Abstract
Electrical rhythmicity (slow waves) in gastrointestinal muscles (GI) is generated by interstitial cells of Cajal (ICC). Cultured ICC from the murine small intestine were studied with the patch-clamp technique to characterize regulation of pacemaker currents by cyclic nucleotides. Cyclic nucleotide agonists were also tested on intact strips of murine small intestine.
Nitric oxide donors slowed the frequency of pacemaker currents in a concentration-dependent manner. These effects depended on cGMP formation and were reduced by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ). The effects of nitric oxide donors were mimicked by membrane-permeable analogues of cGMP. The specific cGMP phosphodiesterase inhibitor zaprinast reduced the frequency of spontaneous pacemaker currents.
The cGMP-dependent effects on pacemaker currents were not affected by okadaic acid or KT-5823, an inhibitor of protein kinase G.
Forskolin, but not dideoxy forskolin, reduced the frequency of spontaneous pacemaker activity and activated a sustained outward current. The latter was likely to be due to ATP-dependent K+ channels because it was blocked by glibenclamide.
The effects of forskolin were not mimicked by membrane-permeable cAMP analogues. A membrane-permeable inhibitor of protein kinase A, myristoylated PKA inhibitor, and the adenylyl cyclase inhibitor SQ-22536, had no effect on responses to forskolin.
Responses of intact muscles to cGMP and cAMP agonists were similar to the responses of pacemaker cells. Changes in resting membrane potential and slow wave amplitude, however, were noted in intact jejunal muscles that were not observed in ICC. Differences in responses may have been due to the effects of cyclic nucleotide agonists on smooth muscle cells that would sum with responses of ICC in intact jejunal muscle strips.
A cGMP-dependent mechanism regulates slow wave frequency, but this occurs through direct action of cGMP not via protein phosphorylation. Regulation of pacemaker currents by cAMP-dependent mechanisms was not observed.
Many regions of the tunica muscularis of the gastrointestinal (GI) tract display spontaneous electrical rhythmicity (see Szurszewski, 1987). It is now known that certain class of interstitial cells of Cajal serve as pacemaker cells in the phasic portions of the GI tract (Langton et al. 1989; Ward et al. 1994; Huizinga et al. 1995; Sanders, 1996; Ordog et al. 1999). Work with isolated and cultured interstitial cells of Cajal (ICC) has shown that discrete ionic mechanisms responsible for pacemaker currents are present in ICC, and GI smooth muscle cells do not express the same conductances (Langton et al. 1989; Lee & Sanders, 1993; Koh et al. 1998; Thomsen et al. 1998). Furthermore, experiments on tissues of mutants lacking pacemaker ICC (Ward et al. 1994, 1995; Huizinga et al. 1995), tissues developmentally deprived of ICC (Torihashi et al. 1995; Ward et al. 1997; Ordog et al. 1999), and muscles with the majority of pacemaker ICC removed by dissection (Sanders et al. 1990) have shown that slow waves are not generated nor propagated through regions lacking ICC. These findings suggest that autorhythmicity in GI muscles is an exclusive property of ICC and the spread of slow waves through GI muscles occurs electrotonically, not by active regeneration (see Horowitz et al. 1999). Smooth muscle cells respond to the depolarization caused by slow waves with the activation of a variety of voltage-dependent ionic conductances. The most important of the voltage-dependent channels, in terms of the mechanical responses of smooth muscles, are the L-type (dihydropyridine-sensitive) Ca2+ channels. Activation of these channels provides a significant portion of the Ca2+ necessary to generate contractions in phasic GI muscles (see Ozaki et al. 1991).
Pacemaker ICC are organized into electrically coupled networks within discrete areas of the stomach, small bowel and colon. The pacemaker areas (i.e. region of the myenteric plexus in the stomach, small bowel and colon, and along the submucosal surface of the circular muscle layer in the colons of some species) are also richly innervated with enteric motor nerves, and it is possible that part of the function of the motor output to the gut is to regulate the amplitude and frequency of electrical slow waves. It is also possible that hormones and/or paracrine substances may also regulate rhythmicity. The amplitude and frequency of slow waves are important parameters for regulation of motility. The frequency of slow waves paces the phasic contractions and the amplitude of the depolarization during slow waves determines the degree of activation of L-type Ca2+ channels in muscle cells.
The effects of many transmitters and hormones are mediated via production of cyclic nucleotides. Previous studies have described the effects of cAMP and cGMP on slow wave amplitude and frequency (Huizinga et al. 1991; Smith et al. 1993; Tsugeno et al. 1995), but past studies have been performed on intact muscles and utilized intracellular recordings from smooth muscle cells. In these experiments it is difficult to discriminate between effects on the pacemaker cells and effects on smooth muscle cells. In the present study we have utilized ICC cultured for 1–3 days after dispersion from small intestinal muscles to study the mechanisms by which cyclic nucleotides regulate pacemaker activity. The cultured ICC retain a rhythmic phenotype (Koh et al. 1998; Thomsen et al. 1998) and generate spontaneous pacemaker potentials similar to the slow waves recorded from intact muscles. These experiments provide a basis for understanding how many regulatory agonists might affect electrical rhythmicity in GI muscles.
METHODS
Preparation of cells and tissues
Balb/C mice (0–15 days old) of either sex were anaesthetized with carbon dioxide and killed by cervical dislocation. Small intestines, from 1 cm below the pyloric ring to the caecum, were removed and opened along the myenteric border. Lumenal contents were washed away with Krebs-Ringer bicarbonate solution (KRB). Tissues were pinned to the base of a Sylgard dish and the mucosa removed by sharp dissection.
Small strips of intestinal muscle were equilibrated in Ca2+-free Hanks' solution for 30 min and cells were dispersed, as previously described (Koh et al. 1998), with an enzyme solution containing: collagenase (Worthington Type II), 1.3 mg ml−1; bovine serum albumin (Sigma, St Louis, MO, USA), 2 mg ml−1; trypsin inhibitor (Sigma), 2 mg ml−1; and ATP, 0.27 mg ml−1. Cells were plated onto sterile glass coverslips coated with murine collagen (2.5 μg ml−1, Falcon/BD) in 35 mm culture dishes. The cells were cultured at 37°C in a 95 % O2-5 % CO2 incubator in SMGM (smooth muscle growth medium; Clonetics Corp., San Diego, CA, USA) supplemented with 2 % antibiotic/antimycotic (Gibco, Grand Island NY, USA) and murine stem cell factor (SCF, 5 ng ml−1, Sigma).
Interstitial cells of Cajal (ICC) were identified immunologically with a monoclonal antibody for Kit protein (ACK2; Nishikawa et al. 1991; and see Koh et al. 1998) labelled with Alexa Fluor 488 (Molecular Probes, Eugene, OR, USA). These studies were performed on isolated ICC and small networks of ICC (< 10 cells). The morphologies of ICC and ICC networks were distinct from other cell types in the cultures, so it was possible to identify the cells with phase-contrast microscopy once the cells were verified with ACK2–Alexa Fluor 488 labelling. As previously reported the pacemaker currents from small clusters of ICC were more robust and more regular than from isolated cells (Koh et al. 1998).
