Abstract
Using transient calcium phosphate transfection into the human embryonic kidney tsa-201 cell line and subsequent whole-cell patch-clamp protocols, we examined the tonic modulation of cloned N- and P/Q-type calcium channels by five different G protein β subunits via strong depolarizing voltage prepulses.
For N- and P/Q-type channels, the magnitude of inhibition was dependent on the Gβ subtype co-expressed.
Both the absolute and relative magnitudes of Gβ subunit-induced inhibition of P/Q-type channels differed from those observed with the N-type channel.
For each calcium channel subtype, kinetics of both the prepulse-mediated recovery from inhibition and the re-inhibition following the prepulse were examined for each of the Gβ subunits by varying either the duration between the pre- and the test pulse or the length of the prepulse.
For each channel subtype, we observed a differential Gβ subunit rank order with regard to the rates of re-inhibition and recovery from inhibition.
On average, P/Q-type channels exhibited more rapid rates of recovery from inhibition than those observed with N-type channels.
Different Gβ subtypes mediated different degrees of slowing of activation kinetics.
The differential modulation of P/Q- and N-type channels by various Gβ subtypes may provide a mechanism for fine tuning the amount of calcium entering the presynaptic nerve termini.
The direct inhibition of voltage-dependent calcium channels via activation of seven helix transmembrane receptors is considered a key factor for regulating calcium entry into presynaptic nerve termini. Molecular biological and biochemical studies have revealed that this inhibition occurs through binding of the G protein βγ dimer (Herlitze et al. 1996; Ikeda, 1996) to multiple sites on the calcium channel α1 subunit, including the domain I-II linker and carboxyl terminal regions (Zamponi et al. 1997; Qin et al. 1997, DeWaard et al. 1997). Furthermore, the N-terminal region of the channel appears to contribute to the overall degree of inhibition (Stephens et al. 1998; Canti et al. 1999). A number of additional factors modulate the ability of G proteins to inhibit channel activity, including protein kinase C (PKC)-dependent phosphorylation (Hamid et al. 1999), co-expression of the calcium channel β subunit (Campbell et al. 1995; Bourinet et al. 1996), and the binding of syntaxin 1A (Jarvis et al. 2000).
One key characteristic of direct Gβγ inhibition of calcium channels is its reversibility following the application of a strong depolarizing prepulse (Bean, 1989; see also Zamponi & Snutch, 1998). During this prepulse (PP), the Gβγ subunits are thought to physically dissociate from the channel, and upon termination of the PP, re-association is thought to occur in a bimolecular fashion (Zamponi & Snutch, 1998; Bertram & Behan, 1999). The PP effect provides convenient access to the G protein-inhibition kinetics (Currie & Fox, 1997; Zamponi & Snutch, 1998).
Recent work by Garcia and colleagues (1998) indicates that N-type calcium channels in rat superior cervical ganglion cells are differentially modulated by different types of Gβ subunits, with Gβ1 and Gβ2 being most effective, Gβ5 showing weaker modulation, and Gβ3 and Gβ4 being ineffective. In contrast, elegant work by Ruiz-Velasco & Ikeda (2000) on the same preparation suggests that all five Gβ isoforms may be capable of modulating N-type channel activity. However, although the G protein species was well defined in both of these studies, the N-type channels present in intact neurons may not form a homogeneous population due to calcium channel β subunit heterogeneity and alternative splicing mechanisms. Furthermore, similar experiments involving P/Q-type channels are still lacking. Finally, the dependence of the inhibition kinetics on the individual G protein β subunit subtypes has not been elucidated for these channels. To complete these gaps in our current understanding of presynaptic calcium channels modulation by G proteins, we have co-expressed individual Gβ isoforms separately with N-type or P/Q-type calcium channels in tsa-201 cells, and examined the properties of the ensuing tonic G protein inhibition of the channel by independently varying either the duration of the PP or the time between the PP and the test pulse. Our data demonstrate for the first time the differential modulation of two different types of presynaptic calcium channels by various Gβ subunit isoforms. Differences are revealed not only in the overall degree of inhibition, but also in the rates of re-inhibition and the rates of recovery from inhibition which underlie Gβγ modulation. The unique inhibition profile seen with individual Gβγ and/or calcium channel combinations may provide a tight regulatory mechanism for precise control of presynaptic calcium levels, and thus, neurotransmission.
Methods
Molecular biology
The cDNAs coding for bovine brain Gβ1 and Gγ2 were the same as those described previously (Jarvis et al. 2000). The bovine Gβ1 sequence is 99 % identical to that of the rat brain isoform. For the remaining Gβ subunits, total brain RNA was isolated from either decapitated adult male rat (Gβ2,3,5) or mouse cDNA from a library (Gβ4- note that the sequence of the rat Gβ4 subunit has not been identified, and hence the mouse isoform was chosen) using Trizol Reagent (GibcoBRL, Life Technologies, Grand Island, NY, USA). RNA integrity was checked and cDNA synthesis was carried out using SUPERSCRIPT II, RNase H and reverse transcriptase (GibcoBRL), with poly(A)-oligo(dT) as the template primer. PCR oligonucleotide primers at either the 5′ and 3′ ends were designed according to the published sequences to span the complete coding regions. Xho I and Kpn I restriction sites were incorporated, respectively, in each forward primer and reverse primer. The primer sequences were as follows:
Gβ2: forward: GGAGCGCTCGAGATGAGTGAGCTGGAGC;
reverse: CGGACGGTACCTTAGTTCCAGATCTTGAGGAAGG;
Gβ3: forward: CCTGGGAGAACTCGAGCTAGAGCCCAAGAGCC;
reverse: GCCTTCATGGCTTCCCATGGCTTCCTCC;
Gβ4: forward CCTGAGGGAAACCTCGAGGCAGGATGAGC;
reverse: GGCTGTAACACGGATTTCTCCAGGTACCATTGG;
Gβ5: forward: GCCCTCGAGATGGCAACCGATGGGC;
reverse: CCAGCACCCTAGTGTGGGATTGCCATGGCCG.
