Abstract
In vivo intracellular recordings were performed from striatal output neurones (SONs) (n = 34) to test the role of their intrinsic membrane properties in the temporal integration of excitatory cortical synaptic inputs.
In a first series of experiments, intracellular injection of a test depolarising current pulse was preceded by a 200 ms suprathreshold prepulse, the two pulses having the same intensity. An increase in intrinsic excitability was observed as a decrease (55 ± 21 ms, n = 13) (mean ± s.d.) in latency to the first action potential of the test response compared to the prepulse response. This value decayed exponentially as a function of the time interval between the current pulses (τ= 364 ± 37 ms, n = 5). The voltage response of SONs was not modified by a prepulse that induced a membrane depolarisation < −62 mV.
The effect of the suprathreshold prepulse was tested on monosynaptic cortically evoked excitatory postsynaptic potentials (EPSPs). The ability to induce suprathreshold EPSPs was markedly increased by the prior depolarisation (n = 11 cells). This facilitation decayed progressively as a function of the time intervals between prepulses and cortical stimuli. The potentiation was not observed on small EPSPs reaching a peak potential < −65 mV (n = 3).
We conclude that SONs can optimise cortical information transfer by modifying their intrinsic excitability as a function of their past activation. It is likely that this time-dependent facilitation results, at least in part, from the kinetics of a striatal slowly inactivating potassium current available around −60 mV that recovers slowly from inactivation.
The striatum, the main input stage of the basal ganglia, integrates monosynaptic glutamatergic inputs originating from widespread areas of cerebral cortex (McGeorge & Faull, 1989; Deniau et al. 1996). Despite this powerful synaptic excitation, striatal output neurones (SONs), the major class of striatal neurones, exhibit a low rate of firing activity (Wilson, 1995; Charpier et al. 1999). It is assumed that this low excitability of SONs is due to a set of voltage-dependent potassium currents (Nisenbaum et al. 1994; Nisenbaum & Wilson, 1995; Wilson, 1995) acting as an inhibitory shunt on excitatory inputs rather than due to competing synaptic inhibition (Jaeger et al. 1994). A distinctive electrophysiological property of SONs is their delayed firing in response to intracellular injection of a threshold current pulse. This has been attributed to a calcium-independent potassium A current (IAs) available around −60 mV that inactivates and recovers from inactivation slowly (Nisenbaum et al. 1994; Gabel & Nisenbaum, 1998). Similar slowly inactivating outward potassium currents have also been described in other central neurones, including hippocampal CA1 pyramidal neurones (ID; Storm, 1988), thalamic lateral geniculate nucleus relay neurones (IAs; McCormick, 1991) and prefrontal cortical neurones (IKs; Hammond & Crepel, 1992). It is proposed that such currents limit the initial response to depolarising inputs and, by inactivating, slowly permit the membrane to depolarise towards the spike threshold. In addition, it has been hypothesised that the slow recovery from inactivation of these currents would produce a time-dependent facilitation in synaptic integration after strong depolarising inputs (Storm, 1988; Turrigiano et al. 1996). However, evidence that synaptic events in an intact brain could be reinforced through use-dependent modulation of such slow potassium currents is lacking.
Therefore, the aim of the present in vivo study was to test whether the efficacy of cortico-striatal connections can be enhanced following strong depolarising inputs. Here, we show that a prior depolarisation of SONs induces a short-term increase in their intrinsic excitability, expressed as a facilitation of action potential firing in response to intracellular current pulses. This use-dependent increase in cell responsiveness can transform subthreshold cortically evoked excitatory postsynaptic potentials (EPSPs) into suprathreshold inputs and thus strongly influence the input-output relationship of the SONs. The putative role of IAs in the time-dependent increase in SON excitability is discussed.
Methods
All animal experimentation was carried out in accordance with the European Community's Council directive 86/609/EEC.
Preparation
Experiments were performed in vivo on 27 adult male Sprague-Dawley rats weighing 230–300 g, initially anaesthetised with sodium pentobarbital (66 mg kg−1, i.p., Sanofi, Libourne, France). A cannula was placed in the trachea before animals were mounted in the stereotaxic apparatus. Wounds and pressure points were infiltrated with lignocaine (Xylocaine 2 %). Electroencephalogram (EEG) waveforms and heart beat were continuously monitored to assess the depth of anaesthesia. Additional doses of sodium pentobarbital (24 mg kg−1, i.p.) were given to maintain a low-frequency synchronised EEG pattern.
The animal's temperature was maintained at 36.5–37.5°C by a homeothermic blanket. To obtain stable recordings, rats were immobilised with gallamine triethiodide (Flaxedil, 160 mg kg−1, i.m., Specia, Paris) and artificially ventilated only after the EEG displayed the usual pattern of deep general anaesthesia. At the end of the experiments, rats were killed with an overdose of pentobarbital (200 mg kg−1, i.p.).
