Abstract
To study post-excitatory changes of conduction velocity, action potentials were recorded from 132 unmyelinated nerve fibres (C fibres) in cutaneous fascicles of the peroneal nerve using microneurography in healthy human subjects. The ‘marking’ technique was used to assess responsiveness to mechanical and heat stimuli or sympathetic reflex provocation.
C fibres were classified into three major classes: mechano-responsive afferent (n = 76), mechano-insensitive afferent (n = 48) and sympathetic efferent C fibres (n = 8).
During regular stimulation at 0.25 Hz, conditioning pulses were intermittently interposed. Changes of conduction velocity were assessed for different numbers of conditioning impulses and varying interstimulus intervals (ISIs). For all three fibre classes the latency shift following conditioning pulses at an ISI of 1000 ms increased linearly with their number (n = 1, 2 and 4). However, the absolute degree of conduction velocity slowing was much higher in the 32 mechano-insensitive fibres as compared with 56 mechano-responsive or 8 sympathetic fibres.
Single additional pulses were interposed at different ISIs from 20 to 2000 ms. For 20 mechano-responsive fibres conduction velocity slowing increased with decreasing ISI (subnormal phase). In contrast, for 16 mechano-insensitive C fibres the conduction velocity slowing decreased with shorter ISIs, and at values lower than 417 ± 49 ms (mean ±s.e.m.) the conduction velocity of the conditioned action potential was faster than before (conduction velocity speeding). This supernormal phase had its maximum at 69 ± 10 ms.
In this study we provide, for the first time, direct evidence of relative supernormal conduction in human mechano-insensitive C fibres. The implications for temporal coding in different afferent C fibre classes are discussed.
An action potential (AP) can be conducted along a nerve fibre before its electrical and ionic equilibrium is fully restored from preceding activation. This second AP will be influenced by the axon's recent impulse discharge history. After-effects of APs have been shown to affect conduction velocity and thereby change impulse discharge frequency in an axon. During a supernormal period induced by one AP, a following AP will close up to this preceding AP (conduction velocity speeding) while the opposite can be observed during a subnormal period. This modulation of effective central frequency by after-effects of APs has been discussed in terms of nervous coding of the peripheral nervous system (Erlanger & Gasser, 1937; Raymond & Lettvin, 1978; Malenka et al. 1983). Not only conduction velocity but also electrical threshold have been shown to be altered by the axon's history. Changes of conduction velocity and excitability are assumed to be a consequence of an altered membrane potential. They offer easy ways to quantify after-effects of APs experimentally.
After-effects in human A fibres and in animal A and C fibres have been divided into several successive phases (Bergmans, 1968; Raymond & Lettvin, 1978; Raymond, 1979; Shin & Raymond, 1991). A short-lasting supernormal period (SNP) immediately after relative refractoriness is characterised by higher conduction velocities and lower activation thresholds, and is followed by a long-lasting hypo-excitable phase (HP). During the hypo-excitable HO phase an early short-lived period (H1) can be distinguished from a long-lasting period (H2), both being dependent on the number of conditioning pulses, the latter even up to large numbers of impulses (Bergmans, 1968; Shin & Raymond, 1991).
Activity-dependent slowing of APs in nociceptive human C fibres has been shown to separate them into two major classes matching the two major receptive classes, mechano-responsive and mechano-insensitive fibres (Weidner et al. 1999b). This is based on a long-lasting HP and was taken as evidence for differences of membrane functions between these two classes. In human C fibres, as in rat, H2 is dependent on the number of conditioning pulses, which is the basis of the ‘marking’ method (Thalhammer et al. 1994; Schmelz et al. 1995).
In the present study it will be shown that mechano-responsive and mechano-insensitive C nociceptors differ with regard to the extent and time course of conduction velocity changes both during supernormal and subnormal periods.
Part of this work has been published in abstract form (Weidner et al. 1999a).
METHODS
The microneurographic recording technique was employed to record 132 C fibres from cutaneous fascicles of the peroneal nerve in our research laboratories in Uppsala and Erlangen. None of the 46 subjects (28 male, 18 female, aged 19–24 years) suffered from any dermatological or neurological disease. All participants gave their informed written consent. The study was performed according to the Declaration of Helsinki and was approved by the local ethics committees.
