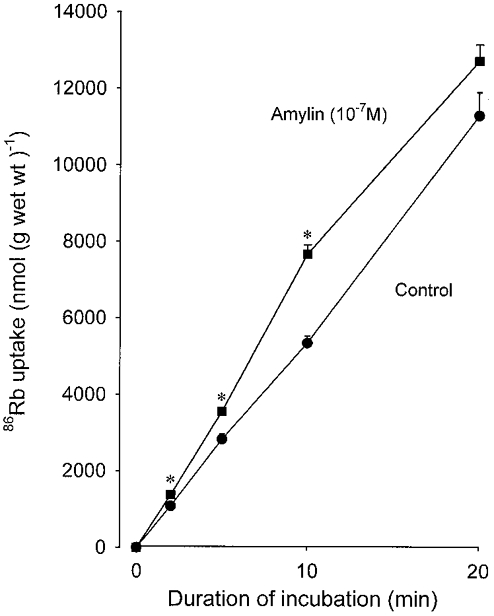
Experimental conditions as described in the legend to Fig. 1. The incubation medium contained 0.1 μCi ml−186Rb. The uptake of K+ was calculated on the basis of the 86Rb activity taken up in the muscles and retained following 4 × 15 min washout in ice-cold Na+-free Tris-sucrose buffer compared with the 86Rb activity in the incubation medium (expressed as nmol (g wet wt)−1). Each point represents the mean of observations on four muscles with bars denoting s.e.m.* Significant difference from control muscles (P < 0.01).
Table 2 shows the effects of supramaximal concentrations of amylin and other peptide hormones on 86Rb uptake as measured over 10 and 15 min incubation periods. It is evident that ouabain (10−3m) completely suppressed the stimulating effect of amylin. By deducting the ouabain-resistant component of 86Rb uptake, it could be calculated that amylin stimulated the ouabain-suppressible component of 86Rb uptake by 53 %. Insulin (7 × 10−7m) produced almost the same increase (55 %) in ouabain-suppressible 86Rb uptake as amylin (10−7m), and when both hormones were present, their effects on ouabain-suppressible 86Rb uptake were additive and a significantly larger increase was observed (90 %, P < 0.05). CGRP (10−7m) produced almost the same increase in 86Rb uptake as amylin. In contrast, the same concentration of adrenomedullin, substance P and IAPP produced no significant change (Table 2). Other experiments showed that, using a 20 min incubation period, neuropeptide Y, C peptide and substance P produced no significant change in 86Rb uptake (data not shown).
Table 2. Effects of peptide hormones and ouabain on 86Rb uptake in rat soleus muscle.
| Additions | 86Rb uptake (nmol (g wet wt)−1 min−1) | P |
|---|---|---|
| 15 min incubation | ||
| Control | 504 ± 11 (8) | — |
| Amylin (10−7m) | 623 ± 20 (8) | <0.05 |
| Insulin (7 × 10−7m) | 643 ± 15 (8) | <0.05 |
| Insulin + amylin | 719 ± 16 (8) | <0.05 |
| Ouabain (10−3m) | 257 ± 4 (4) | <0.05 |
| Ouabain + amylin | 245 ± 12 (4) | <0.05 |
| Ouabain + insulin | 261 ± 8 (4) | <0.05 |
| Ouabain + insulin + amylin | 249 ± 4 (4) | <0.05 |
| 10 min incubation | ||
| Control | 537 ± 26 (8) | — |
| CGRP (10−7m) | 715 ± 20 (8) | <0.05 |
| Substance P (10−7m) | 547 ± 14 (8) | n.s. |
| Control | 595 ± 27 (12) | — |
| IAPP (10−7m) | 596 ± 16 (12) | n.s. |
| Control | 547 ± 44 (4) | — |
| Adrenomedullin (10−7m) | 523 ± 15 (4) | n.s. |
Experimental conditions as described in the legend to Fig. 4, but using incubation periods of 15 or 10 min.86Rb uptake values are given as means ±s.e.m.with the number of observations in parentheses. The significance of the difference between control and experimental values was assessed by multiple comparison procedures (Tukey test).
