Abstract
The effects of tetanus duration on the relaxation rate of extensor digitorum longus (EDL) and flexor digitorum brevis (FDB) muscles were studied in normal (wild-type, WT) and parvalbumin-deficient (PVKO) mice, at 20 °C.
In EDL of PVKO, the relaxation rate was low and unaffected by tetanus duration (< 3.2 s). In contrast, the relaxation rate of WT muscles decreased when tetanus duration increased from 0.2 to 3.2 s. In WT muscles, fast relaxation recovered as the rest interval increased.
Specific effect of parvalbumin was asserted by calculating the difference in relaxation rate between WT and PVKO muscles. For EDL, the rate constant of relaxation slowing was 1.10 s−1 of tetanization; the rate constant of relaxation recovery was 0.05 s−1 of rest.
In FDB, the effects of tetanus duration on WT and PVKO muscles were qualitatively similar to those observed in EDL.
Relaxation slowing as tetanus duration increases, reflects the progressive saturation of parvalbumin by Ca2+, while recovery as rest interval increases reflects the return to Ca2+-free parvalbumin.
At all tetanus durations, relaxation rate still remained slightly faster in WT muscles. This suggested that parvalbumin facilitates calcium traffic from myofibrils to the SR.
No difference was found between WT and PVKO muscles for: (i) the expression of the fast isoforms of myosin heavy chains, (ii) the force-velocity relationship and maximal shortening velocity and (iii) the Ca2+-activated ATPase activity from isolated preparations of the sarcoplasmic reticulum (SR).
The relaxation rate of fast skeletal muscles at the end of tetanic stimulation critically depends on tetanus duration. Rate is maximal after a very short tetanus (< 0.2 s) but is rapidly reduced as tetanus duration is prolonged to 2–3 s, while maximal isometric tension remains unaffected. This early fast decline of relaxation rate has been thoroughly studied in frog muscles but is also present in mouse muscles. It is to be distinguished from further slowing of relaxation, which appears as tetanization is prolonged, and is accompanied by force decline or ‘fatigue’, with numerous underlying biochemical changes (acidosis, increased concentration of Pi and lactate; Westerblad & Allen, 1994).
Fast skeletal muscles from lower vertebrates and from small mammals contain high concentrations (∼0.5 mm) of the cytosolic Ca2+-Mg2+ binding protein parvalbumin (PV) which presents a ∼104 higher affinity for Ca2+ over Mg2+. It has been estimated that, at rest, parvalbumin is essentially in the form of the Mg-PV complex, given the values of cytosolic [Ca2+] and [Mg2+] (Gillis et al. 1982; Maughan & Recchia, 1985). During stimulation, when the cytosolic Ca2+ concentration steeply increases, Ca2+ binding to PV is thought to occur progressively. However, as shown by computer simulation, the formation of the Ca-PV complex is limited by two conditions: (1) the Ca2+ binding rate to PV cannot occur faster than the much slower dissociation of Mg2+ from PV and (2) the Ca2+ binding capacity is limited by the PV concentration (Gillis et al. 1982). It has been proposed that the early slowing of relaxation, as tetanus duration increases, reflects the progressive saturation of PV by Ca2+ and that this saturation is rate limited by the kinetics of the Ca2+-Mg2+ exchange (Gillis et al. 1982; Woledge et al. 1985; Cannell, 1986). Considerable experimental evidence supports this saturation model in frog (Hou et al. 1991; Westerblad & Allen, 1996) and in rat (Garcia & Schneider, 1993; Carroll et al. 1997). Indeed, in frog muscles, the rate constants of relaxation slowing and of the Ca2+-Mg2+ exchange are remarkably similar, have the same temperature dependence and the resulting increase of [Mg2+]i occurs with the expected magnitude (Hou et al. 1991). For reviews of PV in skeletal muscle, see Gillis (1985) and Rall (1996).
This proposed role of PV in tetanus relaxation has been challenged in the case of mammalian muscles (Westerblad & Allen, 1994). The slowing of relaxation has been ascribed either to a reduced rate of Ca2+ dissociation from troponin or to a slowed cross-bridge turnover. PV loading by Ca2+ during tetanic contraction was not disputed but was seen as a non-causal, purely concomitant event, because the authors did not observe a correlation between the slowing of relaxation and the initial decline of [Ca2+]i in the flexor digitorum brevis (FDB) muscle of the mouse. In a more recent study on the rat FDB, however, a class of fibres was identified where the initial decline of [Ca2+]i at the end of the tetanus was markedly reduced as tetanus duration was increased (Carroll et al. 1997).
Recently, we developed a mouse strain where the parvalbumin gene had been knocked out (Schwaller et al. 1999) and reported a decrease of the relaxation rate of the twitch in vivo. Tetanus relaxation, however, was not investigated. PV-free muscles offer a unique opportunity to check if PV saturation does play an important role in the slowing of relaxation with increased tetanus duration.
We report here that, in two different fast muscles of PV-deficient mice, the relaxation rate was slow and unaffected by tetanus duration in the range of 0.2 to 2–3 s, while normal muscles showed a marked decrease. This direct comparison brings new evidence which strongly supports the role of PV saturation in slowing the relaxation rate of fast skeletal muscles in mammals. Moreover, the comparison also suggests that diffusion of Ca2+ from myofibrils to the sarcoplasmic reticulum could be facilitated by formation of the Ca-PV complex. A slowing of the relaxation rate for the longest duration of tetani occurred in both strains, thus was unrelated to the presence of parvalbumin.
Methods
Animals
Fourteen 105- to 159-day-old parvalbumin knockout (PVKO) mice (Schwaller et al. 1999) and 13 control C57Black6xSv129 mice (abbreviated hereafter WT), with genetically identical backgrounds were used. Animals were anaesthetized with an i.p. injection of a mixture of 10 ml kg−1 ketamine (10 mg ml−1) and xylazine (1 mg ml−1), in order to preserve muscle perfusion during dissection of both extensor digitorum longus (EDL) and flexor digitorum brevis muscles (FDB). From the latter, the head connected to the fourth toe was isolated. The depth of anaesthesia was assessed by the complete disappearance of the eyelid, corneal and pedal withdrawal reflexes. After dissection, the animals were killed by rapid neck dislocation. This protocol has been approved by the Animal Ethics Committee of the Faculty of Medicine of the University of Louvain.
