Abstract
Purine and pyrimidine nucleotides evoke changes in the vascular tone of medium to large cerebral vessels through the activation of P2 purinoceptors. We have applied P2 receptor drugs to rat pial arterioles and measured changes in arteriole diameter (o.d. 40–84 μm at rest), and recorded currents from arteriolar smooth muscle cells using patch-clamp techniques.
Transient vasoconstrictions and rapidly inactivating currents were evoked by α,β-methylene ATP (0.1–30 μm) and were sensitive to the P2 receptor antagonists suramin and iso-PPADS.
UTP and UDP (0.1–1000 μm) evoked sustained suramin-sensitive vasoconstrictions.
ATP (0.1–1000 μm) and 2-methylthioATP (2MeSATP, 300 μm) evoked transient vasoconstrictions followed by sustained vasodilatations. ADP application resulted in only vasodilatation (EC50 ∼4 μm). Vasodilator responses to ATP, 2MeSATP or ADP were unaffected by suramin (100 μm).
RT-PCR analysis indicated that P2X1–7 and P2Y1,2,6 RNA can be amplified from the pial sheet. Our results provide direct evidence for the presence of functional P2X receptors with a phenotype resembling the P2X1 receptor subtype on cerebral resistance arterioles. The pharmacological properties of the pyrimidine-evoked responses suggest that a combination of P2Y2- and P2Y6-like receptors are responsible for the sustained vasoconstrictions. It is therefore likely that the nucleotides and their associated receptors are involved in a complicated regulatory system to control cerebral blood pressure.
Purine and pyrimidine nucleotides are released from a variety of sources, act through P2 receptors to modulate vascular tone, and play an important role in the control of blood pressure. Adenosine 5′-triphosphate (ATP) is released from sympathetic nerve terminals, smooth muscle, and endothelial and blood cells (Burnstock, 1997). Diadenosine polyphosphates have been isolated from various preparations including adrenal chromaffin granules (Rodriguez-Del Castilla et al. 1988) and platelets (Schluter et al. 1994). Uridine 5′-triphosphate (UTP) is released from endothelial cells by shear forces during times of increased blood flow and has been isolated from platelets (Ralevic & Burnstock, 1998). This variety of sources of nucleotides in vascular tissue suggests the likelihood of an integrated regulatory system combining locally released and blood-borne vasoactive nucleotides to regulate blood pressure. ATP and UTP have been shown to have vasoconstrictor actions on the cerebral circulation (Ralevic & Burnstock, 1998) but unlike the peripheral circulation, the P2 receptor subtypes mediating these effects, particularly those in cerebral resistance arterioles, remain unclear.
P2 receptors are divided into ligand-gated P2X receptor cation channels and G-protein-coupled P2Y receptors. Seven subtypes of P2X receptor (P2X1–7) and at least five subtypes of P2Y receptor (P2Y1,2,4,6,11) have been identified at the molecular level (Ralevic & Burnstock, 1998). In the peripheral circulation P2X receptors appear to have a particularly important role in resistance arterioles where P2X receptor-mediated neurogenic vasoconstrictions dominate the response to sympathetic nerve stimulation (Evans & Surprenant, 1992). P2X receptor activation leads to membrane depolarisation and calcium influx directly through P2X receptor channels (Benham & Tsien, 1987; Evans & Surprenant, 1996) and through voltage-dependent L-type calcium channels (Lagaud et al. 1996). α,β-Methylene ATP (α,β-meATP) is a full agonist at peripheral arterial smooth muscle P2X receptors and responses rapidly inactivate in the continued presence of the agonist. This native phenotype closely resembles that of recombinant P2X1 receptors which are expressed at high levels by smooth muscle cells in peripheral arteries (Evans & Surprenant, 1996; Vulchanova et al. 1996), and we have shown recently that the P2X1 receptor is essential for the expression of functional P2X receptors in vas deferens smooth muscle (Mulryan et al. 2000). At the molecular level there is evidence suggesting the presence of other P2X receptor isoforms on some arterial smooth muscle (P2X2,4), albeit at a lower level than the P2X1 receptor (Nori et al. 1998). However, their contribution to the functional P2X receptor phenotype remains to be determined. In situ hybridisation studies have indicated that P2X1 receptor mRNA expression in the rat basilar artery or blood vessels in brain sections is below the level of detection (Collo et al. 1996). However, a recent immunohistochemical study has demonstrated the presence of P2X1 receptors on human large cerebral arteries (Bo et al. 1998). ATP and α,β-meATP mediate vasoconstrictions in cerebral arteries from a number of species including rats and humans (Byrne & Large, 1986; Bo et al. 1998), and in a variety of different sized vessels ranging from large cerebral arteries to smaller pial arteries (300–600 μm; Hardebo et al. 1987). These results suggest the presence of functional P2X receptors on medium to large cerebral arteries, although to date there have been no studies on small arteries/arterioles in the cerebral circulation nor has there been direct confirmation that these vasoconstrictions result from the opening of ligand-gated P2X receptor cation channels.
There are four rodent P2Y receptor isoforms, P2Y1,2,4,6, identified at the molecular level (King et al. 1998). P2Y2, P2Y4 and P2Y6 receptors have been detected in vascular smooth muscle and there is functional evidence to suggest that they can mediate vasoconstriction (Erlinge et al. 1998; Hartley et al. 1998; Ralevic & Burnstock, 1998). These receptors can be distinguished based on their pharmacological properties. ATP and UTP are equipotent at recombinant P2Y2 receptors and their effects are sensitive to the P2 receptor antagonist suramin (Boarder & Hourani, 1998; Ralevic & Burnstock, 1998). At the recombinant rat P2Y4 receptor, inosine 5′-triphosphate (ITP), ATP and UTP are equipotent and responses are insensitive to the P2 receptor antagonist suramin (Bogdanov et al. 1998; Webb et al. 1998). P2Y6 receptors are selective for uridine 5′-diphosphate (UDP) and are relatively insensitive to suramin (Boarder & Hourani, 1998; Hartley et al. 1998). UTP is also an agonist at rat P2Y6 receptors (Filippov et al. 1999). The sustained and potent effects of UTP and UDP on cerebral blood flow (Urquilla, 1978) suggest that P2Y2, P2Y4 or P2Y6, or a combination of these receptors may be involved in the control of cerebral vessel tone (Boarder & Hourani, 1998) and may therefore play an important role in chronic cerebral vasospasm associated with subarachnoid haemorrhage (Zhang et al. 1995). P2Y1 receptors are present on endothelial cells and can mediate vasodilatation (Ralevic & Burnstock, 1998). Adenosine 5′-diphosphate (ADP), 2-methylthioATP (2MeSATP), 2-methylthioADP (2MeSADP) and ATP are agonists at recombinant P2Y1 receptors (Palmer et al. 1998; Filippov et al. 2000). In whole-tissue studies it is likely that some of the 2MeSATP and ATP are broken down by nucleotidases to 2MeSADP and ADP.
