Abstract
A putative endogenous excitatory drive to the respiratory system in rapid eye movement (REM) sleep may explain many characteristics of breathing in that state, e.g. its irregularity and variable ventilatory responses to chemical stimuli. This drive is hypothetical, and determinations of its existence and character are complicated by control of the respiratory system by the oscillator and its feedback mechanisms. In the present study, endogenous drive was studied during apnoea caused by mechanical hyperventilation. We reasoned that if there was a REM-dependent drive to the respiratory system, then respiratory activity should emerge out of the background apnoea as a manifestation of the drive.
Diaphragmatic muscle or medullary respiratory neuronal activity was studied in five intact, unanaesthetized adult cats who were either mechanically hyperventilated or breathed spontaneously in more than 100 REM sleep periods.
Diaphragmatic activity emerged out of a background apnoea caused by mechanical hyperventilation an average of 34 s after the onset of REM sleep. Emergent activity occurred in 60 % of 10 s epochs in REM sleep and the amount of activity per unit time averaged approximately 40 % of eupnoeic activity. The activity occurred in episodes and was poorly related to pontogeniculo-occipital waves. At low CO2 levels, this activity was non-rhythmic. At higher CO2 levels (less than 0.5 % below eupnoeic end-tidal percentage CO2 levels in non-REM (NREM) sleep), activity became rhythmic.
Medullary respiratory neurons were recorded in one of the five animals. Nineteen of twenty-seven medullary respiratory neurons were excited in REM sleep during apnoea. Excited neurons included inspiratory, expiratory and phase-spanning neurons. Excitation began about 43 s after the onset of REM sleep. Activity increased from an average of 6 impulses s−1 in NREM sleep to 15.5 impulses s−1 in REM sleep. Neuronal activity was non-rhythmic at low CO2 levels and became rhythmic when levels were less than 0.5 % below eupnoeic end-tidal levels in NREM sleep. The level of CO2 at which rhythmic neuronal activity developed corresponded to eupnoeic end-tidal CO2 levels in REM sleep.
These results demonstrate an endogenous excitatory drive to the respiratory system in REM sleep and account for rapid and irregular breathing and the lower set-point to CO2 in that state.
There is both excitation and inhibition of the respiratory system in rapid eye movement (REM) sleep. Inhibition derives from mechanisms of atonia that affect some respiratory muscles in REM sleep, and it depends on pontine and medullary reticular formation areas that cause either a glycinergic active inhibition or a serotonergic disfacilitation of spinal and cranial motoneurons (Kubin et al. 1993, 1994, 1996). Excitation is evident from irregular breathing at a faster rate (Orem et al. 1977), persistent breathing in the absence of chemical stimuli (Sullivan, 1980) and excitation of some inspiratory and expiratory medullary respiratory neurons (Orem, 1994, 1996, 1998). It has been proposed that endogenous non-metabolic factors are responsible for this excitation (Orem, 1980; Sullivan, 1980). The source and nature of these factors are not known.
If an endogenous excitatory drive exists in REM sleep and is non-chemical, then it should be observed when brainstem respiratory neurons and muscles are not being driven by a rhythmic respiratory drive. In mechanically hyperventilated dogs, Horner et al. (1994) observed bursts of diaphragmatic activity and even rhythmic breathing in REM sleep. The activity occurred when there were twitches of the nose, ears and limbs. The authors suggested that transient fluctuations in respiratory motoneuronal excitability similar to those occurring in other motoneuronal pools in REM sleep account for this emergent activity. Similarly, Orem & Vidruk (1998) studied the behaviour of brainstem respiratory neurons in cats during mechanical hyperventilation and found that there was more activity in REM sleep than in non-REM (NREM) sleep. They stated that this activity appeared as erratic, unrecognizable bursts and that it might be related to periods of highly irregular breathing that occur normally in REM sleep. In both studies the accounts of activity in REM sleep were anecdotal. Horner and colleagues were interested primarily in tonic expiratory muscle activity in sleep and wakefulness, and Orem & Vidruk were studying the neuronal mechanisms of apnoea.