[3H]Ouabain binding
Earlier studies have shown that acute hormonal or non-hormonal stimulation of the Na+–K+ pump leads to an increase in the rate of [3H]ouabain binding (Clausen & Hansen, 1977; Clausen, 1986). Amylin (10−7m) and insulin (7 × 10−7m) increased the rate of [3H]ouabain binding in rat soleus by 54 and 50 %, respectively (n = 4vs. 4, P < 0.05 in both instances). In EDL, amylin (10−7m) and insulin (7 × 10−7m) increased the rate of [3H]ouabain binding by 33 and 41 %, respectively (n = 4vs. 4, P < 0.05). In order to explore whether amylin or insulin induced any change in the pool of Na+–K+ pumps located in the sarcolemma of soleus muscles, experiments were performed using a higher (saturating) concentration of ouabain (5 × 10−6m). Following equilibration to steady state (120 min), there was no significant difference between the amount of ouabain bound in the control muscles (530 ± 81 pmol (g wet wt)−1, n = 4) and in the muscles exposed to 10−7m amylin (510 ± 81 pmol (g wet wt)−1,n = 4) or 7 × 10−7m insulin (505 ± 84 pmol (g wet wt)−1, n = 4).
Contractile performance
In isolated soleus muscles prepared from 4-week-old rats, stimulation with 2 s pulse trains of 30 Hz caused a force development of 37.8 ± 1.2 g. Following around 60 min of exposure to a buffer containing 12.5 mm K+, subtetanic contractions as well as single twitches were reduced to a plateau 85–90 % below the control level (Fig 5 and Fig 6).
Figure 5. Effect of rat amylin and insulin on tetanic contractions of soleus muscle exposed to buffer containing 12.5 mm K+.
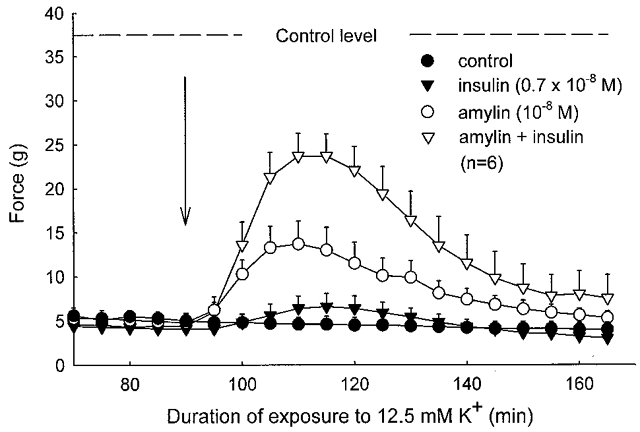
Isolated muscles were mounted in force transducers in a thermostatically controlled (30 °C) chamber containing Krebs-Ringer bicarbonate buffer continuously bubbled with a mixture of 95 % O2 and 5 % CO2. Active force development during 2 s of 30 Hz stimulation was recorded at 5 min intervals, and the mean value of these measurements (the starting control level) is indicated by the dashed line. After 30 min of equilibration, K+ was increased to 12.5 mm, and after 120 min, at the arrow, amylin (10−8m), insulin (0.7 × 10−8m) or the two hormones combined were added, and recordings continued. Each point represents the mean of observations on six muscles with bars denoting s.e.m.
Figure 6. Effects of rat amylin and insulin on twitch contractions of rat soleus muscles exposed to buffer containing 12.5 mm K+.
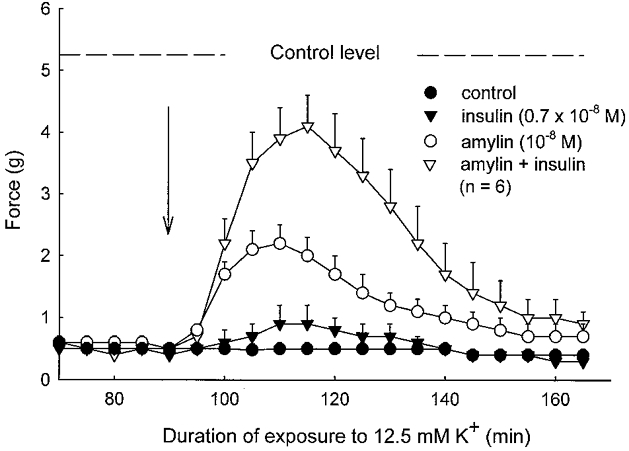
Experimental conditions as described in the legend to Fig. 5. Twitch contractions were elicited using single pulses of 1 ms duration every 5 min as indicated. The mean value of these measurements (the starting control level) is indicated by the dashed line. Each point represents the mean of observations on six muscles with bars denoting s.e.m.
