Abstract
The objective of this study was to determine whether the concentrations of proglycogen (PG) and macroglycogen (MG) in biopsy samples of horse muscle are influenced by extraction time or perchloric acid (PCA) concentration. In study 1, individual muscle-biopsy samples from 10 horses were divided into 4 parts each and then randomly subjected to 4 periods of extraction (10, 20, 60, or 120 min) with 1.5 M PCA. In study 2, individual muscle-biopsy samples from 6 horses were divided into 24 pieces each and then randomly subjected to 12 combinations of extraction time (10, 20, 30, or 40 min) and PCA concentration (0.5, 1.5, or 3.0 M). The results from study 1 indicated that PG and MG concentrations are affected only after extraction for 120 min; the PG concentration decreased significantly (P < 0.05), and the MG concentration increased (not significantly). In study 2, extraction in 3.0 M PCA yielded significantly lower PG and higher MG concentrations (P < 0.05) than extraction in 0.5 or 1.5 M PCA with each of the extraction times. The results of this study further support the existence of 2 glycogen pools and demonstrate that they are not an extraction artifact. The study also suggests that the 2 pools are stable during extraction over a range of extraction times and acid concentrations. However, if the exposure to acid is very long and, or, the acid concentration is high, some of the insoluble PG appears to be hydrolyzed and to enter the MG pool.
Introduction
The metabolism of muscle glycogen during exercise has been intensely studied in both humans and horses. Several earlier studies conducted on human and rat muscle indicated that glycogen is not a uniform molecule (1,2,3). Recent studies have shown that glycogen exists in 2 molecular forms, proglycogen (PG) and macroglycogen (MG) (4,5,6). The 2 forms have identical protein content but differ in the amount of associated carbohydrate. Proglycogen is smaller, with a molecular mass up to 400 kDa; MG has a maximum molecular weight of approximately 10 000 kDa (7). Because of the difference in the ratio of carbohydrate to protein between the 2 molecules, they can be separated on the basis of their solubility in acid. Proglycogen precipitates in trichloroacetic acid (TCA) and perchloric acid (PCA), whereas MG is soluble in these acids (6,7). The relationship between the 2 pools of glycogen in human skeletal muscle is not influenced by the type of acid (PCA vs TCA) or by the strength of the PCA in the range of 0.5 to 3 M (3).
It is not clear whether PG and MG exist as 2 distinct molecular forms or merely represent a continuum of size (4,5,8,9). Several reports have shown that the 2 forms of glycogen can be separated on the basis of their solubility in PCA (6,10,11,12). Recent studies have indicated that the 2 forms function as different metabolic pools in glycogenolysis and resynthesis (10,12,13,14). The magnitude of the glycogenolysis appears to depend on factors such as exercise intensity and duration. Proglycogen is degraded more than MG during brief, intense exercise in humans (13,14), whereas glycogenolysis is greater in the MG pool than the PG pool during extreme energy demands, such as marathon races (15). After glycogen-depleting exercise, the PG resynthesis precedes the MG resynthesis (10). These studies indicate that PG and MG differ not only in terms of acid solubility but also in metabolic regulation.
PG and MG are separated by exposing freeze-dried muscle-biopsy specimens to ice-cooled 1.5 M PCA for 20 min. This extraction time has been used in studies of rat (12,16) and human muscle tissue (6,10,13,14,15). However, PG and MG could be created as an artifact of the extraction process, and separation of the 2 glycogen pools may depend on factors like extraction time and acid concentration. No information is currently available in the literature concerning the stability of the 2 forms of glycogen in relation to extraction time. If the relationship between recovered PG and MG continues to change with extraction time without reaching a stable level, the reliability of the separation technique must be questioned, and the biologic significance of the data would be limited. Information about the effect of the combination of different extraction times and PCA concentrations on the relationship of PG and MG is also lacking. Before studies of the physiological response of glycogenolysis, resynthesis, and regulation of the 2 glycogen forms can be conducted in horses, these issues have to be addressed.
The purpose of this study was to evaluate the influence of extraction time and PCA concentration on the amount of PG and MG recovered from freeze-dried muscle-biopsy specimens from horses. We hypothesized that the PG and MG pools would remain constant over a wide range of extraction times and PCA concentrations.
