Abstract
The purpose of this study was to determine the contributions of central command and the exercise pressor reflex in regulating the cardiovascular response to static exercise in patients with Brown-Séquard syndrome. In this rare condition, a hemisection of the spinal cord typically leaves one side of the body with diminished sensation and normal motor function and the other side with diminished motor function and normal sensation.
Four, otherwise healthy, patients with Brown-Séquard syndrome and varying degrees of motor and sensory dysfunction were studied during four isometric knee extension protocols involving both voluntary contraction and electrically stimulated contractions of each leg. Heart rate, blood pressure, force production and ratings of perceived exertion were measured during all conditions. Measurements were also made during post-contraction thigh cuff occlusion and during a cold pressor test.
With the exception of electrical stimulation of the leg with a sensory deficit, protocols yielded increases in heart rate and blood pressure. Cuff occlusion sustained blood pressure above resting levels only when the leg had intact sensation.
While voluntary contraction (or attempted contraction) of the leg with a motor deficit produced the lowest force, it produced the highest ratings of perceived exertion coupled with the greatest elevations in heart rate and blood pressure.
These data show that the magnitude of the heart rate and blood pressure responses in these patients was greatly affected by an increased central command; however, there were marked cardiovascular responses due to activation of the exercise pressor reflex in the absence of central command.
Despite numerous studies investigating the neural control mechanisms underlying cardiovascular adjustments to static exercise, our understanding of this topic remains incomplete. The two most widely accepted mechanisms, first forwarded by Johansson (1893), are that the stimulus for the cardiovascular response to exercise originates from (i) the exercising muscle (muscle afferent input), and/or (ii) the brain (central command). Both central command (Krogh & Lindhard, 1912; Goodwin et al. 1972) and reflex input from the contracting muscles (Alam & Smirk, 1937; Coote et al. 1971; McCloskey & Mitchell, 1972) are known to elicit increases in cardiovascular function. Furthermore, both central command and the exercise pressor reflex can act as redundant control mechanisms (Mitchell & Schmidt, 1983; Mitchell, 1990). As a means of identifying their individual contributions, investigators have employed numerous strategies to alter either central command or muscle afferent input during exercise; however, few of these studies have explored this interaction utilizing a patient model.
There have been limited studies of patients with sensory and motor disorders and of those studies, the results have been somewhat inconsistent. Alam & Smirk (1938) studied a patient lacking all sensory input from one leg and found that cardiovascular responses during one-legged volitional exercise were similar for both legs, but that blood pressure fell during post-exercise circulatory occlusion in the limb lacking sensation. The authors concluded that the cardiovascular activation must have resulted from a central neural mechanism during exercise. Similarly, Duncan et al. (1981) reported increases in heart rate and arterial pressure during static exercise in two subjects with sensory neuropathies. One subject had a partial Brown-Séquard lesion and the other a congenital sensory neuropathy. Both subjects could perform isometric forearm exercise in the absence of sensory afferent input. They concluded that cardiovascular responses could be elicited without input from the contracting muscle. In contrast to these studies, Lind et al. (1968) exercised a patient with syringomelia that effected the forearm sensory nerves in one arm, and observed no elevation in arterial pressure when the affected arm was used. The investigators concluded that cardiovascular responses to static exercise were indeed dependent upon afferent input from the contracting limb. Taken together these findings do not present a consistent picture of the relationship between central command and the exercise pressor reflex during exercise in these patients. In order to further our understanding in this area, patients with Brown-Séquard syndrome were studied.
The Brown-Séquard syndrome is classically defined as a spinal hemiplegia resulting from the effect of a disease of, or an injury to, the lateral half of the spinal cord (Brown-Séquard, 1868; Fig. 1). The neurological signs caudal to the hemisected region of the spinal cord are utilized by physicians to clinically diagnose the Brown-Séquard syndrome in spinal cord-injured patients. This syndrome, which occurs in less than 1 % of all spinal cord injuries, results in absent or diminished sensation for pain and temperature in one leg because of interruption of the spinothalamic tract and paresis or paralysis of the contralateral leg due to interruption of the corticospinal tract. Of specific relevance to this investigation, signals from group III and IV muscle afferents travelling through the dorsal horn (Light & Perl, 1985) are also disrupted unilaterally. Thus Brown-Séquard patients provide an excellent model for investigating the individual roles of central command and the exercise pressor reflex in determining the cardiovascular response during static exercise. The purpose of this study was to assess the cardiovascular responses during voluntary and electrically stimulated exercise in a group of spinal cord-injured subjects with Brown-Séquard syndrome and to determine the individual contributions of central command and the exercise pressor reflex.
Figure 1. Schematic diagram representing a spinal cord section with selected tracts.
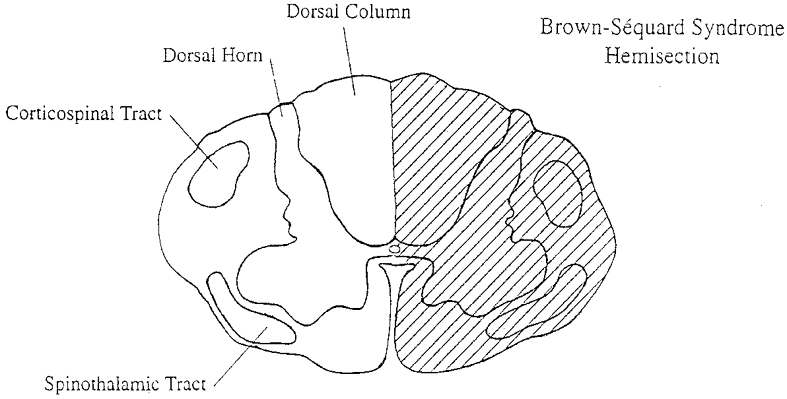
The hatched area represents the classical hemisection of the cord resulting in the Brown-Séquard syndrome.