Muscles were connected at one end to an electromagnetic puller, and at the other end to a force transducer (Maréchal & Beckers-Bleukx, 1998). They were bathed in a continuous flow of oxygenated Krebs solution (95 % O2- 5 % CO2) containing (mm): NaCl 118, NaHCO3 25, KCl 5, KH2PO4 1, CaCl2 2.5, MgSO4 1, glucose 5; maintained at a temperature of 20°C.
Electrical stimuli and isometric force were sampled at 2 kHz through a RTI-815 AD converter and digitized so that the temporal relationship between force and the last stimulus was obtained with an accuracy of 0.5 ms. Platinum electrodes were applied directly on the EDL. For the FDB, stimulation was delivered through electrodes running parallel to the muscles.
A few twitches and 0.2 s tetani were applied, in order to determine L0, the length at which isometric force was maximal. A 10 min rest was allowed, before starting the experimental protocols.
Protocols
The saturation protocol
Each muscle was stimulated to produce a series of tetani of different duration, each delivered at 10 min intervals. Tetanus durations were 0.2, 0.4, 0.8, 1.6, 3.2 and 6.4 s for EDL and 0.3, 0.5, 0.7, 1 and 2 s for FDB. The order of application was randomized. For each type of muscle, the shortest duration studied was imposed by the time needed to get fully developed isometric tension.
The desaturation protocol
This was only tested on EDL. Each muscle was stimulated first to produce a short 0.2 s tetanus to get the 100 % reference for the relaxation rate. Ten minutes later, the following protocol was applied: (i) a long, 3.2 s conditioning tetanus was delivered; (ii) this was followed by a short 0.2 s test tetanus, after one of the five following recovery intervals: 0.2, 0.8, 3.2, 12.8 and 51.2 s, chosen at random. This protocol was repeated four times, separated by 10 min rest, so that each recovery interval was studied on each muscle (this rest interval allowed full recovery of mechanical measurements). Different muscles were used in the saturation and recovery protocols.
The force-velocity relationship of EDL was investigated by the isovelocity method (Maréchal & Beckers-Bleukx, 1998) and the maximal speed of unloaded contraction by the method of Edman (Edman, 1979). In the latter, muscles are released during a well-fused tetanus at a velocity much higher than the maximal velocity of shortening. Then force abruptly falls to zero and rises again after a short delay. The maximal velocity (V0) of unloaded shortening is derived from the slope of the relationship of imposed extent of shortening (ΔL) versus measured delay (Δt).
Myosin heavy chain (MHC) composition
Crude myosin extraction was made from muscles powdered under liquid N2 and isoforms were separated by one-dimensional electrophoresis on 7 % polyacrylamide gels containing 0.1 % SDS as described before (Hämäläinen & Pette, 1996), after extraction in 100 mm pyrophosphate. Gels were silver stained and evaluated by integrating densitometry. Results were expressed as a percentage of the total MHC content.
Parvalbumin content
Two-dimensional electrophoresis (O'Farrell, 1975) was used to isolate PV and myosin light chains (MLC). Isoelectric focusing was obtained with a mixture of ampholines: pH 4–6, 5–8 and 3.5–10. The second dimension was run on 15 % polyacrylamide gels (Fig. 1). Coomasie stained PV spots were measured by integrating densitometry and expressed relative to the sum of the integrated densities of all MLC. The PV/MLC ratios were 1.04 in EDL and 0.71 in FDB (Table 1). PV was undetectable in either muscle from PVKO mice. For the batch of animals used here, it was checked again that the parvalbumin gene was absent and replaced by a neomycin-resistance gene as originally shown in the description of the PVKO mouse by Schwaller et al. (1999).
Figure 1. Parvalbumin identification on 2D-gel electrophoresis of EDL extracts (WT).
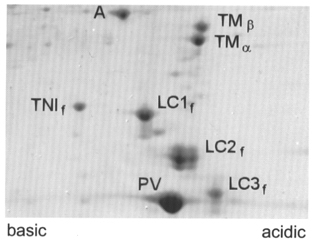
A, actin; TMα and TMβ, isoforms of tropomyosin; LC1–3f, subtypes of fast myosin light chains (MLC); TNIf, fast troponin I; PV, parvalbumin.
Table 1.
Wild-type myosin heavy chain composition and parvalbumin content
| Myosin heavy chain isoforms (% of total) | EDL | FDB |
|---|---|---|
| MHC 1 | 0 | 3.6 ± 4.2 |
| MHC 2a | traces | 60.0 ± 17.8 |
| MHC 2d* | 12.0 ± 4.1 | 34.0 ± 17.0 |
| MHC 2b | 87.8 ± 3.8 | 2.4 ± 0.6 |
| Parvalbumin content (% of MLC) | 104 ± 4.5 | 71 ± 5.0 |
| mg (g wet weight)−1 | 4.86 ± 1.25† | 3.31‡ |
| (n = 5) | (n = 9) |
Values were measured in extensor digitorum longus (EDL) and flexor digitorum brevis (FDB) muscles of the wildtype C57 mouse and are expressed as means ± s.d.
also designated MHC 2x; MLC, myosin light chains
from Berquin & Lebacq (1992), this corresponds to a myoplasmic concentration of 0.59 mmol (l water)−1
calculated from the percentage of MLC.
Ca2+-activated ATPase activity of isolated SR preparations
This was estimated following the procedure of Simonides & van Hardeveld (1990). In short, unfractionated muscle homogenates were studied in conditions that give maximal SR-Ca2+-activated ATPase activity, while eliminating myofibrillar and background ATPase activities. Enzyme activity was determined using the coupled pyruvate kinase-lactate dehydrogenase assay. The reaction was started by adding ATP, in the presence of 10 μM Ca2+ (stabilized by the EGTA/Ca-EGTA buffer), and of the ionophore A23187 to prevent intravesicular Ca2+ accumulation. Results are expressed as nanomoles of ATP hydolysed per minute per milligram wet weight.
Statistical analysis
Two-way analysis of variance (with correction for repeated measures) was used to assess the significance of the effect of tetanus duration, recovery interval and of muscle origin (WT or PVKO mice) on the kinetic parameters of relaxation. This analysis provided the standard errors of least-square means indicated as bars in Figs 5A and C and 7A. For point-to-point comparisons, we used the Tukey test. Analysis of the WT vs. PVKO difference in MHC composition and Ca2+-ATPase activity of SR was assessed by Student's unpaired t tests. The significance level was set at 0.05.
Figure 5. EDL and FDB relaxation rate, after tetanus of increasing duration.