Patch-clamp experiments
The whole-cell configuration of the patch-clamp technique was used to record membrane currents (voltage clamp) and membrane potentials (current clamp) from cultured ICC. Currents or potentials were amplified with an Axopatch 200B patch-clamp amplifier (Axon Instruments, Foster City, CA, USA) or a List EPC-7 (List Electronics) and digitized with a 12 bit A/D converter (Axon Instruments). Data were filtered at 1 kHz using an 8-pole Bessel filter and stored on videotape or digitized on-line using pCLAMP software (Axon Instruments).
The cells were bathed in a solution containing (mm): KCl, 5; NaCl, 135; CaCl2, 2; glucose, 10; MgCl2, 1.2 and Hepes, 10; adjusted to pH 7.4 with Tris. The pipette solution contained (mm): KCl, 140; MgCl2, 5; K2ATP, 2.7; Na2GTP, 0.1; creatine phosphate disodium, 2.5; Hepes, 5; and EGTA, 0.1 adjusted to pH 7.2 with Tris.
8-Bromoguanosine 3′:5′-cyclic monophosphate (8-Br-cGMP), N2, 2′-O-dibutyrylguanosine 3′:5′-cyclic monophosphate (diBu-cGMP), 8-bromoadenosine 3′:5′-cyclic monophosphate (8-Br-cAMP), glibenclamide and isoproterenol (isoprenaline) were purchased from Sigma). 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), forskolin (FSK), dideoxy-forskolin, (±)-S-nitroso-N-acetylpenicillamine (SNAP), myristoylated protein kinase A inhibitor (mPKI), SQ-22536, KT-5823, zaprinast and okadaic acid were purchased from Calbiochem Co. (San Diego, CA, USA). Sodium nitroprusside (SNP, RBI) was dissolved in water immediately prior to use. 8-Br-cGMP, diBu-cGMP and mPKI were dissolved in water. Other drugs tested were dissolved in DMSO. The final concentration of DMSO was less than 0.05 %.
Results were analysed using pCLAMP6 (Axon Instruments, Foster City, CA, USA) and GraphPad Prizm (version 2.01, San Diego, CA, USA) software. All experiments were performed at 29°C.
Intracellular recordings from intact muscles
We studied the electrical activity of jejunal muscles from newborn animals (day 0). These muscles manifest regular slow wave activity (Ward et al. 1997). Sheets of jejunal muscle (5 mm × 5 mm) were pinned to the floor of an electrophysiological chamber with the mucosal aspect of the muscle facing upward. The chamber was constantly perfused with oxygenated KRB of the following composition (mm): NaCl, 118.5; KCl, 4.5; MgCl2, 1.2; NaHCO3, 23.8; KH2PO4, 1.2; dextrose, 11.0; CaCl2, 2.4; pH 7.4 at 37 ± 0.5°C. The pH of the KRB was 7.3–7.4 when bubbled with 95 % O2–5 % CO2. After dissection and pinning, the muscles were allowed to equilibrate for at least 1 h before experiments were begun. Drugs were added to the perfusion solution to make up the concentrations noted in the text.
Impalements of cells were made with glass microelectrodes having resistances of 30–90 MΩ. Transmembrane potential was recorded with a standard electrometer (Intra 767; World Precision Instruments, Sarasota, FL, USA). Data were recorded on digital tape (Vetter, Robersburg, PA, USA), and the tapes were replayed through a polygraph to make hard copies (Gould RS 3200, Cleveland, OH, USA). Nifedipine (1 μm) was included in the perfusion solution to reduce contraction and facilitate the maintenance of impalements to allow exposure to test drugs. Nifedipine had no resolvable effect on slow wave activity as previously reported (Koh et al. 1998).
Statistical analyses
Data are expressed as means ± standard errors of the mean. Differences in the data were evaluated by ANOVA or Student's t test. P values less than 0.05 were taken as a statistically significant difference. The ‘n’ values reported in the text refer to the number of cells used in patch-clamp experiments or the number of tissues used in intracellular electrophysiological experiments.
RESULTS
Effects of nitric oxide donors on pacemaker activity
Under voltage clamp (holding potential −60 mV to simulate the physiological resting potential; e.g. Koh et al. 1998), ICC generated spontaneous inward currents that have been termed ‘pacemaker currents’ (see Koh et al. 1998). The frequency of pacemaker currents was 12.9 ± 0.3 cycles min−1). The ‘resting’ current level and amplitude of pacemaker currents were 127 ± 13 pA and −602 ± 60 pA, respectively (n = 68).
Sodium nitroprusside (10−7 to 10−5m) decreased the frequency of pacemaker currents in a concentration-dependent manner (i.e. controls for these experiments had an average frequency of 11.6 ± 1.6 and the frequency decreased in 10−7m SNP to 11.0 ± 1.7 cycles min−1, in 10−6m to 9.0 ± 2.3 cycles min−1, and in 10−5m to 3.6 ± 2.5 cycles min−1 (n = 6,Fig. 1A and B). The amplitude of the pacemaker currents also decreased on average from a control level of −427 ± 58 pA to −195 ± 45 pA at 10−5m SNP (Fig. 1C). There was no statistically significant change in resting current in response to SNP (Fig. 1C). In general responses could be described as a gradual slowing of pacemaker current frequency and then abrupt cessation at the higher concentrations (i.e. 10−5m). SNAP (10−6m) had the same effect as SNP (n = 3; not shown).
Figure 1. Effects of SNP on pacemaker currents.
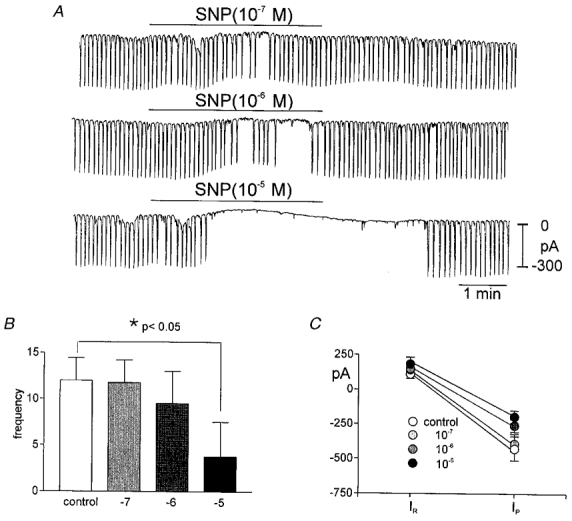
A shows pacemaker currents from the same cell exposed to SNP (10−7 to 10−5m) while holding at −60 mV. SNP caused a concentration-dependent decrease in frequency of pacemaker currents. The scale in the final trace applies to all traces. The responses to SNP are summarized in B and C. The reduction in frequency was significant by ANOVA (*). In C, IR equals resting current at the holding potential and IP is the amplitude of the pacemaker current.
Spontaneous electrical rhythmicity (ICC slow waves) was also recorded in current-clamp mode in a few experiments (holding current, 0 pA). Resting membrane potential (maximum diastolic potential, MDP) averaged −58 ± 1 mV (n = 12). Electrical slow waves recorded from ICC occurred at a frequency of 13.1 ± 0.8 cycles min−1; not significantly different from the frequency of pacemaker currents) and were 29 ± 1 mV in amplitude at 29°C (n = 12). Addition of SNP (10−5m) caused a small hyperpolarization that did not reach statistical significance (i.e. from a control of −58 ± 4 mV to −61 ± 4 mV, n = 4). SNP caused a dramatic decrease in frequency of ICC slow waves, and these events typically ceased after a few minutes of exposure (data not shown). The effects of SNP on ICC slow waves were qualitatively the same as the effects of SNP and SNAP on pacemaker currents.