All PCR protocols used recombinant Taq DNA polymerase (GibcoBRL). For Gβ2, Gβ4 and Gβ5, DNA was first denatured for 30 s at 94°C, followed by a 2 min annealing step at 72°C. These steps were repeated while progressively dropping the annealing temperature to 68°C. Subsequently, DNA was amplified using 15 PCR cycles of 94°C (30 s), 68°C (1 min) and 72°C (2 min). Gβ3 was amplified in 15 PCR cycles of 92°C (30 s), 65°C (90 s) and 72°C (2 min). The PCR products were run on a 0.8 % agarose gel, extracted and purified using QIAquick gel extraction (Qiagen, Mississauga, Ontario, Canada), ligated into a pGEM T-Easy vector (Promega, Madison, WI, USA) and sequenced. Standard automated sequencing methods were carried out using a 373S ABI PRISM sequencer (Perkin-Elmer) on a 5.6 % Long Ranger gel. Both strands of each PCR fragment were sequenced using the ABI PRISM big dye terminator cycle sequencing ready reaction kit (reactions were separately done with both M13 universal and M13 reverse primers) following the instructions supplied by the manufacturer. The sequenced DNA was excised from T-Easy and subcloned into pMT2XS (R) mammalian vector for transient transfection.
Cloned Gβ3 and Gβ5 DNA sequences were identical to those published previously. The amino acid sequence of Gβ4 exhibited two differences relative to the published sequences (D167N and A175P). The Gβ2 construct showed the following differences relative to the sequence published on GenBank (D35A, D60A, A74S, V92A, K101M, N120I, K13E, K139R, D179G, E215D, V233A). To minimize the possibility that the observed differences were due to PCR errors, the occurrence of the amino acid changes relative to published sequences was confirmed in three separate PCR reactions for each Gβ2 and Gβ4, which yielded identical results. Moreover, one additional round of PCR was carried out for Gβ4 using a proof reading enzyme (Pfu) instead of Taq, again confirming the validity of the two amino acid changes. The Gβ2 and Gβ4 sequences were submitted to Genbank (accession numbers AF277892 and AF277893, respectively).
Transient transfection of tsa-201 cells
The human embryonic kidney tsa-201 cell line was transfected transiently with cDNAs encoding for either the N-type calcium channel (α1B+α2−δ+β1b; see Dubel et al. 1992), or the P/Q-type calcium channel (α1A+α2−δ+β1b; Stea et al. 1994) together with green fluorescent protein (EGFP; Clontech, Palo Alto, CA, USA) and as appropriate, the Gγ2 subunit plus the particular Gβ subunit isoform. The exact transient transfection and tissue culture protocols have been described previously (Hamid et al. 1999).
Patch clamp recordings
Glass coverslips carrying transfected cells were transferred to a 3 cm culture dish containing recording solution comprising 20 mm BaCl2, 1 mm MgCl2, 10 mm Hepes, 40 mm TEA-Cl, 10 mm glucose, 65 mm CsCl, (pH 7.2 with TEA-OH). Whole cell patch clamp recordings were performed using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, USA) linked to a personal computer equipped with pCLAMPv6.0. Patch pipettes (Sutter borosilicate glass, BF150-86-15) were pulled using a Sutter P-87 microelectrode puller, fire polished and showed typical resistances of 3–4 MΩ. The internal pipette solution contained 108 mm caesium methanesulphonate, 4 mm MgCl2, 9 mm EGTA, 9 mm Hepes (pH 7.2). When appropriate (i.e. for large currents and/or large cells), series resistance and capacitance were compensated by 80–85 %. Leak currents were negligible. Data were filtered at 1 kHz and recorded directly onto the hard drive of the computer. Unless stated otherwise, currents were evoked by stepping from −100 mV to a test potential of +20 mV. G protein inhibition was assessed by application of a strong depolarizing (+150 mV) PP. With the exception of the data shown in Fig. 4, the degree of inhibition was determined as the ratio of absolute peak current amplitudes in the presence and the absence of the PP. G protein re-inhibition time constants were obtained by varying the duration between the PP and the test pulse (Δt1 = 2, 4, 6, 10, 15, 20, 50 and 1000 ms; Fig. 1A), and the peak current levels were normalized relative to that obtained after a 1 s interpulse duration (i.e. after complete decay of the PP effect). Rates of recovery from Gβγ inhibition during the PP were obtained by varying PP duration (Δt2 = 2, 5, 10, 15, 25, 50 and 75 ms; Fig. 1D) while maintaining a duration of 5 ms between the PP and the test pulse, and the peak current levels were normalized to the level obtained in the absence of the prepulse. The PP protocols were programmed using the ‘train’ and ‘user list’ functions in pCLAMP. In order to obtain a semi-quantitative measure of the activation time constants, we used mono-exponential fits to the late rising phase of the raw current data using Clampfit (Axon Instruments). All other data were analysed using Clampfit and fitted in Sigmaplot 4.0 (Jandel Scientific). All error bars are standard errors of the mean, and P values given reflect either Student's t tests for individual comparisons, or one-way ANOVA for comparisons among multiple data.
Figure 4. Effect of kinetic slowing on the degree of G protein inhibition.
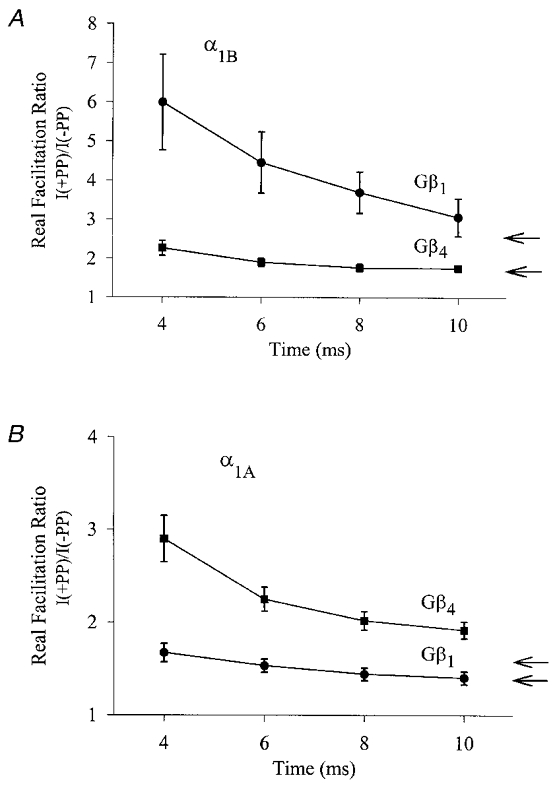
Real facilitation ratios for N-type (A) and P/Q-type (B) channels in the presence of Gβ1γ2 or Gβ4γ2, obtained via isochronal measurements of current amplitude ratios I(+PP)/I(-PP) at various time points, t, into the test depolarization. In each individual experiment, the real facilitation ratio was obtained separately for each time point, t, using the protocols described in Fig. 1. Each point reflects means of 9 to 12 experiments and the error bars reflect standard errors. For comparison, the arrows represent the true facilitation ratios obtained from non-isochronal measurements of the absolute peak current levels (see Fig. 2A and D).