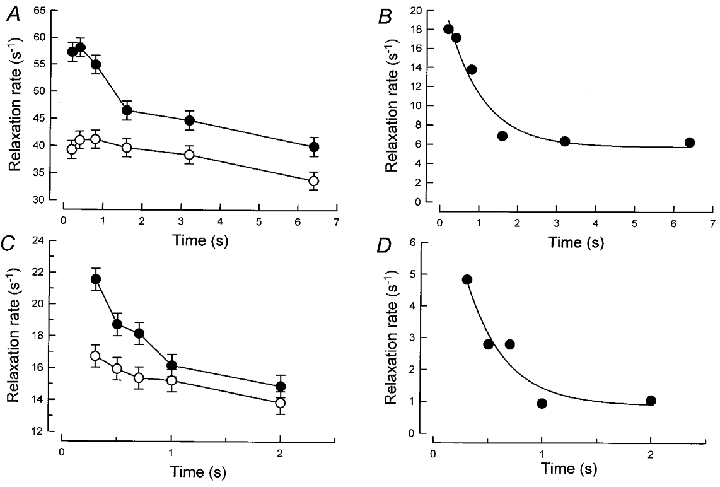
A and C, relaxation rate and tetanus duration. A, EDL; C, FDB. The rates are expressed as 1/(t20–5) for EDL and 1/t5 for FDB. •, WT; ○, PVKO. B and D, difference in relaxation rate (WT – PVKO). B, EDL; D, FDB. The difference was calculated from the data of Fig. 5A and C, respectively.
Figure 7. Recovery protocol on EDL muscle.
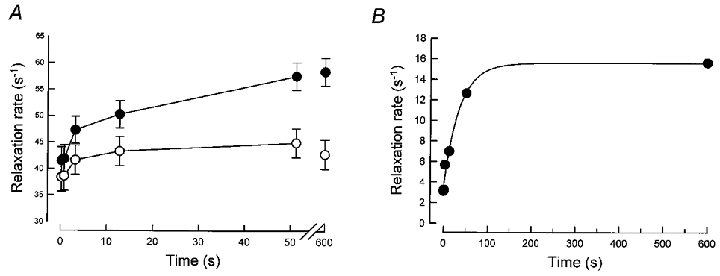
A, recovery of the relaxation rate (expressed as 1/(t20 – 5)) from a 0.2 s test tetanus after increasing recovery intervals after a long conditioning tetanus (see text). •, WT; ○, PVKO. B, difference in relaxation rate (WT – PVKO) calculated from the data of Fig. 7A.
Results
Preservation of the fast phenotype in fast muscles of the PVKO mice
Inactivation of a given gene might induce unexpected side effects in addition to the lack of the protein coded by the inactivated gene. We have already shown that inactivation of the parvalbumin gene in fast muscles preserved the presence of the fast isoforms of myosin heavy chains (MHC), troponin T and troponin I (Schwaller et al. 1999). However, to further document this important point we studied in fast muscles from PVKO mice: (i) the relative expression of the different fast isoforms of MHC, (ii) the kinetic parameters of the contraction which reflect the kinetics of the cross-bridges cycle and (iii) the rate of the Ca2+-activated ATPase activity of the sarcoplasmic reticulum (SR), as these three parameters are the major determinants of the rate of relaxation.
Isoforms of myosin heavy chains
Figure 2 shows an electrophoretic separation of the various MHC isoforms in tibialis anterior muscle, a typical fast muscle, from WT and PVKO mice. The slower isoforms MHC 1 and MHC 2a were undetected, and the relative proportions of the faster MHC 2 (d and b) were unchanged. Quantitative densitometry of gels from three different animals gave the following percentages: MHC 2d: 35.4, 36.3, 35.4 and 32.7, 39.3, 49; MHC 2b: 64.6, 63.7, 64.6 and 67.3, 60.7, 51.0, for PVKO and WT, respectively, with no significant differences between the two mouse lines. Similarly, we detected no significant changes in the MHC proportions in other fast muscles, gastrocnemius and psoas (not shown) and in the slow soleus muscle (gels of the latter are given in Fig. 2 to illustrate the separation of the four different MHCs).
Figure 2. Examples of SDS-polyacrylamide gel electrophoresis of myosin heavy chains (MHC).
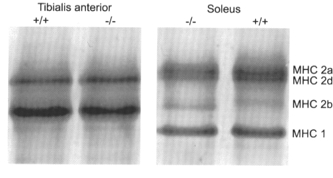
Myosin extracted from tibialis anterior and soleus muscles from WT (+/+) and PVKO(−/−) mice. Separation of the various MHC isoforms: 1, 2a, 2d and 2b. Silver staining.
Kinetics of the contractile response
In a previous series of experiments, isometric twitch and tetanus of PVKO were examined (Schwaller et al. 1999). Maximal tetanus tension (P0) was unchanged, but in unfused, low frequency tetani, the mean tension obtained was increased. Twitch tension and time course were both increased. The two last effects reflected the prolongation of the muscles' active state, as parvalbumin-deficient muscles have lost a fast way to reduce the cytosolic Ca2+ concentration. These studies did not test the proper kinetic characteristics of the contractile apparatus itself.
To evaluate possible differences in these characteristics, the complete force-velocity (F-V) relationship was determined and the maximal shortening velocity in unloaded conditions was obtained using the method of Edman (Edman, 1979). As seen in Fig. 3A, the F–V relationship of PVKO EDL was indistinguishable from that of normal muscle, while Fig. 3B shows that the maximal unloaded velocity, V0, (studied over a large range of shortenings) was also normal: 9.5 and 9.1 fibre lengths s−1 for WT and PVKO, respectively. Thus, neither test indicated the slightest difference in the kinetic parameters of the contractile apparatus, in agreement with the above report that myosin heavy chain composition was not altered in PVKO muscles.
Figure 3. Shortening velocities of EDL from WT and PVKO mice.