Effects of cGMP-dependent pathways on pacemaker activity
The effects of SNP and SNAP on the frequency of pacemaker currents were mimicked by the membrane-permeable cGMP analogues, 8-Br-cGMP and diBu-cGMP. Under voltage clamp (holding potential −60 mV), 8-Br-cGMP (10−5 to 10−3m) and diBu-cGMP (10−3m) reduced the frequency of pacemaker currents. The frequency under control conditions averaged 13.7 ± 1.7 cycles min−1, and the resting current and pacemaker current amplitude were 170 ± 70 pA and −680 ± 118 pA, respectively, for these experiments (n = 5). Addition of 8-Br-cGMP (from 10−5 to 10−3m) to bathing solution decreased the frequency in a dose-dependent manner (10−5m to 11.0 ± 2.0 cycles min−1; 10−4m to 4.7 ± 1.4 cycles min−1; and 10−3m caused total inhibition of pacemaker currents in all cells tested, n = 5; Fig. 2A and B). The resting current and amplitude of pacemaker currents did not change in response to 8-Br-cGMP up to 10−3m (215 ± 72 pA and −738 ± 135 pA, respectively). The effects of diBu-cGMP (n = 3) were identical to the effects of 8-Br-cGMP (data not shown). ICC slow wave activity was also recorded under current clamp (I = 0). ICC slow wave amplitude averaged 30 ± 4 mV and these events occurred at 13.6 ± 1.7 cycles min−1 (n = 3). When cells were treated with 8-Br-cGMP (10−3m), the maximal diastolic potential (MDP) was unchanged but the frequency decreased and ICC slow waves ceased after a few minutes of exposure (Fig. 3).
Figure 2. Effects of 8-Br-cGMP on pacemaker currents.
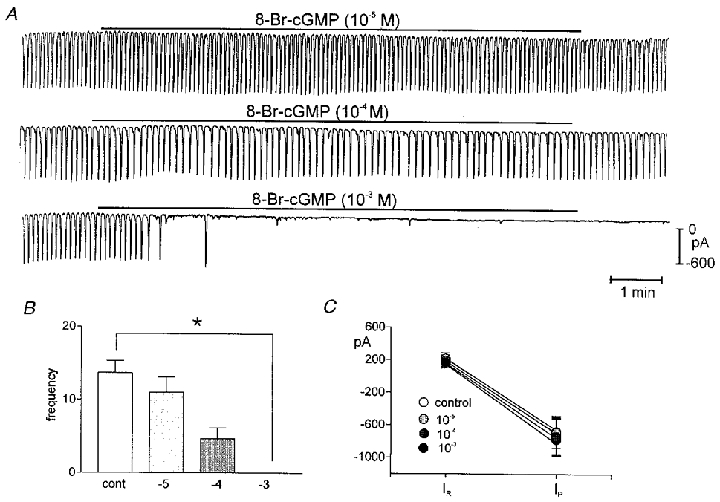
A shows pacemaker currents recorded from the same cell exposed to 8-Br-cGMP (10−5 to 10−3m) held at −60 mV. The higher concentration of 8-Br-cGMP caused cessation of pacemaker currents in all cells tested. The responses to 8-Br-cGMP are summarized in B and C. The reduction in frequency was significant by ANOVA (*). In C, IR equals resting current at the holding potential and IP is the amplitude of the pacemaker current.
Figure 3. Effects of 8-Br-cGMP on ICC slow waves recorded under current clamp (I = 0 pA).
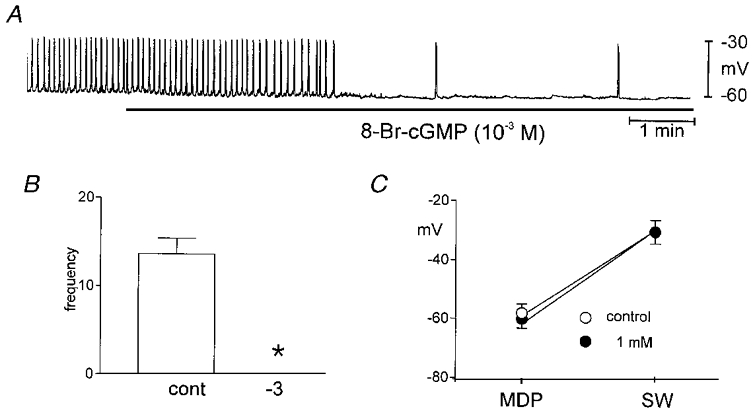
Addition of 8-Br-cGMP (10−3m) inhibited spontaneous slow wave activity. The responses to 8-Br-cGMP are summarized in B and C. The reduction in frequency was significant by Student's t test (*). In C, MDP is the maximum diastolic potential and SW is the maximum potential reached during the slow wave.
Nitric oxide (NO) liberated from SNP or SNAP activates guanylyl cyclase and increases levels of cGMP in many cells (e.g. Rapoport et al. 1983). This can lead to the activation of guanosine 3′,5′-cyclic monophosphate-dependent protein kinase (PKG) and phosphorylation of cellular proteins. We tested the involvement of this pathway in the regulation of pacemaker currents. ODQ (10−6m), an inhibitor of guanylyl cyclase, had no effect on pacemaker currents (Fig. 4A–C), but the effects of SNP were attenuated by pretreatment with ODQ (Fig. 4A–C); SNP (10−5m) decreased the amplitude of pacemaker currents (−475 ± 91 pA to −336 ± 30 pA), but had no significant effect on frequency (13.0 ± 1.4 to 11.8 ± 2.6 cycles min−1; n = 5) in the presence of ODQ. These data suggest that cGMP production is required for the action of SNP on pacemaker currents.
Figure 4. Effects of drugs to block cGMP-dependent pathways.
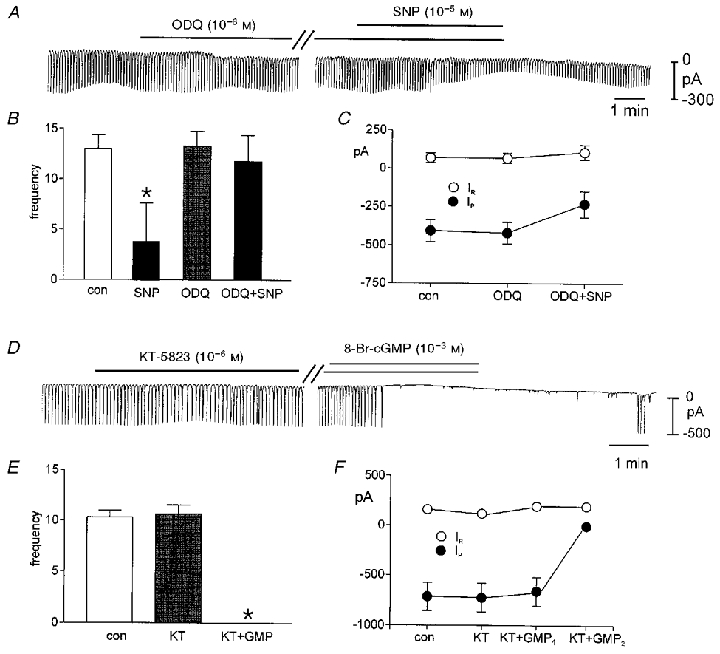
ODQ had no significant effect on pacemaker currents (A). The effects of SNP were attenuated by 15 min of pre-incubation with ODQ. The traces in A are excerpts from a continuous recording (record during parallel lines was omitted for display purposes). B and C summarize data showing the reduced effects of SNP after ODQ. KT-5823 had no significant effect on pacemaker currents, and the effects of 8-Br-cGMP were not affected by 15 min of pre-treatment with KT-5823. The traces in D are excerpts from a continuous recording (record during parallel lines was omitted for display purposes). E and F summarize data showing the response to 8-Br-cGMP after KT-5823. KT + GMP1 denotes the amplitude of the last pacemaker current before inhibition and KT + GMP2 is the amplitude of the currents at steady-state effect. In C and F, IR equals resting current at the holding potential and IP is the amplitude of the pacemaker current.