Figure 1. Determination of G protein inhibition kinetics with PP protocols.
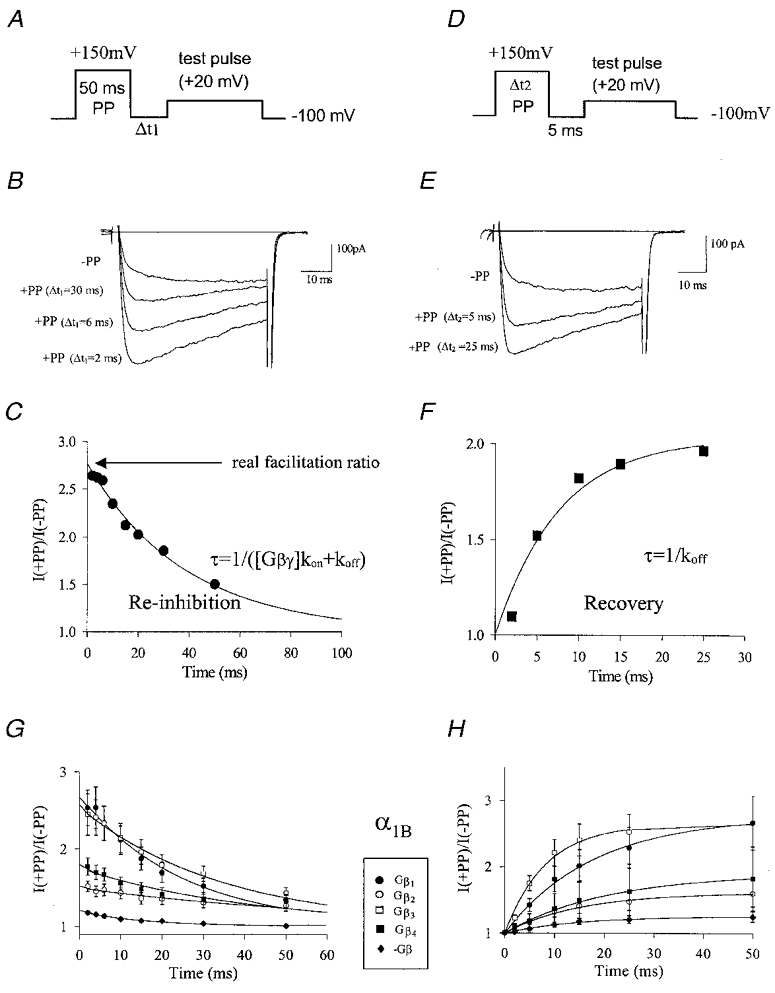
A, pulse protocol used to determine G protein re-inhibition kinetics following a strong depolarizing PP, with Δt1 being varied as outlined in Methods. B, representative current traces obtained from N-type channels co-expressed with Gβ3γ2 either without a PP, or in the presence of a 50 ms PP to +150 mV at variable intervals prior to the test pulse. C, dependence of PP relief on Δt1. The symbols reflect peak current levels normalized to those obtained at Δt1 = 1000 ms. The continuous line is a mono-exponential fit to the data. The time constant obtained from the fit was 38.7 ms, the y intercept (i.e. the real facilitation ratio without contamination from re-inhibition) was 2.76. D, pulse protocol used to determine the rates of recovery from G protein inhibition developing during a strong depolarizing PP. A +150 mV PP of variable duration (see Methods) preceded a test depolarization by a fixed interval of 5 ms. E, current record obtained from N-type channels co-expressed with Gβ3γ2 in the absence of a PP, or in the presence of a PP of either 5 ms or 25 ms duration. F, dependence of PP relief on the duration of the PP (Δt2). Peak currents were normalized to the levels obtained in the absence of the PP and plotted as a function of Δt2. The data were fitted mono-exponentially (continuous line), the time constant obtained from the fit was 7.6 ms. G, re-inhibition kinetics of four different types of Gβ subunits with N-type calcium channels, using the protocol described in Fig. 1A. For comparison, the data obtained in the absence of exogenous Gβγ are included. Error bars are standard errors, and data were fitted mono-exponentially. The time constants/y intercept values obtained from the fits were as follows: Gβ1, 24.9 ms/2.69; Gβ2, 66.4 ms/1.50; Gβ3, 34.8 ms/2.55; Gβ4, 42.0 ms/1.76. H, recovery from inhibition of N-type channels by the various Gβ isoforms using the pulse protocol described in Fig. 1D. The data were fitted exponentially with a single time constant. The time constants obtained from the fit were as follows: Gβ1, 17.9 ms; Gβ2, 15.0 ms; Gβ3, 8.3 ms; Gβ4, 20.61 ms. For comparison, data obtained in the absence of exogenous Gβγ are included.
Results
Determination of the true extent of prepulse relief from G protein inhibition
Figure 1B, C, E and F illustrates a typical experiment for α1B+β1b+α2−δ calcium channels co-expressed with the Gβ3γ2 subtype. In the absence of a PP, the current shows the typical kinetic slowing associated with G protein modulation (Patil et al. 1996). Application of a strong depolarizing PP 2 ms before the test depolarization relieves the tonic inhibition mediated by Gβ3γ2 (Fig. 1A and B). As the duration between the PP and the test pulse is increased, channel re-inhibition by the G protein occurs, and as a result, the degree of PP relief becomes progressively reduced (Fig. 1B and C). The degree of re-inhibition as a function of the inter-pulse duration is nicely described by an exponential fit. The time constant obtained from this fit is inversely proportional to the rate constants for recovery from inhibition and re-inhibition of the channel by the G proteins. Extrapolation of the plot to time zero yields the ‘real facilitation ratio’ which reflects the degree of relief obtained by complete removal of Gβγ inhibition (i.e. in the absence of any re-inhibition occurring after the PP, Fig. 1C). To determine the rate of recovery of Gβ inhibition during the PP, the duration of the PP was varied while leaving the interpulse duration constant at 5 ms (Fig. 1D and F). As illustrated, the degree of PP relief increases exponentially as a function of the PP duration with a time constant inversely proportional to the rate of recovery from G protein inhibition during the PP (Fig. 1E and F). Thus, the two pulse protocols displayed in Fig. 1 provide accurate access to the Gβγ inhibition kinetics of the channel.