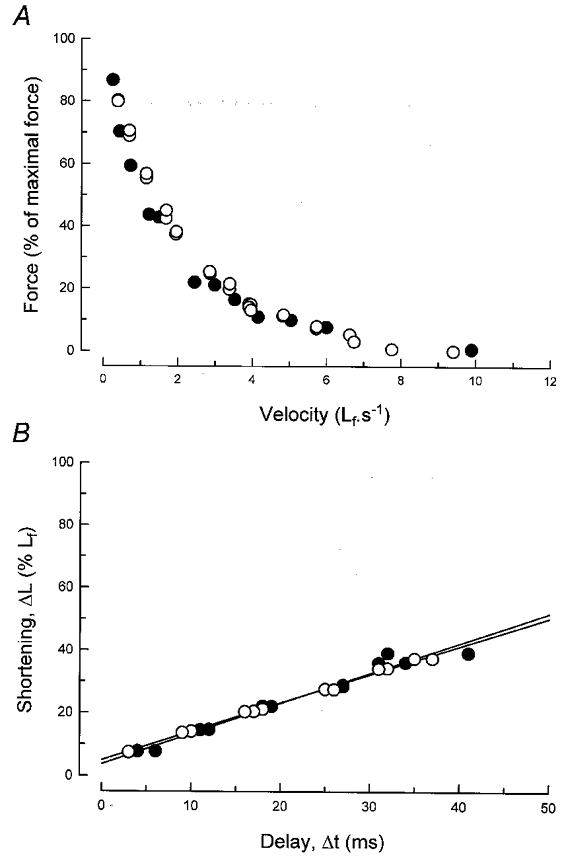
A, force-velocity relationship (velocity is given in fibre lengths s−1). B, Edman's slack test. ΔL, extent of rapid shortening; Δt, delay between rapid shortening and redevelopment of tension. Lines, calculated regression lines. •, WT; ○, PVKO.
Activity of the Ca2+-activated ATPase of the sarcoplasmic reticulum
This activity was studied in four different fast muscles and the results are collected in Table 2. It is clear that no significant changes of the Ca2+-ATPase activity were detected between muscles from WT and PVKO mice. It can thus be excluded that the slowing of relaxation reported for parvalbumin-deficient muscles (Schwaller et al. (1999), and this paper), could be attributed to changes in the Ca2+ pumping rate by the SR.
Table 2.
Ca2+-ATPase activity
| SR Ca2+ATPase activity (nmol P1 min−1 (mg wet weight)−1) | WT | PVKO |
|---|---|---|
| Gastrocnemius | 168 ± 11 | 153 ± 27 |
| Extensor digitorum longus | 201 ± 30 | 236 ± 22 |
| Psoas | 227 ± 46 | 236 ± 24 |
| Tibialis anterior | 240 ± 50 | 207 ± 36 |
| (n = 3) | (n = 3) |
Crude SR fractions from four different mouse fast muscles were used. Values are expressed as means ± s.d.
Effects of tetanus duration on the kinetics of relaxation of the mouse EDL; application of the saturation protocol
Representative records of Fig. 4A and B show the time course of relaxation for various tetanus durations in WT (A) and PVKO (B) EDL muscles. Though in WT muscles, the early phase of relaxation slowed down significantly when tetanus duration was increased from 0.2 to 3.2 s, it is remarkably constant in PVKO muscles, for the first 52 ms after the last stimulus, in the example illustrated. Moreover, even for the shortest duration (0.2 s), this early phase of relaxation of the PVKO muscle is still slower than that of the WT muscle after 3.2 s of stimulation.
Figure 4. Superimposed records of tetanic relaxation of EDL after three durations of tetanization (0.2, 1.6 and 3.2 s).
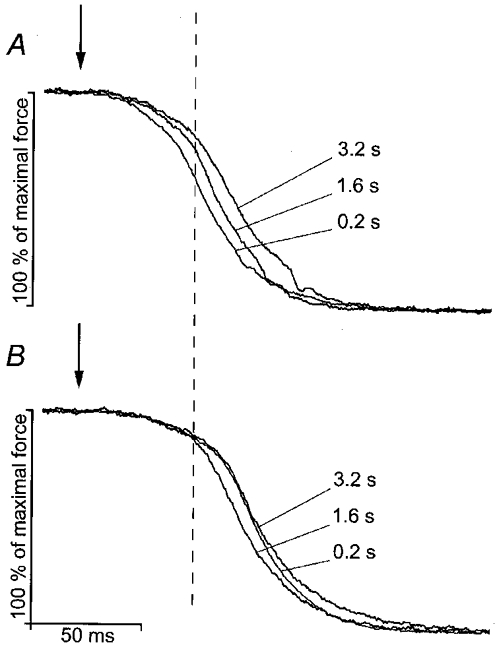
Force expressed as percentage of maximal tetanic force. The dashed vertical line is placed 52 ms after the last stimulus (vertical arrows). A, WT; B,PVKO.
It is customary to quantify the kinetics of relaxation by the time elapsed between the last stimulus (at t0) and a given percentage of tension decrease: e.g. t5, t20 and t50 which correspond to 5, 20 and 50 % of tension decay, respectively. The values of t5, t20 and t50, as tetanus duration was increased from 0.2 s to 6.4 s, are given in Table 3A for the EDL of parvalbumin-deficient mice (PVKO) and the wild-type control (WT). First, the table shows that time intervals t5, t20 and t50 are all larger in PVKO than in WT; these results, obtained after tetanus, extend previous observations that twitch relaxation was increased in mice lacking PV (Schwaller et al. 1999).
Table 3.
Relaxation times after tetanus of increasing duration
| A, EDL | WT | PVKO | ||||
|---|---|---|---|---|---|---|
| tetanus duration (s) | t5 (ms) | t20 (ms) | t50 (ms) | t5 (ms) | t20 (ms) | t50 (ms) |
| 0.2 | 22.7 ± 1.0 | 40.4 ± 1.7 | 54.7 ± 1.3 | 34.6 ± 1.1 | 60.1 ± 0.9 | 76.8 ± 1.3 |
| 0.4 | 24.0 ± 0.7 | 41.4 ± 0.9 | 55.3 ± 1.3 | 34.0 ± 1.1 | 58.6 ± 1.0 | 3.8 ± 1.3 |
| 0.8 | 24.6 ± 0.6 | 42.9 ± 0.9 | 57.1 ± 1.2 | 35.5 ± 0.9 | 60.1 ± 1.0 | 75.1 ± 1.2 |
| 1.6 | 24.4 ± 0.6 | 46.1 ± 1.1 | 59.9 ± 1.6 | 36.5 ± 0.9 | 61.9 ± 1.5 | 77.9 ± 1.6 |
| 3.2 | 27.3 ± 0.8 | 49.9 ± 1.6 | 66.1 ± 2.4 | 37.9 ± 1.6 | 64.0 ± 1.8 | 82.5 ± 2.4 |
| 6.4 | 25.3 ± 1.5 | 50.4 ± 2.2 | 70.1 ± 2.4 | 38.8 ± 1.3 | 68.6 ± 1.6 | 94.1 ± 2.4 |
| (n = 7) | (n = 8) | |||||
| B, FDB | WT | PVKO | ||||
|---|---|---|---|---|---|---|
| tetanus duration (s) | t5 (ms) | t20 (ms) | t50 (ms) | t5 (ms) | t20 (ms) | t50 (ms) |
| 0.3 | 46.6 ± 1.2 | 64.7 ± 1.7 | 79.4 ± 1.8 | 61.0 ± 3.0 | 81.6 ± 1.5 | 95.3 ± 1.6 |
| 0.5 | 54.0 ± 2.2 | 72.7 ± 1.5 | 85.7 ± 1.8 | 63.7 ± 2.8 | 84.1 ± 1.1 | 98.6 ± 1.4 |
| 0.7 | 55.6 ± 2.1 | 76.7 ± 1.9 | 90.7 ± 2.0 | 66.0 ± 2.7 | 85.6 ± 1.0 | 100.1 ± 1.4 |
| 1.0 | 62.1 ± 1.7 | 79.1 ± 1.9 | 93.7 ± 2.0 | 66.6 ± 2.6 | 86.7 ± 1.3 | 102.7 ± 1.5 |
| 2.0 | 67.6 ± 2.0 | 83.6 ± 2.4 | 99.9 ± 2.4 | 72.4 ± 1.5 | 92.1 ± 1.5 | 108.7 ± 1.9 |
| (n = 7) | (n = 7) | |||||
EDL and FDB values are directly calculated from the time after last stimulus, taken as t0. Values are expressed as means ± s.e.m.