METHODS
Subjects
Four spinal cord-injured patients, previously diagnosed with Brown-Séquard syndrome by their neurologists, were recruited for participation in the study. These patients had all undergone physical therapy for their injury and had no other medical complications at the time of the study. All patients signed an informed consent form as approved by The University of Texas Southwestern Medical Center Institutional Review Board and conforming to guidelines set forth in the Declaration of Helsinki. Subjects' descriptive characteristics are presented in Table 1.
Table 1.
Subject descriptive characteristics
| Subject | Age (years) | Sex | Time post injury (years) | Type of injury | Neurological level* |
|---|---|---|---|---|---|
| 01 | 29 | F | 10.5 | Gun shot wound | C5 |
| 02 | 30 | F | 0.5 | Trampoline | C5 |
| 03 | 35 | M | 18.8 | Motor vehicle accident | T4 |
| 04 | 28 | M | 8.0 | Motor vehicle accident | T8 |
Neurological level is defined as the most caudal segment of the spinal cord with normal sensory and motor function on both sides of the body as defined by the International Standards of Neurological and Functional Classification of Spinal Cord Injury (1996).
Patient 01 was a 28-year-old female who sustained an injury to cervical level 5 secondary to a gunshot wound 10 years ago. She presented with motor function (grade 3+/5 or greater) in her right extremities and non-functional motor strength in her left lower extremity. Although she was an independent ambulator in the household setting, she was limited in the community setting secondary to the weakness in her left leg. Sensation to pain and temperature was intact in her left extremities and absent in her right extremities. This profound absence was evident by the numerous numbers of inflammatory insect bites observed on her right upper extremity secondary to reported fire ant bites from a recent picnic outing.
Patient 02 was a 30-year-old female who had recently (6 months) sustained a cervical level 5 injury in a fall from a trampoline. She demonstrated the greatest neurological recovery of the four patients studied. She had normal strength in her left extremities. Although her strength was functional in her right extremities, it tested in the 3+ to 4 range on a 5-point scale. She was an independent ambulator and was able to negotiate all obstacles in the community setting, but was limited in endurance. Sensation both to pinprick and temperature was normal in her right extremities but impaired in her left extremities.
Patient 03 was a 30-year-old male who had injured thoracic level 4 in a motor vehicle accident 19 years ago. He presented with normal strength in the right lower extremity and weakness in his left lower extremity. He could ambulate with the assistance of a left orthosis, but relied on his wheelchair for mobility. Sensation to pinprick and temperature was intact in his left lower extremity and absent in the contralateral right lower extremity.
Patient 04 was a 28-year-old male who had sustained an injury to thoracic level 8 in a motor vehicle accident 8 years previously. Of the four subjects, he presented with the least neurological recovery. He demonstrated absent volitional control in his left leg with only involuntary muscle spasms present. Although voluntary muscle contractions were present in the right lower extremities, these were non-functional. The patient depended on his wheelchair for all mobility. The left lower extremity demonstrated intact sensation to pinprick and temperature with absent sensation to pinprick and temperature in the right lower extremity.
Experimental procedures
To verify the absence of sensory afferent input in the extremity with sensory loss, a cold pressor test was performed. Heart rate (HR) and blood pressure (BP) were measured continuously for 30 s before the foot was submerged to the ankle in a container of crushed ice and water (0–2°C) during the 2 min test period, and for the following 30 s of recovery (Fig. 2). This procedure was repeated on the foot with normal sensation for comparison.
Figure 2. Individual subject responses (01–04) during a 2 min cold pressor test.
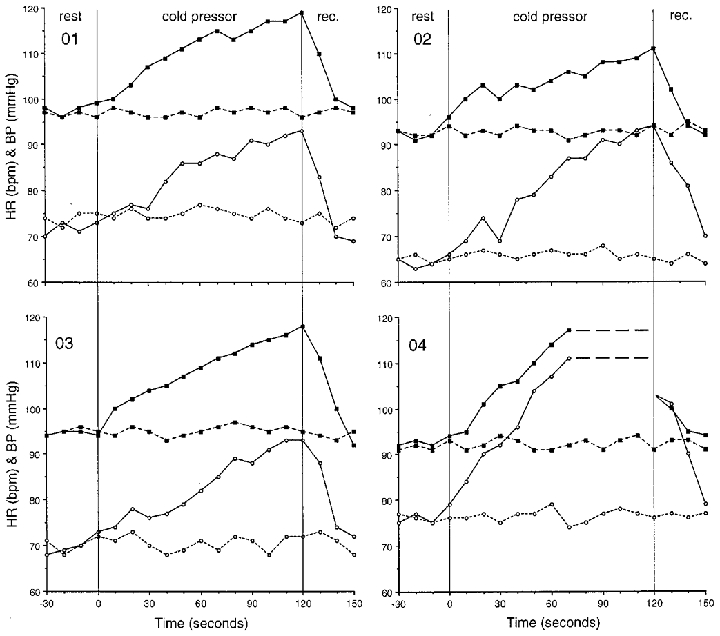
Filled squares represent mean blood pressure (mmHg) and open circles show heart rate (beats min−1). The continuous lines show responses for the leg with intact sensation and motor deficit, while the dashed lines show responses for the leg with sensory deficit and intact motor function. The data points represent 10 s averages across rest, cold pressor exposure, and recovery (rec.)