The control of cerebral vascular tone is an important consideration in determining the aetiology of disease states that occur with stroke, aneurysm, subarachnoid haemorrhage with associated vasospasm and migraine. There is a strong indication for nucleotide regulation of resistance vasculature in the brain integrating dual vasoconstrictor and vasodilator actions of the various nucleotides present in erythrocytes, platelets, structural vascular cells, the bloodstream and neurones. It is likely that a combination of P2X and P2Y receptors would be involved in such a regulatory system. Previously studies have focused on relatively large cerebral vessels; however, small precapillary arterioles < 100 μm in diameter play a key role in cerebrovascular resistance and are vital in the control of blood pressure (Mraovitch & Sercombe, 1996). The functional properties of arteries may vary within a vascular bed depending on the vessel diameter (Lamping et al. 1992; Quayle et al. 1996; Morris et al. 1998). Similar size-dependent differences in the properties of P2X receptor expression have also been demonstrated (D. P. Gitterman & R. J. Evans, unpublished observations). In this study we have used a video-imaging system to measure arteriole diameter changes and patch-clamp techniques to investigate the P2 receptor subtypes present on rat small precapillary cerebral arterioles. In addition we have used the reverse transcription polymerase chain reaction (RT-PCR) to investigate which P2 receptor isoforms are expressed in the pial circulation. Our results suggest that four distinct P2 receptors are involved in the control of cerebral resistance arterioles.
Methods
Pial arteriole dissection and diameter measurement
Male Wistar rats (250–300 g) were killed by exposure to a rising concentration of CO2 and exsanguinated. The brain was removed quickly and the pial connective tissue sheet containing the middle cerebral artery and its branches was dissected free in physiological saline of the following composition (mm): NaCl 146, KCl 5, CaCl2 2.5, MgCl2 2, NaHCO3 25, NaH2PO4 1, and glucose 11 (equilibrated with 95 % O2-5 % CO2). The pial sheet was pinned out under active tension in a Sylgard-coated organ bath (volume 2 ml). The bath was mounted on an inverted microscope and superfused (5 ml min−1) with physiological saline. The superfusate was warmed so that the bath temperature was 30°C. At higher temperatures spontaneous vasoconstrictions and persistent vasomotion interfered with data collection. The outer diameter (o.d.) of pial arterioles was monitored by Diamtrak (Neild, 1989; Galligan et al. 1995) software analysis of a video image (sampling interval 0.125 s) obtained from a JVC TK-S350 video camera mounted on the microscope (Fig. 1A). The integrity of the endothelium was tested with the endothelium-dependent vasodilator bradykinin. In vessels preconstricted with UTP (100 μm), bradykinin (1 μm) produced a 72.7 ± 10.9 % vasodilatation (n = 4). Arterioles had an o.d. of 57.4 ± 1.2 μm (range 40–84 μm, n = 76). The thickness of the arterial wall accounts for ∼35 μm. A 100 mm KCl solution evoked maximal vessel vasoconstriction and a sub-maximal 40 mm KCl solution was used for assessing the effects of calcium channel blockers. These solutions were made by replacing the NaCl in the physiological saline solution with KCl. Vasoconstrictors evoked reproducible responses when added to the superfusate at 30 min intervals. Antagonists were pre-superfused for 15 min before being applied concomitantly with the agonist. UDP can be contaminated with UTP and in whole-tissue studies the enzyme nucleoside diphosphokinase can convert a significant amount of UDP to UTP. Therefore stock solutions of UDP (1 mm) were pre-incubated in physiological saline solution containing hexokinase (10 units ml−1) and glucose (22 mm) for 60 min at 37°C to convert any UTP present to UDP (Matsumoto et al. 1997).
Figure 1. P2X receptor-mediated vasoconstrictor responses evoked by purinergic agonists in rat pial arterioles.
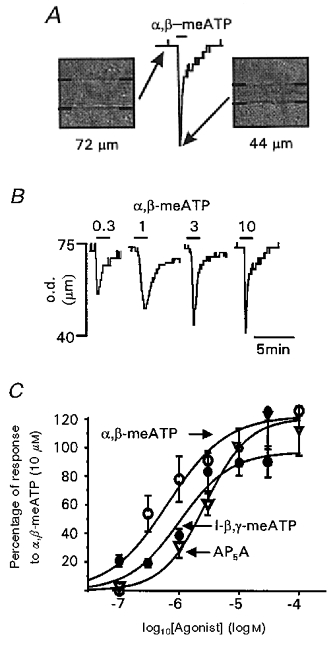
A, Diamtrak screen images and corresponding chart trace of a pial arteriole before and during application of α,β-meATP (10 μm). B, vasoconstrictions evoked by 0.3, 1, 3 and 10 μm α,β-meATP. The agonist was applied for the period indicated by the bars. Arteriole diameter is given as the outer diameter (o.d.) of the vessel (μm). C, concentration-response relationships for vasoconstrictions evoked by α,β-meATP (○), l-β,γ-meATP (•) and AP5A (▿). Data are plotted as a percentage of the response to α,β-meATP (10 μm, n = 4–6 for each point).
Pial arteriole dissociation and patch-clamp recording
Pial connective tissue sheets containing arterioles were dissected at 4°C in Hanks' solution. The pial sheet was then cut into small pieces and transferred into Hanks' solution containing protease (0.092 units ml−1) and collagenase (type 1A, 0.1–0.4 FALGPA hydrolysis units ml−1) at 37°C for 10 min. The tissue was then removed into Hanks' solution on ice and triturated gently until arteriole fragments appeared. The solution was then centrifuged at 1000 r.p.m. for 1 min, the supernatant was discarded and arteriole fragments were resuspended in Hanks' solution, then plated out on coverslips, refrigerated and used on the same day (method adapted from Quinn & Beech, 1998). Arteriole segments (diameter 26.1 ± 2.6 μm, n = 6; length 187 ± 12.8 μm, n = 6; Fig. 3A) were superfused at 2 ml min−1 with physiological solution, drugs were applied using a U-tube perfusion system and amphotericin-permeabilised patch recordings were made as reported previously (Lewis et al. 1998). The holding potential was −60 mV. Reproducible responses were obtained when agonists were applied for 200–500 ms once every 5 min. Antagonists were added to the superfusate for 5 min before being concomitantly applied with the agonist.
Figure 3. P2X receptor-mediated inward currents recorded from pial arteriolar smooth muscle.