Recordings and data analysis
Intracellular recordings were performed using glass microelectrodes filled with 2 m potassium acetate. Resistance of electrodes ranged from 40 to 70 MΩ. All recordings were obtained in current clamp mode using an Axoclamp-2B amplifier (Axon Instruments, Foster City, CA, USA) and were then digitised at a rate of 10 kHz for off-line analysis. As described in detail elsewhere (Charpier et al. 1999), recorded cells were located in the striatal region corresponding to the projection field of the orofacial motor cortex (McGeorge & Faull, 1989; Deniau et al. 1996). Monosynaptic EPSPs (see Results) were evoked (every 1.5 s) in striatal cells by electrical stimulation using a bipolar steel electrode inserted (1.5 mm depth) in the ipsilateral orofacial motor cortex. The amplitude of action potentials was calculated as the potential difference between their voltage threshold, evident as an abrupt increase in slope depolarisation, and the peak of the spike waveform. Their total duration was measured from the threshold to return to this voltage reference. The apparent input resistance of neurones was measured at rest by the voltage changes in response to hyperpolarising current pulses (−0.2 to −0.4 nA, 200 ms duration) intracellularly applied through the recording electrode. The amplitude of cortically evoked responses was measured from the baseline to the peak of the synaptic potential. Numerical values are given as means ±s.d. Student's paired t test (two tailed) or a one way analysis of variance (ANOVA) was used to assess statistical significance.
Morphological methods
Recorded striatal neurones were stained by passing positive current pulses (200 ms, 1 nA) for 5–10 min through the recording microelectrode (50–80 MΩ) filled with neurobiotin (1.5 %, Sigma) dissolved in 2 m potassium acetate. Injected neurones were revealed using the conventional histochemical method (Charpier & Deniau, 1997).
Results
Electrical properties of striatal output neurones in vivo
We recorded intracellularly from 34 SONs in rats anaesthetised with sodium pentobarbital. Labelled cells (see Methods) displayed the morphological features of SONs (not shown), i.e. medium-sized type-I spiny neurones (Chang et al. 1982). Recorded neurones had highly polarised resting potentials (Vm) which ranged from −71 to −92 mV (mean = −79 ± 5 mV, n = 34), a low input resistance (31 ± 8 MΩ, n = 32) and were most often silent at rest. Action potentials had an amplitude which ranged between 50 and 76 mV (mean = 62.4 ± 3 mV, n = 34) with a total duration of 1.4 ms (±0.3 ms, n = 34). Intracellular injection of threshold current pulses induced in SONs a slow ramp-like depolarisation that led to a long latency spike discharge (Fig. 1A). The voltage threshold for this ramp depolarisation (Fig. 1A, arrow) was −60 mV (±1.5 mV, n = 11). This delayed excitation, classically described in SONs, is due to a slowly inactivating potassium outward current (IAs) available around −60 mV that slowly recovers from inactivation (see Introduction). Altogether, these intrinsic membrane properties provide the distinctive electrical features of SONs within the striatum (Nisenbaum et al. 1994; Nisenbaum & Wilson, 1995; Wickens & Wilson, 1998; Charpier et al. 1999).
Figure 1. Activity-dependent increase in intrinsic excitability of SONs.
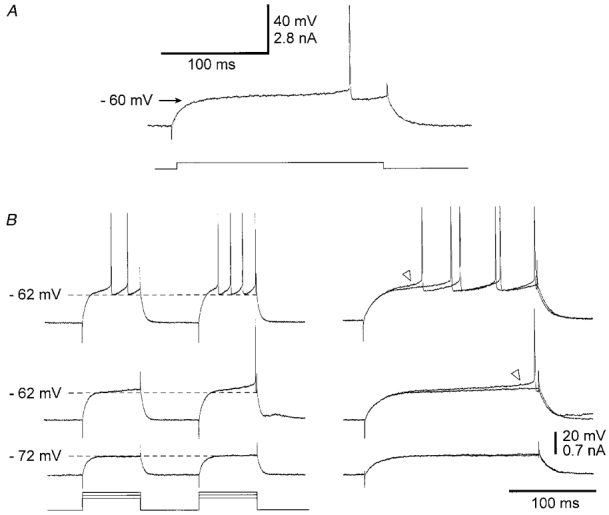
A, intracellular injection in a SON of a threshold current pulse (lower trace) induced a slow ramp of depolarisation leading to a long latency of action potential discharge. The apparent voltage threshold for the ramp depolarisation (arrow) was −60 mV. Vm = −80 mV. B, responses of another SON to two successive depolarising current pulses of same intensity, applied with a time interval of 200 ms. The intensity of the current was progressively increased and the corresponding voltage responses are shown from bottom to top. The right panel shows the superimposition of expanded voltage responses from the corresponding traces on the left. The increase in cell excitability on the test stimulation is shown by an increase in the slope depolarisation (triangles) and a decrease in the latency of the first spike discharge (upper traces). Note the lack of increase in membrane excitability when the membrane depolarisation was below the threshold for the ramp depolarisation (lowest pair of traces). Vm = −82 mV. Here and in the following figures current pulses are shown below the voltage traces.
Activity-dependent increase in intrinsic excitability
What are the changes in excitability of SONs following a prior depolarisation? To answer this question, a depolarising current test pulse was applied 200 ms after a prepulse, the two pulses having the same duration (200 ms) and intensity (+0.5 to +1.2 nA, n = 34). To assess a change in excitability, we compared the test response to the prepulse response. When the intensity of the current was insufficient to lead the membrane potential to the threshold for the ramp depolarisation (< −62 mV), we did not observe any change in the test response (Fig. 1B, lowest traces). In contrast, a prepulse that induced a slow ramp depolarisation was able, on the test stimulation, to bring the cell above threshold for spike generation (Fig. 1B, middle traces). This increase in excitability from the first to the second pulse was always associated with an increase in the slope of ramp depolarisation (see triangles in Fig. 1B). In addition, when the prepulse was already suprathreshold for action potential discharge, we systematically observed a decrease in the first spike latency and the number of spikes was increased by one to three (Fig. 1B, upper traces). This increase in SON excitability by a prior depolarisation, above the voltage threshold for ramp depolarisation, was present in all tested neurones n = 34).