A PKG inhibitor KT-5823 (10−6m) had no effect on spontaneous inward currents, and pretreatment with KT-5823 for 10–15 min (a regime that blocks cGMP-dependent effects in smooth muscle cells; see Murthy & Makhlouf, 1998; S. D. Koh & K. m. Sanders, unpublished observations) had no effect on the frequency and amplitude of spontaneous inward currents. Pacemaker currents occurred at 10.7 ± 0.9 cycles min−1 and averaged −583 ± 69 pA in amplitude after pre-incubation with KT-5823. Addition of 8-Br-cGMP (10−3m) in the continued presence of KT-5823 led to complete inhibition of pacemaker currents (n = 4,Fig. 4D and F). We also tested the selective inhibitor of Type V (cGMP-specific) phophodiesterase, zaprinast (10−4m). Zaprinast decreased the frequency of pacemaker currents to 9.7 ± 0.5 cycles min−1 (from control of 12.7 ± 0.3 cycles min−1) without significantly affecting the amplitude of the pacemaker currents (n = 7,Fig. 5A and B). However, a non-specific phosphatase inhibitor, okadaic acid, did not change any parameter of pacemaker currents (Fig. 5D). These data suggest that inhibition of pacemaker currents by SNP is mediated by cGMP, but the effects of cGMP may be direct rather than via activation of PKG and protein phosphorylation.
Figure 5. Effects of zaprinast and okadaic acid on pacemaker currents.
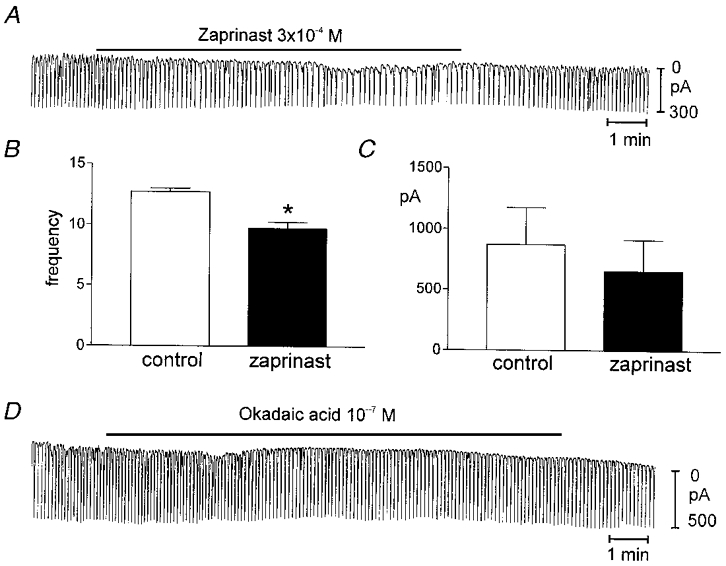
A shows a continuous record and the effects of Zaprinist (3 × 10−4m). This compound reduced the frequency of pacemaker currents. B and C show summary data for the effects of zaprinast; there was a significant reduction in frequency (*). D shows that okadaic acid had no effect on pacemaker currents.
Effects of forskolin on pacemaker activity
To examine possible regulation by cAMP-dependent pathways, we first tested the effects of forskolin (FSK; 10−8 to 10−6m) on pacemaker currents. Under control conditions at a holding potential of −60 mV, the frequency of pacemaker currents was 12.4 ± 1.1 cycles min−1, and the resting current and pacemaker current amplitudes were 123 ± 25 pA and −463 ± 53 pA, respectively, in these experiments (n = 36). FSK caused a concentration-dependent reduction in the frequency of pacemaker currents (Fig. 6A and B). The amplitude of pacemaker currents also decreased as a function of FSK concentration (i.e. 10−8m, −414 ± 74; 10−7m, −200 ± 46; 10−6m, −138 ± 34 pA; n = 5), and resting outward current increased (i.e. 10−8m, 128 ± 31; 10−7m, 224 ± 30; 10−6m, 290 ± 41 pA; n = 5) (Fig. 6C). It should be noted that after application of FSK, the reduction in the amplitude of pacemaker currents occurred before the decrease in frequency became apparent (Fig. 6A). We exposed a total of 31 cells to FSK at a concentration of 10−6m. Complete cessation of pacemaker currents occurred in 14 of these cells. Dideoxy forskolin (10−6m) had no effect on the frequency or amplitude of pacemaker currents (n = 3).
Figure 6. Effects of forskolin (FSK) on pacemaker currents.
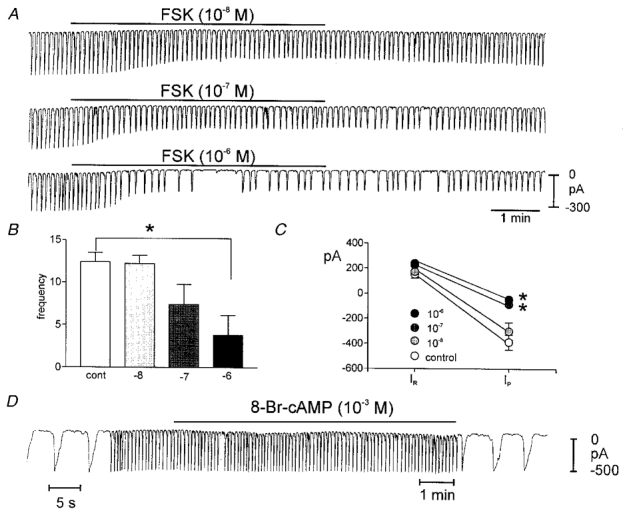
A shows pacemaker currents from the same cell exposed to FSK (10−8 to 10−6m). FSK caused a concentration-dependent decrease in frequency and amplitude of pacemaker currents. The scale in the final trace applies to all traces. The responses to FSK are summarized in B and C. The reduction in frequency was significant by ANOVA; and amplitude significant at concentrations denoted by *. In C, IR equals resting current at the holding potential and IP is the amplitude of the pacemaker current. D shows that 8-Br-cAMP had no effect on pacemaker currents (i.e. did not mimic FSK). Note changes in sweep speed in D showing waveforms of pacemaker currents before and after cAMP administration.
ICC slow waves were recorded under current clamp (I = 0). The MDP of the cultured ICC in these experiments was −58 ± 2 mV, and ICC slow waves averaged 29 ± 0.7 mV in amplitude and 15.7 ± 2.2 cycles min−1 in frequency. Treatment with FSK (10−6m) caused significant hyperpolarization of MDP (to −67 ± 2 mV; P < 0.05) and decreased the amplitude (to 10 ± 1.2 mV) and frequency (to 6.7 ± 1.2 cycles min−1) of ICC slow waves (Fig. 7A–C; all parameters P < 0.05,n = 4).