The approach for this study was to have each subject perform isometric knee extension voluntarily for both legs and then use electrical stimulation to elicit isometric contractions in both legs. Our goal was to match each patient's same absolute force output for each condition, with the exception of the attempted effort at force production in the leg with motor deficit, while recording their cardiovascular responses. Forces recorded during electrical stimulation at maximal tolerance levels were then matched, as closely as possible, during voluntary efforts for each limb. A protocol was created using the four conditions, from which to study the effects of central command and muscle afferent input during exercise: (a) voluntary contraction of the sensory deficit/motor intact leg (activation of central command with diminished input from muscle afferents); (b) electrical stimulation of the sensory deficit/motor intact leg (no activation of central command and diminished input from muscle afferents); (c) voluntary contraction (attempted) of the motor deficit/sensory intact leg (activation of central command and normal input muscle afferents); (d) stimulation of the motor deficit/sensory intact leg (no activation of central command and normal input from muscle afferents). At the end of each condition, a thigh cuff was inflated to a supra-systolic pressure to assess the muscle afferent contributions during post-exercise circulatory occlusion. Subjects were asked to rate their perceived exertion (RPE) according to the Borg 6- to 20-unit scale (Borg, 1970) during the last seconds of the contraction.
For these experiments, the subject was seated with the back reclined and the lower leg fixed at approximately 60 deg of knee flexion. Voluntary or electrically stimulated knee extensor force was measured using a force transducer (Nielsen, Denmark). HR was measured continuously from an ECG tracing (Mingograf 7, Seimans-Elema, Sweden) and blood pressure (systolic and diastolic) was monitored continuously using a Finapres device (Ohmeda, Madison, WI, USA) from a finger positioned at the level of the heart. All signals (force, HR, BP) were input directly into a computer for on-line, beat-by-beat recordings. Mean arterial blood pressure was calculated from the integrated pulse wave as generated by the Finapres.
Neuromuscular electrical stimulation was used to elicit a 2 min knee extensor contraction in the sensory intact, motor deficit leg. Stimulation was delivered to the quadriceps femoris muscle group using paired polymer-based surface electrodes. The quadriceps muscle group was selected so that large electrodes could be used to decrease current density while activating a large muscle mass. Two of the patients were familiar with electrical stimulation of this muscle group as a component of their physical therapy. Symmetrical biphasic square wave pulses of 300 μs duration at a pulse train rate of 50 pulses s−1 were used to produce a tetanic muscle contraction. A maximal level of electrical stimulation was determined for each subject who did not elicit a pain response. The current was adjusted throughout the 2 min contraction to maintain the force output, but never exceeded the maximal tolerable level as determined prior to testing during a familiarization period. This procedure was repeated on the sensory deficit lower extremity using the same amperage. During the last 15 s of the contraction, a thigh cuff was inflated to approximately 250 mmHg. Application of the thigh cuff alone during familiarization trials did not elicit significant cardiovascular responses.
Following a period of rest, the patients performed voluntary isometric knee extension for 2 min in the lower extremity with the sensory deficit. The patients were verbally cued to maintain the same force level achieved during the electrically stimulated contractions. As before, 15 s prior to the end of the volitional contraction, a thigh cuff was inflated to 250 mmHg. This procedure was repeated on the motor deficit/sensory intact leg, with the subject attempting to match the voluntary force produced by the opposite leg.
Data analysis
As data were collected on only four subjects, descriptive analyses are presented for each subject with individual figures. Additionally, some individual responses were averaged for discussion purposes. All data are presented as means ±s.d unless otherwise noted.
RESULTS
Cold pressor test
During the cold pressor test in the leg with intact sensation, HR increased from an average resting value of 70 ± 5 beats min−1 to roughly 95 ± 4 beats min−1 and MAP from a resting level of 94 ± 4 to 117 ± 5 mmHg by the end of the 2 min cold pressor test. The individual responses are shown in Fig. 2. Subject 04 asked to have his foot removed after only 70 s of cold pressor exposure. Following removal of the cold pressor (foot dried with a towel), both heart rate and blood pressure had recovered to resting levels within 30 s. When the leg with a sensory deficit was exposed to the cold pressor test (Fig. 2) no increases in resting HR or MAP were observed, as would be expected for the Brown-Séquard patient.
Cardiovascular responses
Voluntary exercise with sensory deficit
During voluntary contraction of the leg with a sensory deficit (central command with diminished input from muscle afferents), average increases in HR (from 72 ± 3 to 133 ± 8 beats min−1) and MAP (from 94 ± 4 to 117 ± 3 mmHg) were observed after 2 min of sustained contraction. Individual responses for this protocol are presented in Fig. 3. There were also increases in systolic blood pressure (SBP from 120 ± 5 to 165 ± 6 mmHg) and diastolic blood pressure (DBP from 80 ± 6 to 94 ± 4 mmHg). The individual RPE values after 2 min of contraction ranged from 13 to 17 units. The average force produced over the 2 min period to match the force generated via electrical stimulation was 82 ± 25 N (Fig. 7). Post-exercise cuff occlusion did not appear to sustain cardiovascular responses as both HR and MAP approached their respective resting values after 1 min (Fig. 3). During cuff occlusion, all subjects reported a pain value of 6 units on a 6- to 20-unit scale (or no pain) when asked at the end of the test.