This report describes studies designed to analyse the endogenous excitatory drive of REM sleep. We studied diaphragmatic and medullary respiratory neuronal activity during apnoea induced by mechanical hyperventilation. Our objective was to determine the characteristics of the drive and the relation of it to phasic REM sleep activity and the level of carbon dioxide. We propose that the drive causes a lower set-point to carbon dioxide in REM sleep and accounts for the irregularities in breathing that are characteristic of that state.
Methods
Adult cats were prepared for recording electroencephalographic (EEG), electromyographic (EMG) and neuronal activity. In addition, tracheal fistulas were created and a headcap containing a connector for the electrodes was attached to their skulls. The headcap contained standoffs that could be attached to the recording apparatus to immobilize the animal's head during sleep recordings. Surgical and experimental procedures were approved by the Animal Care and Use Committee of Texas Tech University School of Medicine. The welfare of the animal was monitored at all stages of the experiment by a technician whose only responsibility in the laboratory was to ensure the well-being of the animals.
Surgical procedures
The animals were anaesthetized with acepromazine maleate (2.5 mg, i.m.), ketamine (17 mg kg−1, i.m.) and 1–2 % halothane in O2. Surgery was performed under aseptic/antiseptic conditions. A tracheostomy was performed and electrodes for recording crural diaphragmatic and EEG activity were implanted using previously described techniques (Orem & Vidruk, 1998). In addition, tripolar stainless steel electrodes were placed in the lateral geniculate bodies bilaterally (A6.4, L10, H+2.5) to record pontogeniculo-occipital (PGO) waves (Bizzi & Brooks, 1963). Two stainless steel wires were implanted in nuchal muscles to record electromyograms. Electrodes were attached to a connector that was in turn attached to a prefabricated headcap. The headcap was fixed to the skull with anchor screws and dental cement.
Subjects
The animals recovered from surgery for a minimum of 1 month before experimentation. After recovery, they were adapted to the experimental apparatus. Eleven animals were prepared for experimentation but six were rejected because they did not sleep readily in the apparatus.
Recording procedures and mechanical ventilation
On the nights before recording sessions, the animals were housed in a cold (0°C) environment in order to consolidate sleep during the subsequent day. During recordings, the trachea was intubated with a 4.0 mm endotracheal tube that was attached to a Validyne pneumotachograph. Pressure and CO2 levels in the tube were measured using a volumetric pressure transducer and infrared CO2 analyser. Airflow was measured by pneumotachography. A two-position valve switched the animal from breathing ambient air to a ventilator that delivered 40–50 ml tidal volumes at rates of 30–40 min−1. Ventilation produced an adjustable level of hypocapnia that was controlled by an automated system. The computerized system used pulse width modulation of a CO2 injector whose duty cycle was determined by the discrepancy between end-tidal CO2 percentage and the desired CO2 percentage. When studying diaphragmatic activity, end-tidal CO2 was held at a selected percentage throughout the recording session. These experiments were designed to determine if chemical stimulation at CO2 levels below threshold had an effect on emergent diaphragmatic activity. When recording medullary respiratory neurons, CO2 was varied in steps, below NREM sleep eupnoeic threshold values, with each step maintained for approximately 1 min. These experiments were designed to determine the threshold for rhythmic breathing as well as the effect of chemical stimulation on emergent neuronal activity. Mechanical hyperventilation was instituted in NREM sleep or relaxed wakefulness when it readily induced apnoea. Apnoea was defined by the absence of rhythmic diaphragmatic activity, and smooth and regular intratracheal presssure, airflow and tidal CO2 tracings.