Figure 7. Effects of FSK on ICC slow waves recorded under current clamp.
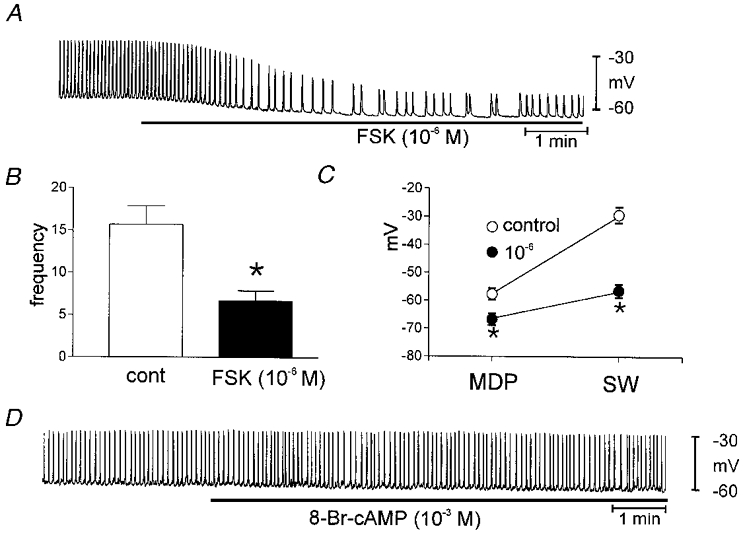
Addition of FSK (10−6m) inhibited spontaneous slow wave activity. The responses to FSK are summarized in B and C. The reduction in frequency was significant using Student's t test (*). In C, MDP is the maximum diastolic potential and SW is the maximum potential reached during the slow wave. D shows that 8-Br-cAMP had no effect on slow waves.
We also tested 8-Br-cAMP (10−3m) and diBu-cAMP (10−3m) to determine whether these membrane-permeable cAMP analogues mimicked the effects of forskolin. Neither cAMP analogue had any effects on membrane potential nor any parameter of pacemaker current. (n = 8,Figs 6D and 7D).
Isoproterenol (10−6m) had no effect on the frequency of pacemaker currents (from 10.8 ± 0.5 to 11.0 ± 0.5 cycles min−1; n = 4), however there was a small reduction in the amplitude of the currents from −375 ± 35 pA to −225 ± 19 pA (P < 0.05). Isoproterenol also induced a mean small, sustained outward current of 56 ± 6 pA (n = 4).
Although membrane-permeable analogues of cAMP did not mimic the effects of FSK, FSK is a potent activator of adenylyl cyclase and increased concentrations of cAMP are known to activate adenosine 3′5′-cyclic monophosphate-dependent protein kinase (PKA). We tested the possible role of PKA in regulating pacemaker activity with myristoylated PKA inhibitor (mPKI). Addition of mPKI (10−7m) did not affect pacemaker currents (i.e. frequency was 14.4 ± 1.6 and 14.0 ± 1.6 cycles min−1; amplitude was −364 ± 59 and −316 ± 56 pA, before and in the presence of mPKI, respectively; n = 5). Application of FSK (10−7m) in the presence of mPKI decreased the amplitude (i.e. to −55 ± 23 pA) and frequency (to 3.2 ± 1.9 cycles min−1; see Fig. 8A–C). These effects were not different from the effects of FSK alone. We also tested KT-5823 (10−6m) the PKG inhibitor, on the effects of FSK to determine whether a ‘cross-over’ effect via PKG might mediate the effects of FSK. After incubation of KT-5823, FSK reduced the pacemaker current amplitude and frequency to −68 ± 5.5 pA and 3.0 ± 3 cycles min−1, respectively (n = 5). These values were not different from the effects of FSK alone (Fig. 8D–F). We also tested the unlikely possibility that FSK might mediate its effects by stimulation of guanylyl cyclase. We found that ODQ (10−5m) had no effect on the inhibition of pacemaker currents by FSK (n = 4,Fig. 9A–C). Finally, we tested the effect of the adenylyl cyclase inhibitor SQ-22536 on pacemaker currents. Treatment with SQ-22536 (10−4m) did not affect pacemaker currents as compared with control currents. Application of FSK (10−6m) in the presence of SQ-22536 (10−4m) decreased the amplitude of pacemaker currents (to −92 ± 52 pA) and frequency (to 3.4 ± 2.4 cycles min−1, n = 5) (Fig. 9D–F).
Figure 8. Effects of drugs to test involvement of protein kinase A and protein kinase G on the effects of FSK.
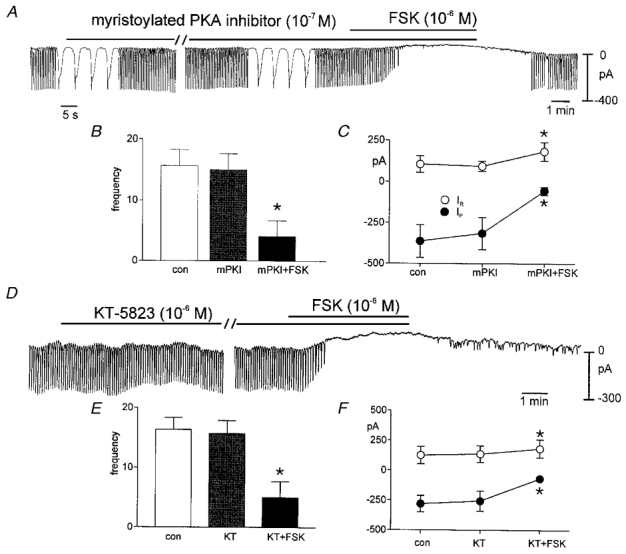
A shows that myristoylated PKA inhibitor (10−7m) had no effect itself on pacemaker currents and this compound did not inhibit the effects of FSK. Data for these experiments are summarized in B and C. D shows that KT-5823 (10−6m) had no effect itself on pacemaker currents and this compound did not inhibit the effects of FSK. Data for these experiments are summarized in E and F. In B, C, E and F*denotes significance using Student's t test.
Figure 9. Effects of drugs to test involvement of guanylyl cyclase and adenylyl cyclase on the effects of FSK.
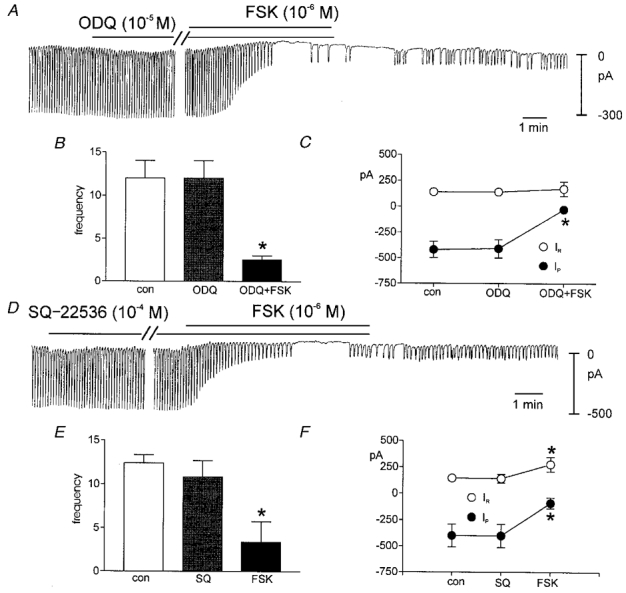
A shows that ODQ (10−5m) had no effect itself on pacemaker currents and this compound did not inhibit the effects of FSK. Data for these experiments are summarized in B and C. D shows that SQ-22536 (10−4m) had no effect itself on pacemaker currents and this compound did not inhibit the effects of FSK. Data for these experiments are summarized in E and F. In B, C, E and F, * denotes significance using Student's t test. In C and F, IRrepresents resting current at the holding potential and IP is the amplitude of the pacemaker current.
