Abstract
The efficacy of pulmonary gas exchange immediately after delivery is inversely related to the volume of liquid in the lung at birth, but aspiration of as much liquid as possible from the lung before Caesarean delivery fails to improve postnatal oxygenation (Pa,O2) to the level achieved after spontaneous term delivery. We hypothesised that the differing respiratory benefit of aspiration and vaginal delivery results from the differing volume of lung liquid remaining after aspiration (17 ml (kg body weight)−1) and labour (7 ml kg−1).
We addressed this hypothesis by reducing lung liquid volume to an estimated 7 ml kg−1 by infusing adrenaline to seven fetal lambs at 140 days gestation (term is 147 days) before performing Caesarean delivery and obtaining postnatal blood gases for comparison with samples from lambs delivered vaginally.
Infusion of adrenaline to fetuses caused a progressive decline in arterial O2 saturation (Sa,O2), pH and base excess, but no change in arterial partial pressure of O2 (Pa,O2) or CO2 (Pa,CO2).
After birth, Pa,O2 rapidly rose to the same level in adrenaline-treated and vaginal-delivery groups. A severe acidosis occurred in the adrenaline-treated group and this appeared to be related to a higher Pa,CO2 and a transiently lower Sa,O2 in this group.
We conclude that adrenaline infusion can enhance postnatal Pa,O2 levels in the newborn lamb, but this beneficial effect may be outweighed by the severe acidosis that develops after prolonged prenatal adrenaline treatment.
The fetal lung actively secretes liquid into the future airspaces via transport of Cl− ions across the epithelium (Olver & Strang, 1974) and by doing so produces a distending pressure that promotes its own growth and differentiation (Alcorn et al. 1977). While distension of the lungs with liquid is imperative for normal fetal development, pulmonary gas exchange after birth is adversely affected unless this liquid is cleared from the airspaces. Thus, when clearance of fetal lung liquid is delayed, infants manifest transient tachypnoea of the newborn, a condition also known as ‘wet lung’. This relatively benign condition takes the form of mild respiratory distress associated with a modest impairment of gas exchange and usually resolves quickly. A recent study showed, however, that the Na+ transport mechanism responsible for clearing lung liquid is diminished in very preterm infants suffering the much more severe problem of hyaline membrane disease (Barker et al. 1997). Thus delayed clearance of lung liquid may play a role in both the mild and serious respiratory disorders of the preterm infant. Physiological evidence also demonstrates the need for liquid clearance from the perinatal lung, since we recently showed that gas exchange in the first hour of newborn life is improved when the volume of liquid in the airspaces of the fetal lungs is reduced by an average of 44 %, to a level of approximately 17 ml kg−1, just before Caesarean delivery (Berger et al. 1996).
Although removal of almost half the liquid present in the fetal lung before Caesarean delivery had a beneficial influence on postnatal gas exchange, the blood gases in newborns after this procedure (Berger et al. 1996) were clearly inferior to those achieved in lambs after normal vaginal delivery (Berger et al. 1990). We outlined a number of possible explanations for pulmonary function being improved more after vaginal delivery than after Caesarean delivery following a reduction in lung liquid volume. Amongst these explanations, we suggested that there might be even less liquid left in the lung at the end of labour than that remaining in the lambs we delivered by Caesarean section after removal of lung liquid (Berger et al. 1996). Our recent measurements have confirmed this suggestion by demonstrating that only a quarter (approximately 7 ml kg−1) of the liquid present in the lungs 1 week before term remained there at the end of labour (Berger et al. 1998).
On the basis of our past work, we hypothesised that the superior blood gas and acid–base status in the first postnatal hour in vaginally delivered lambs compared with Caesarean delivered lambs is a consequence of the differing volumes of liquid occupying the airspaces at birth (7 and 17 ml kg−1, respectively). To examine this hypothesis we aimed to reduce lung liquid volume in a group of lambs before Caesarean delivery to a level as close as possible to that at the end of term labour. However, in our experience the required reduction in volume cannot be achieved by aspiration. Accordingly, we chose to bring about the reduction in lung liquid volume via infusion of adrenaline to the fetal jugular vein at a rate that is known to initiate absorption of liquid from the lungs of the late-gestation fetal lamb via activation of Na+ transport across the pulmonary epithelium (Brown et al. 1983; Olver et al. 1986). In utilising this method we also expected that our lambs would experience additional benefits similar to those ascribed to the high circulating level of adrenaline normally present during labour: namely, increased release of surfactant, increased metabolism, body temperature and alertness, each of which is considered to have a favourable influence on neonatal outcome (Lagercrantz & Slotkin, 1986).
Methods
Adrenaline-infusion group
Surgery
Operations were performed on seven Border-Leicester cross ewes at 132–133 days gestation. Anaesthesia was induced in the ewe with an intravenous injection of propofol (5 mg kg−1; Zeneca). The ewe was intubated and inhalation anaesthesia was established with 1 % halothane, 66 % nitrous oxide and 33 % oxygen. Using standard sterile procedures, the uterus was exposed via a mid-line abdominal incision and the fetus was partially exteriorised through a uterine incision. The tenth intercostal space was opened and a set of bipolar harpoon electrodes (Cooke et al. 1990) was inserted into the costal diaphragm for another study.