EEG, EMG and PGO signals along with CO2 percentages, airflow and intratracheal pressures were amplified and then recorded on paper (Astro-Med 9500) and on magnetic tape. PGO waves were recorded in a bipolar configuration from the electrode that had the largest amplitude waves. Recording sessions lasted about 4 h. NREM and REM sleep and wakefulness were defined on the basis of standard EEG criteria. Animals were humanely killed at the end of the experimental series.
Recordings of medullary respiratory neurons
Medullary respiratory neurons were recorded in one of the five animals. After recording this animal's diaphragmatic activity in one session (Subject I in Table 1), a small craniotomy (5 mm diameter) was made under general anaesthesia (as above) in the occipital bone. The craniotomy was made using stereotaxic coordinates that allowed access to medullary respiratory groups (Orem, 1980).
Table 1.
Charactersitics of emergent diaphragmatic activity in REM sleep during mechnical hyperventilation
| Subjects | No. of REM sleep periods | Duration of REM sleep period (s) | Latency to onset of diaphragmatic activity (s) | Percentage of REM sleep times with diaphragmatic activity | Emergent diaphragmatic activity (% control) |
|---|---|---|---|---|---|
| R | 5 | 385 (170) | 12 (19) | 56 (31) | 46 (36) |
| M | 19 | 311 (112) | 18 (15) | 76 (26) | 60 (18) |
| S | 13 | 432 (205) | 71 (33) | 48 (20) | 30 (12) |
| H | 10 | 432 (213) | 32 (24) | 51 (8) | 17 (2) |
| I | 1 | 670 | 5 | 75 | 16 |
| Mean* | 384 | 34 | 61 | 41 |
Data are means (s.d.).
Mean of all REM sleep periods.
Respiratory neurons were recorded in 26 sessions over a period of 2 months. Only one penetration with the microelectrode was made during each session. Penetrations were defined by reference to a point on the head-restraint plate.
Tungsten microelectrodes (impedances 1–10 MΩ) were used to record single medullary respiratory neurons. The microelectrodes were mounted in a hydraulic microdriver and driven through the cerebellum into the medulla. Signals were led to a high impedance probe (Grass HIP511) and via a preamplifier (Grass p511) to paper and tape recording devices.
Histology was not performed to identify recording sites because the animal was in good health at the end of the recording sessions and was used for further recordings later. Furthermore, reconstruction of recording sites in brains in which there have been many penetrations is difficult or impossible. At best, areas of gliosis in the vicinity of known respiratory areas are evident. The activities of individual respiratory neurons were analysed by computer to determine their relationship to the respiratory cycle. Three-dimensional coordinates of the site of each cell were plotted.
Data analysis
Diaphragmatic activity, PGO activity, EEG activity and airflow were digitized from analog tape records at 1000 samples s−1. Diaphragmatic electromyograms (EMGDIA) were rectified and summed over the REM sleep period and a control period of NREM sleep that occurred in the same session. Control and REM sleep activity values were divided by the time of the sample periods to obtain indices of the amount of activity.
The relation of emergent diaphragmatic activity to the ventilator cycle was analysed quantitatively. For this, the ventilator cycle was divided into 20-iles and diaphragmatic activity (the integral of the electromyographic activity) during each 20-ile was determined by computer. Values across several ventilator cycles (n > 20) were stored in a matrix in which the columns were 20-iles of the ventilatory cycle and rows were cycles. This matrix was evaluated using an analysis of variance to determine the relationship between diaphragmatic activity and ventilator cycle.