Differential inhibition of α1B N-type channels by different Gβ isoforms
Using the PP protocols introduced in Fig. 1A and D, we assessed the abilities of different Gβ isoforms to modulate transiently expressed N-type calcium channels. In the absence of exogenously expressed Gβγ (Fig. 1G and H), the channels exhibited a small degree of tonic inhibition (real facilitation ratio 1.19 ± 0.02), which is presumably due to low background levels of endogenous free Gβγ. Co-expression of the channels with only the Gγ2 subunit did not result in a significant change in PP relief (1.26 ± 0.04, P > 0.05) compared with control conditions. In contrast, co-expression with Gβ1 to Gβ4 inclusively, resulted in significant G protein inhibition which differed among the four Gβ subtypes in both its magnitude of inhibition and its kinetics (Fig. 1G and H). These differences are examined quantitatively in Fig. 2A–C in the form of bar graphs. As seen in Fig. 2A, the PP facilitation observed in the presence of the Gβ5γ2 subunit did not differ from that observed in the absence of exogenous Gβ, indicating that Gβ5 is unable to modulate N-type channel activity under our experimental conditions. In contrast, a large degree of PP relief was observed upon co-expression of Gβ1γ2 (2.83 ± 0.33) and Gβ3γ2 (2.61 ± 0.27) followed by Gβ4γ2 (1.93 ± 0.17) and Gβ2γ2 (1.55 ± 0.07) subunits (P < 0.01vs. control cells), indicating that Gβ1 and Gβ3 inhibit N-type channel activity more effectively compared with the other types of G protein β subunits. In addition to the degree of PP relief, both the rates of re-inhibition after the PP, and recovery from inhibition during the PP were dependent on the Gβ subunit isoform (Fig. 2B and C). Re-inhibition after the PP occurred most rapidly with: Gβ1γ2 < Gβ3γ2≈ Gβ4γ2 < Gβ2γ2, with those of Gβ1γ2 differing significantly from those observed with Gβ2γ2 (P < 0.05; Fig. 2B). However, these values must be viewed cautiously, since the time constant for re-inhibition is predicted to depend on the free Gβγ concentration (Zamponi & Snutch, 1998), which is unknown and may be subject to some variability. In contrast, the rate of recovery from inhibition during the prepulse is independent of G protein concentration, and was fastest with Gβ3γ2 < Gβ1γ2≈ Gβ2γ2 < Gβ4γ2 suggesting that Gβ4γ2 most tightly associates with the N-type channel (P < 0.05, respectively, and vs. other subtypes). Overall, these data indicate the different Gβ subunit isoforms inhibit N-type calcium channels with a unique kinetic profile and an ability to reduce N-type channel activity.
Figure 2. Calcium channel modulation by different Gβ subunit isoforms.
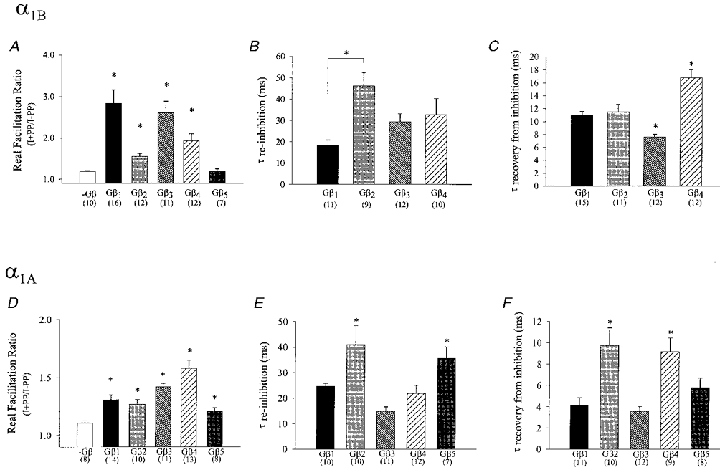
Real facilitation ratios (A and D), mean re-inhibition time constants (B and E) and time constants for recovery from inhibition (C and F) for inhibition of N-type (top row) and P/Q-type (bottom row) calcium channels by various Gβ isoforms. The data were obtained from fits to individual experiments as described in Fig. 1. The numbers in parentheses reflect the number of experiments, error bars indicate standard errors, *Significance (P < 0.05) relative to control (A and D) or among the complete data set (B, C, E and F).
Modulation of N-type and P/Q-type channels is governed by distinct Gβ isoform dependences
Similar to the N-type channel, P/Q-type α1A channels exhibited a small degree of tonic inhibition in the absence of exogenously expressed G proteins, which was not altered upon co-expression with Gγ2 (1.11 ± 0.05 and 1.13 ± 0.02; P > 0.05, respectively). However, unlike with the N-type channel, all five types of Gβ subunits examined mediated a significant (P < 0.05) increase in the degree of PP facilitation of the P/Q-type channel. This effect was most pronounced with Gβ4γ2 (1.58 ± 0.07), followed by Gβ3γ2 (1.42 ± 0.03) Gβ1γ2 (1.31 ± 0.04), Gβ2γ2 (1.27 ± 0.04) and Gβ5γ2 (1.21 ± 0.03) (Fig. 2D). Thus, besides confirming the well-established notion that P/Q-type channels tend to show a smaller degree of G protein-dependent inhibition than their N-type counterparts (i.e. Bourinet et al. 1996; Zhang et al. 1996; Zamponi et al. 1997), the data illustrate the ability of the various Gβ isoforms to differentially modulate the two calcium channel subtypes. As with the N-type channel, the rates of re-inhibition after the PP, and the rates of recovery from inhibition during the PP observed with the P/Q-type channels were dependent on the Gβ subtype (Fig. 2E and F). Whereas the re-inhibition time constants for N- and P/Q-type channels were of comparable magnitude, the rates of recovery from inhibition during the PP were (with the exception of Gβ2γ2) approximately 2 times faster with the P/Q-type channels than those seen with N-type channels. In addition, for the P/Q-type channels the relative order of time constants for recovery from inhibition (Gβ3γ2≈ Gβ1γ2 < Gβ5γ2 < Gβ4γ2≈ Gβ2γ2) and time constants for re-inhibition (Gβ3γ2 < Gβ4γ2≈ Gβ1γ2 < Gβ5γ2≈ Gβ2γ2; but note Gβ concentrations can influence absolute values) differed from those observed with the N-type calcium channels. Overall, our data indicate that P/Q-type and N-type calcium channels exhibit pronounced differences in specific molecular interactions with various G protein β subunit isoforms. Nevertheless, the previously described voltage dependence of Gβγ inhibition (Bean, 1989; Bourinet et al. 1996) was similar for both channel types and independent of Gβ subtype (data not shown).