In these K+-inhibited muscles, insulin (0.7 × 10−8m) and amylin (10−8m) produced a recovery of subtetanic force to, respectively, 18 % (P > 0.05) and 37 % (P < 0.05) of the force developed at 4 mm K+. When combined, the two hormones induced a force recovery of 63 % (P < 0.05, when compared to the untreated controls, and P < 0.01 when compared to amylin or insulin alone).
Figure 6 shows that in the same muscles, insulin, amylin or the two hormones combined produced a slightly larger relative recovery of twitch force (to 19, 40 and 80 % of the force at 4 mm K+, respectively; n.s., P < 0.05 and P < 0.05, respectively). With supramaximal concentrations of insulin (7 × 10−7m), amylin (10−7m) or insulin and amylin added simultaneously at the same concentrations, the recovery of subtetanic contractions was even higher (58 ± 3 %, n = 7,P < 0.05; 67 ± 9 %, n = 4,P < 0.05; and 83 %, n = 2, respectively). Again, the combined effect of amylin and insulin was larger than that of insulin or amylin alone. The force recovery induced by insulin or amylin was completely prevented by ouabain (10−3m) added 1 min before the two hormones (n = 2, data not shown). Another set of experiments showed that the effect of amylin on tetanic force recovery could be detected down to a concentration of 10−9m (38 ± 4 % recovery, n = 4vs. 4, P < 0.05, paired comparison).
In parallel experiments, the effects of amylin and insulin on intracellular Na+ content were examined in muscles incubated in buffer containing 12.5 mm K+. In muscles that had been pre-exposed to 12.5 mm K+ for 45 min, the addition of insulin (0.7 × 10−8m), amylin (10−8m), or the two in combination caused a decrease in the Na+ content by 7 % (P < 0.05), 34 % (P < 0.05) and 39 % (P < 0.05), respectively (4 groups, each n = 8).
Since amylin has been shown to increase the concentration of cAMP in rat soleus, and since cAMP stimulates the Na+–K+ pump in the same preparation (Clausen & Flatman, 1977), it was of general interest to determine the effects of dbcAMP, which enters the muscle cells more readily than cAMP. From Table 3 it can be seen that in muscles exposed to 12.5 mm K+, dbcAMP (1 mm) elicited a significant recovery (106 % increase, P < 0.05) of tetanic force. Theophylline, which inhibits the degradation of cAMP and thus can be expected to increase the intracellular level of cAMP, also induced a significant force recovery (62 % increase, P < 0.05). When theophylline was added together with dbcAMP, force recovery was significantly larger (49 %, P < 0.05) than with theophylline alone. In combination, the two agents produced a 142 % increase in tetanic force, when compared with the control level.
Table 3. Effects of dbcAMP and theophylline, alone or in combination, on force recovery in K+paralysed rat soleus muscle.
| Experimental conditions | Tetanic force (% initial level) | P* | P† |
|---|---|---|---|
| 12.5 mm K+ | 34.6 ± 3.0 (16) | — | — |
| 12.5 mmK++ 1 mm dbcAMP | 71.3 ± 2.7 (4) | <0.05 | n.s. |
| 12.5 mmK++ 1 mm dbcAMP + 0.1 mm theophylline | 83.7 ± 3.9 (6) | < 0.05 | < 0.05 |
| 12.5 mm K++ 0.1 mm theophylline | 56.3 ± 11.5 (4) | < 0.05 |
Experimental conditions as described in the legend to Fig. 5. In each muscle, the tetanic force recorded in the presence of buffer containing 12.5 mm K+ was expressed as a percentage of the initial value measured in buffer containing 4 mm K+. The values shown were recorded after 90 min of exposure to 12.5 mm K+ and again at the peak increase reached after the additions indicated. Relative force is given as means ±s.e.m. with the number of observations in parentheses.
The statistical significance of the difference between values recorded before and after the indicated additions was assessed by multiple comparison procedures (Tukey test).