On single fibres of frog muscles, tension declines linearly for the first 20–25 % drop, while sarcomere length remains constant. This is followed by an acceleration of the tension drop (marked by a ‘shoulder’ in the tension record) and the appearance of sarcomere inhomogeneities (Huxley & Simmons, 1970). Thus, studies of relaxation kinetics should be focused on the early phase, free from possible interferences due to sarcomere disorders. In whole muscle, however, the time course of relaxation is smoothed and is no longer clearly divided into two phases. In this case, the measurement of t20 seems appropriate. However, t20 includes t5 which has a complex origin: the time of full contractile activity elicited by the last stimulus, followed by the very beginning of relaxation. Hou et al. (1991, 1992) proposed to measure the early phase of relaxation as the t20–t5, as this phase corresponds to the largest decline of the Ca2+ transient. Here, when the proper time increments corresponding to the t5–t0, t20–t5 and t50–t20 intervals are calculated, it is evident that the t20–t5 interval is, in relative terms (e.g. percentage change), the parameter most affected by the increase of tetanus duration in WT, as also observed in frog fibres (Hou et al. 1993). Therefore, our study of the influence of parvalbumin on tetanus relaxation will be focused on the t20–t5 interval, in the case of EDL. This will facilitate comparisons with results published by other groups.
The rates of relaxation, expressed as 1/(t20−t5) ratios, are given in Fig. 5A for the six tetanus durations studied (see the saturation protocol). Three main effects are illustrated: (i) there was a sharp decrease of the relaxation rate for the first 2 s of tetanization in WT (difference between 0.2 and 2 s significance: P < 0.001), while this rate remained constant in PVKO during this time (P = 0.7); (ii) between 2 and 6.4 s duration, both preparations showed a small but progressive decrease of relaxation rates, more or less parallel to each other; (iii) values of PVKO always remained smaller than WT values (P < 0.001) and the two sets of values did not merge even after a long tetanus. Thus, the first decline depends on both the presence of parvalbumin and on tetanus duration, the second depends on tetanus duration but not on the presence of parvalbumin and the third effect is related to the presence of parvalbumin, but seems independent of tetanus duration.
Specific effect of parvalbumin on tetanus relaxation
From the data represented in Fig. 5A, it was possible to deduce the specific effect of parvalbumin on the kinetics of relaxation in tetani of various durations. From each value of WT, the corresponding value of PVKO was subtracted (calculated from the mean values plotted in Fig. 5A). This resulted in the graph shown in Fig. 5B. Three important points are illustrated in this figure: first, it is seen that increasing tetanus duration reduces the relaxation rate (defined as 1/(t20–t5)) from about 18 to 6 s−1, i.e. to about 1/3 of the value observed for the shortest duration tested. The decline progressed with an apparent rate constant of 1.10 s−1 of tetanization, and a plateau was reached after about 1.6 s. Second, it is clear that, even after a long tetanus (> 3.2 s), the relaxation rate of WT muscle was still higher than for PVKO; as shown, about 1/3 of the specific effect of parvalbumin seems independent of tetanus duration. This is a new and unexpected observation that could only be obtained by taking the difference between WT and PVKO. In previous work with WT muscles, relaxation rates of tetanus of increasing durations were expressed relative to the relaxation of a long, saturating tetanus of the same WT muscle. As expected using this comparison, the ratio eventually reached 1.0 and an effect of parvalbumin, independent of tetanus duration, could not be observed.
Effects of tetanus duration on the kinetics of relaxation of the mouse FDB
A high content of parvalbumin is classically associated with fast-glycolytic muscles, like EDL, containing a high proportion (> 80 %) of the ‘fastest’ myosin heavy chain isoform MHC 2b. However, FDB, which has a mixed MHC composition where MHC 2a predominates, is also rich in parvalbumin (Table 1). Tension rise and relaxation are slower in FDB, as shown in Fig. 6, and t5 of FDB exceeds t20 of EDL. Figure 6 also shows that 0.3 s of tetanization was needed to reach maximal isometric tension in FDB; this is why shorter durations were not investigated. FDB offered another opportunity to study how parvalbumin affected relaxation after tetanus of increasing duration, in a muscle presenting a slower contraction-relaxation time course. A priori, one could hypothesize that the kinetics of Ca2+-Mg2+ exchange on parvalbumin would be independent from MHC composition and thus from the intrinsic speed of the actin-myosin interaction. Analysis of the EDL relaxation (see Table 3A)showed that the saturation of parvalbumin affected preferentially the t20–t5 interval, which ended about 50 ms after the last stimulus. As seen in Table 3B, these first 50 ms are still included in the t5 time in FDB. Indeed, it is clear from the data of Table 3B that the effect of increasing tetanus duration in WT was a prolongation of the t5 interval. The increase of t5 in WT FDB, when tetanus duration increased from 0.3 to 2 s, is in contrast with its almost constant values of t5 in PVKO FDB. Figure 5C gives the rate of relaxation (expressed as 1/t5). Qualitatively, the three effects of tetanus durations observed in WT and PVKO EDL were also present in WT and PVKO FDB, but with different time scales. An initial sharp decrease which is only seen in WT (P < 0.001) was followed by a slight decrease so that the differences between 0.3 and 2 s were significant in both WT and PVKO (P < 0.001 and < 0.05, respectively). For values smaller than 1 s of tetanization, the difference between WT and PVKO values were highly significant (P < 0.001); later, the PVKO values were always below the WT ones, but the small difference did not reach the significance level.