Materials and methods
Horses
Ten standardbred horses (8 mares and 2 geldings, ages 2 to 10 years) were used in study 1. Six other standardbred horses (5 mares and 1 gelding, ages 2 to 11 years) were used in study 2. All horses were clinically healthy and untrained. They were fed the same diet of grain and hay. The care and use of the animals followed the guidelines of the Canadian Council on Animal Care. All animal experiments were conducted after approval by the Animal Care Committee of the University of Guelph and performed in compliance with the committee's recommendations.
Muscle biopsies
Muscle samples were obtained by needle biopsy from the middle gluteal muscle at rest as described by Lindholm and Piehl (17). The biopsy site was 15 cm from the tuber coxae on a line to the base of the tail and 6 cm deep; 2 samples were obtained from the biopsy site. The same skin incision was used for both biopsies. All samples were frozen immediately in liquid nitrogen and stored at −80°C until analyzed. The samples were dissected free from blood, connective tissue, and fat under a dissecting microscope. The 2 samples from each horse were pooled together and powdered before analysis.
Experimental design of study 1 — Effect of extraction time
To evaluate the effect of extraction time on the recovery of PG and MG, we divided the 10 individually freeze-dried and powdered samples into 4 equal portions each and randomly assigned the portions to 1 of 4 treatment combinations. The 4 pieces were extracted in 1.5 M PCA, as in the original method (6), but for 10, 20, 60, or 120 min. Extraction time was measured from the time PCA was added until the commencement of centrifugation.
Experimental design of study 2 — Effect of the combination of different extraction times and PCA concentrations
To study the influence of extraction time and PCA concentration on the recovery of PG and MG, we divided the 6 individually freeze-dried and powdered muscle samples into 24 pieces each. Each group of 24 pieces was randomly subjected to 12 different combinations of extraction time (10, 20, 30, or 40 min) and PCA molarity (0.5, 1.5, or 3.0 M). Thus, for each treatment combination, 2 pieces from the same muscle sample were analyzed (duplicate analysis). Extraction time was measured from the time PCA was added until the commencement of centrifugation.
Glycogen analysis
The PG and MG fractions were separated with the method described by Adamo and Graham (6), with modification of the extraction times and PCA concentrations as described in “Experimental design”. Briefly, 200 μL of ice-cooled PCA was mixed with 2.0 to 2.5 mg of freeze-dried muscle, using a plastic rod. The extraction was performed on ice followed by centrifugation at 3000 rpm for 15 min at 4°C; 100 μL of the supernatant was kept for the MG determination, and the pellet was used for the PG determination. The fractions of PG and MG were boiled in 1 M hydrochloric acid (HCl) for 2 h, and the formed glucosyl units were measured fluorometrically with the hexokinase method (18). Values are reported as millimoles of glucosyl units per kilogram of dry weight. The total glycogen (Gt) concentration was obtained by adding the PG and MG concentrations.
In study 2, an aliquot was taken from each of the PCA extracts (MG fractions) before hydrolysis in HCl and neutralized with potassium bicarbonate (19). The free glucose concentration was determined by an enzymatic fluorometric method (18).
Statistical analysis and calculations
Data were subjected to analysis of variance (ANOVA). The following model was used for study 1: Yij = μ + τi + ρj + εij, where Yij is the observation, μ is the mean value, τi is the effect of extraction time, ρj is the effect of horse (block effect), and εij is the experimental error. In study 2, the following model was used: Yijk = μ + ρi + αj + βk + (αβ)jk +εijk, where Yijk is the observation, μ is the mean value, ρi is the block effect (horse), αj is the effect of extraction time, βk is the effect of PCA concentration, (αβ)jk is the effect of the interaction between extraction time and PCA concentration, and εijk is the experimental error. The differences between each of the treatments were compared by the Tukey test. In study 2, the reliability of the PG, MG, and Gt measurements was estimated by calculating intraclass correlation coefficients (ICCs) from the results of a 1-way ANOVA for all duplicate analyses, with the following equation:
 |
where S2b is the estimated between-biopsy variance and S2w is the estimated within-biopsy variance (20). The within-biopsy standard deviation (SD) for all duplicate analyses was calculated according to the following formula:
 |
The coefficient of variation (CV) was calculated with the following formula:
 |
where ¯X is the mean value of all duplicates. Intrabiopsy differences between mean values for PG, MG, and Gt were compared with Student's paired t-test. All calculations were performed with the computer software Minitab for Windows (State College, Pennsylvania, USA). The significance level was set at P < 0.05.