An incision was made in the ventral mid-line of the fetal neck, and the trachea, carotid artery and jugular vein were exposed by blunt dissection. The artery and vein were cannulated non-occlusively by inserting a Teflon cannula through a purse-string suture in the adventitial layer of each vessel and then connecting the cannula to a 1.5 m length of Tygon tubing (Tygon, Norton Co., Akron, OH, USA). Two small holes were made in the mid-cervical trachea using electrocautery. A balloon atrioseptostomy catheter (5F, American Edwards Laboratories, Santa Ana, CA, USA) was passed through one hole and directed towards the larynx, making sure that it remained within the trachea. Through the second hole we pushed a Tygon catheter of 1.5 m length (i.d., 1 mm; o.d., 1.8 mm) in the direction of the lung. These two catheters were secured with a purse-string suture (5/0 silk) around their entry points into the trachea. The neck incision was closed and the tracheal and vascular catheters were secured to the skin with a stay suture. An amniotic fluid catheter was also secured at this point.
The fetus was returned to the amniotic sac which was then filled with warm saline. The uterus was closed in a watertight fashion with a blanket stitch. The maternal abdomen was closed and the catheters and leads were tunnelled to the ewe's flank where they were exteriorised through a stab wound in the skin. The maternal skin was sutured, antibiotic powder was applied to all surgical wounds and analgesia given intramuscularly to the ewe (50 mg Finadyne, Schering-Plough, Australia). Antibiotic (500 mg streptomycin and 5 × 106 units penicillin) was administered after the operation and then daily until the end of the study. No fetus or ewe showed any sign of distress or infection throughout the course of the study. All surgical and experimental procedures conformed with the guidelines established by the National Health and Medical Research Council of Australia and had the approval of the Standing Committee in Ethics in Animal Experimentation of Monash University.
Experimental protocol
At 140 days gestation a catheter was positioned in the subarachnoid space of the ewe at the level of lumbar interspace L2–L3 under local anaesthesia (2 % xylocaine). The fetal carotid and amniotic fluid catheters were connected to pressure transducers and amplifiers (Hewlett-Packard 1280 pressure transducers, Hewlett-Packard 8805B carrier amplifiers). Amniotic fluid pressure was electronically subtracted from the systemic blood pressure. The heart rate was obtained from the blood pressure signal using a heart rate meter (Neomedix, Sydney, Australia). All signals were displayed continuously on a chart recorder (Neotrace 800Z, Neomedix) and stored on FM tape running at 2.38 cm s−1 (Hewlett-Packard 3968A).
The atrioseptostomy balloon was inflated to occlude the lumen of the trachea before determination of the lung liquid volume using the standard indicator dilution technique (Brown et al. 1983) with 125I-labelled human serum albumin (Amersham, UK) as the impermeant tracer. A volume of up to 50 ml of lung liquid was withdrawn slowly from the lung into a syringe. A 1–2 μCi sample of 125I-labelled human serum albumin was exposed to an ion-exchange resin (Amberlite, IRA-400) to remove free iodine, and then thoroughly mixed into the withdrawn lung liquid. After taking a small sample, the liquid in the syringe was returned to the lungs and thoroughly mixed with unlabelled lung liquid by repeated withdrawal and installation over a period of 30 min. Six or more samples of lung liquid were taken over the following 72–139 min and the accurately weighed aliquots (approximately 0.5 ml) were counted on a 1282 Compugamma gamma counter (LKB, Turku, Finland) with the energy window set from 10 to 80 keV (1 eV = 1.60219 × 10−19 J).
During the course of the experiment we calculated the approximate volume of liquid in the lung using the radiation counts for each sample derived by counting for 1 min. Assuming a fetal weight of 4 kg, we then infused adrenaline (Astra Pharmaceuticals, Sydney, Australia) at a rate of 1.0 μg min−1, which has been shown to initiate reabsorption of liquid from the airspace in the fetus near term (Brown et al. 1983). Further samples of lung liquid were taken at intervals, and again an approximate lung liquid volume was calculated from 1 min counts of each sample. Some time after adrenaline infusion commenced, it became impossible to withdraw enough lung liquid for effective mixing and sampling. At this stage, we estimated the reabsorption rate by linear regression of the lung liquid volumes obtained after the start of adrenaline infusion. From this value, we calculated the time required for lung liquid volume to fall to 7 ml kg−1. In order to achieve the desired lung liquid volume, the total time of adrenaline infusion ranged from 235 to 410 min.
Once the estimated lung liquid volume had reached 7 ml kg−1, the recording was stopped and all catheters and leads were disconnected before placing the ewe on a delivery table. The adrenaline infusion was restarted and catheters and leads were re-connected to recording equipment to monitor blood pressure and heart and respiratory rates during and after delivery. Three to 5 ml of 0.1 % marcain (Astra Pharmaceuticals) was injected via the indwelling spinal catheter to produce spinal block. The adrenaline infusion was discontinued, the fetus was delivered and the umbilicus tied; this time was defined as birth. A thermistor probe was inserted into the lamb's rectum for measurement of body temperature. The ewe was then killed with an overdose of anaesthetic (Euthatal, 150 mg kg−1; May and Baker). Fetal carotid arterial blood samples were taken 1 min before birth and at 0, 1, 2, 3, 4, 5, 10, 15, 20, 30, and 60 min after birth, at which time the lamb was killed with an overdose of anaesthetic. The lungs were removed, weighed and dried to constant weight in an oven at 95°C over 3 weeks.
Subsequent to the day of the experiment, accurate estimates of lung liquid volume and secretion or reabsorption rate were calculated from the activity level of each lung liquid sample, after allowing for the radiation that had been removed in earlier samples (see Brown et al. 1983). For this calculation, the activity of each sample was derived from the average of three counts, each of which was taken over a 10 min period. A linear relationship between lung liquid volume and time during both the control and the adrenaline-infusion periods indicated that we achieved complete mixing of the tracer.