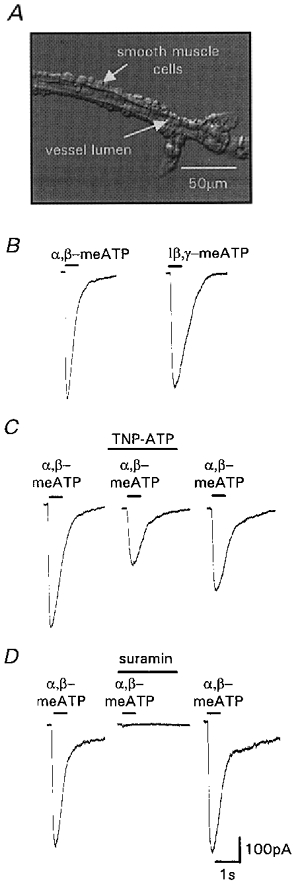
A, photomicrograph of a segment of pial arteriole (image provided by Dr J. Boyle, University of Leicester). Whole-cell patch-clamp recordings were made from smooth muscle cells. B, inward currents evoked by α,β-meATP (10 μm) and l-β,γ-meATP (10 μm). C, TNP-ATP (100 nm) inhibited currents evoked by α,β-meATP (3 μm). Inhibition was partially reversible after 20 min washout. D, suramin (100 μm) abolished inward currents evoked by α,β-meATP (3 μm). Inhibition was totally reversible after 5 min washout. Agonists and antagonists were applied for the period indicated by the bars.
Data analysis
Results are expressed as means ± s.e.m., n = number of observations. The rise time and decay time constants (τ) of P2 receptor-mediated currents were obtained by fitting a single exponential using Clampfit software (pCLAMP, Axon Instruments, USA). Constrictor responses are given as a percentage of the peak response to 10 μm α,β-meATP. The concentration-response data for agonists were pooled and fitted by the least-squares method using Origin software (Microsoft, USA) to:
where α and H are the asymptote and Hill coefficient, [A] is the agonist concentration and EC50 is the concentration evoking 50 % of the maximum agonist response.
RT-PCR analysis of P2X and P2Y receptors
Total RNA was isolated from pial sheets by a scaled down (500 μl extraction volume) version of the method described by Chomczynski & Sacchi (1987). The pial sheet contains a mixture of cell types including vascular smooth muscle, endothelial and neuronal cells. One pial sheet yielded approximately 10 μg of total RNA. First strand cDNA was synthesised from 5 μg total RNA in a 40 μl reaction volume with Superscript reverse transcriptase according to the manufacturer's instructions (Gibco BRL). Control reactions containing all components except reverse transcriptase were also performed to ensure contaminating genomic DNA was not present (no DNase was used). PCR reactions contained 0.5 μl of cDNA, 1.5 mm MgCl2, 12.5 pmol forward and reverse primers, 5 mm nucleotides, 1 × reaction buffer (Bioline) and 2.5 U BioTaq DNA polymerase (Bioline) in a 25 μl reaction volume. PCR conditions for P2X primers consisted of 94°C for 5 min, 30 cycles of 94°C for 45 s, 58°C for 30 s and 72°C for 45 s. Conditions for the P2Y primers were as above apart from the annealing temperature which was 65°C. Primer sequences and expected product sizes for P2X isoforms are shown in Table 1. Primers for P2Y isoforms were a kind gift from Dr T. E. Webb (Dixon et al. 2000) and gave products of 595, 539, 474 and 352 bp for P2Y1, P2Y2, P2Y4 and P2Y6, respectively. Reactions were also run with no reverse transcriptase as a control for genomic DNA contamination. In order to confirm that each primer set amplified the expected gene, the DNA from one PCR reaction for each primer pair was directly sequenced. Bands were cut from the gel and the DNA isolated using a QIAquick kit (Qiagen, Germany) according to the manufacturer's instructions. DNA was directly sequenced (ABI automated sequencing service, Leicester University) using the PCR primers as sequencing primers.
Table 1.
Sequence of P2X receptor primers and expected product size
| Gene | Sequence (5′ to 3′) | Product size (bp) | |
|---|---|---|---|
| P2X1 | Forward | CCTTGGCTATGTGGTGCGAGAGTC | 382 |
| Reverse | AGGCAGGATGTGGAGCAATAAGAG | ||
| P2X2 | Forward | CATCTTCAGGCTGGGTTTCATTG | 477 |
| Reverse | AGGGTCACAGGCCATCTACTTG | ||
| P2X3 | Forward | CCCCATTTTGCCCCATCTTGA | 252 |
| Reverse | ACTCGCTGCCGTTCTCCATCTTAT | ||
| P2X4 | Forward | GTGGGACTGCAACCTGGATAGAGC | 424 |
| Reverse | CTGAGCGGGGTGGAAATGTAACTT | ||
| P2X5 | Forward | CAACCGCCTGGACAACAAACACA | 628 |
| Reverse | CTGAGCAGGCCCCACCGAGAT | ||
| P2X6 | Forward | GACTGGAGAGGGGGTTGGGGTAAT | 311 |
| Reverse | AGGCAGGTGCTTCAGAATAGGTTG | ||
| P2X7 | Forward | GCAACTCTGGCGGCTTCATCC | 557 |
| Reverse | AGGCACAGAGGCGGCTTTTAGT |
Drugs
α,β-MeATP, P1,P5-di(adenosine-5′)pentaphosphate (AP5A), 2MeSATP, UDP, UTP, ADP, ATP, cadmium chloride, ITP, nifedipine, hexokinase and l-NAME were from Sigma; l-β,γ-methylene ATP (l-β,γ-meATP) was from RBI; suramin was from Bayer (UK); pyridoxalphosphate-6-azophenyl-2′,5′-disulphonic acid (iso-PPADS) was from Tocris; and 2′,3′-o-(2,4,6-trinitrophenyl) adenosine 5′-triphosphate (TNP-ATP) was from Molecular Probes.
Results
P2X receptor-mediated vasoconstrictions
The metabolically stable ATP analogue α,β-meATP evoked rapid phasic vasoconstrictions which declined to resting tone in the continued presence of the agonist (Fig. 1A and B). Responses were concentration dependent with an EC50 of 0.7 μm (Fig. 1B and C); maximal responses were 64.2 ± 3 % (n = 41) of the response to 100 mm KCl. α,β-MeATP-sensitive, rapidly inactivating responses are characteristic of recombinant P2X1 and P2X3 receptors. These receptor subtypes may be discriminated pharmacologically using l-β,γ-meATP which is an agonist at P2X1 receptors but ineffective at rat P2X3 receptors (Trezise et al. 1995; Grubb & Evans, 1999). l-β,γ-MeATP evoked concentration-dependent vasoconstrictions (EC50 = 1 μm) which were similar to those evoked by α,β-meATP (Fig. 1C). AP5A evoked similar vasoconstrictions to α,β-meATP and l-β,γ-meATP with an EC50 of 4.3 μm (Fig. 1C).