Figure 6. Comparison of EDL and FDB contraction and relaxation time course.
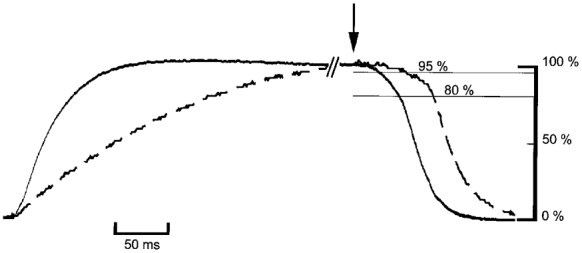
Superimposed traces of EDL (continuous) and FDB (interrupted) isometric force for a 0.3 s tetanus (125 Hz). Traces were adjusted for maximal isometric force, taken as 100 %. Selected part of the records showing tension rises and tension relaxations. The arrow represents the last electrical stimulus.
As shown above, the specific effect of parvalbumin was obtained by the point-to-point difference of the graphs of Fig. 5C and is illustrated in Fig. 5D. The relaxation rate declined from 4.8 to 1 s−1 after about 1 s of tetanization, with an apparent rate constant of 2.7 s−1 of tetanization. The effect of the presence of parvalbumin which seems independent of tetanus duration was present in FDB, but was relatively smaller than in EDL.
Recovery of relaxation rate after a long conditioning tetanus in EDL; application of the recovery protocol
After a 3.2 s conditioning tetanus, the relaxation rate of a short (0.2 s) test tetanus was measured after predetermined recovery intervals. The 1/(t20–t5) values were calculated for these test tetani and are plotted in Fig. 7A. In WT, recovery occurred in two phases: a fast one where about 30 % recovery is achieved within approximately 3 s, and a much slower one which takes about 50 s for completion (values at 50 s are not different from that of a 0.2 s tetanus after 10 min of rest). In PVKO, no significant differences occurred between each relaxation rate compared with the reference (an isolated 0.2 s tetanus). However, there was an indication (yet not significant) that the first fast phase was also present. Relaxation rates returned to 100–110 % of the reference value (equivalent to the 0.2 s points of Fig. 5A, obtained from muscles of different animals). The kinetics of the specific recovery of the parvalbumin effect were, as before, computed from the point-to-point differences between the graphs of Fig. 7A and are given in Fig. 7B. The recovery of the parvalbumin-dependent relaxation has an apparent rate constant of 0.05 s−1 of rest.
Discussion
In this paper, we report how the presence of parvalbumin, a cytosolic Ca2+ binding protein, affects the rate of relaxation in muscles submitted to tetanus stimulations of increasing duration. The study is based on the comparison of muscles (EDL and FDB) coming from either normal (wild-type) mice, thus with a high parvalbumin content, or from genetically modified mice where the parvalbumin gene had been inactivated. This modification does not significantly alter the fast phenotype of the muscles. The major determinants of the rate of relaxation, MHC composition, cross-bridge kinetics and Ca2+ pumping rate by the SR, were all found to be unaffected by the inactivation of the parvalbumin gene. The comparison of WT versus PVKO thus offered far better conditions to study the role of parvalbumin in muscle relaxation than by comparing muscles in which different parvalbumin contents occur together with different myosin isoform compositions, e.g. the comparison of EDL versus soleus.
We first confirmed previous studies that in two mouse muscles, namely EDL and FDB, increased tetanus duration slowed down the rate of relaxation until an approximately constant value was obtained (Berquin & Lebacq, 1992; Westerblad & Allen, 1994). Similar results have been observed in fast frog muscles (Hou et al. 1991). Here a stable value for relaxation was obtained after 1.6 s of tetanic stimulation (125 Hz) of the EDL, and somewhat earlier (∼1 s) in FDB. It is remarkable that while the relaxation process is clearly slower in FDB than in EDL (due to different myosin isoform composition), the effect on relaxation with increasing tetanus duration concerned essentially the same first 50 ms period after the last stimulus, affecting the t20–t5 interval in EDL and mainly the t5–t0 interval in FDB.
In contrast to normal muscles, parvalbumin-deficient muscles (EDL and FDB) display a slow relaxation rate, even after the shortest tetanus duration studied, and this rate remained essentially constant over the duration range where slowing of relaxation was observed in normal muscles. For tetanus duration longer than 3 s, relaxation rate and isometric tension (not shown) slowly decreased in a similar way, in EDL from both WT and PVKO mice (Fig. 5A). This late effect on relaxation was thus unrelated to the parvalbumin presence and probably results from other, yet unidentified, effects which, according to Berquin & Lebacq (1992), become significant after 2 s (see also Westerblad & Allen, 1994).
The present results fully support, at least qualitatively, the saturation model where, as tetanus duration is prolonged, progressive Ca2+ binding to parvalbumin diminishes its ability to contribute to Ca2+ removal from the cytosol and thus to promote fast relaxation. The simplest form of the PV saturation model supposes that the rate constant of relaxation slowing cannot be faster than the rate constant of Mg2+ dissociation from PV (as subsequent Ca2+ binding is very fast). As the rate constant of Mg2+ dissociation has not been determined for mouse PV, this hypothesis could not be tested in this study, but in frog muscles, Rall and his collaborators (Hou et al. 1991, 1992) have shown that, at 0°C, the rate constants of relaxation slowing and of Mg2+ dissociation from PV were almost identical (1.18 vs. 0.93 s−1, respectively). At 10°C however, some differences seemed to appear: 2.96 vs. 1.76 s−1; thus, in frog, at higher temperatures, the slowing of relaxation appeared to advance with a faster rate than the PV saturation. Most probably, at a higher temperature, the effect of tetanus duration on the relaxation rate is more complex: parvalbumin saturation will depend not only on the effect of temperature on the Mg2+ dissociation constant, but also on the rate at which cytosolic Ca2+ is actively removed by the SR, together with the contribution of other factor(s) with a high Q10 (H+ and Pi accumulation, change of the free energy available from ATP) which tends to slow down relaxation.