Comparison groups
Two groups of lambs were used for comparison in this study. The first group comprised animals whose surgical preparation and age at Caesarean delivery were identical to the adrenaline-infusion group. The experimental protocol used in this comparison group (aspiration group) was also identical to that used for the adrenaline-infusion group except that lung liquid volume was reduced by aspiration just before delivery rather than through the action of adrenaline; the findings from this group of animals have already been reported (Berger et al. 1996). The second comparison group delivered spontaneously without assistance, although each of the lambs in this group had undergone surgery for implantation of catheters and electrodes, as described for the adrenaline-infusion group, at an age of approximately 130 days gestation; blood gas data from this group (vaginal-delivery group) have already been reported (Berger et al. 1990, 1996). In this group, blood gases were taken at irregular times over the first hour of postnatal life.
Data analysis
To test the effect of adrenaline infusion on fetal heart rate and blood pressure we compared the mean values before and after commencement of infusion using Student's paired t test. To do so, we calculated a mean value for each variable in each fetus from 12 control values taken at 5 min intervals in the hour before adrenaline infusion, and from 24 samples taken in the first 2 h of infusion.
For each lamb in the adrenaline-infusion group we estimated the volume of liquid in the lungs at the time of delivery, based upon the accurate estimates of lung liquid volume and reabsorption rate determined by indicator dilution, together with the time for which the infusion continued. For postnatal data, we calculated the average value for each blood gas and acid–base variable and for body temperature for each lamb over the 60 min study period. These average values were then used to calculate the mean value for each variable for the group as a whole; similar values have already been published for the aspiration group (Berger et al. 1996). Differences between means were tested with Student's unpaired t test. In addition, at each sampling time a mean value for each variable was calculated for the adrenaline-infusion group for comparison with the aspiration group, as shown in Fig. 3. Differences between groups were tested with 2-way ANOVA followed by a post hoc Student-Newman-Keuls test. At the time each blood sample was taken, we determined blood pressure and heart rate, while respiratory rate was counted over the following minute. Comparison of the present results with those in the vaginal-delivery group (Berger et al. 1990) required the data to be put into appropriate time bins. When any lamb in the three groups had two or more blood samples in a bin, the average value was computed for each blood gas and acid–base variable; differences between groups were tested with Student's unpaired t test.
Figure 3. Postnatal respiratory variables in lambs delivered with reduced lung liquid.
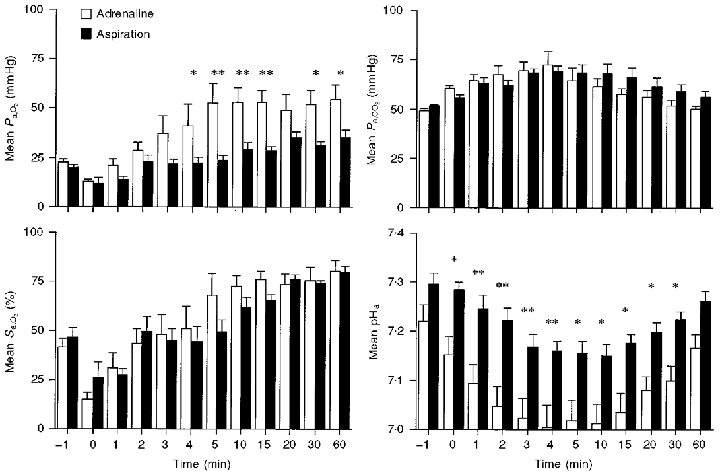
Time course of changes in Pa,O2, Pa,CO2, Sa,O2 and carotid arterial pH in the first 60 min after delivery in the adrenaline (n = 7) and aspiration (n = 5) groups. *P < 0.05, **P < 0.01.
All values are given as the mean ± s.e.m., with P < 0.05 accepted as the critical level.
Results
Effect of adrenaline infusion on the fetus
In the seven lambs studied, liquid was secreted under control conditions at 4.9 ± 0.6 ml min−1 kg−1, and in the presence of adrenaline infusion (1.0 μg min−1) liquid was reabsorbed at 5.9 ± 0.9 ml kg−1 h−1; these rates are comparable to values reported earlier (Brown et al. 1983). Adrenaline infusion was continued for between 235 and 410 min in order to achieve an estimated lung liquid volume of approximately 7 ml kg−1 in each fetus (see below). During the first 2 h of the infusion, blood pressure and heart rate rose significantly (Fig. 1; Table 1). At the end of the adrenaline infusion there was no change from control values in arterial partial pressures of O2 (Pa,O2) and CO2 (Pa,CO2) or arterial haemoglobin concentration, but arterial O2 saturation (Sa,O2), base excess and pH had fallen significantly (Table 1). In four fetuses in which we had at least five blood samples for analysis, there was a progressive decline in Sa,O2, pH and base excess during the course of the adrenaline infusion, with a rate of decline of 1.7 % h−1, 0.016 unit h−1 and 1.0 mmol l−1 h−1, respectively (P < 0.05 in each case).
Figure 1. Blood pressure and heart rate after adrenaline infusion.