Effects of the P2 receptor antagonists suramin and iso-PPADS on α,β-meATP-evoked vasoconstrictions
Suramin (100 μm) abolished vasoconstrictions evoked by α,β-meATP (3 μm, n = 4; Fig. 2A); this effect was reversible within the 30 min drug addition cycle. Iso-PPADS (30 μm) reduced the vasoconstriction by 74.4 ± 9.4 % (n = 9; Fig. 2B) and this effect was irreversible after 60 min.
Figure 2. Effects of the purinergic antagonists suramin and iso-PPADS and the L-type calcium channel blocker nifedipine on P2X receptor-mediated vasoconstrictions.
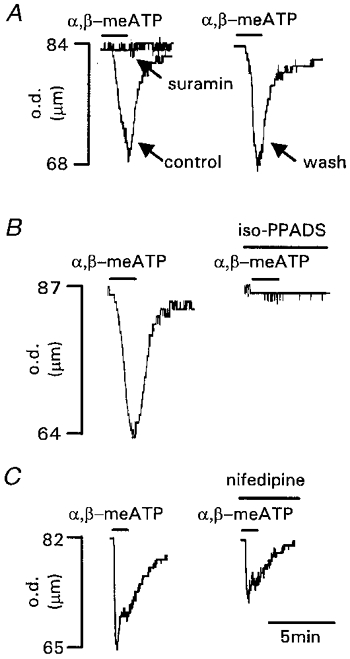
A, suramin (100 μm) abolished vasoconstrictions evoked by α,β-meATP (3 μm). Inhibition was reversed after 30 min washout. B, iso-PPADS (30 μm) inhibited vasoconstrictions evoked by α,β-meATP (3 μm). Inhibition was irreversible after 60 min washout. C, nifedipine (1 μm) inhibited vasoconstrictions evoked by α,β-meATP (3 μm). Inhibition was partially reversible after 30 min washout. Agonist and antagonists were applied for the period indicated by the bars. Arteriole diameter is given as the outer diameter (o.d.) of the vessel (μm).
Effects of calcium channel blockers on P2X receptor-mediated vasoconstrictions
Vasoconstrictions evoked by α,β-meATP (10 μm) were abolished in calcium-free solution (n = 4) demonstrating that calcium influx is essential. To determine the contribution of calcium influx through voltage-dependent calcium channels vs. direct calcium influx through the P2X receptor, the calcium channel blockers cadmium (1 mm) and nifedipine (1 μm) were used. There was no significant difference between these two treatments and they reduced the vasoconstriction evoked by α,β-meATP (3 μm) by 54.2 ± 10.9 % (n = 5) and 72.6 ± 11.9 % (n = 4; Fig. 2C), respectively. These effects were reversible after a 60 min washout period. The 40 mm KCl solution evoked vasoconstrictions with an equal amplitude to those evoked by α,β-meATP (3 μm). Cadmium and nifedipine reduced the 40 mm KCl-evoked vasoconstriction by 100 % (n = 4) and 77.1 ± 5.8 % (n = 5), respectively. These effects were reversible after a 60 min washout period.
P2X receptor-mediated ionic currents
The properties of the α,β-meATP-evoked vasoconstrictions are indicative of a P2X receptor-mediated response. Direct confirmation of the activation of P2X receptors by α,β-meATP was obtained from patch-clamp studies on acutely dissociated pial arteriolar segments. Following a short delay due to solution exchange, α,β-meATP (10 μm) evoked transient inward currents (peak amplitude 523 ± 71 pA, rising phase τ = 34.9 ± 5.8 ms, n = 4) which inactivated in the continued presence of the agonist (decay τ = 702.4 ± 26.8 ms, n = 4; Fig. 3B). ATP and the selective P2X1 receptor agonist l-β,γ-meATP (10 μm) also evoked inward currents with a similar time course and amplitude to the α,β-meATP-evoked current (peak amplitude was 78.4 ± 6.5 and 75.8 ± 4 % of the α,β-meATP current, respectively, n = 4; Fig. 3B). Following desensitisation of the α,β-meATP-sensitive P2X receptor (10 μm α,β-meATP), ATP or l-β,γ-meATP failed to evoke inward currents (n = 3 for both). These results indicate that α,β-meATP, ATP and l-β,γ-meATP act at the same P2X receptor to evoke inward currents and mediate vasoconstriction. TNP-ATP (100 nm), an antagonist at P2X1, P2X3 and P2X2/3 receptors (Virginio et al. 1998) reduced the α,β-meATP (3 μm)-evoked current by 60.6 ± 4.8 % (n = 3). This effect was partially reversible (87.9 ± 14.1 %, n = 3) after a 20 min wash (Fig. 3C). The inhibition by TNP-ATP (100 nm) of the pial arteriole responses to α,β-meATP (3 μm) is less than the ∼95 % block we reported for the mesenteric artery (Lewis et al. 1998) and may suggest that there are some minor differences between the P2X receptor channels in these arteries. The P2 receptor antagonist suramin (100 μm) abolished the α,β-meATP (3 μm)-evoked current (n = 3) and this effect was reversed within the 5 min washout period (Fig. 3D).
Pyrimidine nucleotide-evoked vasoconstrictions
UTP and UDP evoked sustained concentration-dependent vasoconstrictions in small pial arterioles (Fig. 4A–C). A clear maximum response was not reached using concentrations up to 1 mm (Fig. 4C). The concentration- response curves are very shallow and do not appear to plateau over a change in concentration of 4 orders of magnitude; this suggests that UTP and UDP may activate at least two receptors. UTP and UDP (1 mm) evoked responses that were 158.3 ± 38 and 104.5 ± 24.3 % of the maximal α,β-meATP (10 μm)-induced vasoconstriction (n = 4). In the presence of hexokinase, UDP (100 μm)-evoked responses were slightly potentiated by 18.2 ± 4.4 % (n = 4), which demonstrates that UDP was not being interconverted to, or contaminated by, UTP. ITP (100 μm)-evoked vasoconstrictions in the pial arterioles were 24.2 ± 11.8 % of the UTP (100 μm)-evoked response (n = 5).
Figure 4. P2Y receptor-mediated vasoconstrictions evoked by pyrimidines in rat pial arterioles.