Outward current activated by FSK in ICC
We noted a persistent outward current activated by FSK (see above). We tested the identity of this current using glibenclamide, an inhibitor of ATP-dependent K+ channels. Glibenclamide (10−5m) decreased the resting current in ICC from 71 ± 21 to 30 ± 26 pA (P < 0.05,n = 5), and inhibited the outward current in response to FSK (10−6m; i.e. from 241 ± 56 to 49 ± 28 pA; before and after glibenclamide treatment). Thus, a glibenclamide-sensitive conductance is active at rest in ICC and this conductance is enhanced by FSK (Fig. 10A–E), as previously shown in smooth muscle cells (Zhang et al. 1994).
Figure 10. The outward current activated by FSK was inhibited by glibenclamide.
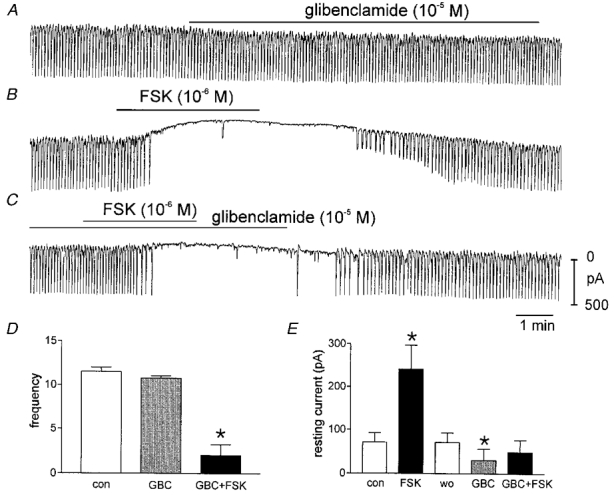
A shows effects of glibenclamide (10−5m) on pacemaker currents. This caused a significant reduction in outward current suggesting that KATP is activated tonically in these cells. B shows the effects of FSK, and a persistent outward current is activated by FSK. C shows that pre-treatment with glibenclamide (15 min) inhibited the outward current activated by FSK. All traces were recorded from the same cell. D and E summarize the effects of glibenclamide (GBC) and FSK added after glibenclamide. wo denotes washout of FSK, and *, significance by Student's t test.
Effects of SNP and FSK on intact small intestinal muscles
Cyclic nucleotide agonists were also applied to jejunal muscle strips to determine whether effects noted on cultured ICC were manifest in intact muscles. SNP (10−6m) and SNAP (10−6m) caused small hyperpolarizations of membrane potential (i.e. from −58.0 ± 2.0 to −61.0 ± 4.0 mV for SNP (n = 6) and from −55.0 ± 2.0 to −61.0 ± 2.0 mV for SNAP; n = 6; P < 0.05; Fig. 11A and B). The hyperpolarization for SNP did not reach statistical significance for the tissues tested. Both NO donors reduced the amplitude and frequency of slow waves. The effects of SNP and SNAP were mimicked by 8-Br-cGMP (10−3m): resting membrane potential hyperpolarized from −56.0 ± 1.0 to −61.0 ± 1.0 mV and slow wave amplitude and frequency reduced from 10.8 ± 1.4 to 6.0 ± 2.0 mV and 13.6 ± 0.7 to 3.4 ± 1.3 cycles min−1, respectively (n = 5; P < 0.05 for slow wave amplitude and frequency; Fig. 10C). Forskolin (10−6m) also reduced frequency (from 11.1 ± 1.3 to 3.1 ± 1.4 cycles min−1; n = 8,P < 0.001) and caused a reduction in amplitude of slow waves from 11 ± 1.2 to 1.4 ± 0.6 mV (P < 0.05), and hyperpolarization in membrane potential (−61.0 ± 2.0 to −67.0 ± 3.0 mV; Fig. 11D). Isoproterenol (10−6m) mimicked the effects of FSK in terms of the hyperpolarization response (−53.0 ± 5.0 to −60.0 ± 6.0 mV) and reduction in the amplitude of slow waves (9.5 ± 1.1 to 4.3 ± 1.3 mV; P < 0.05), however there was no reduction in the frequency of slow waves in response to isoproterenol (14.3 ± 1.3 to 13.0 ± 2.6 cycles min−1; P > 0.05; Fig. 11E). No effects were noted on membrane potential (−59.0 ± 1.0 to −59.0 ± 1.0 mV), slow wave amplitude (11.0 ± 1 to 11.0 ± 1 mV), or frequency (10.8 ± 1 to 10.0 ± 1 cycles min−1n = 5; P > 0.05) with 8-Br-cAMP (10−3m; Fig. 11F). Data from these experiments are summarized in Fig. 11G–L.
Figure 11. Effects of cyclic nucleotide agonists on slow waves from intact small intestinal muscles.
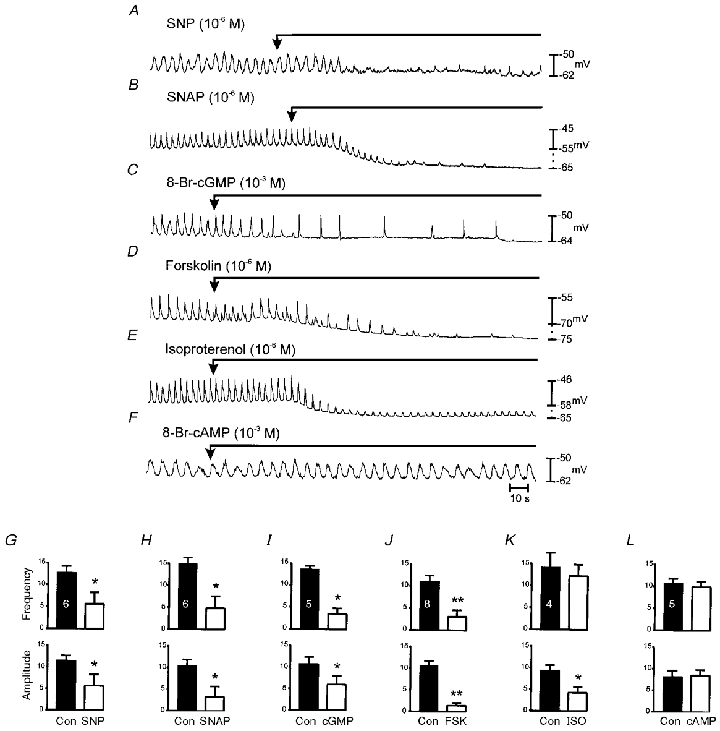
A and B show the effects of SNP (10−6m) and SNAP (10−6m) on slow waves. SNP and SNAP caused a small degree of hyperpolarization of membrane potential, and examples showing the range of little effect to extreme effect are presented. Both compounds caused a dramatic reduction in frequency and amplitude of slow waves. The effects of SNP and SNAP were mimicked by 8-Br-cGMP (10−3m; C). FSK (10−6m) also reduced frequency and caused a reduction in amplitude of slow waves and hyperpolarization (D). Isoproterenol (ISO; 10−6m) mimicked the effects of FSK in terms of the hyperpolarization response and reduction in the amplitude of slow waves; however, there was no reduction in the frequency of slow waves (E). No changes in amplitude and frequency of slow waves were noted with 8-Br-cAMP (10−3m; F). The bar graphs (G–L) show the mean effects of these drugs on slow wave frequency and amplitude. Numbers of each experiment performed are denoted by the numbers in the control bar in each panel and significance is denoted by * (P < 0.05) or ** (P < 0.005). Numbers and labels in G and designations for control and drugs along bottom pertain to other graphs along the same rows and columns.