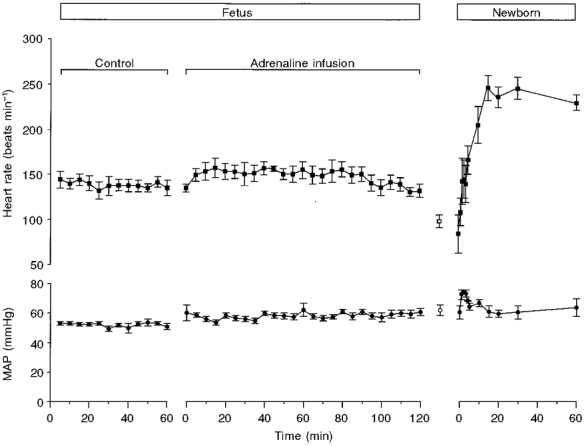
Heart rate and mean arterial blood pressure (MAP) over the control period and the first 2 h following adrenaline infusion to the fetus, and in the first 60 min after delivery. The values shown as open symbols represent data taken for the minute preceding delivery of the fetus. Note the rapid rise in heart rate after birth.
Table 1.
Effect of adrenaline infusion on the fetal lamb
| Control | Adrenaline | Significance | |
|---|---|---|---|
| Heart rate (beats min−1) | 138 ± 7 | 148 ± 7 | < 0.05 |
| MAP (mmHg) | 52.0 ± 1.0 | 58.2 ± 1.5 | < 0.001 |
| [Haemoglobin](g dl−1) | 11.1 ± 0.4 | 11.3 ± 0.4 | n.s. |
| Pa,O2 (mmHg) | 25.4 ± 1.7 | 23.7 ± 1.3 | n.s. |
| Pa,CO2 (mmHg) | 49.0 ± 1.6 | 49.9 ± 1.6 | n.s. |
| Sa,O2 (%) | 55.3 ± 3.9 | 47.2 ± 4.0 | < 0.001 |
| pHa | 7.350 ± 0.009 | 7.232 ± 0.036 | < 0.01 |
| Base excess (mmol l−1) | 0.9 ± 0.7 | −6.5 ± 1.9 | < 0.05 |
Values are means ±s.e.m. under control conditions and at the end of the period of infusion. MAP, mean arterial blood pressure; Pa,O2, arterial partial pressure of O2; Pa,CO2, arterial partial pressure of CO2; Sa,O2, arterial O2 saturation; pHa, arterial pH.
Comparison of adrenaline-infusion and aspiration groups
The adrenaline-infusion (n = 7) and aspiration (n = 5) groups had similar body weights (3.99 ± 0.37 versus 3.57 ± 0.11 kg, respectively) and similar lung liquid volumes (29.2 ± 4.2 and 27.0 ± 1.8 ml kg−1, respectively) at the start of the study. As planned, the volume of liquid remaining in the lungs of the adrenaline-infusion group at the time of delivery (6.7 ± 3.0 ml kg−1), as calculated from the reabsorption rate and the duration of adrenaline infusion, was significantly lower (P < 0.05) than that in the aspiration group (17.3 ± 0.5 ml kg−1), and similar to the volume of 6.8 ± 1.0 ml kg−1 measured in the lungs of eight lambs just before delivery at the end of labour (Berger et al. 1998). As in an earlier study (Berger et al. 1996), we estimated the efficacy of pulmonary gas exchange in the first hour of newborn life by averaging the 10 values for each variable measured in blood samples taken between 1 and 60 min after delivery. These values are shown in Table 2, together with the comparable values for heart rate and carotid arterial blood pressure determined at the same time as the blood samples were taken. Lung water, indicated by the wet/dry weight ratio, was significantly reduced by adrenaline infusion compared with aspiration (Table 2). Adrenaline infusion led to a higher Pa,O2 (but not Sa,O2), a higher heart rate, a substantially lower pH, and a greater base deficit than in the aspiration group (Table 2).
Table 2.
Lung weights and postnatal blood gas and cardiovascular variables over the first 60 min following delivery of lambs after prenatal adrenaline infusion or lung liquid aspiration
| Adrenaline (n = 7) | Aspiration (n = 5) | Significance | |
|---|---|---|---|
| Heart rate (beats min−1) | 195 ± 11 | 147 ± 12 | < 0.05 |
| MAP (mmHg) | 64.6 ± 2.2 | 65.3 ± 2.7 | n.s. |
| [Haemoglobin] (g dl−1) | 11.4 ± 1.0 | 10.4 ± 0.2 | n.s. |
| Respiratory rate (breaths min−1) | 57 ± 2 | 79 ± 14 | n.s. |
| Pa,O2 (mmHg) | 44.1 ± 6.1 | 26.5 ± 2.0 | < 0.05 |
| Pa,CO2 (mmHg) | 61.3 ± 3.2 | 63.6 ± 2.6 | n.s. |
| Sa,O2 (%) | 62.1 ± 6.0 | 57.5 ± 3.3 | n.s. |
| pHa | 7.056 ± 0.035 | 7.196 ± 0.018 | < 0.01 |
| Base excess (mmol l−1) | −13.8 ± 1.6 | −4.1 ± 1.0 | < 0.001 |
| Wet lung/body wt (g kg−1) | 24.8 ± 4.4 | 29.8 ± 2.3 | n.s. |
| Dry lung/body wt (g kg−1) | 3.5 ± 0.4 | 3.5 ± 0.4 | n. s. |
| Wet/dry wt ratio | 7.09 ± 0.4 | 9.06 ± 0.8 | < 0.05 |
Values are means ± s.e.m.