Kinetic slowing of N-type and P/Q-type channels is dependent on the Gβ subunit isoform
In order to elucidate putative differences in the abilities of different Gβ isoforms to mediate kinetic slowing of the N-and P/Q-type channels, we compared the time course of channel activation in the absence of a PP to that observed 2 ms subsequent to a PP. Figure 3A illustrates the slowing of the activation kinetics of α1A and α1B channels in the presence of Gβ1γ2 and Gβ4γ2. As evident from the current records displayed in Fig. 3, Gβ1γ2 was much more effective in slowing channel activation of the N-type channel compared with Gβ4γ2, whereas the exact opposite was observed with the P/Q-type channels. In order to obtain a semi-quantitative measure of the slowing effect, mono-exponential fits to the rising phase of the currents were used as an arbitrary index of the rate of channel activation (see inset, Fig. 3). As seen in Fig. 3B, a substantial slowing was observed in the presence of the Gβ1γ2 and Gβ3γ2 complexes, (2.6 (± 0.3)- and 2.1 (± 0.2)-fold, respectively), whereas other Gβ subunits were less effective. The α1A channels (Fig. 3C) were on average subject to a much more pronounced G protein-induced slowing of the activation kinetics, particularly in the presence of Gβ4γ2 and Gβ3γ2 (by 4.9 (± 0.6)- and 3.0 (± 0.4)-fold, respectively) while the remaining Gβ isoforms mediated a smaller effect. These observations appear to be correlated with the facilitation data shown in Fig. 2, and further support the notion that P/Q-type and N-type calcium channels exhibit differential modulation by the various Gβ isoforms. Moreover, in a native neuronal setting, such pronounced kinetic slowing observed with certain Gβ isoforms would result in a substantially decreased amount of calcium influx during the relatively brief membrane depolarization, which occurs during a typical neuronal action potential. The contribution of this kinetic slowing to the overall inhibition is shown in Fig. 4A and B. Here, rather than determining the degree of inhibition by comparing absolute peak current levels (as in Figs 1, 2 and 3), inhibition was instead measured isochronally at several fixed durations (4, 6, 8 and 10 ms) into the test depolarization. As shown in Fig. 4, the isochronal measurement unmasks a selective amplification of the facilitation ratios for those calcium channel/Gβγ combinations which exhibit a large degree of kinetic slowing. Furthermore, as expected, the degree of PP facilitation was much larger in the early phase of the test depolarization. A similar analysis for the remaining calcium channel/Gβγ combinations revealed that the relative rank order of potency was not altered (not shown). For the briefest duration examined, the degree of PP relief reached as much as 599 ± 120 % (N-type channel plus Gβ1γ2) and 290 ± 25 % (P/Q-type channel plus Gβ4γ2), and hence, over the time course of a neuronal action potential, certain Gβ isoforms may be capable of inhibiting a predominant portion of the calcium influx via P/Q-type and/or N-type channels.
Figure 3. Kinetic slowing of channel kinetics by different Gβ subunit isoforms.
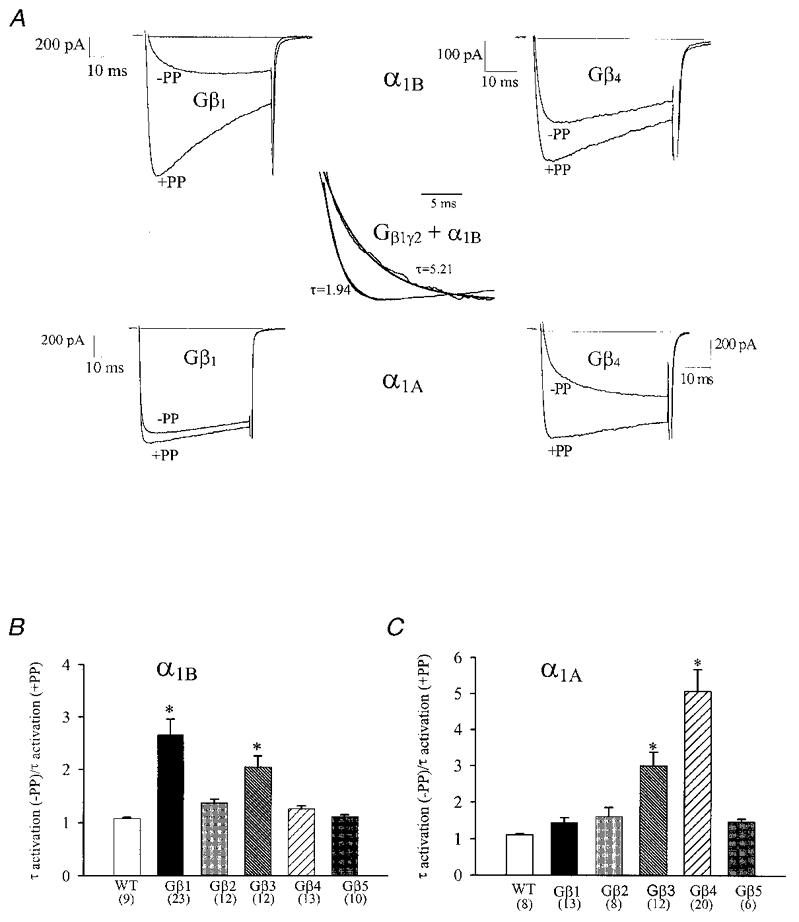
A, current traces illustrating the kinetic slowing of N-type (top row) and P/Q-type (bottom row) channels by Gβ1γ2 and Gβ4γ2. Records shown were obtained either in the absence of a PP, or 2 ms subsequent to a 50 ms long PP to +150 mV. Inset: mono-exponential fits of the activation time course as a semi-quantitative measure of activation time constants. The records shown in the inset are from the same experiment as that in A (α1B/Gβ1γ2), but here the currents have been arbitrarily scaled to further illustrate the kinetic slowing. B and C, the Gβγ mediated slowing of the activation properties of (α1B) N-type (B) and (α1A) P/Q-type channel (C) is demonstrated for each Gβ subtype. The bar graphs reflect the mean change in activation time constant following the PP from 50 ms to +150 mV obtained from mono-exponential fits to the raw current data as illustrated in the inset. *Significance (P < 0.05) within the data set (ANOVA).