The statistical significance of differences between muscles exposed to dbcAMP, dbcAMP plus theophylline and theophylline alone was assessed by nonpaired comparison.
DISCUSSION
The present results demonstrate that in rat soleus muscle, amylin produces a rapid and pronounced stimulation of K+ influx and Na+ efflux, leading to a new steady state with decreased intracellular Na+ and an almost equivalent rise in K+ content. This is in keeping with the observation that in rat soleus and EDL muscles, amylin at concentrations down to 10−8m induces a significant decrease in the intracellular Na+/K+ ratio (James et al. 1999b). Since all these effects were abolished by ouabain, it is justifiable to assume that amylin stimulates the Na+–K+ pump in skeletal muscle. The early onset of the effect on K+ influx (2 min) as well as the rise in intracellular K+ indicates that this stimulation is not the result of the opening of K+ channels and the ensuing Na+–K+ pump activation induced by elevated extracellular K+. Moreover, a rise in extracellular K+ would inhibit the rate of [3H]ouabain binding. On the contrary, amylin was found to increase the rate of [3H]ouabain binding, a specific indication that the rate of Na+–K+ pumping had been accelerated (Clausen & Hansen, 1977). It should be noted that amylin and insulin produced similar increases in the rate of [3H]ouabain binding, both in soleus and in EDL muscles. This indicates that the Na+–K+ pump stimulation elicited by the two hormones is not restricted to muscles with predominantly type 1 fibres.
Neither amylin nor insulin caused any change in the binding capacity for [3H]ouabain measured under saturating conditions. Thus, the stimulating effects of these hormones on active Na+–K+ transport are not associated with any increase in the pool of Na+–K+ pumps associated with the sarcolemma and therefore are not likely to reflect translocation of Na+–K+ pumps from an intracellular pool to the plasma membrane. Taken together, the data provide strong evidence that amylin stimulates the Na+–K+ pump within a few minutes, eliciting a transient increase in the efflux of Na+ and the influx of K+, leading to a new steady state with decreased intracellular Na+. As previously proposed for other hormones (Clausen, 1986, 1996), this indicates that amylin increases the affinity of the Na+–K+ pump for intracellular Na+, allowing the muscle cells to maintain a steeper gradient for Na+ across the plasma membrane. As intracellular Na+ is reduced, less activation of the Na+–K+ pump takes place and the rate of active Na+–K+ transport returns towards the control level.
As first described by James et al. (1999b), the stimulating effect of amylin on aerobic lactate production in rat skeletal muscle can to a large extent be attributed to Na+–K+ pump stimulation. A similar relation was observed for the effect of adrenaline on lactate production. In rat diaphragm, the glycogenolytic effects of adrenaline and dbcAMP were shown to be markedly inhibited by cardiac glycosides (Kypson et al. 1968). Moreover, in the same preparation, the stimulating effect of insulin on aerobic lactate production was found to be suppressed by ouabain (Clausen, 1965). Taken together, these observations support the general concept that in skeletal muscle, stimulation of the Na+–K+ pump is closely associated with activation of aerobic glycolysis (James et al. 1999a).
The amylin-induced stimulation of the Na+–K+ pump is similar to that exerted by calcitonins and CGRP (Andersen & Clausen, 1993), indicating that it could be related to the structural similarity between these peptides. High affinity CGRP receptors have been demonstrated on the sarcolemma (Popper & Micevych, 1989), and might mediate the effect of these peptides on active Na+–K+ transport. Indeed, amylin displaces CGRP from its receptors, albeit with an affinity 100–1000 times lower than that of CGRP (Pittner et al. 1994). In keeping with this, the minimum concentration of amylin (10−8m) required to produce a significant decrease in intracellular Na+ was in this study found to be around 10 times larger than that earlier reported for CGRP (Andersen & Clausen, 1993).