The time course of change in key physiological variables in the first 60 min after delivery for the adrenaline-infusion and aspiration groups is shown in Figs 1–3. It is clear from Fig. 1 that the heart rate in the adrenaline group rose rapidly after birth, just as has been reported in vaginally delivered lambs (Berger et al. 1990). Body temperature was significantly lower in the aspiration group from the third minute onwards (Fig. 2). From the perspective of blood gases and pH (Fig. 3), the picture was less clear-cut, but by 4 min after birth the adrenaline group had a higher Pa,O2 than the aspiration group, and the difference between the two groups remained at 60 min after birth (54.6 ± 7.2 versus 35.3 ± 3.9 mmHg). For Pa,CO2 there was a trend for the adrenaline group to have lower values than the aspiration group from 5 to 60 min after delivery, and the values at 30 and 60 min just failed to reach significance when compared with Student's unpaired t test (P = 0.07 and 0.055, respectively). Compared with the aspiration group, the adrenaline group was relatively acidotic just before delivery and a more profound acidosis developed in this group postnatally, persisting throughout the 60 min studied.
Figure 2. Body temperature in lambs delivered with reduced lung liquid volume.
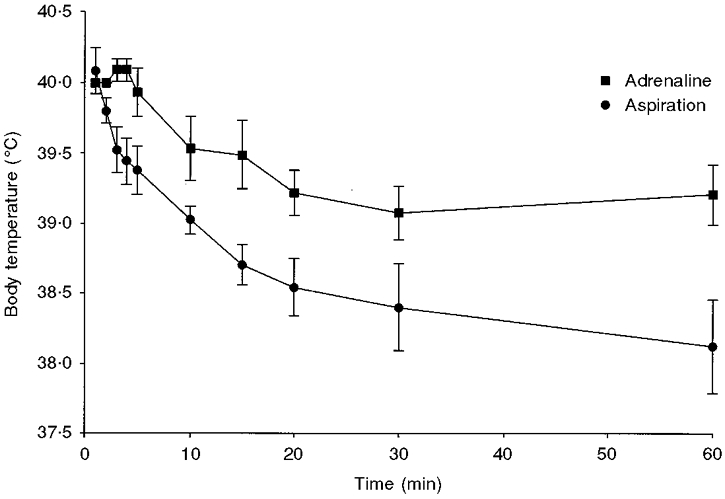
Comparison of changes in body temperature in the aspiration (n = 5) and adrenaline-infusion (n = 7) groups in the 60 min following delivery. Temperature in the aspiration group was significantly lower than that in the adrenaline-infusion group from the third minute onwards (P < 0.05).
Comparison of adrenaline and vaginal-delivery groups
Heart rate rose rapidly after delivery in the adrenaline group (Fig. 1), reaching values similar to those observed after vaginal delivery (see Fig. 1 of Berger et al. 1990). During the first 30 min after birth, the time for which values were available for both the vaginal-delivery and adrenaline groups, only heart rates at 20 min were significantly different (284 ± 10 vs. 232 ± 16 beats min−1, respectively; P < 0.01). The postnatal blood gas and acid–base data for the adrenaline group were grouped into five time bins to enable comparison with lambs that had undergone a normal vaginal delivery at term (Berger et al. 1990). As can be seen from Fig. 4, the only significant difference between the Pa,O2 level in the vaginal-delivery and adrenaline groups was in the 1–3 min bin. For Pa,CO2, the adrenaline group had a significantly higher value in all but one time bin (11–20 min). The Sa,O2 of the adrenaline group remained significantly less than that of the vaginal-delivery group until the 11–20 min bin. The pHa level of the adrenaline group was significantly lower than that in the vaginal-delivery group in all time bins.
Figure 4. Comparison of adrenaline treatment and vaginal delivery on postnatal blood gases and pH.
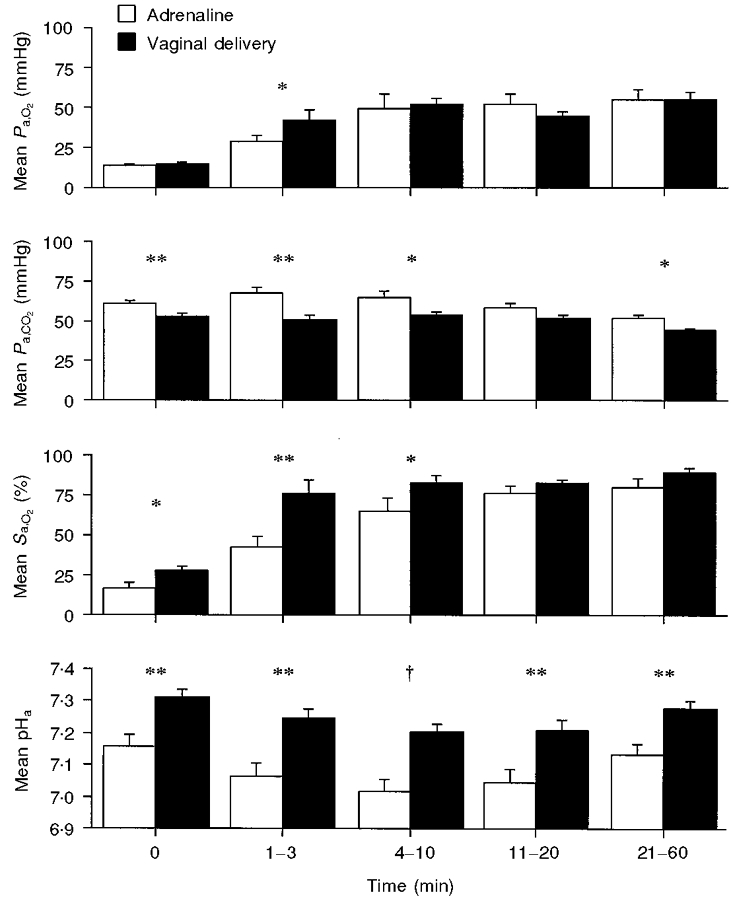
Comparison of blood gas and pH levels in the adrenaline group (n = 7) and in a group of lambs that were delivered vaginally at term (n = 9 animals, with data from ≥ 6 animals in each time bin). Note that Pa,O2 was similar in the two groups from the fourth minute onwards, and that pH was significantly lower in the adrenaline group throughout the 60 min period. *P < 0.05, **P < 0.01, †P < 0.001.