This emphasizes the importance of comparing WT versus PVKO, by a point-to-point subtraction, which allows us to isolate the specific effect of parvalbumin, in otherwise identical experimental conditions, information that previous studies were not able to provide. With this approach, it is assumed that the rate constant of the relaxation slowing is an indirect measurement of the rate-limiting reaction in parvalbumin saturation i.e. the rate constant of Mg2+ dissociation. Here, for the EDL (20°C), this was calculated to be 1.10 s−1 (see Fig. 5B). Direct measurements of the rate constant of Mg2+ dissociation for frog parvalbumin is around 3 s−1 at 20°C (Hou et al. 1992), but no value for mouse parvalbumin is available so far.
The tetanus duration necessary to eliminate the contribution of parvalbumin to relaxation is expected to increase with the PV concentration. This duration was about 1 s in FDB and 1.5 s in EDL, in agreement with the fact that the average parvalbumin content of EDL muscle was about 1.4 times higher. Similarly, the initial fast removal of Ca2+ levelled off after 0.5 s of stimulation in the rat FDB (Carroll et al. 1997) in accordance with the fact that the PV content of rat fast muscles is about one-half of that of the mouse (Heizmann et al. 1982).
The recovery protocol is the counterpart of the saturation protocol. Progressive dissociation of the PV-Ca complex as Ca2+ is re-accumulated actively in the SR during the recovery interval, regenerates the pool of the PV-Mg complex and therefore allows fast relaxation again. Thus the recovery kinetics depend both on the rate constant of Ca2+ dissociation from PV and on the turnover rate of the Ca2+-ATPase of the SR. However, during the recovery intervals, other effects of tetanic stimulation are also progressively reversed and this too may contribute to the recovery of the original fast relaxation. Here again, the point-to-point subtraction of PVKO results from those of WT allows us to determine the specific effect of PV recovery on the return to a fast relaxation (Fig. 7B). This procedure yielded a rate constant of 0.05 s−1. This value is close to the rate constant of 0.12 s−1 calculated for the recovery of the 1/(t20–t5), in frog muscles at 0°C (Hou et al. 1991). This suggests that Ca2+ dissociation from mouse PV is either genuinely slower or shows only a small temperature dependence.
The WT versus PVKO comparisons (Fig. 5B and D) have revealed an effect of parvalbumin on relaxation which was not expected from the ‘saturation-recovery model’. We observed that in all conditions, the relaxation rates of muscles from PVKO were always slower than from WT. Relaxation rates of WT and PVKO did not merge, even after long tetani when PV was supposed to be Ca2+-saturated. This suggests that calcium removal from the cytosol was accelerated by the mere presence of parvalbumin. We previously proposed that parvalbumin could act as a ‘shuttle’, transporting calcium from myofibrils to the sarcoplasmic reticulum (Gillis & Gerday, 1977; Gillis et al. 1979), a model inspired by the role of myoglobin in accelerating oxygen diffusion from capillaries to muscle mitochondria (Wittenberg, 1970). Parvalbumin was seen dynamically as a diffusible Ca2+ buffer ‘commuting’ between two immobile Ca2+-binding sites: troponin C on myofibrils and the Ca2+-ATPase of the sarcoplasmic reticulum (calcium uptake within the latter imposing the direction of the calcium flux). The kinetics of calcium traffic in situations involving both diffusible and immobile calcium buffers or binding sites have been studied by computer simulations to mimic the intracellular [Ca2+] response to a short Ca2+ pulse (Nowycky & Pinter, 1993). It turned out that, in the absence of diffusible buffers, the presence of fixed Ca2+ binding sites greatly retards calcium diffusion and that, once the Ca2+ pulse is over, these binding sites act as a source of calcium which prolongs the time when Ca2+ is elevated in their close surroundings. On the contrary, the presence of competing diffusible buffers speeds up calcium diffusion (in a bound state). Similar conclusions were reached after modelling the facilitated diffusion of calcium by the intestinal calcium binding protein in aiding calcium entry at the luminal side and calcium exit at the serosal side of enterocytes (Kretsinger et al. 1982). We thus propose that the increase of relaxation rate in the presence of parvalbumin, irrespective of tetanus duration, reflects its effect in speeding up calcium traffic between myofibrils and the SR, an effect which may be further enhanced if parvalbumin interacts with the SR and stimulates calcium uptake as proposed by Ushio & Watabe (1994). Most probably, such calcium traffic, in a parvalbumin-bound state, would largely escape detection by the usual calcium indicators and it would be rate limited by the value of the diffusion coefficient of parvalbumin. Indeed, direct measurements of the diffusion coefficient of 45Ca2+ injected into frog fibres (thus containing PV) (Kushmerick & Podolsky, 1969) and of parvalbumin are identical (Maughan & Godt, 1999).
In summary, the comparison between parvalbumin-deficient muscles and the corresponding normal, parvalbumin-containing muscles has demonstrated the specific contribution of parvalbumin in accelerating the rate of tetanus relaxation of fast mouse muscles. This contribution seems adequately described by the combination of the ‘saturation-recovery model’ together with the ‘shuttle model’. The physiological advantage conferred by an elevated parvalbumin content is to provide very fast relaxation for very short bursts of activity.
Acknowledgments
The authors wish to thank G. Maréchal for critical discussions and F. Uytterhoeven for skilled technical assistance. J.M.R is a research fellow and P.G is a research associate of the FNRS (Belgium). This work was supported by the Programme of Concerted Research Action, Belgium (no. 95/00–188) and by the Swiss National Science Foundation (grant 3100–047291.96 to M.R.C.).