To quantify a possible relationship between PGO waves and EMGDIA, we used empirical entropy measurements. To do this, rectified EMGDIA and PGO wave densities were averaged in 5 s bins. The range of each of these variables was divided into 18 equal segments. All records available for all REM periods for each animal were used to construct frequency histograms of each variable and two-dimensional matrices showing the number of 5 s bins of the possible joint occurrences of the two variables. Empirical entropy was defined as the sum of standard entropy terms, -pilog2pi, where instead of probabilities we used empirical frequencies of variables within the segments. The two-dimensional matrices were used to obtain conditional amplitude distributions of EMGDIA amplitude at a given level of PGO wave density. The average conditional empirical entropy for the EMGDIA was defined as the weighted average of conditional values of entropy with weights equal to empirical frequencies of the corresponding PGO wave density. Dependence between PGO waves and EMGDIA would produce a gain in information, i.e. a lowering of entropy, in the weighted conditional EMGDIA distribution compared to the entropy of the EMG signal without conditions. To obtain a statistical evaluation of this difference, we randomly permuted the order of the EMGDIA samples to obtain eleven synthetic sets of data containing PGO wave and EMGDIA pairs. A mean and standard error of the mean of unconditional entropy were calculated from these sets of permuted data, and we compared the averaged conditional entropy to the means and standard errors of the mean to obtain a measure of the informational gain.
Respiratory neuronal activity was analysed off-line. Data were played back from the tape recorder into a PS/2 486 computer with a LabWindows data acquisition system (National Instruments). Cycle-triggered histograms and the signal strength and consistency of the respiratory component of the activity of a cell (η2 value) were determined for each cell. Procedures for constructing cycle-triggered histograms and calculating the η2 value of the activity of a cell have been published (Orem & Dick, 1983). In addition, neuronal activity, the CO2 and airflow signals and electroencephalographic recordings from the thalamus and cerebral cortex were digitized at 12 kHz using a National Instruments data acquisition board running under VisualBasic in a pentium personal computer. The cumulative sum of action potentials of the neurons was plotted for the NREM sleep period before the onset of REM sleep and throughout the REM sleep period. The slope of this plot was measured to determine the discharge rate of the cell during NREM and REM sleep and, in the case of neurons that were excited in REM sleep, the time of the change in slope was measured to determine the time of occurrence of the excitation.
Results
Results were obtained from more than 100 REM sleep periods recorded in five adult cats (4 females, 1 male). Ninety REM sleep periods were studied during mechanical ventilation. The rest of the REM sleep periods were recorded during spontaneous breathing. The mean length of the REM sleep periods was 364 ± 188 s (s.d.).
Spontaneous breathing in REM sleep
Figure 1 shows spontaneous breathing in REM sleep while recording the activity of a respiratory neuron. The activity of the respiratory neuron increased from a mean rate of 8 impulses s−1 in NREM sleep to approximately 14 impulses s−1 in REM sleep. End-tidal CO2 percentages were less during REM sleep than NREM sleep or wakefulness. Analysis of 14 REM sleep periods in one animal showed that end-tidal CO2 percentages averaged 95.5 ± 3.62 % (s.d.) of those of NREM sleep.
Figure 1. End-tidal CO2 percentage and activity of a respiratory neuron during spontaneous breathing in NREM and REM sleep and wakefulness.
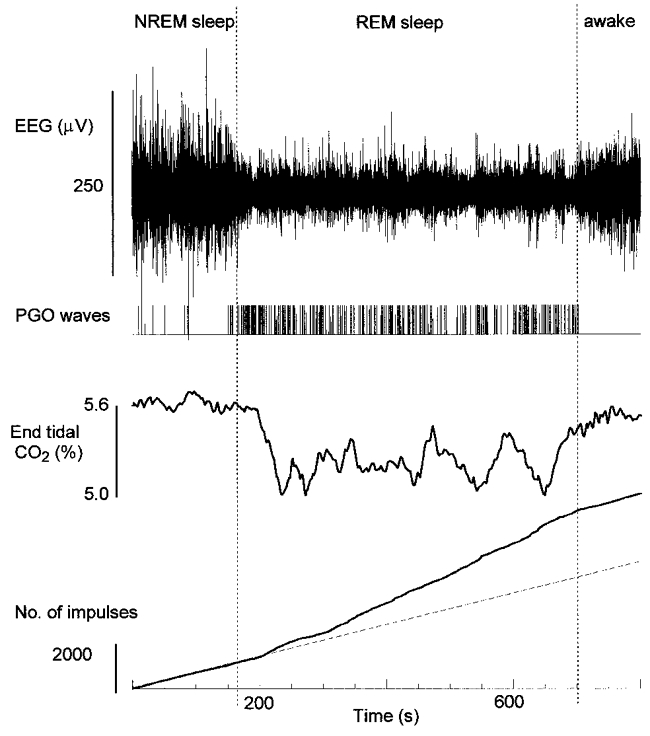
Neuronal activity is shown as cumulative impulses from the beginning of the record. The dashed line is 8 impulses s−1. In REM sleep the mean discharge rate increased to 14 impulses s−1. The neuron was an augmenting inspiratory cell with an η2 value of 0.9.