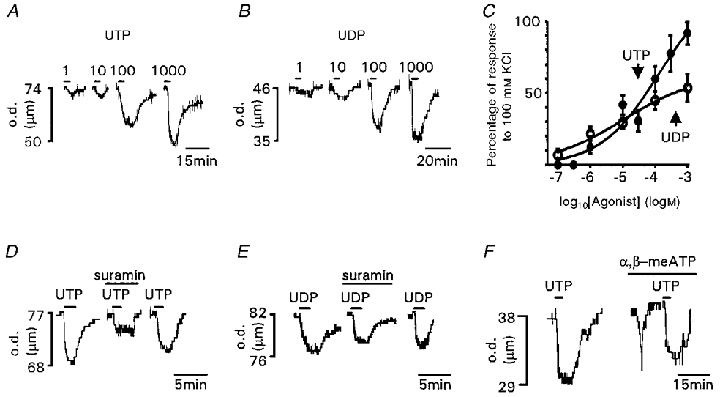
A, vasoconstrictions evoked by 1, 10, 100 and 1000 μm UTP. B, vasoconstrictions evoked by 1, 10, 100 and 1000 μm UDP. C, concentration-response relationships for vasoconstrictions evoked by UTP (•) and UDP (○). Data are expressed as a percentage of the response to 100 mm KCl (n = 3–5 for each point). D, suramin (100 μm) inhibited vasoconstrictions evoked by UTP (60 μm). Inhibition was reversible after 30 min washout. E, suramin (100 μm) inhibited vasoconstrictions evoked by UDP (100 μm). Inhibition was reversible after 30 min washout. F, inactivation of P2X receptors by α,β-meATP (10 μm) does not affect the amplitude of the UTP (100 μm)-evoked vasoconstriction. Agonists and antagonists were applied for the period indicated by the bars. Arteriole diameter is given as the outer diameter (o.d.) of the vessel (μm).
To classify further the receptors mediating the response to pyrimidine nucleotides, the P2 receptor antagonists suramin and iso-PPADS and inactivation of P2X receptors with α,β-meATP were used. The amplitude of UTP-evoked vasoconstrictions was unaffected following inactivation of P2X receptors by α,β-meATP (response 100.1 ± 11.2 % of the control UTP vasoconstriction, n = 5; Fig. 4F). Suramin (100 μm) reduced the UTP (60 μm)-evoked vasoconstriction by 59.4 ± 5.7 % (n = 5; Fig. 4D) and the UDP (100 μm)-evoked vasoconstriction (of similar magnitude to that evoked by 60 μm UTP) by 40.4 ± 10 % (n = 6; Fig. 4E). At a lower concentration (10 μm), suramin was essentially ineffective; it reduced responses to UTP (60 μm) by 13.4 ± 16 % and had no effect on responses to UDP.
P2Y receptor-mediated vasodilatation
ATP activates both P2X and P2Y receptors and it has a dual vasoconstrictor and vasodilator action on rat pial arterioles. Low concentrations (< 10 μm) of ATP evoked vasoconstriction, while 30–1000 μm ATP evoked a transient vasoconstriction followed by a sustained vasodilatation (n = 5; Fig. 5A). The rapid transient vasoconstriction evoked by ATP resembled that evoked by α,β-meATP and was abolished following desensitisation by α,β-meATP (10 μm, n = 3) indicating that ATP and α,β-meATP act through the same receptor to mediate vasoconstriction. ATP (300 μm) evoked a reponse that was 58.2 ± 7.7 % (n = 6) of the maximal KCl-evoked vasoconstriction. Dual vasoconstrictor and vasodilator actions were also observed with the P2 receptor agonist 2MeSATP (300 μm, n = 7). 2MeSATP (300 μm) evoked a vasodilatation that was 83.6 ± 16.2 % (n = 5) of that evoked by ATP (300 μm). It is possible that the dilator actions of ATP and 2MeSATP could be due to the actions of ADP and 2MeSADP produced by nucleotidase breakdown of the agonists in the pial sheet. ADP evoked concentration-dependent vasodilatations (EC50 = 3.7 μm) of similar maximum amplitude to those in response to ATP (Fig. 5C). The P2 receptor antagonist suramin (100 μm) had no effect on vasodilatations evoked by 2MeSATP (300 μm) or ADP (10 μm) (n = 9). In contrast, suramin inhibited the vasoconstrictions evoked by 2MeSATP by 95.8 ± 4.2 % (n = 6). The effects of suramin on 2MeSATP-evoked vasoconstrictions were reversible after a 30 min washout period (Fig. 5B). The nitric oxide synthase inhibitor N-nitro-L-arginine methyl ester (l-NAME, 100 μm) evoked a vasoconstriction in all preparations studied indicating that there was some basal nitric oxide-mediated dilator effect in the vessels. Following l-NAME treatment (2–8 μm constriction), vasodilatations in response to ATP (300 μm) or ADP (100 μm) were 144 ± 22.6 % (n = 5) of control responses. This apparent potentiation probably reflects the increased vasoconstrictor tone in the vessels following application of l-NAME. These results suggest that the P2 receptor-mediated dilatation in these vessels is not due to the production of nitric oxide.
Figure 5. P2Y receptor-mediated vasodilatations in pial arterioles.
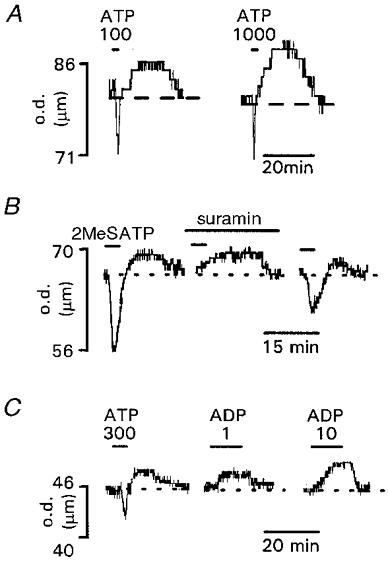
A, biphasic vasoconstrictor and vasodilator responses evoked by 100 and 1000 μm ATP. B, biphasic vasoconstrictor and vasodilator responses evoked by 2MeSATP (300 μm). Suramin (100 μm) inhibited vasoconstriction but not vasodilatation. Inhibition was reversible after 30 min washout. C, biphasic vasoconstrictor and vasodilator responses evoked by 300 μm ATP compared to vasodilator responses evoked by 1 and 10 μm ADP. Agonists and antagonists were applied for the period indicated by the bars. Arteriole diameter is given as the outer diameter (o.d.) of the vessel (μm).
RT-PCR analysis of P2 receptor subtypes expressed in the pial sheet
P2Y receptor transcripts for P2Y1, P2Y2 and P2Y6 were amplified from pial sheet total RNA (Fig. 6A). The sequences of the amplified products corresponded to the appropriate P2Y receptor isoform. P2Y4 receptor transcripts were not detected in the pial sheet although they were detected in rat brain.