Discussion
Infusion of adrenaline to the fetus resulted during the first hour of postnatal life in a higher Pa,O2 than that of lambs from which liquid had been aspirated before delivery. In addition, within 4 min of delivery, Pa,O2 in the adrenaline group was equal to that in vaginally delivered lambs. Since adrenaline initiated reabsorption of lung liquid, our results add support to the physiological (Lagercrantz & Slotkin, 1986; Berger et al. 1996) and epidemiological (Hales et al. 1993; Morrison et al. 1995) evidence that clearance of liquid from the fetal lungs before birth plays an important role in adapting the lungs for postnatal function. However, we also found that adrenaline infusion leads to a substantial decline in arterial pH in the fetus, and exaggerates the normal fall in pHa that follows vaginal delivery (Koch, 1968; Comline & Silver, 1972; Tunell, 1975; Berger et al. 1990). We consider that the fall in pHa may account for Sa,O2 being lower in the first 10 min after delivery, and Pa,CO2 being higher, than in lambs delivered vaginally. The acidosis we report after prenatal adrenaline treatment, and also observed at birth in the human infant after maternal treatment with a selective β-agonist (Eisler et al. 1999), may limit the clinical utility of strategies employing β-stimulation to reduce lung liquid volume before Caesarean delivery.
This study is part of a series designed to investigate the fate of fetal lung liquid in the perinatal period and to establish the impact of this liquid on the function of the lung after Caesarean and vaginal birth. Our goal was to reduce lung liquid volume to the level present at the end of labour in term fetuses (Berger et al. 1998), and then to assess the efficacy of gas exchange during the first hour of postnatal life after Caesarean delivery. We could not directly establish whether we achieved the intended lung liquid volume of 7 ml kg−1, because it becomes impossible to obtain samples of liquid from the lung once its volume falls below about 15 ml kg−1. However, we have two reasons for considering that adrenaline infusion reduced the volume to approximately the intended level. First, as observed by others (Brown et al. 1983; Olver et al. 1986), adrenaline initiated a reabsorption of liquid which continued at a steady rate for as long as we could make volume determinations, making it reasonable to extrapolate volume as we have done. Second, the wet/dry weight ratio of the lung 1 h after birth was significantly less in the adrenaline than in the aspiration group, consistent with adrenaline treatment reducing the volume of liquid in the lung substantially below what remained in the aspiration group at the time of delivery (17.3 ml kg−1).
Comparison of adrenaline and aspiration groups
Our results show that infusion of adrenaline leads to a faster rise in Pa,O2 than occurs after aspiration of lung liquid. This finding appears to confirm a prediction we made in an earlier study (Berger et al. 1996) that the efficacy of pulmonary gas exchange would continue to improve if lung liquid volume could be reduced below 17 ml kg−1 at delivery. By contrast, the fact that Sa,O2 and Pa,CO2 were not different in the adrenaline and aspiration groups suggests that there was no difference in gas transfer across the lung in the two groups. However, we suspect that improved gas transfer is obscured by a shift to the right in the oxygen dissociation curve secondary to acidosis in the adrenaline group. We also suspect that the higher body temperature in the adrenaline group results from a higher metabolic rate and hence CO2 production. Thus, we suggest that there was increased CO2 excretion even though Pa,CO2 was not lower in the adrenaline group. Furthermore, the addition of acid to the blood after birth titrated a substantial amount of HCO3− to dissolved CO2, increasing still further the CO2 that had to be excreted across the lung in the adrenaline group after delivery. Finally, at the 30th and 60th minute after delivery, the Pa,CO2 level in the adrenaline group just failed to be significantly lower than that in the aspiration group, suggesting that a larger study group may have revealed a lower Pa,CO2 in the adrenaline group at these times.
As discussed in an earlier study (Berger et al. 1996), reduced lung liquid volume after delivery could improve gas exchange by facilitating aeration of the lung (Milner et al. 1978; Vyas et al. 1981; Lee et al. 1999) and by reducing ventilation/perfusion mismatch. However, there are two alternative explanations. First, postnatal gas exchange could have improved because prenatally administered adrenaline produced arousal and wakefulness after delivery (Lagercrantz & Slotkin, 1986), thereby generating a powerful ventilatory drive (Phillipson, 1978). Such an effect is unlikely, however, as the half-life of circulating adrenaline approximates 0.25–1 min in fetal and adult sheep (Jones & Robinson, 1975). A second possibility is that adrenaline treatment improved gas exchange after delivery by promoting release of surfactant in the lung (Ballard, 1986). While we cannot rule out this possibility, the powerful effect of lung liquid independent of surfactant is evident from the observation that halving lung liquid volume by aspiration results in improved gas exchange despite half of the luminal surfactant being discarded (Berger et al. 1996).