Figure 3. Individual responses during volitional contraction of the leg with intact motor function and sensory deficit.
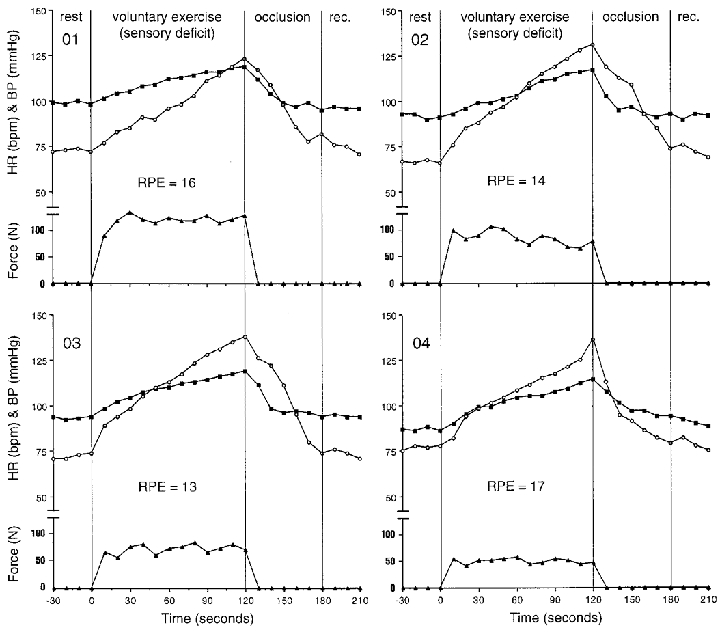
Filled squares represent mean blood pressure (mmHg); open circles show heart rate (beats min−1) and filled triangles show force (N) during rest, isometric knee extension (volitional exercise), thigh cuff occlusion (occlusion), and recovery (rec.). Individual ratings of perceived exertion (RPE) are shown in units (6–20 range).
Figure 7. Average responses across the four protocols.
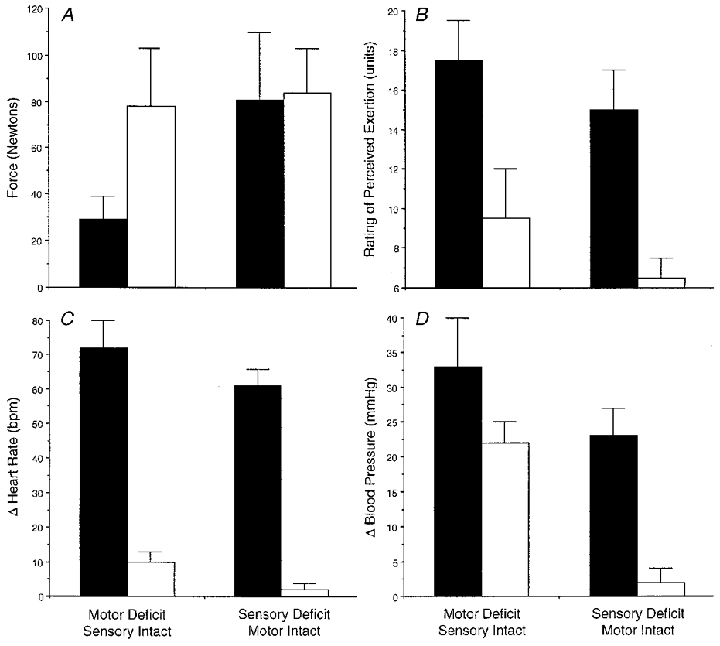
The filled bars represent voluntary exercise and the open bars stimulated exercise. Mean responses (±s.d) are shown for: force (N;A);perceived exertion (RPE units; B); change in responses for peak heart rate (beat min−1) from rest (C); and change in responses for peak mean blood pressure (mmHg) from rest (D).
Stimulated exercise with sensory deficit
The electrically stimulated contractions of the sensory deficit leg (no central command and diminished input from muscle afferents) did not produce any changes in HR or blood pressure. Individual data are shown in Fig. 4. There appeared to be a slight upward trend in HR for subject 02 (from 71 to 75 beats min−1), who was the only one to have a RPE value greater than 6. The average force generated over the 2 min contraction at a maximal tolerable level of stimulation was 84 ± 21 N (Fig. 7). There was no effect of post-stimulation cuff occlusion on HR or MAP. Accordingly, all subjects rated the pain at 6 units using the 6–20 unit scale (no pain).
Figure 4. Individual responses during electrical stimulation of the leg with intact motor function and sensory deficit.