Figure 6. RT-PCR analysis of total RNA isolated from rat pial sheet.
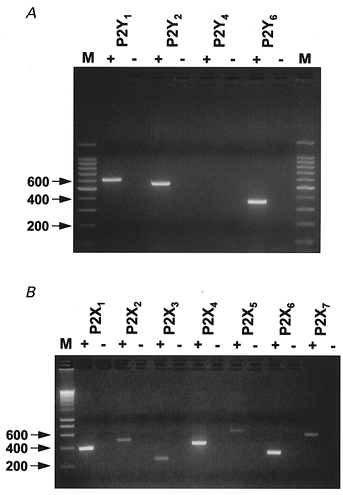
Primers used corresponded to the rat P2Y1,2,4,6 (A) and P2X1–7 receptors (B). Control reactions without reverse transcriptase (-) were run alongside cDNA templates (+) to verify that amplification was not from genomic DNA. M, molecular mass marker (size in base pairs).
RNA transcripts for all seven P2X receptors were amplified from the pial sheet preparation. P2X1–7 receptors were detected (Fig. 6B). The sequences of the amplified products corresponded to the appropriate P2X receptor isoform. Similar amplification patterns of P2X receptor subunits have been described for other arteries using a PCR-based approach (Phillips & Hill, 1999).
Discussion
This study has investigated the expression and role of P2 receptors in the control of cerebral arterioles. Phenotypes corresponding to P2X1, P2Y2 and P2Y6 receptors mediate vasoconstriction and an ADP-sensitive P2Y receptor mediates vasodilatation.
The involvement of P2X receptors in mediating vasoconstrictions of rat pial arterioles is indicated by the agonist actions of α,β-meATP and l-β,γ-meATP (EC50 values of 0.7 and 1 μm, respectively), and the sensitivity of these responses to the P2 receptor antagonists suramin and iso-PPADS (Boarder & Hourani, 1998; Ralevic & Burnstock, 1998). Similar results have been reported for a number of larger diameter peripheral and cerebral arteries (Byrne & Large, 1986; Hardebo et al. 1987; Evans & Surprenant, 1996; Vulchanova et al. 1996; Bo et al. 1998) but this is the first time a role for P2X receptors in the control of cerebral precapillary arterioles has been demonstrated. Confirmation that P2X receptors mediate these vasoconstrictions comes from the electrophysiological studies which demonstrated that α,β-meATP directly gated a P2X receptor ion channel in rat pial arteriolar segments. The presence of multiple P2X receptor subtypes on arterial smooth muscle has been indicated at the molecular level in various vessels (Burnstock, 1997; Nori et al. 1998; this study). However, the role of P2X receptors other than P2X1 receptors in the control of smooth muscle function remains unclear. In the present study the properties of P2X receptor-mediated inward currents, in particular the rapid inactivation, the sensitivity to the P2X1 receptor agonist l-β,γ-meATP (Trezise et al. 1995; Lewis et al. 1998) and the potent inhibition by TNP-ATP (Lewis et al. 1998; Virginio et al. 1998) strongly suggest that P2X1 receptors dominate the P2X receptor-mediated response in rat pial arterioles, as has been shown for vas deferens smooth muscle P2X receptors using P2X1 receptor-deficient mice (Mulryan et al. 2000).
P2X receptor activation in smooth muscle leads to membrane depolarisation and calcium influx. Calcium can enter directly through calcium-permeable P2X receptor channels (Benham & Tsien, 1987; Evans & Surprenant, 1996) and voltage-dependent calcium channels opened in response to membrane depolarisation. In the present study calcium entry through nifedipine-sensitive L-type calcium channels accounted for ∼50 % of the P2X receptor-mediated vasoconstriction. A similar sensitivity to nifedipine has been reported for some peripheral arteries (Bulloch et al. 1991; Cheung, 1991). However, in guinea-pig submucosal arterioles (Galligan et al. 1995) and rat mesenteric arteries (diameter 250 μm; D. P. Gitterman & R. J. Evans, unpublished observations) P2X receptor-mediated vasoconstrictions are unaffected following blockade of L-type calcium channels. The peak amplitude of α,β-meATP (10 μm)-evoked inward currents for pial arteriolar segments and rat mesenteric arteries (authors' unpublished observations) were 523 ± 71 pA (n = 7) and 1140 ± 55 pA (n = 90), respectively. Thus the degree of calcium influx through P2X receptors vs. L-type calcium channels may therefore simply reflect differences in the level of expression of P2X receptor channels relative to voltage-dependent calcium channels in different vascular preparations.
The pyrimidines UTP and UDP evoked sustained suramin-sensitive vasoconstrictions of rat pial arterioles through the activation of metabotropic P2Y receptors. Similar P2Y receptor-mediated vasoconstrictions where UTP and UDP are equipotent have been reported for other arterial preparations including the rabbit basilar arteries (von Kugelgen & Starke, 1990). Three of the currently identified P2Y receptors (P2Y2,4,6) are sensitive to pyrimidine nucleotides (Boarder & Hourani, 1998). mRNA transcripts for P2Y1, P2Y2 and P2Y6 but not P2Y4 receptors were amplified from the pial sheet preparation. The shallow slope of the concentration-response curves to UTP and UDP indicates that at least two receptors for these agonists are present on the pial arterioles. UTP is an agonist at suramin-sensitive rat P2Y2 and P2Y6 receptors and suramin-insensitive P2Y4 receptors. The suramin sensitivity of the UTP-evoked pial arteriole vasoconstriction and expression of P2Y2 receptor RNA transcripts indicates the involvement of P2Y2 receptors. UDP can evoke responses through the activation of P2Y6 receptors (Hartley et al. 1998; Ralevic & Burnstock, 1998). Thus the response to pyrimidines could be accounted for by the expression of a mixture of P2Y2 and P2Y6 receptors; however, the contribution of a P2Y receptor not yet identified at the molecular level cannot be excluded.
The presence of a P2Y receptor-mediated vasodilatation was demonstrated by the actions of the purinergic agonists ADP, ATP and 2MeSATP. In the pial arteriole preparation used in this study the endothelium was left intact and it is considered likely that the vasodilator P2Y receptors are associated with the endothelium, as has been demonstrated in the majority of other arteries (Ralevic & Burnstock, 1998). The agonist actions of ADP and the expression of P2Y1 receptor mRNA transcripts in the pial sheet is suggestive of a P2Y1 receptor mediating the vasodilatation. However, it has been suggested that functional P2Y1 receptor activity may not always correlate with mRNA transcript levels (Park et al. 1997). Recombinant P2Y1 receptor-mediated responses are suramin sensitive and the insensitivity to suramin of the vasodilatations would appear to discount the possibility that P2Y1 receptors are involved. Nucleotides are susceptible to nucleotidase breakdown in whole-tissue studies, and it is probable in this study that some of the vasodilator actions of ATP and 2MeSATP are due to their metabolic breakdown products ADP and 2MeSADP. Thus the vasodilator P2Y receptor present in the pial arteriolar circulation may be a novel P2Y receptor that has yet to be identified at the molecular level.