Results
Study 1 — Effect of extraction time
The PG and MG concentrations were not affected by an extraction time of 10 to 60 min (Table I). With extraction for 120 min, the PG concentration decreased significantly (P < 0.05), and the MG concentration increased, though not significantly. The average decrease in PG was 37 mmol/kg, which is very similar to the average increase in MG of 17 mmol/kg. The total glycogen values were similar and not significantly different with the various extraction times.
Table I.
Study 2 — Effect of the combination of different extraction times and PCA concentrations
Similar values for PG and MG were obtained with extraction in PCA at concentrations of 0.5 and 1.5 M at each extraction time (10 to 40 min) (Tables II and III). However, an extraction time of 40 min had a significant effect (P < 0.05) on the PG concentration when the specimens were extracted in 3.0 M PCA. In addition, extraction in 3.0 M PCA yielded significantly lower PG concentrations and higher MG concentrations (P < 0.05) than did extraction in 0.5 or 1.5 M with each extraction time.
Table II.
Table III.
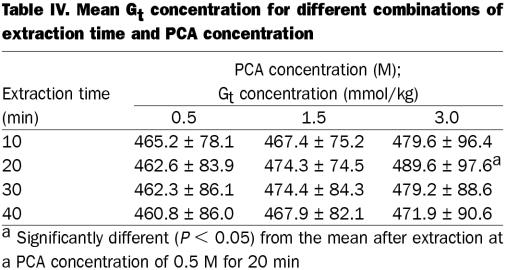
The Gt concentrations were similar over the range of extraction times and PCA concentrations, with 1 exception: extraction for 20 min yielded slightly but significantly higher Gt values when a PCA concentration of 3.0 M was used compared with 0.5 M (Table IV). Analysis of glucose in all aliquots from the MG fractions before hydrolysis in HCl yielded low values (0.14 to 0.89 mmol/kg). The mean free glucose concentrations in the PCA extracts before hydrolysis were similar for the 12 treatment combinations of extraction time and PCA concentration, ranging from 0.45 to 0.54 mmol/kg. Thus, glycogen was not hydrolyzed into glucose residues during PCA extraction, regardless of extraction time or PCA concentration.
Table IV.
Mean values and estimates of reproducibility for the duplicate muscle samples from the same biopsy are given in Table V. There were no significant differences in mean values between the 1st and 2nd analyses for any of the measurements.
Table V.
Discussion
The purpose of this study was to determine whether the 2 structural forms of muscle glycogen are stable during extraction. Our data indicate that extraction time has little effect on the concentrations of PG and MG obtained from freeze-dried muscle samples. There was no detectable time effect on either concentration after extraction with 0.5 or 1.5 M PCA in study 2; however, the maximum extraction time was limited to 40 min in this study. The concentrations of PG and MG were also stable after extraction in 3.0 M PCA for up to 30 min and 40 min, respectively. It appears that very long extraction times are necessary to affect the relationship between the 2 pools of glycogen in muscle tissue when extraction is performed with 1.5 M PCA. In study 1, after 120 min of extraction, there was a small decrease in the PG concentration and a similar increase in the MG concentration, but the change was significant only for PG. Considering that extraction of muscle metabolites in 0.5 M PCA is usually performed for 10 to 20 min (19), an extraction time of 120 min must be considered extremely long and probably does not have any physiological relevance. Our data demonstrate that, under normal circumstances, there is no evidence that the extraction technique generates artifacts that change the concentrations of the 2 pools.
We were not able to identify any differences for PG or MG between extraction in 0.5 M and 1.5 M PCA. However, extraction in 3.0 M yielded significantly lower PG and higher MG concentrations with all extraction times (between 10 and 40 min). It may be that the stronger acid or the longer exposure caused a modest amount of hydrolysis of small chains of polysaccharides in the insoluble PG fraction and that they entered the MG fraction. This appears to be the case because the change in each pool tended to be inversely related. These results differ from the data presented by Jansson (3), who showed that the relationship between PCA-soluble and PCA-insoluble glycogen was not influenced by the PCA concentration in the range of 0.5 to 3 M. It is possible that species variation accounts for the differences: the study by Jansson was performed on human skeletal muscle, whereas ours was conducted on equine skeletal muscle. In addition, Jansson's study was performed with an extraction time of only 20 min. Extraction in 3 M PCA for longer periods might have resulted in elevated MG and lower PG concentrations.