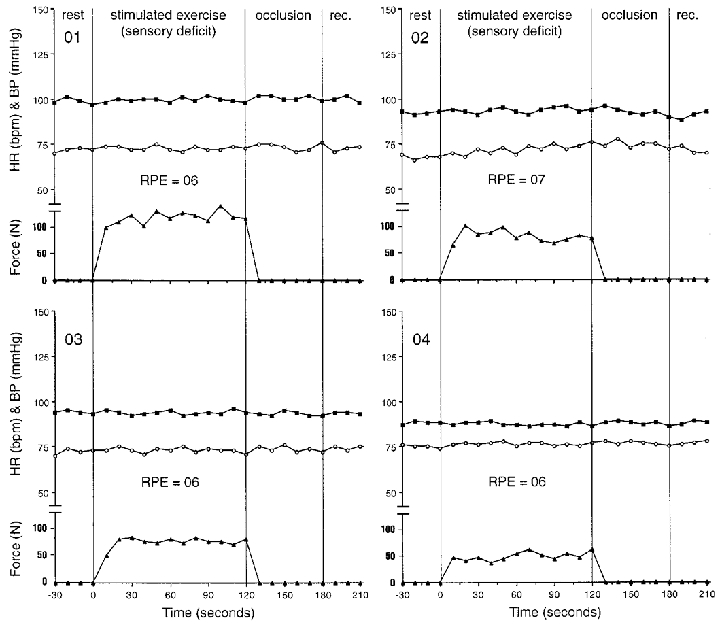
Filled squares represent mean blood pressure (mmHg); open circles show heart rate (beats min−1) and filled triangles show force (N) during rest, isometric knee extension (volitional exercise), thigh cuff occlusion (occlusion), and recovery (rec.). Individual ratings of perceived exertion (RPE) are shown in units (6–20 range).
Voluntary exercise with motor deficit
All subjects demonstrated increases in HR and BP during voluntary (attempted) contraction of the sensory intact, motor deficit leg over the 2 min of exercise (activated central command with normal input from muscle afferents). The individual responses are presented in Fig. 5. Subject 04 could only maintain the contraction for 90 s. The average peak HR increase was from 73 ± 4 to 144 ± 11 beats min−1 and MAP from 94 ± 4 to 127 ± 3 mmHg. SBP increased from 121 ± 5 to 171 ± 9 mmHg and DBP from 80 ± 5 to 105 ± 4 mmHg. Individual RPE values ranged from 16 to 19 units although the average force production was only 27 ± 9 N (Fig. 7), and represented only 33 % of force produced during the other three protocols. Following 1 min of post-exercise cuff occlusion, the MAP (average of 104 ± 3 mmHg) appeared to remain higher than the resting MAP values of 94 ± 4 mmHg for all subjects (Fig. 5). The subjects rated their pain during post-exercise cuff occlusion as 11 ± 1 units (using a 6–20 scale) and the sensation could be described as very tolerable.
Figure 5. Individual responses during volitional contraction of the leg with intact sensation and a motor deficit.
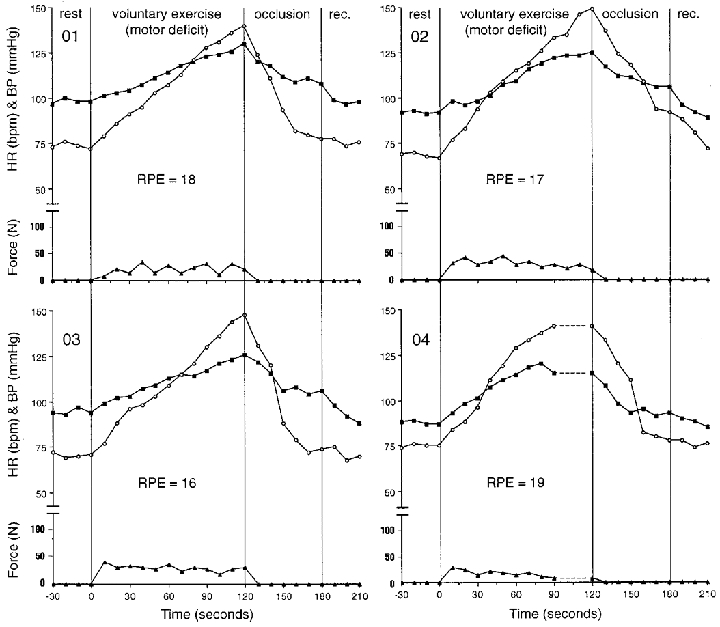
Filled squares represent mean blood pressure (mmHg); open circles show heart rate (beat min−1) and filled triangles show force (N) during rest, isometric knee extension (volitional exercise), thigh cuff occlusion (occlusion), and recovery (rec.). Individual ratings of perceived exertion (RPE) are shown in units (6–20 range).
Stimulated exercise with motor deficit
After 2 min of electrical stimulation of the leg with intact sensation and motor deficit (no central command with normal input from muscle afferents), there were overall elevations in HR (from 70 ± 4 to 88 ± 4 beats min−1) and MAP (from 93 ± 5 to 116 ± 7 mmHg). Individual HR and MAP data are shown in Fig. 6. The SBP increased from 121 ± 5 to 139 ± 10 mmHg and DBP increased from 80 ± 6 to 105 ± 5 mmHg. The RPE values ranged from 6 to 12 units, with subject 04 rating the exertion as 12 units. The average force generated was 78 ± 26 N (Fig. 7). Following electrical stimulation, when the post-stimulation cuff occlusion was employed, MAP appeared to be sustained above resting values (Fig. 6). During post-stimulation cuff occlusion, subjects rated their pain as 10 ± 2 units (6–20 scale) which corresponded to a very tolerable sensation.
Figure 6. Individual responses during electrical stimulation of the leg with intact sensation and a motor deficit.