Acid–base effects of adrenaline
Administration of adrenaline resulted in a progressive acidosis before delivery, and a severe acidosis after birth. Since there was no rise in Pa,CO2 in the fetus, the acidosis before birth is purely metabolic. It was recently suggested that the rise in circulating glucose, lactate and free fatty acids after adrenaline, isoprenaline, ritodrine or noradrenaline infusion in the fetal lamb (Apatu & Barnes, 1991; Milley, 1997; Bassett & Hanson, 1998; Bassett & Symonds, 1998; Gournay et al. 1999) leads to the metabolic rate increasing above the capacity of the placenta to transfer oxygen to the fetus, resulting in anaerobic production of lactate, and a fall in pH (Gournay et al. 1999). This hypothesis is consistent with the decline in Sa,O2 we observed after adrenaline infusion.
The cause of the acidosis we observed after birth is unknown. Although there is a respiratory component, as Pa,CO2 rose after birth, the severe acidosis was predominantly metabolic. A possible explanation for the acidosis is that the oxygenation of the newborn may be insufficient to sustain its high metabolic rate. Alternatively, prenatally produced lactic acid may be released into the blood stream from tissues that had been vasoconstricted before delivery, a suggestion put forward to account for the transient acidosis that follows normal vaginal delivery in the lamb (Comline & Silver, 1972).
Effects of prenatal β-stimulation on the newborn
Responses to the adrenaline surge in labour, which include promotion of the reabsorption of lung liquid in the near-term fetus (Brown et al. 1983) and increases in the production and secretion of surfactant (Ballard, 1986), are known to adapt the fetus for newborn life (Lagercrantz & Bistoletti, 1977). These actions led Lagercrantz & Slotkin (1986) to propose that antenatal administration of a β-agonist could be useful to compensate for the lack of an adrenaline surge in babies delivered by elective Caesarean section. If we interpret our results in relation to this proposition, a dose of adrenaline which raises the circulating level to that observed in spontaneous labour (Brown et al. 1983) does indeed appear to bestow a benefit, at least in terms of the arterial Pa,O2 achieved immediately after birth. However, it also gives rise to a severe acid–base disturbance after delivery.
A recent controlled trial sought to test directly whether use of a more selective β-agonist helps in adapting the fetus to postnatal life. In the trial, mothers were treated with terbutaline for 2 h before elective Caesarean delivery at term. Treated infants had improved pulmonary mechanics (Eisler et al. 1999), apparently confirming the expectation that β-stimulation would benefit the newborn (Lagercrantz & Slotkin, 1986). However, treated infants also had a greater base deficit in cord blood than controls. The existence of a base deficit in babies after terbutaline, and in the lambs we studied after adrenaline, suggests that the two agents influenced the metabolism of the fetus in similar ways, even though adrenaline has broader actions than terbutaline and was infused for a longer time. We have shown that the acid–base effects of adrenaline are most severe following delivery, reaching their maximum 10 min after birth. No acid–base data were determined in the terbutaline-treated babies at this time, so it is not known whether terbutaline had an adverse impact in the period immediately after birth. However, the postnatal acidosis observed in adrenaline-treated lambs and in terbutaline-treated infants, together with the increased maternal bleeding caused by terbutaline (Eisler et al. 1999), constitute potential dangers in the use of this form of therapy prior to delivery. These findings limit the extent to which such a therapeutic strategy can be justified before Caesarean delivery at term, especially since its goal is to prevent transient tachypnoea of the newborn, a relatively benign condition.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia. We are grateful to Mr Vojta Brodecky for his expert assistance, especially during deliveries.
References
- Alcorn D, Adamson TM, Lambert TF, Maloney JE, Ritchie BC, Robinson PM. Morphological effects of chronic tracheal ligation and drainage in the fetal lamb lung. Journal of Anatomy. 1977;123:649–660. [PMC free article] [PubMed] [Google Scholar]
- Apatu RSK, Barnes RJ. Release of glucose from the liver of fetal and postnatal sheep by portal vein infusion of catecholamines or glucagon. The Journal of Physiology. 1991;436:449–468. doi: 10.1113/jphysiol.1991.sp018560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard PL. Hormones and Lung Maturation. Berlin, Heildelberg: Springer-Verlag; 1986. [Google Scholar]
- Barker PM, Gowen CW, Lawson EE, Knowles MR. Decreased sodium ion absorption across nasal epithelium of very premature infants with respiratory distress syndrome. Journal of Pediatrics. 1997;130:373–377. doi: 10.1016/s0022-3476(97)70198-7. [DOI] [PubMed] [Google Scholar]
- Bassett JM, Hanson C. Catecholamines inhibit growth in fetal sheep in the absence of hypoxemia. American Journal of Physiology. 1998;274:R1536–1545. doi: 10.1152/ajpregu.1998.274.6.R1536. [DOI] [PubMed] [Google Scholar]
- Bassett JM, Symonds ME. β2-Agonist ritodrine, unlike natural catecholamines, activates thermogenesis prematurely in fetal sheep. American Journal of Physiology. 1998;275:R112–119. doi: 10.1152/ajpregu.1998.275.1.R112. [DOI] [PubMed] [Google Scholar]