The microneurographic technique for recording from human C fibres has been described in detail elsewhere (Torebjörk, 1974) and will only be summarised briefly in this report. A microelectrode of 0.2 mm diameter (MNG active/2 MΩ, Frederik Haer Inc., Bowdoinham, ME, USA) was manually inserted into the peroneal nerve dorsolateral to the fibular head for recording nerve signals, and a reference microelectrode was placed subcutaneously nearby. The uninsulated tip of the recording electrode was inserted into a cutaneous fascicle while its output signal was passed through an audio-amplifier. Positioning of the electrode was guided by the characteristic sound of multifibre discharges evoked by gently stroking the skin in the innervation territory (lower leg or foot dorsum).
Strong painful electrical impulses (0.2 ms, 50 mA) from an insulated constant current stimulator (Digitimer DS7, Digitimer, Hertfordshire, UK) were used as search stimuli to minimise bias towards mechano-responsive C fibres. These pulses are known to exceed even the high electrical thresholds of mechano-insensitive fibres (Weidner et al. 1999b). By moving a pointed steel probe with a small contact surface (1 mm in diameter) on the skin, single C fibres were localised and identified by the long and reproducible latency of their responses. When the skin innervation territory of a C fibre was found, two needle electrodes of 0.2 mm shaft diameter were inserted 5 mm apart in this territory for repetitive intracutaneous electrical stimulation (0.25 Hz, 0.2 ms, 10–150 V, from an insulated constant voltage Grass S 88 stimulator). C fibres were then characterised by the ‘marking’ technique (Torebjörk & Hallin, 1974; Schmidt et al. 1995), i.e. any sudden increase of response latency was regarded as a sign of activation of the respective fibre. Sympathetic C fibres were identified by their ‘marking’ response during activation related to arousal stimuli which are known to elicit sympathetic reflex responses in human skin nerves (Hallin & Torebjörk, 1970, 1974; Hagbarth et al. 1972). Afferent C fibres were identified by their ‘marking’ response to natural stimulation (i.e. mechanical or heat stimuli) of their innervation territories in the skin (Torebjörk, 1974).
Heat stimuli were delivered from a halogen lamp, with feedback controlled by a thermocouple gently touching the skin. Skin temperature was increased by 0.25°C s−1 from an adapting level of 32°C to tolerance level of 52°C.
After a rest period of at least 2 min the latencies of C fibre responses to the first electrical impulse delivered from the intracutaneous needle electrodes were used for calculating the initial conduction velocity. The shortest distance between the stimulating needles in the skin and the recording electrode in the nerve was assessed with a measuring tape to the nearest millimetre. Room temperature was kept constant at 22–24°C. After-effects of additional action potentials were assessed after a period of ongoing intracutaneous electrical stimulation at 0.25 Hz for at least 2 min in which a stable latency of the C fibre response was obtained.
Two protocols were used.
Protocol 1
To assess the dependency of after-effects on the number of conditioning pulses, additional pulses were interposed between the regular stimulus pulses (n = 1, 2 and 4). The interval between the last interpolated pulse and the next regular pulse was 1000 ms; the interval between additional pulses was 500 ms. The initial latency increase was allowed to recover before another test with additional impulses was performed. Each fibre was tested at least twice with 1, 2 and 4 additional pulses. The mean latency increase of the next regular pulse after a given number of additional pulses was evaluated (latency ‘shift’; Fig. 1).
Figure 1. Response of a CMH fibre to repetitive electrical stimulation with varying number of interpolated conditioning stimuli.