We obtained the time course of the depolarisation-induced change in excitability by progressively increasing the time intervals between the pre- and the test pulses (Fig. 2A). In this set of experiments n = 13), the conditioning pulse elicited one to three action potentials. The modification in intrinsic excitability was quantified by the decrease in the first spike latency (Δt, Fig. 2B) observed during the test stimulus. In the range of interstimulus intervals (ISIs) tested (200–1400 ms), the maximal increase in cell excitability was obtained with the 200 ms protocols, the mean value of Δt being 55 ms (±21 ms, n = 13). A complete recovery was systematically observed for an ISI of 1.2 s n = 13). In five SONs, we have been able to explore the complete range of ISIs. We found that Δt decreased in an exponential fashion as a function of the time interval between current pulses (Fig. 2C). The mean time constant calculated from these five cells was 364 ± 37 ms.
Figure 2. Time course of activity-dependent changes in intrinsic excitability.
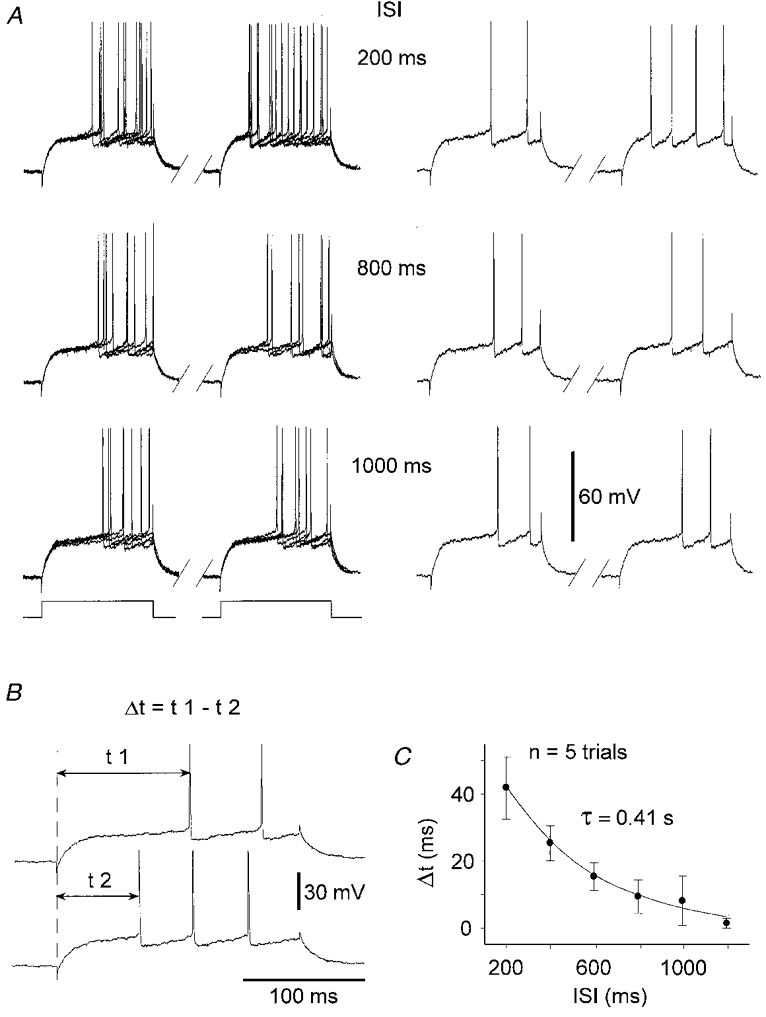
A, superimposition of voltage responses of a SON to 5 successive, paired, depolarising current pulses (left panel). The single paired responses (right panel) are from the superimposed records shown on the left. From top to bottom, the inter-stimulus intervals (ISIs) were progressively increased. For each paired stimulation, current pulses had a duration of 200 ms and an intensity of +0.7 nA. B, the increase in cell excitability was assessed by measuring the decrease (Δt) in the first spike latency between the first (t1, upper trace) and the second response (t2, lower trace). Traces are from one of the 200 ms protocols shown in A. C, recovery from increased excitability as a function of the ISIs. Each data point represents the mean value (error bars, s.d.) of Δt obtained from 5 successive trials. The time course of recovery was best fitted by a single exponential (τ= 0.41 s; n = 5 trials).
The increase in the responsiveness of SONs could be mediated by an increase in input resistance in the period following the prepulse. To test this, we performed n = 4 cells) protocols where negative current pulses n = 5 trials, 200 ms duration, −0.2 to −0.4 nA) were applied (every 1.5 s) in a control situation (i.e. without prior depolarisation), then 200 ms after a suprathreshold current prepulse. In this set of experiments, while the prepulse was able to facilitate the cell firing in response to a depolarising current (Fig. 3A), the voltage changes induced by weak negative pulses were not significantly modified (P > 0.5 in each experiment) during pairing protocols compared to control responses (Fig. 3B and C). This result indicated that the prepulse was without effect on the input resistance in the period where we observed the maximal increase in excitability.
Figure 3. The increase in excitability of SONs was not associated with a change of input resistance.