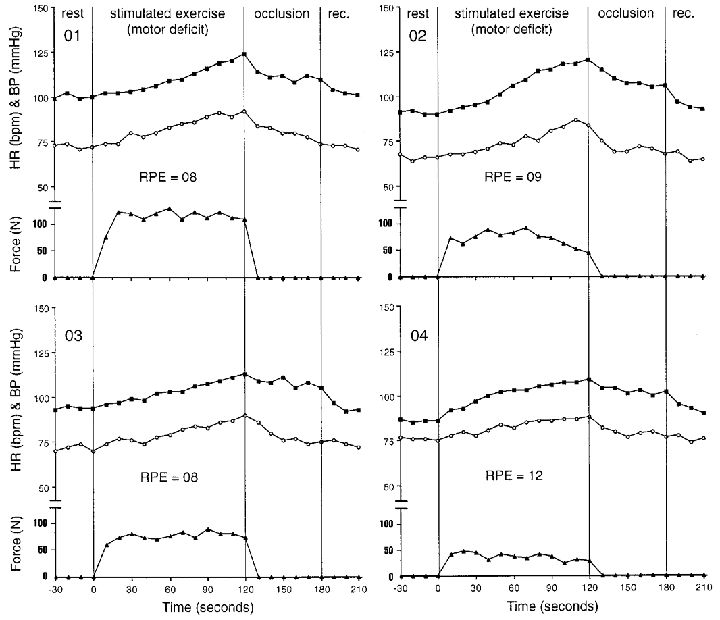
Filled squares represent mean blood pressure (mmHg); open circles show heart rate (beat min−1) and filled triangles show force (N) during rest, isometric knee extension (volitional exercise), thigh cuff occlusion (occlusion), and recovery (rec.). Individual ratings of perceived exertion (RPE) are shown in units (6–20 range).
DISCUSSION
This study was designed to evaluate the contributions of central command and the exercise pressor reflex in determining the cardiovascular responses to static leg exercise in a specific patient model. Of note, while the patients studied presented with classic Brown-Séquard symptoms, their lesions were, in all likelihood, not an exact hemisection of the spinal cord as depicted in Fig. 1. Subjects demonstrated marked weakness in the leg with motor deficit as evidenced by their limited force production. There is also the possibility of some degree of sensation in the leg with sensory deficit. However, it should be noted that there were no cardiovascular or perceptual changes during the cold pressor tests of the sensory deficit leg, nor cardiovascular responses to electrical stimulation of the sensory deficit leg, with the possible exception of a small heart rate change noted in patient 02 (Fig. 4). During static contractions, whether produced voluntarily or by electrical stimulation, cardiovascular changes were typically observed with the exception of the condition in which a contraction of the leg with a sensory deficit was elicited via electrical stimulation.
Voluntary exercise with sensory deficit
During the volitional contraction of the leg with sensory deficit (activated central command with diminished input from muscle afferents), there were elevations in all variables (Fig. 3). Since post-exercise cuff occlusion did not sustain the blood pressure responses above resting values, the cardiovascular changes appear to be mediated primarily by central command. These findings are similar to those reported by Gandevia et al. (1993) when subjects were requested to contract their muscles following administration of curare. Likewise, significant increases in heart rate and blood pressure were noted during attempted static contraction following epidural anaesthesia (Mitchell et al. 1989b). The mean rating of perceived exertion for the Brown-Séquard patients was 15 out of 20 units (range 13–17 units), suggesting a significant level of central command. Increases in central command are closely related to increases in HR (Mitchell, 1990). Central command-induced increases in heart rate can increase cardiac output (Williamson et al. 1996), which in turn can contribute to elevations in blood pressure. It is not clear whether or not the observed blood pressure increases were mediated solely by changes in cardiac output or also involved vascular resistance changes. Olesen et al. (1995) have reported that central command can elicit arterial constriction which is thought to be mediated by centrally induced increases in muscle sympathetic nerve activity (Victor et al. 1995). Further, it has been reported that central command can act to reset the arterial baroreflex during static exercise (Querry et al. 1999) which would function to raise blood pressure.
Stimulated exercise with sensory deficit
Electrical stimulation of the leg with sensory deficit (no activation of central command and diminished input from muscle afferents) produced no cardiovascular changes although the force produced was similar to the voluntary effort (Fig. 7A). This lack of cardiovascular change in response to electrical stimulation was in line with what would be expected from the pure Brown-Séquard patient. Coupled with the absence of heart rate or blood pressure change during the cold pressor, this lack of significant cardiovascular response during stimulation further suggests a completeness of the spinal lesion with regards to interruption of pathways involving afferent feedback from the skeletal muscle. This also suggests that these pathways capable of eliciting a pressor response are confined to only one side of the spinal cord and do not appear to travel bilaterally as noted in the cat (Iwamoto et al. 1984).
However, it is also possible that the heart rate responses to a stimulated static contraction may differ from those during stimulated dynamic exercise, when muscle afferent input is blocked. Using epidural anaesthesia to elicit cutaneous sensory anaesthesia and complete paralysis of both legs, Strange et al. (1993) found that electrically stimulated dynamic exercise resulted in heart rate increases. These HR increases tended to parallel increases in work rate and oxygen consumption. It is not known if higher levels of stimulated dynamic exercise would also elicit HR increases in the Brown-Séquard patients.