Plasma amylin in rats and mice is in the range 0.3 × 10−10 to 1.0 × 10−10m, and in diabetic animals it may reach 6 × 10−10m (Rink et al. 1993). It seems unlikely, therefore, that the circulating levels of amylin alone are sufficient to produce much stimulation of the Na+–K+ pump in normal skeletal muscle. On the other hand, 10−9m amylin produced a significant force recovery in muscles exposed to 12.5 mm K+. More detailed studies are required to determine whether the combination of physiological concentrations of insulin and amylin is sufficient to elicit Na+–K+ pump stimulation and force recovery. This is of particular interest since the two hormones are co-secreted from the β-cells. It remains to be determined whether amylin participates in the insulin-mediated regulation of plasma K+. Intravenous infusion of amylin into rats was found to elicit a significant drop (16 %) in plasma K+ (Vine et al. 1998) which was interpreted as the result of increased Na+–K+ pump-mediated K+ uptake in skeletal muscle. Thus, amylin might be used for the treatment of hyperkalaemia.
Amylin increases cAMP in rat soleus (Pittner et al. 1994, 1995). The present results show that the effect of amylin on force recovery can be mimicked by dbcAMP and that this effect was augmented by inhibiting the degradation of cAMP with theophylline. Taken together, the data provide further support to the general concept that hormonal activation of adenylate cyclase in skeletal muscle leads to a cAMP-mediated activation of the electrogenic Na+–K+ pump (Clausen & Flatman, 1977). This, in turn, induces hyperpolarization and allows the maintenance of steeper concentration gradients for Na+ and K+. This explains why not only catecholamines, but also calcitonins, CGRP and amylin elicit a considerable recovery of contractility in muscles that have been depolarized by high extracellular K+ (Andersen & Clausen, 1993; Clausen et al. 1993).
The effects of amylin on force recovery as well as on intracellular Na+ and 86Rb uptake were additive to those induced by insulin. Thus, there seem to be two different pathways for the hormonal stimulation of the Na+–K+ pump in muscle cells. This is in keeping with earlier observations that the effects of insulin and adrenaline on the Na+–K+ pump in rat soleus are additive (Flatman & Clausen, 1979). It should be noted that in buffer containing 12.5 mm K+, the force recovery induced by hormones was transient, possibly because once the new steady state is established, intracellular Na+ is reduced leading to a decreased electrogenic contribution of the Na+–K+ pump with an ensuing reduction of the hyperpolarization. Several experiments have shown that at 10 mm K+, hormonal and non-hormonal stimulation of the Na+–K+ pump lead to more complete force recovery, which can be maintained for a considerably longer time (Andersen & Clausen, 1993; Clausen et al. 1993; Nielsen et al. 1998). This might be related to the fact that at 10 mm K+, the depolarization is less pronounced than at 12.5 mm K+ and therefore is more efficiently counterbalanced by the hormonal Na+–K+ pump stimulation.
The present study adds one more example to what seems to be a growing category of agents exerting acute stimulating actions on active Na+–K+ transport in skeletal muscle (Clausen, 1996). The stimulating effect of insulin is well documented. More recently, insulin-like growth factor I was shown to exert virtually the same effect on the Na+–K+ pump as insulin (Dørup & Clausen, 1995). On the other hand, cAMP and hormones eliciting a rise in the cellular content of cAMP exert a somewhat more pronounced and rapid stimulation (Clausen, 1986). It is interesting that the endocrine factors shown to elicit stimulation of the Na+–K+ pump also seem capable of inducing force recovery of muscles exposed to inhibitory concentrations of K+. This adds a functional dimension to the present observations and further substantiates the idea that the Na+–K+ pump plays an essential role in the maintenance of excitability and contractility in skeletal muscle.
Acknowledgments
I thank Ann Charlotte Andersen, Tove Lindahl Andersen, Ebba de Neergaard, Bente Mortensen and Marianne Stürup-Johansen for technical assistance and Professor Jon Wahren for the gift of C peptide. This study was supported by grants from the Danish Biomembrane Research Centre and the Danish Medical Research Council (grant no. 12–1336).