In summary this study has shown for the first time that P2 receptors can mediate the control of small (< 100 μm diameter) precapillary arterioles in the cerebral circulation. P2X1 receptors mediate transient vasoconstrictions which can be activated by circulating ATP and AP5A. The pyrimidines UTP and UDP evoke sustained P2Y2 and P2Y6 receptor-mediated vasoconstrictions and P2Y receptors, probably on the endothelium, can mediate a vasodilator response. Thus P2 receptors can play a regulatory role in the cerebral circulation to increase or decrease blood flow. Peripheral arteries receive a dense sympathetic innervation and a substantial component of the vasoconstrictor response to nerve stimulation can be mediated by nerve-released ATP acting at P2X receptors (Evans & Surprenant, 1992). In contrast, pial arterioles are poorly innervated (Hill et al. 1986) and it is likely that the nucleotides acting at P2 receptors are those present in the circulation or released locally. Thus local tissue damage may lead to the activation of P2X receptors and pyrimidine-sensitive P2Y receptors to mediate vasoconstriction. Under normal circumstances nucleotides circulating in the blood stream probably act through vasodilator P2Y receptors on the endothelium. However, if the endothelium is damaged circulating nucleotides may result in vasoconstriction by direct actions of for example AP5A, ATP and UTP on vasoconstrictor P2X and P2Y receptors. Thus the complement of P2 receptors on pial arterioles may allow for the regulation of blood flow in response to a variety of physiological challenges.
Acknowledgments
We would like to thank Dr J. P. Boyle (University of Leicester) for the dissociation of the pial arteriole segments and for the photomicrograph of the arteriole segment used in Fig. 3, and Professor N. B. Standen for useful comments on the manuscript. This work was supported by the Wellcome Trust and the British Heart Foundation.
References
- Benham CD, Tsien RW. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987;328:275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Bo J, Karoon P, Nori SL, Bardini M, Burnstock G. P2X purinoceptors in postmortem human cerebral arteries. Journal of Cardiovascular Pharmacology. 1998;31:794–799. doi: 10.1097/00005344-199805000-00020. [DOI] [PubMed] [Google Scholar]
- Boarder MP, Hourani SMO. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends in Pharmacological Sciences. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- Bogdanov YD, Wildman SS, Clements MP, King BF, Burnstock G. Molecular cloning and characterization of rat P2Y4 nucleotide receptor. British Journal of Pharmacology. 1998;124:428–430. doi: 10.1038/sj.bjp.0701880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulloch JM, Macdonald A, McGrath JC. Different sensitivities of rabbit isolated blood vessels exhibiting co-transmission to the slow calcium channel blocker, nifedipine. British Journal of Pharmacology. 1991;103:1685–1690. doi: 10.1111/j.1476-5381.1991.tb09847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. The past, present and future of purine nucleotides as signaling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Byrne NG, Large WA. The effect of α,β-methylene ATP on the depolarization evoked by noradrenaline (gamma-adrenoceptor response) and ATP in immature rat basilar artery. British Journal of Pharmacology. 1986;88:6–8. doi: 10.1111/j.1476-5381.1986.tb09464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung DW. Neuropeptide Y potentiates specifically the purinergic component of the neural responses in the guinea pig saphenous artery. Circulation Research. 1991;68:1401–1407. doi: 10.1161/01.res.68.5.1401. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Annals of Biochemistry. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. Journal of Neuroscience. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CJ, Woods NM, Webb TE, Green AK. Evidence that rat hepatocytes co-express functional P2Y1 and P2Y2 receptors. British Journal of Pharmacology. 2000;129:764–770. doi: 10.1038/sj.bjp.0703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlinge D, Hou M, Webb TE, Barnard E, Moller S. Phenotype changes of the vascular smooth muscle cell regulate P2 receptor expression as measured by quantitative RT-PCR. Biochemical and Biophysical Research Communications. 1998;248:864–870. doi: 10.1006/bbrc.1998.9083. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Surprenant A. Vasoconstriction of guinea-pig submucosal arterioles following sympathetic nerve stimulation is mediated by the release of ATP. British Journal of Pharmacology. 1992;106:242–249. doi: 10.1111/j.1476-5381.1992.tb14323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RJ, Surprenant A. P2X receptors in autonomic and sensory neurons. Seminars in the Neurosciences. 1996;8:217–223. [Google Scholar]
- Filippov AK, Brown DA, Barnard EA. The P2Y1 receptor closes the N-type Ca2+ channel in neurones, with both adenosine triphosphates and diphosphates as potent agonists. British Journal of Pharmacology. 2000;129:1063–1066. doi: 10.1038/sj.bjp.0703185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov AK, Webb TE, Barnard EA, Brown DA. Dual coupling of heterologously-expressed rat P2Y6 nucleotide receptors to N-type Ca2+ and M-type K+ currents in rat sympathetic neurones. British Journal of Pharmacology. 1999;126:1009–1017. doi: 10.1038/sj.bjp.0702356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galligan JJ, Herring A, Harpstead T. Pharmacological characterization of purinoceptor-mediated constriction of submucosal arterioles in guinea pig ileum. Journal of Pharmacology and Experimental Therapeutics. 1995;274:1425–1430. [PubMed] [Google Scholar]
- Grubb BD, Evans RJ. Characterization of cultured dorsal root ganglion neuron P2X receptors. European Journal of Neuroscience. 1999;11:149–154. doi: 10.1046/j.1460-9568.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- Hardebo J, Kahrstrom J, Owman C. P1- and P2-purine receptors in brain circulation. European Journal of Pharmacology. 1987;144:343–352. doi: 10.1016/0014-2999(87)90387-6. [DOI] [PubMed] [Google Scholar]
- Hartley SA, Kato K, Salter KJ, Kozlowski RZ. Functional evidence for a novel suramin-insensitive pyrimidine receptor in rat small pulmonary arteries. Circulation Research. 1998;83:940–946. doi: 10.1161/01.res.83.9.940. [DOI] [PubMed] [Google Scholar]