References
- Berquin A, Lebacq J. Parvalbumin, labile heat and slowing of relaxation in mouse soleus and extensor digitorum longus muscles. The Journal of Physiology. 1992;445:601–616. doi: 10.1113/jphysiol.1992.sp018942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell MB. Effect of tetanus duration on the free calcium during the relaxation of frog skeletal muscle fibres. Journal of Physiology. 1986;376:203–218. doi: 10.1113/jphysiol.1986.sp016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SL, Klein MG, Schneider MF. Decay of calcium transients after electrical stimulation in rat fast- and slow-twitch skeletal muscle fibres. The Journal of Physiology. 1997;501:573–588. doi: 10.1111/j.1469-7793.1997.573bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KA. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. The Journal of Physiology. 1979;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Schneider MF. Calcium transients and calcium release in rat fast-twitch skeletal muscle fibres. The Journal of Physiology. 1993;463:709–728. doi: 10.1113/jphysiol.1993.sp019618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis JM. Relaxation of vertebrate skeletal muscle. A synthesis of the biochemical and physiological approaches. Biochimica et Biophysica Acta. 1985;811:97–145. doi: 10.1016/0304-4173(85)90016-3. [DOI] [PubMed] [Google Scholar]
- Gillis JM, Gerday C. Calcium movements between myofibrils, parvalbumins and sarcoplasmic reticulum in muscle. In: Wasserman RM, Corradino RA, Carafoli E, Kretsinger RH, MacLennan DH, Siegel FL, editors. Calcium-Binding Proteins and Calcium Functions. New York: North Holland; 1977. pp. 193–196. [Google Scholar]
- Gillis JM, Piront A, Gosselin-Rey C. Parvalbumins. Distribution and physical state inside the muscle cell. Biochimica et Biophysica Acta. 1979;585:444–450. doi: 10.1016/0304-4165(79)90089-8. [DOI] [PubMed] [Google Scholar]
- Gillis JM, Thomason D, Lefèvre J, Kretsinger RH. Parvalbumins and muscle relaxation: a computer simulation study. Journal of Muscle Research and Cell Motility. 1982;3:377–398. doi: 10.1007/BF00712090. [DOI] [PubMed] [Google Scholar]
- Hämäläinen N, Pette D. Slow-to-fast transitions in myosin expression of rat soleus muscle by phasic high-frequency stimulation. FEBS Letters. 1996;399:220–222. doi: 10.1016/s0014-5793(96)01325-7. [DOI] [PubMed] [Google Scholar]
- Heizmann CW, Berchtold MW, Rowlerson AM. Correlation of parvalbumin concentration with relaxation speed in mammalian muscles. Proceedings of the National Academy of Sciences of the USA. 1982;79:7243–7247. doi: 10.1073/pnas.79.23.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou TT, Johnson JD, Rall JA. Parvalbumin content and Ca2+ and Mg2+ dissociation rates correlated with changes in relaxation rate of frog muscle fibres. The Journal of Physiology. 1991;441:285–304. doi: 10.1113/jphysiol.1991.sp018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou TT, Johnson JD, Rall JA. Effect of temperature on relaxation rate and Ca2+, Mg2+ dissociation rates from parvalbumin of frog muscle fibres. The Journal of Physiology. 1992;449:399–410. doi: 10.1113/jphysiol.1992.sp019092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou TT, Johnson JD, Rall JA. Role of parvalbumin in relaxation of frog skeletal muscle. Advances in Experimental Medicine and Biology. 1993;332:141–151. doi: 10.1007/978-1-4615-2872-2_13. [DOI] [PubMed] [Google Scholar]
- Huxley AF, Simmons RM. Rapid ‘give’ and the tension ‘shoulder’ in the relaxation of frog muscle fibres. The Journal of Physiology. 1970;210:32–33P. [PubMed] [Google Scholar]
- Kretsinger R, Mann J, Simmonds J. Model of facilitated diffusion of calcium by the intestinal calcium binding protein. In: Norman A, editor. Proceedings of the Fifth Workshop on Vitamin D. New York: Walter de Gruyter & Co.; 1982. pp. 233–248. [Google Scholar]
- Kushmerick MJ, Podolsky RJ. Ionic mobility in muscle cells. Science. 1969;166:1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Maréchal G, Beckers-Bleukx G. Effect of nitric oxide on the maximal velocity of shortening of a mouse skeletal muscle. Pflügers Archiv. 1998;436:906–913. doi: 10.1007/s004240050722. [DOI] [PubMed] [Google Scholar]
- Maughan DW, Godt RE. Parvalbumin concentration and diffusion coefficient in frog myoplasm. Journal of Muscle Research and Cell Motility. 1999;20:199–209. doi: 10.1023/a:1005477002220. [DOI] [PubMed] [Google Scholar]
- Maughan D, Recchia C. Diffusible sodium, potassium, magnesium, calcium and phosphorus in frog skeletal muscle. Journal of Physiology. 1985;368:545–563. doi: 10.1113/jphysiol.1985.sp015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky MC, Pinter MJ. Time courses of calcium and calcium-bound buffers following calcium influx in a model cell. Biophysical Journal. 1993;64:77–91. doi: 10.1016/S0006-3495(93)81342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell PH. High resolution two-dimensional electrophoresis of proteins. Journal of Biological Chemistry. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rall JA. Role of parvalbumin in skeletal muscle relaxation. News in Physiological Sciences. 1996;11:249–255. [Google Scholar]
- Schwaller B, Dick J, Dhoot G, Carroll S, Vrbova G, Nicotera P, Pette D, Wyss A, Bluethmann H, Hunziker W, Celio MR. Prolonged contraction- relaxation cycle of fast-twitch muscles in parvalbumin knockout mice. American Journal of Physiology. 1999;276:C395–403. doi: 10.1152/ajpcell.1999.276.2.C395. [DOI] [PubMed] [Google Scholar]
- Simonides WS, Van Hardeveld C. An assay for sarcoplasmic reticulum Ca2+-ATPase activity in muscle homogenates. Analytical Biochemistry. 1990;191:321–331. doi: 10.1016/0003-2697(90)90226-y. [DOI] [PubMed] [Google Scholar]
- Ushio H, Watabe S. Carp parvalbumin binds to and directly interacts with the sarcoplasmic reticulum for Ca2+ translocation. Biochemical and Biophysical Research Communications. 1994;199:56–62. doi: 10.1006/bbrc.1994.1193. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Relaxation, [Ca2+]i and [Mg2+]i during prolonged tetanic stimulation of intact, single fibres from mouse skeletal muscle. The Journal of Physiology. 1994;480:31–43. doi: 10.1113/jphysiol.1994.sp020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Slowing of relaxation and [Ca2+]i during prolonged tetanic stimulation of single fibres from Xenopus skeletal muscle. The Journal of Physiology. 1996;492:723–736. doi: 10.1113/jphysiol.1996.sp021341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg JB. Myoglobin-facilitated oxygen diffusion: role of myoglobin in oxygen entry into muscle. Physiological Reviews. 1970;50:559–636. doi: 10.1152/physrev.1970.50.4.559. [DOI] [PubMed] [Google Scholar]
- Woledge R, Curtin N, Homcher E. Energetic Aspects of Muscle Contraction. London: Academic Press; 1985. Heat production and chemical change; pp. 167–275. [Google Scholar]