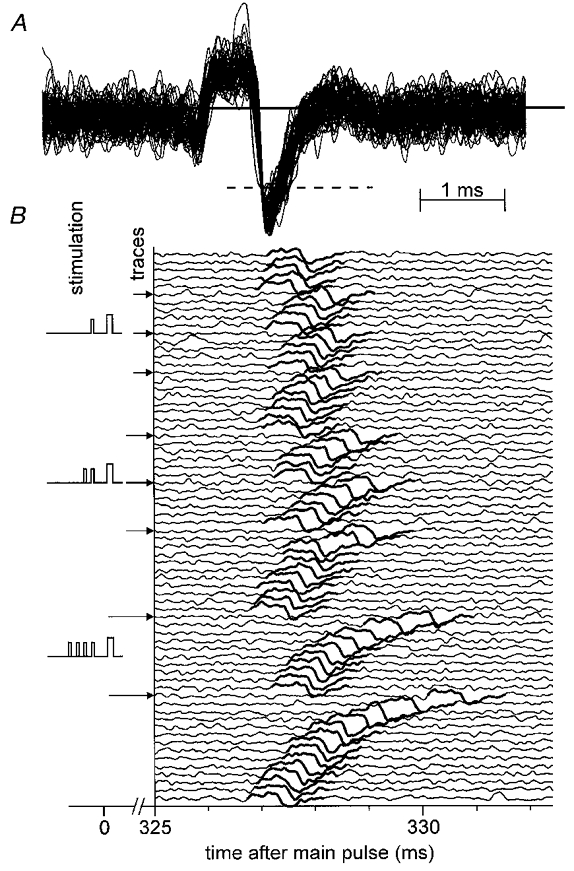
In A, spikes of a single C fibre recorded in 70 consecutive sweeps are superimposed showing the form of the action potential and signal-to-noise ratio. In B, the traces are displayed from top to bottom in successive order. Intracutaneous electrical stimuli were applied at 4 s intervals (0.25 Hz). Segments 325–332 ms after each regular stimulus are shown. The left part of the diagram indicates the pattern for conditioning stimulation. One, 2 or 4 pulses were interpolated; the interval from the last conditioning pulse to the next regular pulse was 1000 ms and the intervals between conditioning pulses were 500 ms (protocol 1).
Protocol 2
To assess the time course of after-effects, we intermittently applied one additional pulse at an ISI varying from 2000 to 20 ms (2000, 1000, 500, 250, 100, 50 and 20 ms). The latency shifts of the first and of the second following ‘regular’ action potentials were analysed.
Data acquisition and analysis
Signals from the recording electrodes were amplified and recorded on line by a PC through an interface card (DAP, Microstar, USA) and the SPIKE/SPIDI software package (Forster & Handwerker, 1990). For the purpose of statistical testing we used Statistica's (StatSoft Inc., Tulsa, OK, USA) analysis of variance (ANOVA). Where appropriate, non-parametric statistics were applied. Data are presented as means ±s.e.m. Differences were regarded to be significant at P < 0.05. The appropriate corrections (Bonferroni) for repetitive testing were employed.
RESULTS
A total of 132 C fibres were recorded with innervation territories on the lower leg and foot. Conduction distances were 200–565 mm (363 ± 7 mm, mean ±s.e.m.).
Of these 132 fibres, 76 responded to stimulation with von Frey hairs and had a mean conduction velocity (CV) of 0.98 ± 0.01 m s−1. Seventy-one of them also responded to heat up to 50°C and were classified as mechano-heat-responsive (CMH, ‘polymodal’ nociceptors) (Schmidt et al. 1995). The remaining five fibres could not be tested for heat because of deterioration in recording quality. Forty-eight fibres did not respond to mechanical forces up to 750 mN and had a mean conduction velocity of 0.80 ± 0.02 m s−1. Six of them could not be tested for heat, while 29 of them were found responsive to heat and were classified as CH fibres. Thirteen fibres responded to neither strong mechanical nor to heat stimuli in non-inflamed skin and were classified as mechano-heat-insensitive C fibres (CMiHi). They were proven to be afferent by their responsiveness to intracutaneously injected capsaicin or their pronounced activity-dependent slowing, which has been shown to clearly separate them from sympathetic efferents or mechano-responsive afferents (Weidner et al. 1999b). Eight fibres responded to arousal stimuli and were classified as sympathetic efferent fibres.
Figure 1 shows a specimen record of a fibre responding to 1, 2 and 4 conditioning stimulus pulses interposed into the repetitive main stimulation of 0.25 Hz (protocol 1). Taking all C fibre classes together, the delay induced by two additional pulses was 2.1 ± 0.1 times larger (P < 0.01, Student's t test) than that after one additional pulse. Four pulses again doubled (2.1 ± 0.1-fold, P < 0.01,t test) the latency shift observed after two pulses, in agreement with previous results obtained with an identical stimulus protocol (Schmelz et al. 1995). In the present material we found that this relative increase of response latency with the number of conditioning pulses was identical for all fibre classes. Therefore, the delay per additional pulse can be calculated for each fibre (‘shift per pulse’).