Emergent diaphragmatic activity in REM sleep during mechanical ventilation
EMGDIA was recorded in five cats during 63 sleep-wake cycles that included a period of REM sleep. Figure 2 illustrates the basic finding. Mechanical ventilation caused hypocapnia and apnoea (Fig. 2). There was little or no diaphragmatic activity during relaxed wakefulness and NREM sleep, but in REM sleep diaphragmatic activity emerged despite the hypocapnia (Fig. 2). Emergent diaphragmatic activity occurred in 58 of 63 REM sleep periods. In 48 of these the activity was quantified (Table 1). REM sleep periods were divided into 10 s epochs and scored for the occurrence of any diaphragmatic activity in the epoch. Sixty-one per cent of the epochs contained some activity (Table 1). The amount of emergent activity averaged 41 % of the amount of activity in a similar time period during normal breathing (Table 1). The delay to the onset of emergent diaphragmatic activity from the onset of REM sleep varied from more than 1 min to only a few seconds and averaged, across all REM periods in all subjects, 34 s (Table 1).
Figure 2. Emergence of diaphragmatic activity during REM sleep.
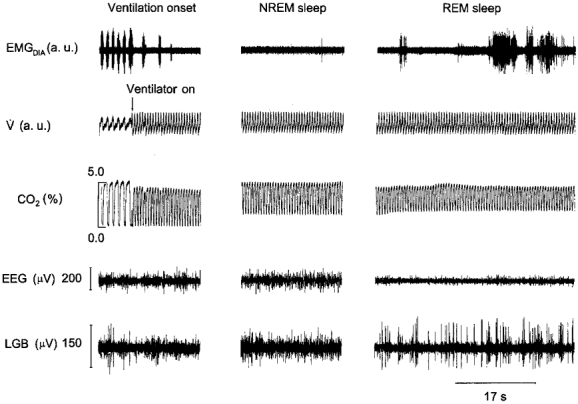
The traces shown are EMGDIA, airflow (V̇), tidal CO2 percentage, electroencephalographic (EEG) and pontogeniculo-occipital activity recorded from the lateral geniculate body (LGB) of the thalamus before and during mechanical ventilation.
Emergent activity occurred variously as tonic activity (Fig. 3) or rhythmic activity that was either out of phase or in phase (Fig. 4) with the ventilator cycle. The latter was seen in 2 of the 5 animals. In one of the animals, rhythmic activity in phase with the ventilator occurred in 2 of 5 REM sleep periods; the other animal showed this activity in 2 of 19 REM sleep periods. The strength of the relationship to the ventilator was assessed with the η2 statistic which averaged 0.35 for one animal and 0.12 for the other.
Figure 3.
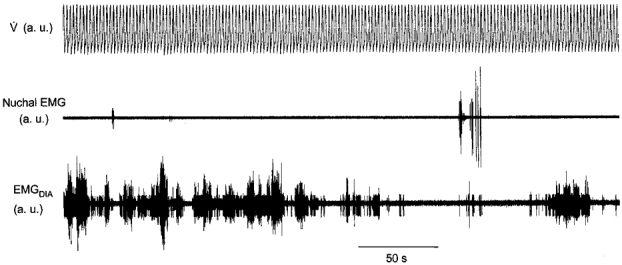
Non-rhythmic emergent diaphragmatic and nuchal activity during mechanical hyperventilation in REM sleep
Figure 4. Rhythmic diaphragmatic activity during hyperventilation in REM sleep.