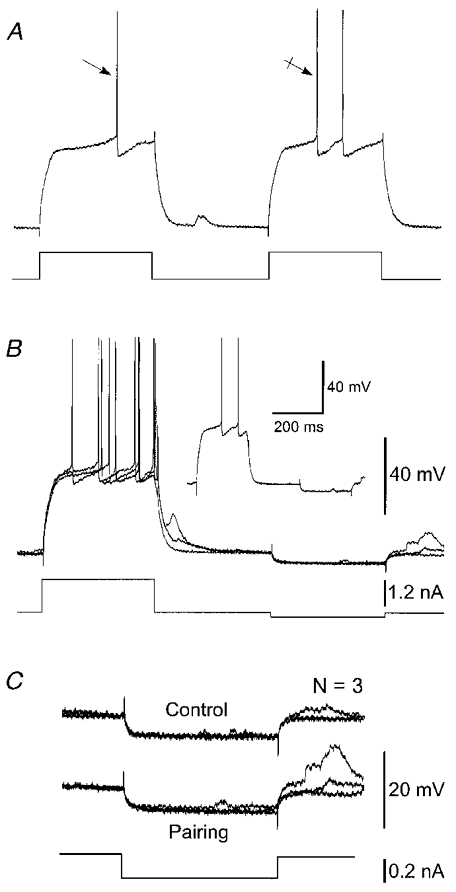
Results shown in A–C are from the same SON. A, example of an increase in SON excitability during a 200 ms pairing procedure. The decrease of the first spike latency of the test response (crossed arrow) compared to the prepulse response (arrow) was accompanied by an additional action potential. B, superimposition (n = 3) of voltage responses induced by a pairing protocol consisting of an intracellular injection of a negative current pulse of weak intensity (−0.2 nA, 200 ms) preceded by a 200 ms suprathreshold current pulse. The single voltage response shown in the inset is from the superimposed records. C, superimposition (n = 3) of the cell responses to hyperpolarising current pulses in control (without prior depolarisation) and during the pairing protocols shown in B. Note the constancy in the changes of membrane potential indicating that input resistance of the SON (28 MΩ) was not altered by the prepulse. The voltage calibration bar in B also applies in A. Vm = −92 mV.
Short-term facilitation in cortico-striatal transmission
The depolarisation-induced prolonged change in excitability might provide an intrinsic mechanism to increase the gain of cortico-striatal connections as a result of past depolarisation of the SONs. We tested this hypothesis on 13 SONs located in the striatal projection field of the orofacial motor cortex (Deniau et al. 1996; see also Charpier et al. 1999). Electrical stimulation of this cortical region induced, in the recorded SONs, EPSPs (see Fig. 6A and Ca and b) with latencies ranging between 2 and 3.1 ms (mean = 2.6 ± 0.3 ms, n = 13) and a time to peak of 11.4 ms (±3 ms, n = 13). These properties are consistent with the well-characterised monosynaptic glutamatergic cortically evoked synaptic potentials in SONs (Jiang & North, 1991; Kita, 1996; see also Charpier et al. 1999).
Figure 6. Properties of cortico-striatal facilitation.
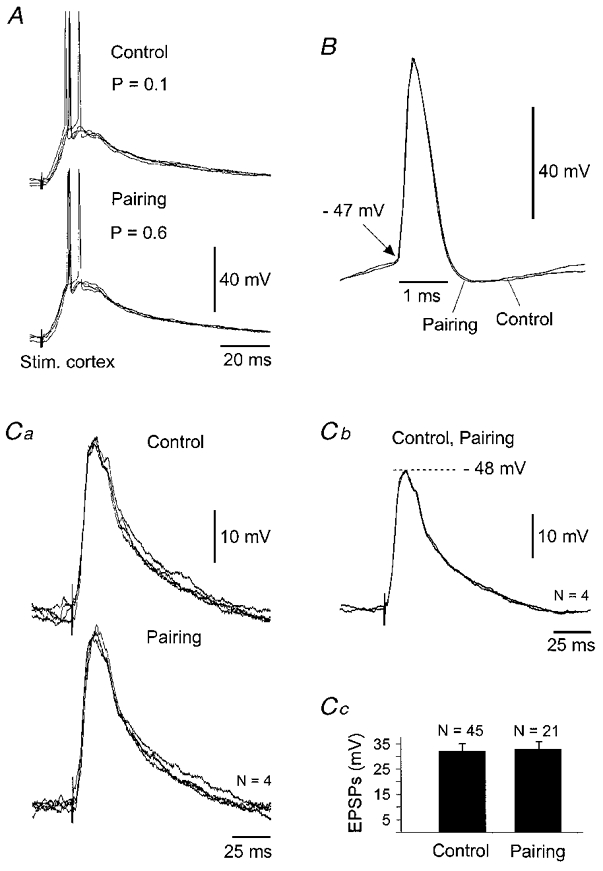
Results presented in A-C are from the facilitated neurone shown in Fig. 4A. A, superimposition (n = 4) of suprathreshold EPSPs recorded in control and during the 200 ms pairing procedure. The probability (P) of evoking a suprathreshold cortically evoked response in both periods is indicated in the figure. B, DC superimposition of expanded single action potentials induced by cortical stimulations in control and during the pairing protocol. Note the constancy of the voltage firing threshold (arrow) and of the kinetics of action potentials. Spikes are from records shown in A. C, subthreshold EPSPs are not modified by the prepulses. Ca, superimposition of 4 successive subthreshold EPSPs in control and during the pairing protocol. Cb, superimposition of averaging of traces shown in Ca indicating that the time to peak and the decay time of subthreshold synaptic potentials remained stable. The membrane potential at the peak of EPSPs is indicated in the figure (dashed line). Cc, histogram (error bars, s.d.) showing the absence of significant (P= 0.3) changes in subthreshold EPSP amplitude during the pairing procedure.