Discussion
Comparison with previous work
The direct G protein modulation of presynaptic calcium channels via a membrane delimited pathway was first described almost two decades ago (Dunlap & Fischbach, 1981), and has since been the subject of intense study (for review, see Hille, 1994; Dolphin 1998). It is now well established that channel inhibition is mediated by binding of the G protein βγ complex directly to the domain I-II linker region and possibly also to the carboxyl terminal region of the calcium channel α1 subunit with the Gβ subunit being the active species (Ikeda, 1996; Herlitze et al. 1996; Zhang et al. 1996; Zamponi et al. 1997; DeWaard et al. 1997; Qin et al. 1997). The mammalian brain expresses at least five different Gβ subunit isoforms, suggesting the possibility of differential modulation of calcium channel activity by endogenous Gβ subunit isoforms. Indeed, the work of Garcia et al. (1998) indicates that native N-type channels in cervical ganglion neurons are most effectively inhibited by the Gβ1 and Gβ2 subunit isoforms, whereas Gβ5 mediates a weaker inhibition, and Gβ3 and Gβ4 are ineffective. Surprisingly, in the same experimental system and with the same Gγ subunit (i.e. Gγ2) present, data of Ruiz-Velasco & Ikeda (2000) indicate that all five Gβ isoforms are capable of mediating N-type channel inhibition, albeit to varying degrees. Although our present data agree with this general concept, our results reveal a rather different relative order of efficacy of N-type channel inhibition, with Gβ1 and Gβ3 being more effective than Gβ4 and Gβ2, and no significant modulation being induced by Gβ5.
This apparent discrepancy with previous work is not surprising. Our results were obtained through use of a transient expression system, which permits us to determine the interactions between a single defined population of calcium channels (i.e. α1B+β1b+α2−δ) and a single known G protein β subunit species. In contrast, due to calcium channel β subunit heterogeneity (for review, see Stea et al. 1995) and alternative splicing in the putative G protein binding regions (i.e. Lu & Dunlap, 1999), native neurons are likely to express a mixed population of N-type channels with potentially different G protein modulation properties. This may account, at least in part, for the differences in our experimental results compared with those obtained in rat cervical ganglion neurons (Garcia et al. 1998; Ruiz-Velasco & Ikeda, 2000; see also Jeong & Ikeda, 2000). In addition, both groups assessed the degree of PP relief using a protocol in which the PP preceded the test depolarization by a fixed duration. As this does not take into consideration any putative differential G protein re-inhibition occurring between the PP and the test pulse, PP relief data obtained under those conditions may not faithfully represent the relative effects of individual Gβ subtypes on calcium channel inhibition. Thus, the data presented here and the work reported in cervical ganglion neurons are not directly comparable.
Rates of re-inhibition and recovery from inhibition
A number of groups have reported G protein re-inhibition time constants in various experimental settings. The values obtained here closely reflect those reported by Stephens et al. (1998) for Gβ1 modulation of the N-type calcium channel, whereas the re-inhibition time constants reported by Currie & Fox (1997) for native neurons after activation of G protein coupled receptors were substantially slower (> 100 ms), which may be due to a lower effective Gβγ concentration after receptor activation compared to the levels obtained after transient overexpression in a heterologous system. Interestingly, Currie & Fox reported similar re-inhibition time constants for both N-type and P/Q-type channels, whereas Zhang and colleagues (1996) obtained slower re-inhibition kinetics for N-type channels compared with the P/Q-types upon transient expression in Xenopus oocytes. This latter observation also contrasts with our current work and may be due to the peculiarities of the Xenopus oocyte expression system or the particular channel constructs used by Zhang et al. (1996). Time constants of approximate 20 to 50 ms as reported here have also been obtained after application of 5 to 10 nm of purified bovine brain Gβγ subunits to transiently expressed N-type calcium channels (Zamponi & Snutch 1998).
Our present observations may well reflect intrinsic differences in the molecular interactions between the channels and the individual Gβ isoforms. However, as re-inhibition kinetics are dependent on the free Gβγ concentration (Zamponi & Snutch, 1998), we cannot rule out the influence of differential expression levels of the individual Gβ isoforms on our data. For example, with both P/Q-type and N-type channels, Gβ2 yielded the slowest re-inhibition time constants among all Gβ subtypes examined. This could, in principle, be due to lower levels of expression, however, the relative rank order of re-inhibition time constants differed between the P/Q-type and N-type channels. This cannot be explained solely by different expression levels of the individual Gβ subunit subtypes, and suggests the existence of intrinsic differences in the re-inhibition kinetics between certain Gβ isoforms and the channels. Nonetheless, we reiterate that the absolute values presented in Fig. 2B and E need to be interpreted cautiously.
Unlike the re-inhibition time constant, the rate of recovery from G protein inhibition during the PP is predicted to be largely independent of the G protein concentration (Patil et al. 1996; Zamponi & Snutch, 1998). Therefore, the Gβ subunit dependence of the rate of recovery from inhibition can probably be attributed to the bimolecular interactions between the channel and the individual Gβ subunits (Zamponi & Snutch, 1998). Of particular note, different Gβ subunit isoforms differentially affected the two channel types with recovery from inhibition occurring, on average, twice as rapidly for the P/Q-type channels, indicating that Gβ binding to this channel subtype is less stable. In addition, the relative order of the rates of recovery from inhibition determined for various Gβ subunits differed among the two channel types. Both of these observations may be due to differential interactions of individual Gβ subtypes with the I-II linker regions of the two channels as shown recently for opioid modulation of α1A calcium channel splice variants (Bourinet et al. 1999).