Analysis of glucose in the PCA extracts (MG fractions) before hydrolysis in HCl in study 2 showed that none of the glycogen fractions were hydrolyzed into glucose residues by the PCA, regardless of extraction time or PCA concentration. These results exclude the possibility of prehydrolysis of glycogen by the 3.0 M PCA as an explanation for the differences in the relationship between PG and MG at this PCA concentration. It is possible that the higher PCA concentration (3.0 M) extracted more MG from the samples and that the extraction in 0.5 and 1.5 M PCA was incomplete. This is, however, not likely. Extraction of muscle metabolites from freeze-dried muscle is considered to be complete after extraction for 10 to 20 min in 0.5 M of PCA (19). If the extraction had been incomplete, one would not expect such a stable relationship between PG and MG as that obtained with the different extraction times. The relationship between the 2 forms of glycogen was also very stable with both 0.5 and 1.5 M PCA.
Reproducibility of the PG and MG determinations as reflected by the ICC, which takes the within- and between-sample variability into account, has not been reported previously. In contrast to the CV, the ICC has the advantage of not being dependent on either the mean or the SD. An ICC of 1.0 indicates that there is no within-subject variance associated with an analysis (20). Conversely, an ICC of zero indicates that there is no reliability associated with an analysis. The reliability of the PG, MG, and Gt determinations was considered very good (ICC = 0.92, 0.97, and 0.95, respectively) when data from all extraction times and PCA concentrations were combined. Reproducibility of the PG, MG, and Gt determinations as reflected by the SD and the CV was in good agreement with those of previous studies (6,11).
The concentrations of free glucose (21) and glucose-6-phosphate (G6P) (22) are low in muscle tissue during rest, but with intense exercise they rise. Since these metabolites are recovered in the MG fraction during extraction, they could cause a significant overestimation of the MG concentration. When the glucose residues formed after HCl hydrolysis were measured in the MG fraction by the hexokinase method, G6P was excluded from the MG fraction by incorporation of the enzyme G6P-dehydrogenase in the reagent and not adding the enzyme hexokinase until after the initial reading of nicotinamide adenine dinucleotide phosphate. Separate analysis of free glucose in postexercise samples is, however, required to enable exclusion of this error by subtraction of the glucose concentration from the MG concentration.
There is controversy in the literature about whether PG and MG exist as distinct molecular entities or if the 2 are a continuum of molecular sizes, from glycogenin up to a large MG molecule of approximately 10 000 kDa (4,5,8,9). Irrespective of the range of molecular sizes, several studies have demonstrated that the 2 fractions can be separated on the basis of acid solubility (6,10,11,12). Recent studies have indicated that the 2 glycogen forms function as different metabolic pools in glycogenolysis and resynthesis (10,12,13,14). Furthermore, the wide variation between tissues in the proportion of PG to MG indicates that there are factors that control the relative amounts of PG and MG (5). The fact that the PG and MG pools are very stable during extraction with various acid concentrations for various times further supports the theory of the existence of 2 molecular forms of glycogen that can be reliably separated.
The separation technique allows further understanding of the metabolism of muscle glycogen and permits determination of the concentrations of total glycogen, PG, MG, and metabolites in the same piece of tissue, which eliminates the variation between muscle pieces. It is also possible that this technique could be of value in the investigation of equine glycogen-storage myopathies.
In summary, our study demonstrated that the technique for separating PG and MG is reliable and does not simply reflect an artifact of extraction time and, or, acid concentration. The concentrations of the 2 glycogen pools were very stable after extraction with PCA at concentrations of 0.5 and 1.5 M. Extraction with 3.0 M PCA yielded significantly different values for PG and MG, and this concentration is therefore not considered to be useful for separation of the 2 glycogen pools in equine skeletal muscle.
Footnotes
Acknowledgments
The E.P. Taylor Equine Trust Fund generously supported this study. We thank Kim Kultiman, Swedish Veterinary Institute, for advice with the statistical analyses.
Address correspondence and reprint requests to Dr. Johan T. Bröjer, Department of Large Animal Clinical Sciences, Faculty of Veterinary Medicine, Box 7018, 750 07, Uppsala, Sweden, tel: + 46 18 671890, fax: + 46 18 672610, e-mail: johan.brojer@kirmed.slu.se
Received July 20, 2001. Accepted March 25, 2002.