DISCUSSION
In previous studies it was shown that specific loss of pacemaker ICC in the mouse small intestine did not disrupt inputs from motor neurons (Ward et al. 1994, 1995). The experiments here suggest that pacemaker ICC in the myenteric regions of the small intestine are not required for motor inputs from intrinsic nerves, but the results do not exclude the possibility that pacemaker ICC are innervated in normal animals and that neural inputs can affect the amplitude and frequency of slow waves. ICC within the myenteric plexus region of several species have been shown to have close association with varicose nerve endings, in some cases forming apparent synaptic-like contacts (e.g. Faussone-Pellegrini & Cortesini, 1983; Thuneberg, 1989; Rumessen et al. 1993). The specific chemical coding of neurons that innervate pacemaker ICC in the small intestine has not been clarified, but if inhibitory neurons impinge upon these cells, part of the regulation of slow waves might be mediated by cyclic nucleotides. Hormonal and paracrine substances may also exert effects on pacemaker ICC via cyclic nucleotide-dependent pathways.
Previous studies have shown that SNP reduces slow wave frequency in canine gastric antrum (Ozaki et al. 1992a). The frequency of slow waves in the small intestine is relatively more resistant to changes in response to neural or hormonal effects, however some studies have shown that NO-dependent neurotransmission can increase the period between slow waves, and application of NO can delay subsequent slow waves (e.g. Stark et al. 1991). These findings suggest that regulation of slow wave frequency could be a factor in modulating motility responses of the small intestine. The amplitude of slow waves in the small intestine is highly regulated by neural inputs, and particularly inputs from nitrergic motor neurons that would be mediated via cGMP formation (e.g. Stark et al. 1991; Ward et al. 1994). It is unclear from whole tissue experiments whether nitrergic effects on slow wave amplitude occur primarily at pacemaker cells by reducing the amplitude of pacemaker currents or by enhancing overall membrane conductance of the smooth muscle syncytium. The latter would increase the decay of slow waves as a function of distance. We found some reduction in pacemaker current in response to SNP but little or no decrease in response to cGMP. Both compounds caused abrupt cessation of pacemaker currents at higher concentrations. These data suggest that regulation of slow wave amplitude by nitrosovasodilators may not be accomplished by cGMP-dependent effects in pacemaker cells. The major influence of cGMP-dependent mechanisms appears to be on pacemaker frequency.
Our data suggest that cGMP-dependent regulation of slow waves is due to the direct actions of cGMP rather than to actions mediated by cGMP-dependent protein kinase (PKG). SNP and membrane-permeable analogues of cGMP reduced the frequency of pacemaker currents and then abruptly inhibited spontaneous currents. The effects of SNP were reduced by ODQ, an inhibitor of guanylyl cyclase (Garthwaite et al. 1995), suggesting that cGMP formation is necessary for the effects of nitric oxide donors. An inhibitor of PKG, however, did not block the effects of 8-Br-cGMP, suggesting the effects were direct rather than mediated by phosphorylation. The site of action of cGMP is not yet understood. Pacemaker currents appear to be due to activation of a non-selective cation conductance and it is possible that this conductance is inhibited by intracellular cGMP. Non-selective cation conductances stimulated by cGMP are well known (e.g. Fesenko et al. 1985), but we are unaware of a cation conductance inhibited by this nucleotide. Other sites of potential action are on the oscillatory mechanism within ICC that regulates the cation current. Future experiments will attempt to elucidate the mechanism of cGMP action.
The decrease in frequency of pacemaker currents in ICC caused by forskolin was similar to the whole tissue responses recorded in murine small intestine (this study), canine colon (Huizinga et al. 1991), and guinea-pig and canine stomachs (Ozaki et al. 1992b; Tsugeno et al. 1995). In the present study we also noted that a sustained outward current developed in response to forskolin, and this was associated with a hyperpolarization response in current-clamp mode. Others have also reported this effect of forskolin on cultured ICC (Lee et al. 1999). Some authors have reported that forskolin did not affect resting potential in intact muscles of canine colon (Huizinga et al. 1991) and guinea-pig stomach (Tsugeno et al. 1995), but Smith et al. (1993) observed hyperpolarization in response to forskolin in canine colon. The amplitude of this response was small, but this may have been because the resting potentials before forskolin were close to the K+ equilibrium potential. The outward current (and hyperpolarization) activated by forskolin in ICC was probably due to ATP-sensitive K+ channels in ICC since this response was blocked by glibenclamide.
A major difference between the present study and some of the previous studies on intact muscles was that membrane-permeable cAMP analogues had no effect on pacemaker currents in ICC. We also found that the responses to forskolin were not blocked by inhibitors of adenylyl cyclase, protein kinase A, or guanylyl cyclase, and that isoproterenol, a β receptor agonist with effects mediated by cAMP, also had no effect on the frequency of pacemaker currents recorded from ICC. Others have reported that dibutyryl cyclic AMP, 8-bromo-cyclic AMP, and 3-isobuty-1-methylxanthine (IBMX) mimicked the effects of forskolin in canine colon (Huizinga et al. 1991) and guinea-pig stomach (Tsugeno et al. 1995). Our observations on ICC suggest that the effects of forskolin on pacemaker currents may be direct rather than effects mediated by stimulation of adenylyl cyclase and protein kinase A. Tsugeno et al. (1995) also acknowledged that some of the effects of forskolin and IBMX on slow waves could have been due to cAMP-independent effects. It should also be noted that other studies of the canine colon have not confirmed the observations of Huizinga et al. (1991). In another study 8-bromo-cAMP (up to 3 × 10−3m) did not mimic the effects of forskolin on slow wave frequency (Smith et al. 1993). Canine gastric muscles have variable frequency responses to drugs known to enhance cAMP (i.e. isoproterenol reduced slow wave frequency only at concentrations in which more than 80 % of contractile force was inhibited; calcitonin gene-related peptide had no effect on frequency at concentrations high enough to block contractions; and vasoactive intestinal polypeptide enhanced frequency; Ozaki et al. 1992b). Taken together these data suggest that regulation of slow-wave frequency by cAMP-dependent mechanisms is variable and possibly questionable. It is also possible that species or organ differences could explain the disparity in the responses to agents that enhance cAMP and that pacemaker cells in different regions of the GI tract are regulated differently by cyclic nucleotides.