In the control situation, cortical stimuli n = 50) were applied without prior depolarisation (Fig. 4Aa, Control) every 1.5 s. The conditioning procedure consisted of pairing protocols where the cortical stimuli were delivered 200 ms after the offset of a suprathreshold prepulse (Fig. 4Aa, Pairing). Paired stimuli were applied 50 times at the same rate as in the control period.
Figure 4. Facilitation of cortico-striatal transmission by a prior postsynaptic suprathreshold depolarisation.
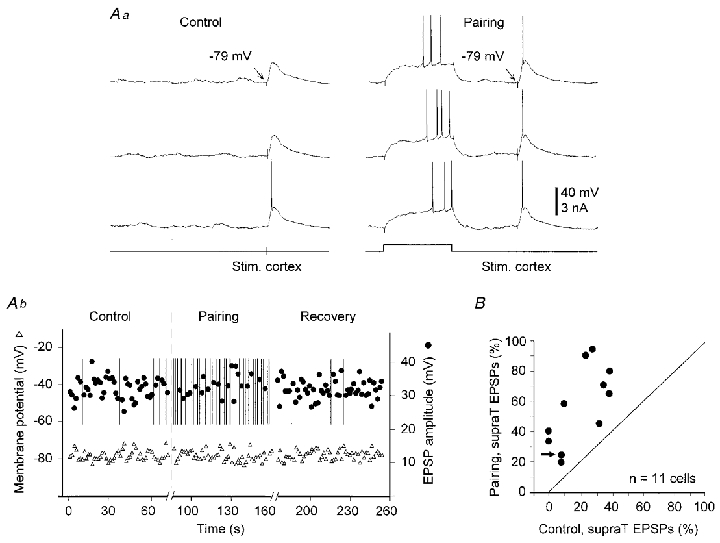
Aa, Control, three successive responses evoked in a SON by cortical stimulation without prior depolarisation. Pairing, three successive responses when the cortical stimulation was applied 200 ms after the offset of a suprathreshold prepulse. Arrows indicate the membrane potential measured immediately before the cortical stimulation. Ab, time course of membrane potential (measured as shown in Aa) and amplitude of subthreshold cortically evoked EPSPs during the control period, the pairing protocol and a following period of control cortical stimulation (Recovery). Vertical bars indicate the occurrence of a cortically evoked suprathreshold response. Note the dramatic increase in the occurrence of suprathreshold potentials, from 5 in control to 29 during the pairing procedure. In A and in the following figures, the time of cortical stimulation is shown below the voltage recordings. B, relationship between the number of suprathreshold (supraT) EPSPs evoked in control and during the pairing procedures in 11 experiments. Values were normalised with respect to the total number of stimuli (n = 50 in each experiment). The arrow indicates a successful experiment in a cell which was previously unaffected by a pairing protocol using a weak cortical stimulation (see Fig. 7).
In eight cells, some suprathreshold EPSPs (from 4 to 20 out of 50 stimuli, mean = 13 ± 6) could be produced in control (Fig. 4Aa and b). In this set of experiments, during the pairing protocols, the number of cortical stimuli that induced spike discharge was enhanced by 180 ± 146 % (between 38 and 480 %) (Fig. 4Ab and B) compared to the control values. The long duration of the intracellular recording period allowed us to apply after the pairing procedure another set of control cortical stimulation (n = 5 cells). A recovery to control values in the efficacy of the cortico-striatal transmission (Fig. 4Ab) was observed in all cases. The membrane potential measured immediately before the synaptic activation (see Fig. 4Aa) was not significantly modified (P > 0.5 in each experiment) during the pairing protocol (Fig. 4Ab). This result indicates that the facilitation did not result from a steady membrane depolarisation.
An important result is that cortical stimuli that remained subthreshold throughout the control period became effective for SON firing during the pairing procedure. In two neurones, where the resting potential was particularly low (−83 and −89 mV), while the control EPSPs were of large amplitude, 29 ± 1 and 35 ± 1 mV, respectively, they reached a peak potential (about −54 mV) close to but always below the threshold for spike discharge. In these two cells, the cortical stimuli were able during the pairing protocol to evoke 18 and 20 suprathreshold synaptic potentials, respectively.
The increased probability of inducing firing depended on the time interval between the offset of the depolarising prepulse and the cortical stimulation (Fig. 5A). Similarly with the time course of change in intrinsic excitability (see Fig. 2C), the efficacy of the cortico-striatal transmission decayed progressively as a function of the ISIs (Fig. 5B) and recovered to its control level for a time interval of 1.2 s (n = 5).
Figure 5. Time course of cortico-striatal facilitation.
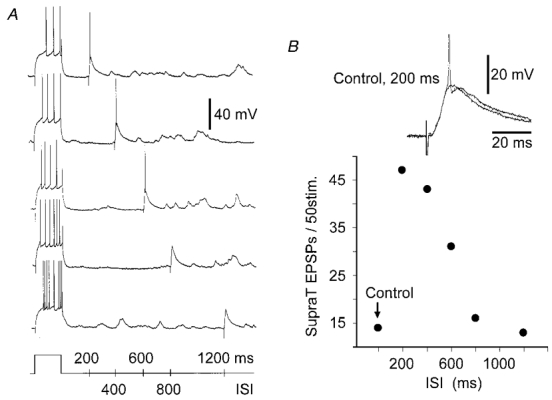
A, responses of a SON to injections of current pulses followed by cortical stimulation with progressively increasing time intervals. B, from the same experiment, number of suprathreshold EPSPs evoked by cortical stimulation in control and during the pairing protocol as a function of ISI. The inset shows an example of a subthreshold control EPSP and the suprathreshold synaptic potential from the 200 ms protocol shown in A. The spike is truncated. Vm = −79 mV.