Shifts per pulse
Values were 0.66 ± 0.07 ms (all mean ±s.e.m.) in 56 CMH fibres, 1.87 ± 0.13 ms in 26 CH fibres, 1.88 ± 0.25 ms in 6 CMiHi fibres and 1.31 ± 0.17 ms in 8 efferent sympathetic fibres. Shifts per pulse were larger in the mechano-insensitive (CH and CMiHi) than in the mechano-responsive (CMH) fibre population (t test, P < 0.01), but there was no significant difference between CH and CMiHi fibres. The difference between sympathetic fibres and both mechano-insensitive and mechano-responsive fibres was significant (t test, P < 0.05). Mean shifts per 1, 2 and 4 additional impulses in the three classes of mechano-responsive, sympathetic and mechano-insensitive fibres are shown in Fig. 2.
Figure 2. Encoding properties for additional pulses during the hyponormal period.
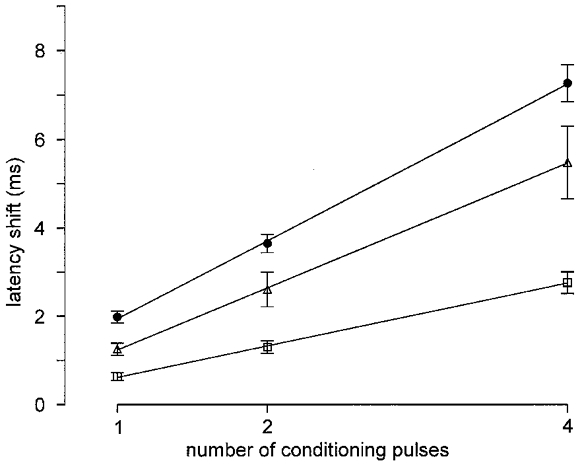
The average latency shifts (error bars show s.e.m.) after 1, 2 and 4 additional pulses at 1000 ms before main pulse are depicted for mechano-insensitive (•, n = 32) and mechano-responsive afferents (□, n = 56) and for sympathetic efferents (▵, n = 8). Linear regression functions are added. For stimulus protocol see Fig. 1.
Influence of interstimulus interval
Twenty mechano-responsive and 16 mechano-insensitive fibres were tested at least twice with one additional pulse interposed before the regular pulse at ISIs from 20 to 2000 ms (protocol 2). Figure 3 shows a specimen record of a mechano-responsive and a mechano-insensitive fibre responding to single conditioning pulses at various ISIs. The induced latency shift of the mechano-responsive fibre in Fig. 3 increased with decreasing ISI which was consistent with the behaviour of all tested mechano-responsive fibres. The shift induced by additional pulses at ISIs longer than 250 ms was correlated with the conduction distance (all P < 0.024). For all mechano-insensitive fibres, however, the latency decreased with decreasing ISI until a minimum was reached. For 14 of 16 fibres a speeding of conduction velocity (negative latency shift) was observed at this minimum. The extent of this speeding was not correlated with conduction distance as was the slowing at long ISIs. The ISI at which short-lasting CV speeding and long-lasting CV slowing mutually cancelled out their effects on the following AP was interpolated to be at 417.2 ± 49.1 ms.
Figure 3. Response of a CMH and a CMiHi fibre to repetitive electrical stimulation with interpolated conditioning stimuli at different interstimulus intervals.