References
- Andersen SLV, Clausen T. Calcitonin gene-related peptide stimulates active Na+-K+ transport in rat soleus muscle. American Journal of Physiology. 1993;264:C419–429. doi: 10.1152/ajpcell.1993.264.2.C419. [DOI] [PubMed] [Google Scholar]
- Cairns S, Flatman JA, Clausen T. Relation between extracellular [K+], membrane potential and contraction in rat soleus muscle: modulation by the Na+-K+ pump. Pflügers Archiv. 1995;430:909–915. doi: 10.1007/BF01837404. [DOI] [PubMed] [Google Scholar]
- Chinet A, Clausen T, Girardier L. Microcalorimetric determination of energy expenditure due to active sodium-potassium transport in the soleus muscle and brown adipose tissue of the rat. The Journal of Physiology. 1977;265:43–61. doi: 10.1113/jphysiol.1977.sp011704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. II. Effects of K+-free medium, ouabain and insulin upon the fate of glucose in rat diaphragm. Biochimica et Biophysica Acta. 1965;120:361–368. doi: 10.1016/0926-6585(66)90303-7. [DOI] [PubMed] [Google Scholar]
- Clausen T. Regulation of active Na+-K+ transport in skeletal muscle. Physiological Reviews. 1986;66:542–580. doi: 10.1152/physrev.1986.66.3.542. [DOI] [PubMed] [Google Scholar]
- Clausen T. Long- and short-term regulation of the Na+-K+ pump in skeletal muscle. News in Physiological Sciences. 1996;11:24–30. [Google Scholar]
- Clausen T, Andersen SLV, Flatman JA. Na+-K+ pump stimulation elicits recovery of contractility in K+-paralysed rat muscle. The Journal of Physiology. 1993;472:521–536. doi: 10.1113/jphysiol.1993.sp019960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Everts ME. K+-induced inhibition of contractile force in rat skeletal muscle: role of active Na+-K+ transport. American Journal of Physiology. 1991;261:C799–807. doi: 10.1152/ajpcell.1991.261.5.C799. [DOI] [PubMed] [Google Scholar]
- Clausen T, Flatman JA. The effect of catecholamines on Na-K transport and membrane potential in rat soleus muscle. The Journal of Physiology. 1977;270:383–414. doi: 10.1113/jphysiol.1977.sp011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Hansen O. Active Na-K transport and the rate of ouabain binding. The effect of insulin and other stimuli on skeletal muscle and adipocytes. The Journal of Physiology. 1977;270:415–430. doi: 10.1113/jphysiol.1977.sp011959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Kohn PG. The effect of insulin on the transport of sodium and potassium in rat soleus muscle. The Journal of Physiology. 1977;265:19–42. doi: 10.1113/jphysiol.1977.sp011703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GJS, Willis AC, Clark A, Turner RC, Sim RB, Reid KBM. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proceedings of the National Academy of Sciences of the USA. 1987;84:8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppock HA, Owji AA, Bloom SR, Smitch DM. A rat skeletal muscle cell line (L6) expresses specific adrenomedullin binding sites but activates adenylate cyclase via calcitonin gene-related peptide receptors. Biochemical Journal. 1996;318:241–245. doi: 10.1042/bj3180241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dørup I, Clausen T. Insulin-like growth factor I stimulates active Na+-K+ transport in rat soleus muscle. American Journal of Physiology. 1995;268:E849–857. doi: 10.1152/ajpendo.1995.268.5.E849. [DOI] [PubMed] [Google Scholar]
- Dørup I, Clausen T. 86Rb is not a reliable tracer for potassium in skeletal muscle. Biochemical Journal. 1997;302:745–751. doi: 10.1042/bj3020745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman JA, Clausen T. Combined effects of adrenaline and insulin on active electrogenic Na+-K+ transport in rat soleus muscle. Nature. 1979;281:580–581. doi: 10.1038/281580a0. [DOI] [PubMed] [Google Scholar]
- James JH, Luchette FA, McCarter FD, Fischer JE. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet. 1999a;354:505. doi: 10.1016/S0140-6736(98)91132-1. [DOI] [PubMed] [Google Scholar]