Voluntary exercise with motor deficit
Voluntary contraction of the leg with motor deficit and intact sensation (activation of central command and input from muscle afferents) elicited the greatest cardiovascular responses. Although force production was the lowest of any condition (on average, only 33 % of other contractions), the patient's rating of perceived exertion was the highest of any condition (Fig. 7B) averaging 17.5 units. When comparing the voluntary conditions, the RPE values were 2–3 units higher on average and HR responses were also elevated by 15–20 beats min−1. Again, this tends to verify the relationship between central command and heart rate during static exercise in these patients. The combination of a relatively low force output and an exaggerated HR response are in agreement with previous work (Goodwin et al. 1972; Mitchell et al. 1989a; Gandevia et al. 1993) suggesting that the HR and BP responses to static exercise are more dependent upon the subject's perception of effort as opposed to the actual force generated. However, blood pressure can also be increased by muscle afferent activity from exercising muscles (Coote et al. 1971; McCloskey & Mitchell, 1972; Mitchell et al. 1977, 1983). While there was intact sensation, and the potential for muscle afferent input, the blood pressure responses were not sustained during post-exercise circulatory occlusion at the level of the 2 min exercise value; they were sustained at a level higher than the resting value. These observations suggest that the exercise pressor reflex contributed, at least in part, to the observed blood pressure changes.
Stimulated exercise with motor deficit
Elevations in cardiovascular responses during electrical stimulation of the leg with motor deficit and intact sensation (no activation of central command and input from muscle afferents) occurred without a major influence of central command. Krogh & Lindhard (1917) first suggested that the increases in heart rate observed during electrically stimulated work were produced reflexly, most probably a mechanical or metabolic reflex originating in the contracting skeletal muscle (Hollander & Bouman, 1975; Williamson et al. 1995). The present heart rate changes during this condition, although elevated from rest, appear to be much lower in magnitude as compared with the voluntary contractions (Fig. 7C). This observation serves to confirm that central command has a greater effect on heart rate (Mitchell, 1990) in the Brown-Séquard patient. The cardiovascular increases are consistent with previous findings of increased HR and BP when electrical stimulation was used to match the force produced during a voluntary contraction in normal subjects (Hultman & Sjöholm, 1982; Friedman et al. 1992).
Cuff occlusion maintained the blood pressure above resting values, suggesting that the BP increase was attributable to an exercise pressor response from the working muscle. This finding is inconsistent with previous findings in normal subjects in that blood pressure responses are generally not sustained during post-exercise muscle ischaemia following one-legged static exercise (Leonard et al. 1985; Mitchell et al. 1989b). One plausible explanation may involve differences between patients and normal subjects with regard to adaptation of compensatory mechanisms that may develop in patients with chronic spinal cord injuries. Interestingly, the patients did sense that they were exerting a minimal effort (RPE ranged from 8–12) although they were not trying to voluntarily contract the muscle.
In conclusion, these findings further our knowledge of cardiovascular regulation in that the cardiovascular responses to voluntary and electrically stimulated exercise in subjects with Brown-Séquard syndrome appear to be similar to those reported in normal subjects undergoing partial neuromuscular blockade. Overall, the magnitude of heart rate and blood pressure responses were greater when central command, as indexed by the RPE, was higher, yet there were significant changes in cardiovascular responses in the absence of central command increases across the static exercise protocols. While such responses reflect the redundancy of the cardiovascular control system (Mitchell, 1990), the observed differences in the magnitude of responses between conditions appear to be related to different mechanisms responsible for the resulting cardiovascular changes. While it is difficult to assess the specific types of neurological adaptations that may have occurred in these patients, this patient model appears to be a viable means of further assessing the roles of central command and the exercise pressor reflex towards overall cardiovascular regulation during various types and intensities of exercise.
Acknowledgments
We would like to thank our subjects for their co-operation and Jack Wrobbel for his technical and manuscript production assistance. This study was supported in part by the Lawson and Rogers Lacy Research Funds in Cardiovascular Diseases (J.H.M.), an American Heart Association Grant-in-Aid from the Texas Affiliate, Inc. (96G-085; J.W.W.) and NIH R01 HL 59145 (J.W.W.).