References
- 1.Kits Van Heijningen AJM, Kemp A. Free and fixed glycogen in rat muscle. Biochem J 1955;59:487–491. [DOI] [PMC free article] [PubMed]
- 2.Stetten D Jr, Stetten MR. Glycogen metabolism. Physiol Rev 1960;40:505–537. [DOI] [PubMed]
- 3.Jansson E. Acid soluble and insoluble glycogen in human skeletal muscle. Acta Physiol Scand 1981;113:337–340. [DOI] [PubMed]
- 4.Alonso MD, Lomako J, Lomako WM, Whelan WJ. A new look at the biogenesis of glycogen. FASEB J 1995;9:1126–1137. [DOI] [PubMed]
- 5.Lomako J, Lomako WM, Whelan WJ, Dombro RS, Neary JT, Norenberg MD. Glycogen synthesis in the astrocyte: from glycogenin to proglycogen to glycogen. FASEB J 1993;7: 1386–1393. [DOI] [PubMed]
- 6.Adamo KB, Graham TE. Comparison of traditional measurements with macroglycogen and proglycogen analysis of muscle glycogen. J Appl Physiol 1998;84:908–913. [DOI] [PubMed]
- 7.Lomako J, Lomako WM, Whelan WJ. Proglycogen: a low-molecular-weight form of muscle glycogen. FEBS Lett 1991;279:223–228. [DOI] [PubMed]
- 8.Lomako J, Lomako WM, Whelan WJ. Glycogen metabolism in quail embryo muscle. The role of the glycogenin primer and the intermediate proglycogen. Eur J Biochem 1995;234:343–349. [DOI] [PubMed]
- 9.Roach PJ, Skurat AV. Self-glucosylating initiator proteins and their role in glycogen biosynthesis. Prog Nucleic Acid Res Mol Biol 1997;57:289–316. [DOI] [PubMed]
- 10.Adamo KB, Tarnopolsky MA, Graham TE. Dietary carbohydrate and postexercise synthesis of proglycogen and macroglycogen in human skeletal muscle. Am J Physiol Endocrinol Metab 1998;275:E229–234. [DOI] [PubMed]
- 11.Bröjer JT, Stämpfli HR, Graham TE. Analysis of proglycogen and macroglycogen content in muscle biopsy specimens obtained from horses. Am J Vet Res 2002;63:570–575. [DOI] [PubMed]
- 12.Derave W, Gao S, Richter EA. Pro- and macroglycogenolysis in contracting rat skeletal muscle. Acta Physiol Scand 2000;169: 291–296. [DOI] [PubMed]
- 13.Graham TE, Adamo KB, Shearer J, Marchand I, Saltin B. Pro- and macroglycogenolysis: relationship with exercise intensity and duration. J Appl Physiol 2001;90:873–879. [DOI] [PubMed]
- 14.Shearer J, Marchand I, Tarnopolsky MA, Dyck DJ, Graham TE. Pro- and macroglycogenolysis during repeated exercise: roles of glycogen content and phosphorylase activation. J Appl Physiol 2001;90:880–888. [DOI] [PubMed]
- 15.Asp S, Daugaard JR, Rohde T, Adamo K, Graham T. Muscle glycogen accumulation after a marathon: roles of fiber type and pro- and macroglycogen. J Appl Physiol 1999;86: 474–478. [DOI] [PubMed]
- 16.Hansen BF, Derave W, Jensen P, Richter EA. No limiting role for glycogenin in determining maximal attainable glycogen levels in rat skeletal muscle. Am J Physiol Endocrinol Metab 2000;278: E398–404. [DOI] [PubMed]
- 17.Lindholm A, Piehl K. Fibre composition, enzyme activity and concentrations of metabolites and electrolytes in muscles of standardbred horses. Acta Vet Scand 1974;15:287–309. [DOI] [PMC free article] [PubMed]
- 18.Bergmeyer H. Methods of Enzymatic Analysis. Vol. 3. New York: Academic Press, 1974:1128–1131.
- 19.Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 1974;33:109–120. [PubMed]
- 20.Bland M. An Introduction to Medical Statistics. 3rd ed. Oxford, England: Oxford University Press, 2000:204–205,269–272.
- 21.Bröjer J, Jonasson R, Schuback K, Essén-Gustavsson B. Pro- and macroglycogenolysis in skeletal muscle during maximal treadmill exercise. Equine Vet J 2002: in press. [DOI] [PubMed]
- 22.Schuback K, Essen-Gustavsson B, Persson SGB. Effect of creatine supplementation on muscle metabolic response to maximal treadmill exercise test in standardbred trotters. Equine Vet J 2000;32:533–540. [DOI] [PubMed]