- James JH, Wagner KR, King J-K, Leffler RE, Upputuri RK, Balasubramaniam A, Friend LA, Shelly DA, Paul RJ, Fisher JE. Stimulation of both aerobic glycolysis and Na+-K+-ATPase activity in skeletal muscle by epinephrine and amylin. American Journal of Physiology. 1999b;277:E176–186. doi: 10.1152/ajpendo.1999.277.1.E176. [DOI] [PubMed] [Google Scholar]
- Kohn PG, Clausen T. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. VI. The effect of insulin, ouabain, and metabolic inhibitors on the transport of 3-0-methylglucose and glucose in rat soleus muscle. Biochimica et Biophysica Acta. 1971;225:277–290. doi: 10.1016/0005-2736(71)90221-5. [DOI] [PubMed] [Google Scholar]
- Kypson J, Triner L, Nahas GG. The effects of cardiac glycosides and their interaction with catecholamines on glycolysis and glycogenolysis in skeletal muscle. Journal of Pharmacology and Experimental Therapeutics. 1968;164:22–30. [PubMed] [Google Scholar]
- Nielsen OB, Hilsted L, Clausen T. Excitation-induced force recovery in potassium-inhibited rat soleus muscle. The Journal of Physiology. 1998;512:819–829. doi: 10.1111/j.1469-7793.1998.819bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novials A, Sarri Y, Casamitjana R, Rivera F, Gomis R. Regulation of islet amyloid polypeptide in human pancreatic islets. Diabetes. 1993;42:1514–1519. doi: 10.2337/diab.42.10.1514. [DOI] [PubMed] [Google Scholar]
- O'Brien TD, Butler PC, Westermark P, Johnson KH. Islet amyloid polypeptide: A review of its biology and potential roles in the pathogenesis of diabetes mellitus. Veterinary Pathology. 1993;30:317–332. doi: 10.1177/030098589303000401. [DOI] [PubMed] [Google Scholar]
- Owji AA, Smith DM, Hedlay AC, Morgan DGA, Bhogal R, Ghatei MA, Bloom SR. An abundant and specific binding site for the novel vasodilator adrenomedullin in the rat. Endocrinology. 1995;136:2127–2134. doi: 10.1210/endo.136.5.7720662. [DOI] [PubMed] [Google Scholar]
- Pieber TR, Stein DT, Ogawa A, Alam T, Ohneda M, McCorkle K, Chen L, McGarry JD, Unger RH. Amylin-insulin relationships in insulin resistance with and without diabetic hyperglycemia. American Journal of Physiology. 1993;265:E446–453. doi: 10.1152/ajpendo.1993.265.3.E446. [DOI] [PubMed] [Google Scholar]
- Pittner RA, Albrandt K, Beaumont K, Gaeta LSL, Koda JR, Moore CA, Rittenhouse J, Ring TJ. Molecular physiology of amylin. Journal of Cellular Biochemistry. 1994;55(suppl.):19–28. doi: 10.1002/jcb.240550004. [DOI] [PubMed] [Google Scholar]
- Pittner RA, Beaumont K, Young A, Rink TJ. Dose-dependent elevation of cyclic AMP, activation of glycogen phosphorylase, and release of lactate by amylin in rat skeletal muscle. Biochimica et Biophysica Acta. 1995;1267:75–82. doi: 10.1016/0167-4889(95)00033-o. [DOI] [PubMed] [Google Scholar]
- Popper P, Micevych PE. Localization of calcitonin gene-related peptide from nerve terminals in rat skeletal muscle. The Journal of Physiology. 1989;496:180–186. doi: 10.1016/0006-8993(89)91064-0. [DOI] [PubMed] [Google Scholar]
- Rink TJ, Beaumont K, Koda J, Young A. Structure and biology of amylin. Trends in Pharmacological Sciences. 1993;14:113–118. doi: 10.1016/0165-6147(93)90081-t. [DOI] [PubMed] [Google Scholar]
- Stace PB, Fatania HR, Jackson A, Kerbey AL, Randle P. Cyclic AMP and free fatty acids in the longer-term regulation of pyruvate dehydrogenase kinase in rat soleus muscle. Biochimica et Biophysica Acta. 1992;1135:201–206. doi: 10.1016/0167-4889(92)90137-z. [DOI] [PubMed] [Google Scholar]
- Vine V, Smith P, LaChappell R, Blase E, Young A. Effects of rat amylin on renal function in the rat. Hormone and Metabolic Research. 1998;30:518–522. doi: 10.1055/s-2007-978924. [DOI] [PubMed] [Google Scholar]
- Westermark P, Wernstedt C, Wilander E, Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochemical and Biophysical Research Communications. 1986;140:827–831. doi: 10.1016/0006-291x(86)90708-4. [DOI] [PubMed] [Google Scholar]