In summary, cultured ICC have provided an important opportunity for investigating the regulation of pacemaker activity in the GI tract. Our data suggest that nitrergic inputs would result in negative chronotropic effects. These effects appear to be mediated by cGMP directly and not via protein phosphorylation. Forskolin also inhibits pacemaker currents, but our data show that these effects may be a direct effect of the diterpene and not via downstream cAMP-dependent mechanisms. It will be necessary to test these observations further in ICC from other regions of the GI tract and from other species before a generalized concept of cyclic nucleotide regulation of electrical rhythmicity can be formulated.
Acknowledgments
This project was supported by DK40569 from NIDDK. We are grateful to Mark M. Zenker and Nancy Horowitz for preparation of the cultured ICC. J.Y.J. was supported by Chosun University (Republic of Korea) and KOSEF postdoctoral fellowship programme.
References
- Faussone-Pellegrini MS, Cortesini C. Some ultrastructural features of the muscular coat of human small intestine. Acta Anatomica. 1983;115:47–68. doi: 10.1159/000145676. [DOI] [PubMed] [Google Scholar]
- Fesenko EE, Kolesnikov SS, Lyubarsky AL. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985;313:310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Molecular Pharmacology. 1995;48:184–188. [PubMed] [Google Scholar]
- Horowitz BM, Ward SM, Sanders KM. Cellular and molecular basis for electrical rhythmicity in gastrointestinal muscles. Annual Review of Physiology. 1999;61:19–43. doi: 10.1146/annurev.physiol.61.1.19. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Farraway L, DenHertog A. Effect of voltage and cyclic AMP on frequency of slow-wave-type action potentials in canine colon smooth muscle. The Journal of Physiology. 1991;442:31–45. doi: 10.1113/jphysiol.1991.sp018780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. The Journal of Physiology. 1998;513:203–213. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proceedings of the National Academy of Sciences of the USA. 1989;86:7280–7284. doi: 10.1073/pnas.86.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Sanders KM. Comparison of ionic currents from interstitial cell and smooth muscle cells of canine colon. The Journal of Physiology. 1993;460:135–152. doi: 10.1113/jphysiol.1993.sp019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Thuneberg L, Berezin I, Huizinga JD. Generation of slow waves in membrane potential is an intrinsic property of interstitial cells of Cajal. American Journal of Physiology. 1999;277:G409–423. doi: 10.1152/ajpgi.1999.277.2.G409. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Makhlouf GM. Differential regulation of phospholipase A2 (PLA2)-dependent Ca2+ signalling in smooth muscle by cAMP- and cGMP-dependent protein kinases. Inhibitory phosphorylation of PLA2 by cyclic nucleotide-dependent protein kinases. Journal of Biological Chemistry. 1998;273:34519–34526. doi: 10.1074/jbc.273.51.34519. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Kusakabe M, Yoshinaga K, Ogawa M, Hayashi S, Kunisada T, Era T, Sakakura T, Nishikawa SI. In utero manipulation of coat color formation by a monoclonal anti-c-kit antibody: two distinct waves of c-kit-dependency during malanocyte development EMBO. Journal. 1991;10:2111–2118. doi: 10.1002/j.1460-2075.1991.tb07744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ördög T, Ward SM, Sanders KM. Interstitial cells of Cajal generate electrical slow waves in the murine stomach. The Journal of Physiology. 1999;518:257–269. doi: 10.1111/j.1469-7793.1999.0257r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Blondfield DP, Hori M, Publicover NG, Kato I, Sanders KM. Spontaneous release of nitric oxide inhibits electrical, Ca2+ and mechanical transients in canine gastric smooth muscle. The Journal of Physiology. 1992a;445:231–247. doi: 10.1113/jphysiol.1992.sp018921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Blondfield DP, Hori M, Sanders KM, Publicover NG. Cyclic AMP-mediated regulation of excitation-contraction coupling in canine gastric smooth muscle. The Journal of Physiology. 1992b;447:351–372. doi: 10.1113/jphysiol.1992.sp019006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Blondfield DP, Stevens RJ, Publicover NG, Sanders KM. Simultaneous measurement of membrane potential, cytosolic calcium and muscle tension in smooth muscle tissue. American Journal of Physiology. 1991;260:C917–925. doi: 10.1152/ajpcell.1991.260.5.C917. [DOI] [PubMed] [Google Scholar]
- Rapoport RM, Draznin MB, Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983;306:174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Rumessen JJ, Mikkelsen HB, Qvortrup K, Thuneberg L. Ultrastructure of interstitial cells of Cajal in circular muscle of human small intestine. Gastroenterology. 1993;104:343–350. doi: 10.1016/0016-5085(93)90400-7. [DOI] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Stevens R, Burke EP, Ward SM. Slow waves actively propagate at submucosal surface of circular layer in canine colon. American Journal of Physiology. 1990;259:G258–263. doi: 10.1152/ajpgi.1990.259.2.G258. [DOI] [PubMed] [Google Scholar]
- Smith TK, Ward SM, Zhang L, Buxton ILO, Gerthoffer WT, Sanders KM, Keef KD. β-Adrenergic inhibition of electrical and mechanical activity in canine colon: Role of cyclic AMP. American Journal of Physiology. 1993;264:G708–717. doi: 10.1152/ajpgi.1993.264.4.G708. [DOI] [PubMed] [Google Scholar]
- Stark ME, Bauer AJ, Szurszewski JH. Effect of nitric oxide on circular muscle of the canine small intestine. The Journal of Physiology. 1991;444:743–761. doi: 10.1113/jphysiol.1991.sp018904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurszewski JH. Electrical basis for gastrointestinal motility. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 2. New York: Raven Press; 1987. pp. 383–422. [Google Scholar]
- Thomsen L, Robinson TL, Lee JC, Farraway LA, Hughes MJ, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nature Medicine. 1998;4:848–851. doi: 10.1038/nm0798-848. [DOI] [PubMed] [Google Scholar]
- Thuneberg L. Interstitial cells of Cajal. In: Wood JD, editor. Handbook of Physiology section 6, The Gastrointestinal System. part 1. Vol. 1. Bethesda, MD, USA: American Physiological Society; 1989. pp. 349–386. [Google Scholar]
- Torihashi S, Ward SM, Nishikawa S -I, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell and Tissue Research. 1995;280:97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- Tsugeno M, Huang SM, Pang YW, Chowdhury JU, Tomita T. Effects of phosphodiesterase inhibitors on spontaneous electrical activity (slow waves) in the guinea-pig gastric muscle. The Journal of Physiology. 1995;485:493–502. doi: 10.1113/jphysiol.1995.sp020745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in Steel mutants. American Journal of Physiology. 1995;269:C1577–1585. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. The Journal of Physiology. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Harney SC, Bayguinov YJ, Sanders KM. Development of electrical rhythmicity in the gastrointestinal tract is specifically encoded in the tunica muscularis. The Journal of Physiology. 1997;505:241–258. doi: 10.1111/j.1469-7793.1997.241bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Bonev AD, Mawe GM, Nelson MT. Protein kinase A mediates activation of ATP-sensitive K+ currents by CGRP in gallbladder smooth muscle. American Journal of Physiology. 1994;267:G494–499. doi: 10.1152/ajpgi.1994.267.3.G494. [DOI] [PubMed] [Google Scholar]