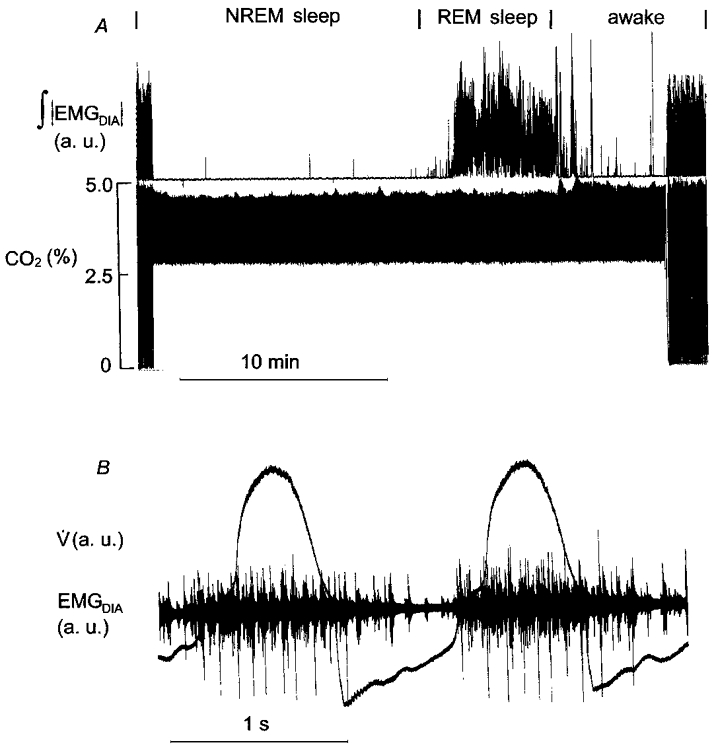
A, diaphragmatic activity during eupnoea, and mechanical hyperventilation during NREM and REM sleep and wakefulness. B, two ventilator cycles and rhythmic, entrained diaphragmatic activity in REM sleep.
Once developed, emergent activity tended to continue for several minutes before subsiding. This is evident in Fig. 4 and particularly in Fig. 5, which shows two distinct episodes within a REM sleep period. In each of the two episodes, activity developed slowly to maximal levels and then subsided.
Figure 5. Excitation of the diaphragm and PGO density in REM sleep.
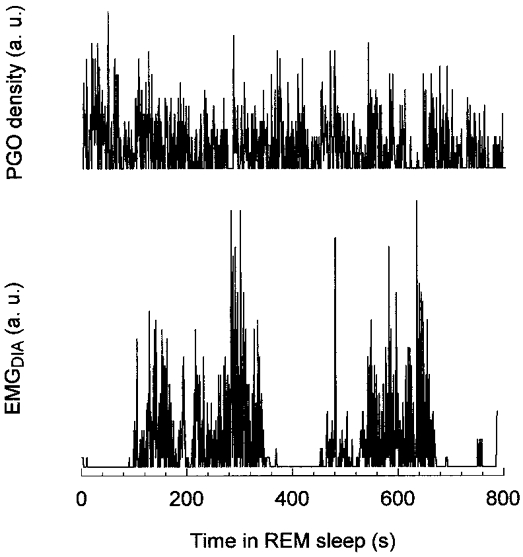
PGO wave density and EMGDIA averaged over 0.8 s bins versus time in REM sleep.