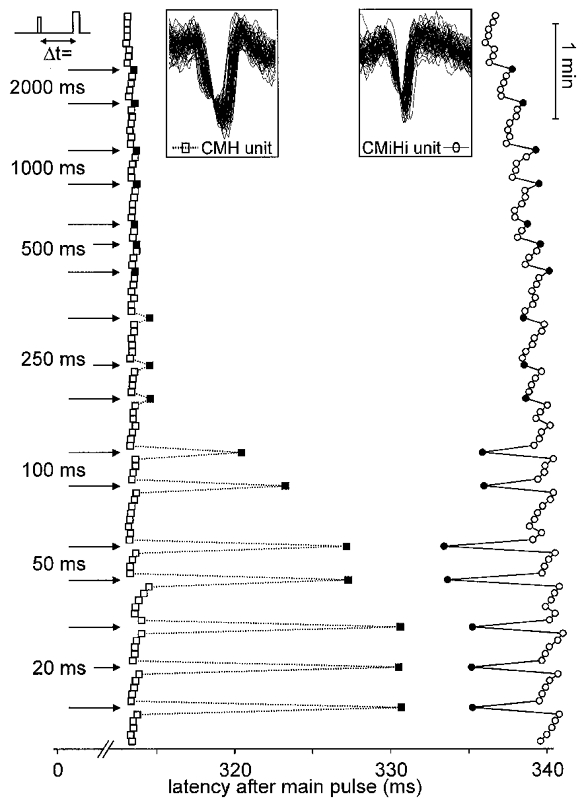
Only action potentials (APs) are displayed, from top to bottom in successive order of traces symbolised by squares (CMH fibre) or circles (CMiHi fibre) The superposition of all APs in the insets shows their stable shape. Segments 313–342 ms after each stimulus are shown. Intracutaneous electrical stimuli were applied at 4 s intervals (0.25 Hz). Arrows on the left indicate additional pulse interposition at the given ISIs (Δt). Filled symbols represent the first AP after the additional pulse.
The minimum latency shift (i.e. the most pronounced speeding) of the 16 mechano-insensitive fibres was found at ISIs between 50 and 250 ms (mean 69.0 ± 9.6 ms). On average the latency shift at the individual minima was −12.1 ± 3.1 ms. However, the CV speeding of mechano-insensitive fibres was relative to the stable CV at 0.25 Hz stimulus frequency. The CV at 0.25 Hz was slower than the initial CV assessed after a resting period of at least 2 min by 0.61 ± 0.12 % for 20 mechano-responsive fibres and by 6.75 ± 1.02 % for 16 mechano-insensitive fibres. For none of the fibres was the CV during speeding faster than its initial value. The mean latency shift for mechano-responsive and mechano-insensitive fibres is depicted in Fig. 4.
Figure 4. Latency shift as a function of time after the additional pulse.
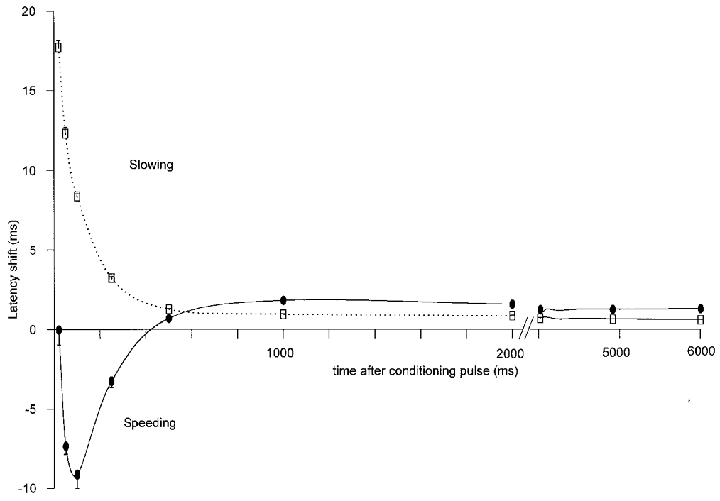
Mean and s.e.m. of latency shifts as obtained in protocol 2 (Fig. 3) are depicted as a function of time after the additional pulse for mechano-responsive (□, n = 20) and mechano-insensitive (•, n = 16) afferents. After-effects between 4020 and 6000 ms were evaluated from the second regular AP after the additional pulse. Latency increase is termed ‘slowing’, latency decrease ‘speeding’ of conduction velocity.