As illustrated by the superimposed records shown in Fig. 6A (same experiment as in Fig. 4A), the pairing protocol did not affect the kinetics of suprathreshold cortically evoked EPSPs. In addition, in this experiment, action potential latencies on evoked EPSPs showed similar fluctuations in control (from 9 to 15 ms, n = 5) and during the pairing procedure (10 to 15 ms, n = 29). To assess possible changes in action potential properties that could accompany the increase in the probability of firing, we measured from the 11 facilitated neurones different spike parameters in control, such as voltage threshold (−47.6 ± 2.8 mV), rise time (366 ± 63 μs), half-width (604 ± 85 μs), decay time (481 ± 363 μs) and amplitude (58.5 ± 7.5 mV). These values were not statistically modified during the pairing session compared to the control period (P > 0.3 for each spike parameter). This relative stability of action potential properties is exemplified in Fig. 6B. Moreover, we did not notice any change in kinetics (Fig. 6Ca and b) or in amplitude (Fig. 6Cc) of subthreshold EPSPs recorded in control and during the pairing protocol.
In three additional experiments, the intensity of cortical stimulation was adjusted to induce EPSPs of small amplitude which depolarised the cell to potentials of −66, −70 and −71 mV, respectively. Under this condition, pairing protocols did not affect the cortico-striatal synaptic strength (Fig. 7A and B, Control 1 and Pairing 1). However, in these cells, the facilitation was restored by increasing the control EPSP amplitude (Fig. 7A and B, Control 2 and Pairing 2), demonstrating that the lack of facilitation was not associated with a physiologically distinct set of striatal neurones. Moreover, these results indicated that only strong cortical synaptic inputs could be reinforced.
Figure 7. Demonstration that cortico-striatal facilitation depended on the amplitude of evoked EPSPs.
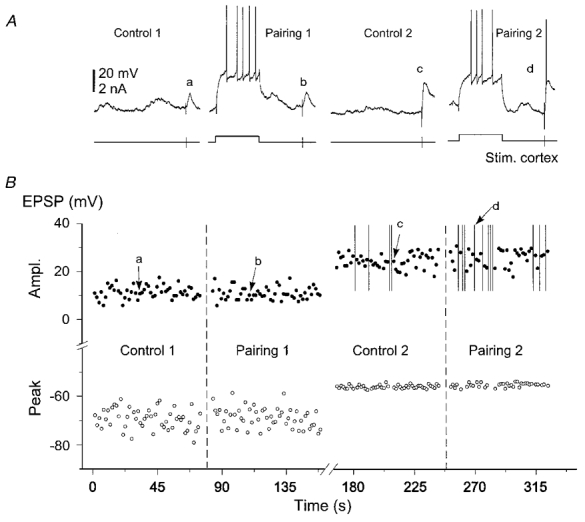
A, example of records, in a single SON (see arrow in Fig. 4B), from control and during the corresponding 200 ms pairing protocol using cortical stimuli that induced small (Control 1, Pairing 1) then large (Control 2, Pairing 2) amplitude EPSPs. B, plot of EPSP amplitude and of the corresponding peak potential versus time. Vertical bars indicate suprathreshold responses. Letters correspond to the cortically evoked responses shown in A. Note that prepulses became effective at inducing the cortico-striatal facilitation when the peak potential of synaptic response was above −60 mV. Vm = −80 mV.
Discussion
The main conclusion of this in vivo investigation is that a strong depolarisation of SONs causes a time-dependent increase in their excitability which facilitates the input-output relationship at cortico-striatal synaptic connections. Therefore, these findings demonstrate that in an intact brain the dynamics of intrinsic electrical properties of the postsynaptic cell are potentially important in the temporal integration of synaptic inputs.
Mechanism of activity-dependent increase in SON excitability: putative role of IAs
Our in vivo model does not allow exploration of the cellular mechanisms of the facilitation described here. However, several arguments support the hypothesis that the short-term increase in SON excitability could result from use-dependent modulation of the striatal slowly inactivating potassium outward current, termed IAs. From in vitro studies (Nisenbaum et al. 1994; Gabel & Nisenbaum, 1998), it has been demonstrated that this current underlies the slow ramp depolarisation and the delayed firing of SONs in response to threshold current pulses. In addition, IAs is available at subthreshold potentials (around −60 mV), exhibits rapid kinetics of activation, slow kinetics of inactivation and slow kinetics of recovery from inactivation. The facilitation we described here shows voltage dependence and kinetic properties consistent with a role of IAs. First, prepulses that depolarised the cell below the expected voltage threshold of IAs, as indicated by the absence of the slow ramp depolarisation (see Fig. 1B, lower traces), did not induce an increase in cell responsiveness. Second, the prepulse inducing the facilitation was suprathreshold for spike discharge, a level of potential where IAs is ∼90 % inactivated (Gabel & Nisenbaum, 1998). Third, the change in intrinsic excitability observed on the current test pulse, was associated with an increase in the slope of membrane depolarisation and with a decrease in firing latency, indicating substantial inactivation of IAs. It is important to stress that in other central neurones, recorded in vitro, a similar facilitation during repetitive membrane depolarisation has been attributed to a cumulative inactivation of slowly inactivating potassium currents (Storm, 1988; McCormick, 1991; Hammond & Crepel, 1992; Turrigiano et al. 1996). Fourth, the time course of the facilitation, observed with current pulses as well as with cortically evoked EPSPs, is consistent with the recovery kinetics of IAs. In SONs recorded in vitro, the time constant of recovery from inactivation at −80 mV ranged between 0.9 and 2.2 s (mean = 1.2 s) (Gabel & Nisenbaum, 1998). Since these values were obtained at 22°C, an adjustment was needed to take into account the temperature of our in vivo experiments (37°C). Assuming a conservative Q10 value of 2.5, this results in a corrected time constant ranging between 0.23 and 0.56 s (mean = 0.3 s) that is in accordance with the decay kinetics of the facilitation described here at 37°C. Finally, the cortico-striatal transmission could be enhanced if the peak of synaptic depolarisation was within the voltage window where IAs is available (> −60 mV, see Figs 6Cc and 7). While we cannot exclude a possible involvement of inward sodium or calcium currents, the kinetics and the voltage dependence of such currents described in SONs (Cepeda et al. 1995; Song & Surmeier, 1996) are not compatible with the properties of the prepulse-induced facilitation observed in the present study.