- Hill CE, Hirst GDS, Silverberg GD, Van Helden D F. Sympathetic innervation and excitability of arterioles originating from the rat middle cerebral artery. The Journal of Physiology. 1986;371:305–316. doi: 10.1113/jphysiol.1986.sp015976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Townsend-Nicholson A, Burnstock G. Metabotropic receptor for ATP and UTP: exploring the correspondence between native and recombinant nucleotide receptors. Trends in Pharmacological Sciences. 1998;19:506–514. doi: 10.1016/s0165-6147(98)01271-1. [DOI] [PubMed] [Google Scholar]
- Lagaud GJL, Stoclet JC, Andriantsitohaina R. Calcium handling and purinoceptor subtypes involved in ATP-induced contraction in rat small mesenteric arteries. The Journal of Physiology. 1996;492:689–703. doi: 10.1113/jphysiol.1996.sp021338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamping KG, Clothier JL, Eastham CL, Marcus ML. Coronary microvascular response to endothelin is dependent on vessel diameter and route of administration. American Journal of Physiology. 1992;263:H703–709. doi: 10.1152/ajpheart.1992.263.3.H703. [DOI] [PubMed] [Google Scholar]
- Lewis C, Surprenant A, Evans RJ. 2′,3′-O-(2,4,6-trinitrophenyl) adenosine 5′-triphosphate (TNP-ATP) – a nanomolar affinity antagonist at rat mesenteric artery P2X receptor ion channels. British Journal of Pharmacology. 1998;124:1463–1466. doi: 10.1038/sj.bjp.0702001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Nakane T, Chiba S. UTP induces vascular responses in the isolated and perfused canine epicardial coronary artery via UTP-preferring P2Y receptors. British Journal of Pharmacology. 1997;122:1625–1632. doi: 10.1038/sj.bjp.0701559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JL, Cunnane TC, Hirst GD. Regional differences in sympathetic neurotransmission to cutaneous arteries in the guinea-pig isolated ear. Journal of the Autonomic Nervous System. 1998;10:115–124. doi: 10.1016/s0165-1838(98)00122-2. [DOI] [PubMed] [Google Scholar]
- Mraovitch S, Sercombe R. Neurophysiological Basis of Cerebral Blood Flow Control. 1. London: John Libbey; 1996. [Google Scholar]
- Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, Brown JE, Conley EC, Buell G, Pritchard CA, Evans RJ. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- Neild TO. Measurement of arteriole diameter changes by analysis of television images. Blood Vessels. 1989;26:48–52. [PubMed] [Google Scholar]
- Nori SL, Fumagalli L, Bo X, Bogdanov Y, Burnstock G. Coexpression of mRNAs for P2X1, P2X2 and P2X4 receptors in rat vascular smooth muscle: an in situ hybridization and RT-PCR study. Journal of Vascular Research. 1998;35:179–185. doi: 10.1159/000025582. [DOI] [PubMed] [Google Scholar]
- Palmer RK, Boyer JL, Schachter JB, Nicholas RA, Harden TK. Agonist action of adenosine triphosphates at the human P2Y1 receptor. Molecular Pharmacology. 1998;54:1118–1123. [PubMed] [Google Scholar]
- Park MK, Garrad RC, Weisman GA, Turner T. Changes in P2Y1 nucleotide receptor activity during the development of rat salivary glands. American Journal of Physiology. 1997;41:C1388–1393. doi: 10.1152/ajpcell.1997.272.4.C1388. [DOI] [PubMed] [Google Scholar]
- Phillips JK, Hill CE. Neuroreceptor mRNA expression in the rat mesenteric artery develops independently of innervation. International Journal of Developmental Neuroscience. 1999;17:377–386. doi: 10.1016/s0736-5748(99)00032-5. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Dart C, Standen NB. The properties and distribution of inward rectifier potassium currents in pig coronary arterial smooth muscle. The Journal of Physiology. 1996;494:715–726. doi: 10.1113/jphysiol.1996.sp021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn K, Beech DJ. A method for direct patch-clamp recording from smooth muscle cells embedded in functional brain microvessels. Pflügers Archiv. 1998;435:564–569. doi: 10.1007/s004240050553. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacological Reviews. 1998;50:413–492. [PubMed] [Google Scholar]
- Rodriguez-Del Castilla A, Torres M, Delicado FG, Miras-Portugal M-T. Subcellular distribution studies of diadenosine polyphosphates – Ap4A and Ap5A in bovine adrenal medulla: presence in chromaffin granules. Journal of Neurochemistry. 1988;51:1696–1703. doi: 10.1111/j.1471-4159.1988.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Schluter H, Offers E, Bruggemann G, Van der Geit M, Tepel M, Nordhoff E, Karas M, Spieker C, Witzel H, Zidek W. Diadenosine phosphates and the physiological control of blood pressure. Nature. 1994;367:186–188. doi: 10.1038/367186a0. [DOI] [PubMed] [Google Scholar]
- Trezise DJ, Michel AD, Grahames CBA, Khakh BS, Surprenant A, Humphrey PPA. The selective P2X purinoceptor agonist, beta,gamma-methylene-L-adenosine 5′-triphosphate, discriminates between smooth muscle and neuronal P2X purinoceptors. Naunyn-Schmiedeberg's Archives of Pharmacology. 1995;351:603–609. doi: 10.1007/BF00170159. [DOI] [PubMed] [Google Scholar]
- Urquilla PR. Prolonged contraction of isolated human and canine cerebral arteries induced by uridine 5′-triphsophate. Stroke. 1978;9:133–136. doi: 10.1161/01.str.9.2.133. [DOI] [PubMed] [Google Scholar]
- Virginio C, Robertson G, Surprenant A, North RA. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3 and heteromeric P2X2/3 receptors. Molecular Pharmacology. 1998;53:969–973. [PubMed] [Google Scholar]
- von Kugelgen I, Starke K. Evidence for two separate vasoconstriction-mediating nucleotide receptors, both distinct from the P2X receptor, in rabbit basilar artery: a receptor for pyrimidine nucleotides and a receptor for purine nucleotides. Naunyn- Schmiedeberg's Archives of Pharmacology. 1990;341:538–546. doi: 10.1007/BF00171734. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Arvidsson U, Riedl M, Buell G, Surprenant A, North RA, Elde RP. Differential distribution of two ATP-gated ion channels (P2X receptors) determined by immunohistochemistry. Proceedings of the National Academy of Sciences of the USA. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TE, Henderson DJ, Roberts JA, Barnard EA. Molecular cloning and characterization of the rat P2Y4 receptor. Journal of Neurochemistry. 1998;71:1348–1357. doi: 10.1046/j.1471-4159.1998.71041348.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Weir BKA, Marton LS, Macdonald RL, Bindokas VP, Miller RJ, Brorson JR. Mechanisms of hemolysate-induced [Ca2+]i elevation in cerebral smooth muscle cells. American Journal of Physiology. 1995;269:H1874–1890. doi: 10.1152/ajpheart.1995.269.6.H1874. [DOI] [PubMed] [Google Scholar]