The AP induced by the second regular pulse after the additional pulse still appeared at a latency significantly different from its stable value before conditioning stimulation for both fibre classes (t test, P < 0.01, see Fig. 4). Only slowing of conduction velocities could be observed in this time interval (4000 ms + ISI after the conditioning pulse). For both afferent classes of fibres these late after-effects differed only quantitatively. Mechano-insensitive fibres slowed down significantly more than mechano-responsive fibres from 1000 to 6000 ms after the additional pulse (Scheffé's post hoc test, P < 0.05 each). At 500 ms no significant difference could be observed (no values were obtained between 500 and 1000 ms or after 6000 ms).
DISCUSSION
The results of this study demonstrate that both early and late phases of after-effects of action potentials separate mechano-responsive from mechano-insensitive C fibres.
Several studies in different species have shown that long-lasting subnormality induced by repetitive stimulation at increasing frequencies can separate afferent fibre classes in accordance with their receptive qualities (Raymond et al. 1990; Thalhammer et al. 1994; Gee et al. 1996). In humans, CV slowing separates cold fibres from nociceptive fibres (Serra et al. 1999) and, without overlap, mechano-responsive from mechano-insensitive nociceptors (Weidner et al. 1999b). These long-lasting and cumulative changes have convincingly been attributed to after-hyperpolarisation of the axonal membrane and are most probably induced by enhanced activity of Na+–K+ pumps and limited by inward rectification (Rang & Ritchie, 1968; Jansen & Nicholls, 1973; Van Essen, 1973; Grafe et al. 1997). Two periods of hypo-excitability have been described in rat C fibres and human motor fibres (Bergmans, 1968; Shin & Raymond, 1991). In animal C fibres the early H1 (< 100 ms) period has been shown to encode for small numbers of conditioning pulses, while the late H2 period is additive for a large number of conditioning pulses (Shin & Raymond, 1991). In human C fibres this property of late after-effects is the basis of the ‘marking’ method and was shown to correlate well with numbers of conditioning pulses (Schmelz et al. 1995). The minimal activity-dependent change of late HP in A motor fibres fits with the finding that for afferent human A fibres CV slowing and thereby marking is much less pronounced (Torebjörk & Hallin, 1974).
The short-lasting supernormality directly following the relative refractory period has been described by many authors for different human and animal nerve types and has been explained by after-depolarisation (Gilliatt & Willison, 1963; Swadlow & Waxman, 1976; Raymond & Lettvin, 1978; Kocsis et al. 1979; Bowe et al. 1987). Many authors suggested that spike-induced accumulation of extracellular potassium was responsible for the after-depolarisation in both myelinated and unmyelinated fibres (Kocsis et al. 1983; Shin & Raymond, 1991). However, there is good evidence, at least for myelinated axons, that the depolarising after-potentials represent a passive capacitative discharge rather than potassium accumulation (Barrett & Barrett, 1982).
Both conduction velocity speeding and slowing would be expected to cumulate with the axonal length. However, such correlation was only found for slowing of mechano-responsive but not for slowing or speeding of mechano-insensitive fibres in this study. This is most probably explained by the assumption that for mechano-insensitive fibres most of the (pronounced) conduction modulation occurs in the terminal branches rather than homogeneously along the whole axon.
Since conduction velocity slowing and speeding are due to different mechanisms, the extent of the hypo-excitable period (HP) does not necessarily predict the extent of a supernormal period. We found that mechano-insensitive fibres showed more CV slowing than mechano-responsive fibres during their HP late after the conditioning pulse. In addition the mechano-insensitive fibres exhibited CV speeding as a sign of a SNP, which was absent for the mechano-responsive fibres with the stimulus parameters we used. Probably pre-existing slowing is a prerequisite for speeding of human C fibres. Under the conditions of a regular stimulus rate of 0.25 Hz mechano-insensitive fibres showed an average CV slowing of 6.75 %, but for mechano-responsive fibres the value was only 0.61 % (see Results). Mechano-insensitive fibres can only increase their CV within the margin given by the pre-existing CV slowing (‘relative speeding’).
According to preliminary findings (authors' unpublished observations) mechano-responsive fibres also show SNP when a similar amount of slowing is achieved as induced by 0.25 Hz stimulation in mechano-insensitive fibres.