In vivo studies have shown that the threshold for spike discharge in SONs is related to the voltage trajectory preceding the action potential firing, a faster rise of depolarisation leading to a lower threshold for spike discharge (Sugimori et al. 1978; Wickens & Wilson, 1998). This effect was attributed to non-isopotentiality at somatic recording and spike-initiation regions (Sugimori et al. 1978) or by the kinetics of potassium currents acting close to the firing threshold (Wickens & Wilson, 1998). In the present study, the cortico-striatal facilitation was obtained for synaptic depolarisation close to cell firing but was not associated with a lowering of spike threshold or any obvious increase in the rate of rise of synaptic potentials. Presuming a role of IAs, it is possible that the inactivation of this current will favour sharp depolarisations at the trigger zone and therefore will increase the probability of inducing action potential firing.
Functional implications
It has been shown that SONs recorded in urethane-anaesthetised rats exhibit step-like fluctuations in membrane potential consisting of recurring sustained depolarising ‘up-states’ alternating with hyperpolarising periods (‘down-states’) (Wilson & Kawagushi, 1996). The transitions to ‘up-states’, which require powerful excitatory synaptic inputs lead to a membrane potential around −55 mV (Wilson & Kawagushi, 1996; Stern et al. 1997). It is likely that ‘up-states’ would produce the same type of time-dependent increase in SON excitability as we obtained by direct stimulation. Therefore, if the timing of ‘down’ and ‘up’ transitions and inter-‘up-state’ durations is appropriate, we could expect an increase in SON firing during successive ‘up-states’.
Due to their low excitability, it is assumed that SONs perform coincidence detection of synchronised converging cortical inputs (Houk, 1995). The short-term use-dependent facilitation described here could prolong the temporal window for detection of uncorrelated synaptic inputs, arising either from the same or from spatially separated cortical areas. In the behaving animal, SONs process information from functionally associated cortical regions that are implicated in a given behavioural response (Flaherty & Graybiel, 1991). For instance, SONs in the sensorimotor sector of the striatum receive converging inputs from the cortical representation of homologous body parts. During a sensorimotor task, the use-dependent increase in excitability of the related SONs is expected to maintain their responsiveness to the serial sensorimotor cortical information which occurs in the correct execution of the task.
The short-term increase in intrinsic excitability may also be critical for the induction of in vivo cortico-striatal long-term plasticity that requires suprathreshold postsynaptic depolarisation (Charpier & Deniau, 1997; Charpier et al. 1999). In particular, we recently showed that cortico-striatal long-term potentiation (LTP) could be induced in vivo following cortical stimulation at 5 Hz which mimics episodes of spontaneous cortical synchronisation in our preparation (Charpier et al. 1999). This frequency of cortical activity should produce in SONs the optimal pattern of membrane depolarisation to induce the use-dependent increase in excitability. Therefore, it is likely that an increase in SON responsiveness during successive cortical inputs at 5 Hz will facilitate the induction of cortico-striatal LTP.
A new form of short-term synaptic facilitation
Most forms of short-term synaptic enhancement result from an activity-dependent accumulation of calcium in presynaptic terminals that lead to an increase in neurotransmitter release (Zucker, 1999). Here, we describe a form of short-term enhancement in the input-output relationship at cortico-striatal synapses resulting from use-dependent modulation of intrinsic properties of the postsynaptic cell. While this phenomenon differs from the ‘classical’ presynaptic mechanisms, it shows decay kinetics similar to the short-term synaptic ‘facilitation’ that exhibits time constants from tens to hundreds of milliseconds (Fisher et al. 1997). Therefore, the present time-dependent increase in the synaptic gain provides a new form of ‘facilitation’ based on postsynaptic mechanisms. In addition, assuming a role of a slowly inactivating potassium current, we propose that such a short-term use-dependent increase in neuronal electrical output should occur in many other neurones where potassium currents, similar to the striatal IAs, are present.
Acknowledgments
We thank Drs J. C. Behrends and N. Leresche for thoughtful comments and Dr R. Miles for critical reading of the manuscript. This work was supported by the BIOMED 2 programme, PL 962215.