Relationship between PGO activity and diaphragmatic activity during mechanical ventilation
Four of the five animals had recordings of both PGO waves and diaphragmatic activity. In one of these four animals only one period of REM sleep was recorded, and the small number of samples was not suitable for analysis of entropy. Values of entropy are given in Table 2 for the other three animals. Information gain was defined as the difference between conditional entropy calculated from the experimental data and conditional entropy calculated with permuted EMGDIA data. The difference was surprisingly constant for all animals despite large differences in the number of 5 s samples. Table 2 shows that there was a relation between PGO and diaphragmatic activity but that this relation was weak. A measure of this relation can be obtained from the ratio of information gain to entropy of the EMGDIA, i.e. 0.0392/2.6827 = 0.0146. In other words, 1/70th of the information about EMGDIA can be obtained by knowledge of the density of PGO wave activity. The weakness of this relation, and the necessity of quantitative analysis to reveal it, is apparent in Fig. 5 in which there appears to be no relation between these variables.
Table 2.
Empirical entropy and averaged conditional empirical entropy for PGO wave intensity and diaphragmatic activity
| Averaged conditional entropy | ||||||
|---|---|---|---|---|---|---|
| Subjects | No.of 5 s samples | Entropy of PGO waves | Entropy of EMGDIA | EMGDIA | EMGDIA for permuted samples* | Information gain |
| M | 1152 | 3.8469 | 2.9685 | 2.7640 | 2.8187±0.0025 | 0.0487±0.0025 |
| S | 481 | 3.8961 | 2.2385 | 1.7422 | 1.7855±0.0083 | 0.0433±0.0083 |
| R | 296 | 3.4031 | 2.8411 | 2.4461 | 2.4718±0.0092 | 0.0257±0.0092 |
Means ±s.e.m.
Effect of CO2 on emergent diaphragmatic activity during mechanical ventilation
Emergent diaphragmatic activity was studied in two subjects at different levels of hypocapnia. In these subjects, a given level of hypocapnia was maintained by computer control throughout a REM sleep period. The level of CO2 was varied from session to session. Figure 6 illustrates the data from these two animals. Each point is the average obtained in a single REM sleep period. Carbon dioxide had little effect on emergent activity when the difference between apnoeic end-tidal CO2 and eupnoeic (NREM sleep) end-tidal CO2 was greater than 0.5 %. When the difference was less than 0.5 %, emergent diaphragmatic activity was equal to that during spontaneous breathing. This activity was rhythmic breathing. It occurred at CO2 percentages that were below NREM sleep eupnoeic end-tidal CO2 percentages but that were comparable to REM sleep eupnoeic end-tidal CO2 percentages.
Figure 6. Diaphragmatic activity in REM sleep at different levels of CO2.
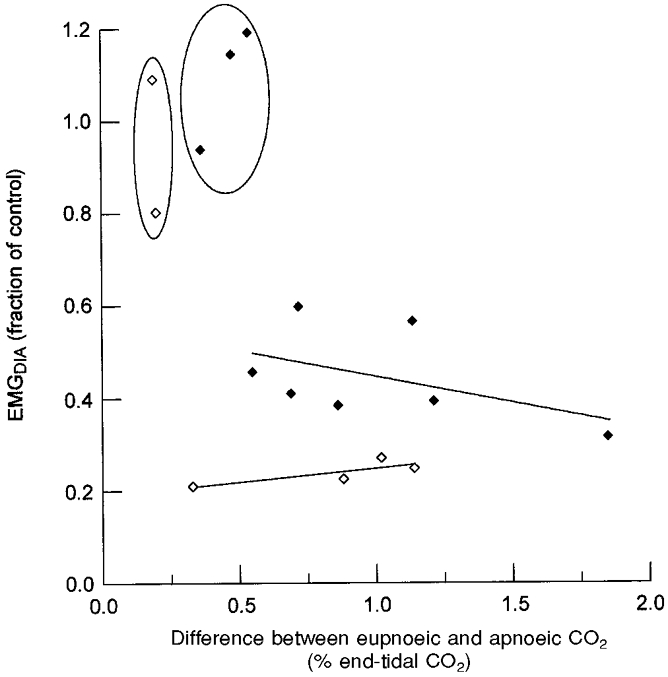
Plotted data are means obtained in separate REM periods in two subjects shown as ♦ and ⋄, respectively. The abscissa is the difference between eupnoeic end-tidal CO2 levels in NREM sleep and end-tidal CO2 level during mechanical ventilation in REM sleep. Regression lines for each of the data sets are plotted with exclusion of data enclosed by the ovals.