These differential post-excitatory effects will affect nervous coding (Raymond & Lettvin, 1978) in the form of contrast enhancement. An AP evoked by a stimulus during the SNP will close up to the preceding AP that caused the SNP as its after-effect. A peripheral stimulus frequency of more than 2.4 Hz (ISI < 417 ms) will therefore be enhanced on the way to the CNS in mechano-insensitive fibres with a given history (0.25 Hz regular discharges). The opposite happens if the peripheral frequency is below 2.4 Hz and the second of two APs is conducted during the hypo-excitable period. Contrast enhancement in this frequency range occurs apparently only in mechano-insensitive fibres.
The functional significance of this peripheral contrast enhancement has still to be regarded as hypothetical and should be tested with a different experimental approach. In this study, supernormal conduction is only shown for pairs of action potentials. Preliminary tests showed that it also extends to trains of four at 20 Hz but it will probably not extend to much longer trains. This indicates that the major physiological impact has to be expected in the beginning of a high-frequency burst discharge. Irregular bursts during tonic activation are typical for C fibre responses to natural stimulation.
Acknowledgments
This work was supported by a Max-Planck award to H.E.T., Deutsche Forschungsgemeinschaft Grant SFB 353, Swedish Medical Research Council Project 5206 and a grant to R.S. from the Swedish Foundation for Brain Research. We thank FHC Inc., Bowdoinham, ME, USA for their specially quality-controlled recording electrodes.
References
- Barrett EF, Barrett JN. Intracellular recording from vertebrate myelinated axons: mechanism of the depolarizing afterpotential. The Journal of Physiology. 1982;323:117–144. doi: 10.1113/jphysiol.1982.sp014064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmans J. Active and passive mechanisms in the recovery of single human motor axons from activity. Archives Internationales de Physiologie et de Biochimie. 1968;76:135–138. doi: 10.3109/13813456809058990. [DOI] [PubMed] [Google Scholar]
- Bowe CM, Kocsis JD, Waxman SG. The association of the supernormal period and the depolarizing afterpotential in myelinated frog and rat sciatic nerve. Neuroscience. 1987;21:585–593. doi: 10.1016/0306-4522(87)90144-8. [DOI] [PubMed] [Google Scholar]
- Erlanger J, Gasser HS. Electrical Signs of Nervous Activity. Philadelphia: University of Pensylvania Press; 1937. [Google Scholar]
- Forster C, Handwerker HO. Automatic classification and analysis of microneurographic spike data using a PC/AT. Journal of Neuroscience Methods. 1990;31:109–118. doi: 10.1016/0165-0270(90)90155-9. [DOI] [PubMed] [Google Scholar]
- Gee MD, Lynn B, Cotsell B. Activity-dependent slowing of conduction velocity provides a method for identifying different functional classes of C-fibre in the rat saphenous nerve. Neuroscience. 1996;73:667–675. doi: 10.1016/0306-4522(96)00070-x. [DOI] [PubMed] [Google Scholar]
- Gilliatt RW, Willison RG. The refractory and supernormal periods of the human median nerve. Journal of Neurology, Neurosurgery and Psychiatry. 1963;26:136–143. doi: 10.1136/jnnp.26.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe P, Quasthoff S, Grosskreutz J, Alzheimer C. Function of the hyperpolarization-activated inward rectification in nonmyelinated peripheral rat and human axons. Journal of Neurophysiology. 1997;77:421–426. doi: 10.1152/jn.1997.77.1.421. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Hallin RG, Hongell A, Torebjörk HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiologica Scandinavica. 1972;84:164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Hallin RG, Torebjörk HE. Afferent and efferent C units recorded from human skin nerves in situ. A preliminary report. Acta Societatis Medicorum Upsaliensis. 1970;75:277–281. [PubMed] [Google Scholar]
- Hallin RG, Torebjörk HE. Single unit sympathetic activity in human skin nerves during rest and various manoeuvres. Acta Physiologica Scandinavica. 1974;92:303–317. doi: 10.1111/j.1748-1716.1974.tb05749.x. [DOI] [PubMed] [Google Scholar]