References
- Cepeda C, Chandler SH, Shumate LW, Levine MS. Persistent Na+ conductance in medium-sized neostriatal neurons: characterization using infrared videomicroscopy and whole cell patch-clamp recordings. Journal of Neurophysiology. 1995;74:1343–1348. doi: 10.1152/jn.1995.74.3.1343. [DOI] [PubMed] [Google Scholar]
- Chang HT, Wilson CJ, Kitai ST. A Golgi study of rat neostriatal neurons: light microscopic analysis. Journal of Comparative Neurology. 1982;208:107–126. doi: 10.1002/cne.902080202. [DOI] [PubMed] [Google Scholar]
- Charpier S, Deniau J-M. In vivo activity-dependent plasticity at cortico-striatal connections: evidence for physiological long-term potentiation. Proceedings of the National Academy of Sciences of the USA. 1997;94:7036–7040. doi: 10.1073/pnas.94.13.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpier S, Mahon S, Deniau J-M. In vivo induction of striatal long-term potentiation by low-frequency stimulation of the cerebral cortex. Neuroscience. 1999;91:1209–1222. doi: 10.1016/s0306-4522(98)00719-2. [DOI] [PubMed] [Google Scholar]
- Deniau J-M, Menetrey A, Charpier S. The lamellar organization of the rat substantia nigra pars reticulata: segregated patterns of striatal afferents and relationship to the topography of corticostriatal projections. Neuroscience. 1996;73:761–781. doi: 10.1016/0306-4522(96)00088-7. [DOI] [PubMed] [Google Scholar]
- Fisher SA, Fischer TM, Carew TJ. Multiple overlapping processes underlying short-term synaptic enhancement. Trends in Neurosciences. 1997;20:170–177. doi: 10.1016/s0166-2236(96)01001-6. [DOI] [PubMed] [Google Scholar]
- Flaherty AW, Graybiel AM. Corticostriatal transformations in the primate somatosensory system. Projections from physiologically mapped body-part representations. Journal of Neurophysiology. 1991;66:1249–1263. doi: 10.1152/jn.1991.66.4.1249. [DOI] [PubMed] [Google Scholar]
- Gabel LA, Nisenbaum ES. Biophysical characterization and functional consequences of a slowly inactivating potassium current in neostriatal neurons. Journal of Neurophysiology. 1998;79:1989–2002. doi: 10.1152/jn.1998.79.4.1989. [DOI] [PubMed] [Google Scholar]
- Hammond C, Crepel F. Evidence of a slowly inactivating K+ current in prefrontal cortical cells. European Journal of Neuroscience. 1992;4:1087–1092. doi: 10.1111/j.1460-9568.1992.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Houk JC. Information processing in modular circuits linking basal ganglia and cerebral cortex. In: Houk JC, Davies JL, Beiser DG, editors. Models of Information Processing in the Basal Ganglia. Cambridge, USA: MIT Press; 1995. pp. 3–9. [Google Scholar]
- Jaeger D, Kita H, Wilson CJ. Surround inhibition among projection neurons is weak or nonexistent in the rat neostriatum. Journal of Neurophysiology. 1994;72:2555–2558. doi: 10.1152/jn.1994.72.5.2555. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, North RA. Membrane properties and synaptic responses of rat striatal neurones in vitro. The Journal of Physiology. 1991;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H. Glutamatergic and GABAergic postsynaptic responses of striatal spiny neurons to intrastriatal and cortical stimulation recorded in slice preparations. Neuroscience. 1996;70:925–940. doi: 10.1016/0306-4522(95)00410-6. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Functional properties of a slowly inactivating potassium current in guinea pig dorsal lateral geniculate relay neurons. Journal of Neurophysiology. 1991;66:1176–1189. doi: 10.1152/jn.1991.66.4.1176. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RLM. The organization from the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Wilson CJ. Potassium current responsible for inward and outward rectification in rat neostriatal spiny projection neurons. Journal of Neuroscience. 1995;15:4449–4463. doi: 10.1523/JNEUROSCI.15-06-04449.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum ES, Xu ZC, Wilson CJ. Contribution of a slowly inactivating potassium current to the transition to firing of neostriatal spiny projection neurons. Journal of Neurophysiology. 1994;71:1174–1189. doi: 10.1152/jn.1994.71.3.1174. [DOI] [PubMed] [Google Scholar]
- Song WJ, Surmeier DJ. Voltage-dependent facilitation of calcium channels in rat neostriatal neurons. Journal of Neurophysiology. 1996;76:2290–2306. doi: 10.1152/jn.1996.76.4.2290. [DOI] [PubMed] [Google Scholar]
- Stern EA, Kincaid AE, Wilson CJ. Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. Journal of Neurophysiology. 1997;77:1697–1715. doi: 10.1152/jn.1997.77.4.1697. [DOI] [PubMed] [Google Scholar]
- Storm JF. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988;336:379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- Sugimori M, Preston RJ, Kitai ST. Response properties and electrical constants of caudate nucleus neurons in the cat. Journal of Neurophysiology. 1978;41:1662–1675. doi: 10.1152/jn.1978.41.6.1662. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Marder E, Abbott LF. Cellular short-term memory from a slow potassium conductance. Journal of Neurophysiology. 1996;75:963–966. doi: 10.1152/jn.1996.75.2.963. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Wilson CJ. Regulation of action-potential firing in spiny neurons of the rat neostriatum in vivo. Journal of Neurophysiology. 1998;79:2358–2364. doi: 10.1152/jn.1998.79.5.2358. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. The contribution of cortical neurons to the firing pattern of striatal spiny neurons. In: Houk JC, Davies JL, Beiser DG, editors. Models of Information Processing in the Basal Ganglia. Cambridge, USA: MIT Press; 1995. pp. 29–50. [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. Journal of Neuroscience. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS. Calcium- and activity-dependent synaptic plasticity. Current Opinion in Neurobiology. 1999;9:305–313. doi: 10.1016/s0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]