In summary, despite the caveat concerning the re-inhibition kinetics, our kinetic analysis suggests that the molecular interactions, which occur during Gβγ modulation of presynaptic calcium channels, are dependent on both the channel type and the Gβ subunit isoform present.
Possible mechanism of differential inhibition
Our data clearly indicate that different types of exogenously expressed Gβ subunits differentially modulate N-type and P/Q-type channels. What might underlie the large differences in real facilitation ratios observed with the various Gβγ/channel combinations? In view of the relatively narrow range in the re-inhibition and recovery rates observed with the various channel/Gβγ combinations, it seems unlikely that the wide range in the degree of PP relief can be attributed exclusively to the G protein binding affinities for the channel. Instead, we can envision several alternative mechanisms. For example, Stephens et al. (1998; see also Canti et al. 1999) showed that the N-terminus of the N-type calcium channel α1 subunit is a critical determinant of the degree of G protein inhibition, despite not necessarily being directly involved in the physical binding interaction between the channel and the G protein. In such a scenario, the N-terminus might perhaps serve to transduce G protein binding to the domain I-II linker (Zamponi et al. 1997; DeWaard et al. 1997) and carboxyl terminal (Qin et al. 1998) regions of the channel to alter channel gating. If so, it is possible that different types of Gβ subunits differentially couple to this ‘translation machinery’, thereby accounting for the observed effects.
The observation that expression of Gγ2 alone did not result in significant G protein inhibition supports the notion that the observed effects were due to the exogenously expressed Gβ subunits rather than a Gγ dependent recruitment of endogenous Gβ. It is, however, possible that certain types of Gβ subunits may not as effectively couple to the Gγ2 subunit used throughout our experiments (Schmidt et al. 1992; Yan et al. 1996). Work of Ruiz-Velasco & Ikeda (2000) indicates that G protein inhibition of native neurons is, for the most part, relatively independent of the Gγ subunit isoform and seems to occur readily in the presence of Gγ2. Nonetheless, in the case of Gβ4, co-expression of Gγ3 instead of Gγ2 appeared to result in larger inhibition (Ruiz-Velasco & Ikeda, 2000) suggesting the possibility of a modulatory role of the Gγ subunit in G protein inhibition. Thus, although the effects of each Gβ subunit on calcium channel function were assessed under identical conditions (i.e. Gγ2) in our experiments, the molecular determinants underlying the Gβ dependence of calcium channel inhibition may well include a contribution from a putative differential coupling of the Gγ2 subunit to certain Gβ subunit isoforms.
Despite our earlier point concerning differences in the experimental settings between the present study and earlier work (i.e. Garcia et al. 1998; Ruiz-Velasco & Ikeda, 2000), it is surprising to note that both groups observed effective N-type channel inhibition in the presence of Gβ2, whereas in our experiments Gβ2 was a relatively poor N-type channel inhibitor. In principle, it is possible that the lower degree of N-type channel inhibition observed with Gβ4 and Gβ2 (compared to Gβ1) could be due to lower expression levels. If this were the case, one might expect P/Q-type channels to exhibit a similarly reduced susceptibility to inhibition by these two Gβ subtypes. On the contrary, however, for P/Q-type channels, Gβ4 mediated the largest inhibition, and unlike the N-type channels, the effects of Gβ2 and Gβ1 on the P/Q-type were of similar magnitude. Thus, the less effective inhibition of N-type channels by the Gβ2 and Gβ4 subunits is probably not due to poor expression of these constructs. Nonetheless, we cannot rule out the possibility that variability in Gβ expression levels might contribute to the overall Gβ dependence of PP relief. Along these lines, it is possible that effective G protein inhibition requires colocalization of the channels and the G proteins, which may be dependent on the Gβ isoform. However, additional experiments such as the use of flag epitopes will ultimately be required to substantiate such a putative mechanism.
Although the exact molecular mechanisms underlying our observations will require further study, our data indicate that both the putative kinetic slowing, as well as the magnitude of PP facilitation of the two channels, differentially depend on the Gβ subtype present. This may suggest a possible mechanism by which the relative activities of N- and P/Q-type calcium channels could be tightly regulated by selective activation of a subset of Gβ subunits. For example, recent work of Jeong & Ikeda (2000) shows that coupling of seven helix transmembrane receptors to N-type calcium channels is dependent on specific G protein heterotrimer subunit compositions. In view of the involvement of P/Q and N-type calcium channels in synaptic transmission (i.e. Wheeler et al. 1994), the differential modulation of two types of presynaptic calcium channels by different Gβ subunits may ultimately contribute to the fine-tuning of neurotransmission.
Acknowledgments
This work was supported by an operating grant to G.W.Z. from the Medical Research Council of Canada (MRC) and through a Scholarship Award to G.W.Z. from the EJLB Foundation. G.W.Z. also holds faculty Scholarships from the Alberta Heritage Foundation for Medical Research (AHFMR) and the MRC, and is the Novartis Investigator in Schizophrenia Research. M.I.A. holds a Postdoctoral Fellowship award from the Savoy Foundation. S.C.S. and S.E.J. are recipients of AHFMR studentships. We would like to thank Dr Z. P. Feng for helpful discussions and Dr Terry Snutch for providing calcium channel cDNAs.