- Jansen JK, Nicholls JG. Conductance changes, an electrogenic pump and the hyperpolarization of leech neurones following impulses. The Journal of Physiology. 1973;229:635–655. doi: 10.1113/jphysiol.1973.sp010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis JD, Malenka RC, Waxman SG. Effects of extracellular potassium concentration on the excitability of the parallel fibres of the rat cerebellum. Journal of Physiology. 1983;334:225–244. doi: 10.1113/jphysiol.1983.sp014491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis JD, Swadlow HA, Waxman SG, Brill MH. Variation in conduction velocity during the relative refractory and supernormal periods: a mechanism for impulse entrainment in central axons. Experimental Neurology. 1979;65:230–236. doi: 10.1016/0014-4886(79)90263-2. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Kocsis JD, Waxman SG. The supernormal period of the cerebellar parallel fibres effects of [Ca2+]o and [K+]o. Pflügers Archiv. 1983;397:176–183. doi: 10.1007/BF00584354. [DOI] [PubMed] [Google Scholar]
- Rang HP, Ritchie JM. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. The Journal of Physiology. 1968;196:183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond SA. Effects of nerve impulses on threshold of frog sciatic nerve fibres. The Journal of Physiology. 1979;290:273–303. doi: 10.1113/jphysiol.1979.sp012771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond SA, Lettvin JY. Aftereffects of activity in peripheral axons as a clue to nervous coding. In: Waxman SG, editor. Physiology and Pathobiology of Axons. New York: Raven Press; 1978. pp. 203–225. [Google Scholar]
- Raymond SA, Thalhammer JG, Popitz BF, Strichartz GR. Changes in axonal impulse conduction correlate with sensory modality in primary afferent fibres in the rat. Brain Research. 1990;526:318–321. doi: 10.1016/0006-8993(90)91239-d. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Forster C, Schmidt R, Ringkamp M, Handwerker HO, Torebjörk HE. Delayed responses to electrical stimuli reflect C-fibre responsiveness in human microneurography. Experimental Brain Research. 1995;104:331–336. doi: 10.1007/BF00242018. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjörk HE, Handwerker H. Novel classes of responsive and unresponsive C nociceptors in human skin. Journal of Neuroscience. 1995;15:333–341. doi: 10.1523/JNEUROSCI.15-01-00333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra J, Campero M, Ochoa J, Bostock H. Activity-dependent slowing of conduction differentiates functional subtypes of C fibres innervating human skin. The Journal of Physiology. 1999;515:799–811. doi: 10.1111/j.1469-7793.1999.799ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HC, Raymond SA. Excitability changes in C fibres of rat sciatic nerve following impulse activity. Neuroscience Letters. 1991;129:242–246. doi: 10.1016/0304-3940(91)90471-5. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Waxman SG. Variations in conduction velocity and excitability following single and multiple impulses of visual callosal axons in the rabbit. Experimental Neurology. 1976;53:128–150. doi: 10.1016/0014-4886(76)90288-0. [DOI] [PubMed] [Google Scholar]
- Thalhammer JG, Raymond SA, Popitz BF, Strichartz GR. Modality-dependent modulation of conduction by impulse activity in functionally characterized single cutaneous afferents in the rat. Somatosensory and Motor Research. 1994;11:243–257. doi: 10.3109/08990229409051392. [DOI] [PubMed] [Google Scholar]
- Torebjörk HE. Afferent C units responding to mechanical, thermal and chemical stimuli in human non-glabrous skin. Acta Physiologica Scandinavica. 1974;92:374–390. doi: 10.1111/j.1748-1716.1974.tb05755.x. [DOI] [PubMed] [Google Scholar]
- Torebjörk HE, Hallin RG. Responses in human A and C fibres to repeated electrical intradermal stimulation. Journal of Neurology, Neurosurgery and Psychiatry. 1974;37:653–664. doi: 10.1136/jnnp.37.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D. The contribution of membrane hyperpolarization to adaptation and conduction block in sensory neurones of the leech. The Journal of Physiology. 1973;230:509–534. doi: 10.1113/jphysiol.1973.sp010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner C, Schmelz M, Hilliges M, Schmidt R, Handwerker H, Torebjörk E. Supernormal conduction in C-nociceptors in human. Society for Neuroscience Abstracts. 1999a;25:685, 272.13. [Google Scholar]
- Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker H, Torebjörk E. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. Journal of Neuroscience. 1999b;19:10184–10190. doi: 10.1523/JNEUROSCI.19-22-10184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