References
- Alam M, Smirk FH. Observation in man upon a blood pressure raising reflex arising from the voluntary muscles. The Journal of Physiology. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M, Smirk FH. Unilateral loss of a blood pressure rising, pulse accelerating, reflex from the voluntary muscle due to a lesion of the spinal cord. Clinical Science. 1938;3:247–258. [Google Scholar]
- Borg GA. Perceived exertion as an indicator of somatic stress. Scandanavian Journal of Rehabilitation Medicine. 1970;23:92–98. [PubMed] [Google Scholar]
- Brown-Séquard C E. Lectures on the physiology and pathology of the nervous system and on the treatment of organic nervous affections. Lancet. 1868;2:593–821. [Google Scholar]
- Coote JH, Hilton SM, Perez-Gonzalez JF. The nature of the pressor response to muscular exercise. The Journal of Physiology. 1971;215:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan G, Johnson RH, Lambie DG. Role of sensory nerves in the cardiovascular and respiratory changes with isometric forearm exercise in man. Clinical Science. 1981;60:145–155. doi: 10.1042/cs0600145. [DOI] [PubMed] [Google Scholar]
- Friedman DB, Peel C, Mitchell JH. Cardiovascular responses to voluntary and non-voluntary static exercise in humans. Journal of Applied Physiology. 1992;73:1982–1985. doi: 10.1152/jappl.1992.73.5.1982. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Killian K, McKenzie DK, Crawford M, Allen GM, Gorman RB, Hales JP. Respiratory sensations, cardiovascular control, kineasthesia, and transcranial stimulation during paralysis in humans. The Journal of Physiology. 1993;470:85–107. doi: 10.1113/jphysiol.1993.sp019849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GA, McCloskey DI, Mitchell JH. Cardiovascular responses to changes in central command during isometric exercise at a constant muscle tension. The Journal of Physiology. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander AP, Bouman LN. Cardiac acceleration in man elicited by a muscle-heart reflex. Journal of Applied Physiology. 1975;38:272–278. doi: 10.1152/jappl.1975.38.2.272. [DOI] [PubMed] [Google Scholar]
- Hultman E, Sjöholm H. Blood pressure and heart rate responses to voluntary and non-voluntary static exercise in man. Acta Physiologica Scandinavica. 1982;115:499–501. doi: 10.1111/j.1748-1716.1982.tb07110.x. [DOI] [PubMed] [Google Scholar]
- Iwamoto GA, Botterman BR, Waldrop TG. The execise pressor reflex: evidence for an afferent pathway outside the dorsolateral sulcus region. Brain Research. 1984;292:160–164. doi: 10.1016/0006-8993(84)90901-6. [DOI] [PubMed] [Google Scholar]
- Johansson JE. Uber die Einwirkung der Musdeltaatigiceit anf die atmun und die herzafatigkeit. Skandinavica Archives Physiology. 1893;5:20–66. [Google Scholar]
- Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. The Journal of Physiology. 1912;47:112–136. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A, Lindhard J. A comparison between voluntary and electrically induced muscular work in man. The Journal of Physiology. 1917;51:182–201. doi: 10.1113/jphysiol.1917.sp001795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard B, Mitchell JH, Mizuno M, Rube N, Saltin B, Secher NH. Partial neuromuscular blockade and cardiovascular responses to static exercise in man. The Journal of Physiology. 1985;359:365–379. doi: 10.1113/jphysiol.1985.sp015590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. Journal of Comparative Neurology. 1985;186:133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- Lind AR, McNicol GW, Bruce RA, MacDonald HR, Donald KW. The cardiovascular responses to sustained contractions of a patient with unilateral syringomyelia. Clinical Science. 1968;35:45–53. [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. The Journal of Physiology. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH. Neural control of the circulation during exercise. Medicine and Science in Sports and Exercise. 1990;22:141–154. [PubMed] [Google Scholar]
- Mitchell JH, Kaufmann MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annual Review of Physiology. 1983;43:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Reardon WC, McCloskey DI. Reflex effects on circulation and respiration from contracting skeletal muscle. American Journal of Physiology. 1977;233:H374–378. doi: 10.1152/ajpheart.1977.233.3.H374. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Reeves DR, Jr, Rogers HB, Secher NH, Victor RG. Autonomic blockade and cardiovascular response to static exercise in partially curarized man. The Journal of Physiology. 1989a;413:433–445. doi: 10.1113/jphysiol.1989.sp017662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Reeves DR, Jr, Rogers HB, Secher NH. Epidural anaesthesia and cardiovascular responses to static exercise in man. The Journal of Physiology. 1989b;417:13–24. doi: 10.1113/jphysiol.1989.sp017787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Schmidt RF. Handbook of Physiology, Cardiovascular Reflexes and Circulatory Integration. part 2. Bethesda, MD, USA: American Physiological Society; 1983. Cardiovascular control by afferent fibers from skeletal muscle receptors; pp. 623–658. chapter 17. [Google Scholar]
- Olesen HL, Mitchell JH, Friedman DB, Iversen HL, Secher NH. Reduced arterial diameter during static exercise in humans. Acta Physiologica Scandinavica. 1995;153:335–341. doi: 10.1111/j.1748-1716.1995.tb09871.x. [DOI] [PubMed] [Google Scholar]
- Querry R, Gallagher KM, Smith SA, Stromstad M, Ide K, Secher NH, Saltin B, Raven PB. Effects of partial neuromuscular blockade on carotid baroreflex function during static exercise in humans. Medicine and Science in Sports and Exercise. 1999;31:s225. [Google Scholar]
- Strange S, Secher NH, Pawelczyk JA, Karpakka J, Christensen NJ, Mitchell JH, Saltin B. Neural control of cardiovascular responses and of ventilation during dynamic exercise in man. The Journal of Physiology. 1993;470:693–704. doi: 10.1113/jphysiol.1993.sp019883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor RG, Secher NH, Lyson T, Mitchell JH. Central command increases muscle sympathetic nerve activity during intense intermittent isometric exercise in humans. Circulation Research. 1995;76:127–131. doi: 10.1161/01.res.76.1.127. [DOI] [PubMed] [Google Scholar]
- Williamson JW, Nóbrega ACL, Winchester P, Zim S, Mitchell JH. Instantaneous heart rate increase with dynamic exercise: central command and muscle-heart reflex contributions. Journal of Applied Physiology. 1995;73:1273–1279. doi: 10.1152/jappl.1995.78.4.1273. [DOI] [PubMed] [Google Scholar]
- Williamson JW, Olesen HL, Pott F, Mitchell JH, Secher NH. Central command increases cardiac output during static exercise in humans. Acta Physiologica Scandinavica. 1996;156:429–434. doi: 10.1046/j.1365-201X.1996.472186000.x. [DOI] [PubMed] [Google Scholar]