References
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage-dependence. Nature. 1989;340:153–155. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Bertram R, Behan M. Implications of G-protein-mediated Ca2+ channel inhibition for neurotransmitter release and facilitation. Journal of Computational Neuroscience. 1999;7:197–211. doi: 10.1023/a:1008976129832. [DOI] [PubMed] [Google Scholar]
- Bourinet E, Soong T, Stea A, Snutch TP. Determinants of the G protein-dependent opioid modulation of neuronal calcium channels. Proceedings of the National Academy of Sciences of the USA. 1996;93:1486–1491. doi: 10.1073/pnas.93.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourinet E, Soong TW, Sutton K, Slaymaker S, Mathews E, Monteil A, Zamponi GW, Nargeot J, Snutch TP. Splicing of alpha 1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nature Neuroscience. 1999;2:407–15. doi: 10.1038/8070. [DOI] [PubMed] [Google Scholar]
- Campbell V, Berrow NS, Fitzgerald EM, Brickley K, Dolphin AC. Inhibition of the interaction of G-protein Go with calcium channels by the calcium channel β-subunit in rat neurons. The Journal of Physiology. 1995;485:365–372. doi: 10.1113/jphysiol.1995.sp020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canti C, Page KM, Stephens GJ, Dolphin AC. Identification of residues in the N terminus of alpha1β critical for inhibition of the voltage-dependent calcium channel by Gβγ. Journal of Neuroscience. 1999;19:6855–6864. doi: 10.1523/JNEUROSCI.19-16-06855.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie KPM, Fox AP. Comparison of N- and P/Q- type voltage-gated calcium channel current inhibition. Journal of Neuroscience. 1997;17:4570–4579. doi: 10.1523/JNEUROSCI.17-12-04570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWaard M, Liu H, Walker D, Scott VES, Gurnett CA, Campbell KP. Direct binding of G-protein βγ complex to voltage-dependent calcium channels. Nature. 1997;385:446–450. doi: 10.1038/385446a0. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. Mechanisms of modulation of voltage-dependent calcium channels by G proteins. The Journal of Physiology. 1998;506:3–11. doi: 10.1111/j.1469-7793.1998.003bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubel SJ, Starr TV, Hell J, Ahlijanian MK, Enyeart JJ, Catterall WA, Snutch TP. Molecular cloning of the α-1 subunit of an ω-conotoxin-sensitive calcium channel. Proceedings of the National Academy of Sciences of the USA. 1992;89:5058–5062. doi: 10.1073/pnas.89.11.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K, Fischbach GD. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurons. The Journal of Physiology. 1981;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia DE, Li B, Garcia-Ferreiro RE, Hernandez-Ochoa EO, Yan K, Gautam N, Catteral WA, Mackie K, Hille B. G-protein β-subunit specificity in the fast membrane-delimited inhibition of Ca2+ channels. Journal of Neuroscience. 1998;18:9163–9170. doi: 10.1523/JNEUROSCI.18-22-09163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid J, Nelson D, Spaetgens R, Dubel SJ, Snutch TP, Zamponi GW. Identification of an integration center for cross-talk between protein kinase C and G protein modulation of N-type calcium channels. Journal of Biological Chemistry. 1999;274:6195–6202. doi: 10.1074/jbc.274.10.6195. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall W A. Modulation of Ca2+ channels by G-protein βγ subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends in Neurosciences. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature. 1996;380:255–262. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- Jarvis SE, Magga JM, Beedle AM, Braun JEA, Zamponi GW. G protein modulation of N-type calcium channels is facilitated by physical interactions between syntaxin 1A and Gβγ. Journal of Biological Chemistry. 2000;274:6388–6394. doi: 10.1074/jbc.275.9.6388. [DOI] [PubMed] [Google Scholar]
- Jeong SW, Ikeda SR. Effect of G protein heterotrimer composition on coupling of neurotransmitter receptors to N-type Ca2+ channel modulation in sympathetic neurons. Proceedings of the National Academy of Sciences of the USA. 2000;97:907–912. doi: 10.1073/pnas.97.2.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Dunlap K. Cloning and functional expression of novel N-type Ca2+ channel variants. Journal of Biological Chemistry. 1999;274:34566–34575. doi: 10.1074/jbc.274.49.34566. [DOI] [PubMed] [Google Scholar]
- Patil PG, de Leon M, Reed RR, Dubel S, Snutch TP, Yue DT. Elementary events underlying voltage-dependent G-protein inhibition of N-type calcium channels. Biophysical Journal. 1996;71:2509–2521. doi: 10.1016/S0006-3495(96)79444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Platano D, Olcese R, Stefani E, Birnbaumer L. Direct interaction of Gβγ with a C-terminal Gβγ-binding domain of the Ca2+ channel α1 subunit is responsible for channel inhibition by G protein-coupled receptors. Proceedings of the National Academy of Sciences of the USA. 1997;94:8866–8871. doi: 10.1073/pnas.94.16.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Velasco V, Ikeda SR. Multiple G-protein βγ combinations produce voltage-dependent inhibition of N-type calcium channel in rat superior cervical ganglion neurons. Journal of Neuroscience. 2000;20:2183–2191. doi: 10.1523/JNEUROSCI.20-06-02183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CJ, Thomas TC, Levine MA, Neer EJ. Specificity of G protein beta and gamma subunit interactions. Journal of Biological Chemistry. 1992;267:13807–13810. [PubMed] [Google Scholar]
- Stea A, Tomlinson WJ, Soong TW, Bourinet E, Dubel SJ, Vincent SR, Snutch TP. Localization and functional properties of a rat brain α1A calcium channel reflects similarities to neuronal Q- and P-type channels. Proceedings of the National Academy of Sciences of the USA. 1994;91:10576–10580. doi: 10.1073/pnas.91.22.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stea A, Soong TW, Snutch TP. Determinants of PKC-dependent modulation of a family of neuronal calcium channels. Neuron. 1995;15:929–940. doi: 10.1016/0896-6273(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Stephens GJ, Brice NL, Berrow NS, Dolphin AC. Facilitation of rabbit α1B calcium channels: involvement of endogenous Gβγ subunits. The Journal of Physiology. 1998;509:15–27. doi: 10.1111/j.1469-7793.1998.015bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- Yan K, Kalyanarman V, Gautam N. Differential ability to form the G protein βγ complex among members of the β and γ subunit families. Journal of Biological Chemistry. 1996;271:7141–7146. doi: 10.1074/jbc.271.12.7141. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Bourinet E, Nelson D, Nargeot J, Snutch TP. Crosstalk between G-proteins and protein kinase C mediated by the calcium channel α1 subunit. Nature. 1997;385:442–446. doi: 10.1038/385442a0. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Snutch TP. Decay of prepulse facilitation of N type calcium channels during G protein inhibition is consistent with binding of a single Gβγ subunits. Proceedings of the National Academy of Sciences of the USA. 1998;95:4035–4039. doi: 10.1073/pnas.95.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ellinor PT, Aldrich RW, Tsien RW. Multiple structural elements in voltage-dependent Ca2+ channels support their inhibition by G proteins. Neuron. 1996;17:991–1003. doi: 10.1016/s0896-6273(00)80229-9. [DOI] [PubMed] [Google Scholar]