- Berger PJ, Horne RSC, Soust M, Walker AM, Maloney JE. Breathing at birth and the associated blood gas and pH changes in the lamb. Respiration Physiology. 1990;82:251–266. doi: 10.1016/0034-5687(90)90039-2. [DOI] [PubMed] [Google Scholar]
- Berger PJ, Kyriakides MA, Smolich JJ, Ramsden CA, Walker AM. Massive decline in lung liquid before vaginal delivery at term in the fetal lamb. American Journal of Obstetrics and Gynecology. 1998;178:223–227. doi: 10.1016/s0002-9378(98)80004-5. [DOI] [PubMed] [Google Scholar]
- Berger PJ, Smolich JJ, Ramsden CA, Walker AM. Effect of lung liquid volume on respiratory performance after Caesarean delivery in the lamb. The Journal of Physiology. 1996;492:905–912. doi: 10.1113/jphysiol.1996.sp021356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MJ, Olver RE, Ramsden CA, Strang LB, Walters DV. Effects of adrenaline and of spontaneous labour on the secretion and absorption of lung liquid in the fetal lamb. The Journal of Physiology. 1983;344:137–152. doi: 10.1113/jphysiol.1983.sp014929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comline RS, Silver M. The composition of foetal and maternal blood during parturition in the ewe. The Journal of Physiology. 1972;222:233–256. doi: 10.1113/jphysiol.1972.sp009795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke IRC, Brodecky V, Berger PJ. Easily-implantable electrodes for chronic recording of electromyogram activity in small fetuses. Journal of Neuroscience Methods. 1990;33:51–54. doi: 10.1016/0165-0270(90)90081-p. [DOI] [PubMed] [Google Scholar]
- Eisler G, Hjertberg R, Lagercrantz H. Randomised controlled trial of effect of terbutaline before elective caesarean section on postnatal respiration and glucose homeostasis. Archives of Disease in Childhood, Fetal and Neonatal Edition. 1999;80:F88–92. doi: 10.1136/fn.80.2.f88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gournay VA, Roman C, Rudolph AM. Effect of β-adrenergic stimulation on oxygen metabolism in the fetal lamb. Pediatric Research. 1999;45:432–436. doi: 10.1203/00006450-199903000-00023. [DOI] [PubMed] [Google Scholar]
- Hales KA, Morgan MA, Thurnau GR. Influence of labor and route of delivery on the frequency of respiratory morbidity in term neonates. International Journal of Gynaecology and Obstetrics. 1993;43:35–40. doi: 10.1016/0020-7292(93)90271-w. [DOI] [PubMed] [Google Scholar]
- Jones CT, Robinson RO. Plasma catecholamines in foetal and adult sheep. The Journal of Physiology. 1975;248:15–33. doi: 10.1113/jphysiol.1975.sp010960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G. Lung function and acid-base balance in the newborn infant. Acta Paediatrica Scandinavica. 1968;S181:2–45. doi: 10.1111/j.1651-2227.1968.tb05654.x. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H, Bistoletti P. Catecholamine release in the newborn infant at birth. Pediatric Research. 1977;11:889–893. doi: 10.1203/00006450-197708000-00007. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H, Slotkin TA. The ‘stress’ of being born. Scientific American. 1986;254:92–102. doi: 10.1038/scientificamerican0486-100. [DOI] [PubMed] [Google Scholar]
- Lee S, Hassan A, Ingram D, Milner AD. Effects of different modes of delivery on lung volumes of newborn infants. Pediatric Pulmonology. 1999;27:318–321. doi: 10.1002/(sici)1099-0496(199905)27:5<318::aid-ppul4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Milley JR. Ovine fetal metabolism during norepinephrine infusion. American Journal of Physiology. 1997;273:E336–347. doi: 10.1152/ajpendo.1997.273.2.E336. [DOI] [PubMed] [Google Scholar]
- Milner AD, Saunders RA, Hopkin IE. Effects of delivery by caesarean section on lung mechanics and lung volume in the human neonate. Archives of Disease in Childhood. 1978;53:545–548. doi: 10.1136/adc.53.7.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JJ, Rennie JM, Milton PJ. Neonatal respiratory morbidity and mode of delivery at term: influence of timing of elective caesarean section. British Journal of Obstetrics and Gynaecology. 1995;102:101–106. doi: 10.1111/j.1471-0528.1995.tb09060.x. [DOI] [PubMed] [Google Scholar]
- Olver RE, Ramsden CA, Strang LB, Walters DV. The role of amiloride-blockable sodium transport in adrenaline-induced lung liquid reabsorption in the fetal lamb. The Journal of Physiology. 1986;376:321–340. doi: 10.1113/jphysiol.1986.sp016156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olver RE, Strang LB. Ion fluxes across the pulmonary epithelium and the secretion of lung liquid in the foetal lamb. The Journal of Physiology. 1974;241:327–357. doi: 10.1113/jphysiol.1974.sp010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson EA. Respiratory adaptations in sleep. Annual Review of Physiology. 1978;40:133–156. doi: 10.1146/annurev.ph.40.030178.001025. [DOI] [PubMed] [Google Scholar]
- Tunell R. The influence of different environmental temperatures on pulmonary gas exchange and blood gas changes after birth. Acta Paediatrica Scandinavica. 1975;64:57–68. doi: 10.1111/j.1651-2227.1975.tb04380.x. [DOI] [PubMed] [Google Scholar]
- Vyas H, Milner AD, Hopkin IE. Intrathoracic pressure and volume changes during the spontaneous onset of respiration in babies born by Cesarean section and by vaginal delivery. Journal of Pediatrics. 1981;99:787–791. doi: 10.1016/s0022-3476(81)80412-x. [DOI] [PubMed] [Google Scholar]