Emergent respiratory neuronal activity in REM sleep during mechanical ventilation
Ninety respiratory neurons were recorded in one animal in 26 recording sessions. Of these, 27 were studied in REM sleep during mechanical hyperventilation. Eighteen of the 27 were excited in REM sleep; three showed no change in activity; and six could not be evaluated for various reasons. The neurons that were excited in REM sleep had an average η2 value of 0.72 ± 0.17 (s.d.) in NREM sleep and included inspiratory throughout (n = 8), augmenting (n = 2), and decrementing inspiratory (n = 3) cells, expiratory- inspiratory phase-spanning cells (n = 3), and expiratory cells (n = 2) (Table 3). Respiratory cells that showed no change in activity had low η2 values (0.1–0.4).
Table 3.
Respiratory cells that were excited during REM sleep with mechanical hyperventilation
| Cell | Type | η2 | NREM rate(impulses s−1) | REM rate (impulses s−1) | Delay to increased activity in REM (s) |
|---|---|---|---|---|---|
| I75 | I | 0.9 | 15.62 | 19.32 | 70 |
| I52 | I | 0.9 | 0.62 | 2.33 | 50 |
| I82 | I | 0.8 | 0.37 | 2.00 | 40 |
| I40 | I | 0.8 | 0.00 | 18.26 | 40 |
| I61 | I | 0.7 | 3.45 | 4.96 | 70 |
| I69 | I | 0.5 | 9.60 | 16.92 | 0 |
| I89 | I | 0.5 | 1.00 | 11.00 | — |
| I84 | I | 0.4 | 1.00 | 5.38 | 80 |
| I90 | Iaug | 0.9 | 0.00 | 7.89 | — |
| I70 | Iaug | 0.8 | 5.88 | 33.00 | 0 |
| I32 | Idec | 0.8 | 18.20 | 20.51 | 50 |
| I58 | Idec | 0.8 | 0.00 | 1.25 | 28 |
| I62 | Idec | 0.6 | 12.50 | 25.00 | — |
| I79 | EI | 0.9 | 16.67 | 32.14 | 70 |
| I33 | EI | 0.9 | 5.00 | 32.50 | 40 |
| I88 | EI | 0.7 | 17.14 | 31.79 | 0 |
| I27 | E | 0.6 | 0.29 | 11.99 | 5 |
| I38 | E | 0.5 | 0.36 | 3.55 | 60 |
| Mean | 5.98 | 15.54 | 40.2 | ||
| s.d. | 6.97 | 11.59 | 28.1 |
Cell type and η2 value were determined in eupnoea. NREM rate and REM rate are discharge rates during mechanical hyperventilation. Abbreviations: I, inspiratory; Iaug, augmenting inspiratory; Idec, decrementing inspiratory; EI, expiratory-inspiratory; E, expiratory.
The 27 respiratory cells were located in an area having a volume of 1.71 mm3 (0.9 mm anterior to posterior × 0.5 mm medial to lateral × 3.8 mm dorsal to ventral) (Fig. 7). The volume was 0.81 mm3 when one cell located 0.5–2 mm above the others was excluded. The various cell types, inspiratory, expiratory and phase spanning, were intermixed within this cluster (Fig. 7). The dorsal outlier was the augmenting inspiratory cell shown in Fig. 1. It will be argued in Discussion that this cell was in the dorsal respiratory group of the medulla whereas the other cells were within the ventral respiratory group. Cells that showed no change in activity in REM sleep were located within the same cluster as cells that were excited (Fig. 7).
Figure 7. Coordinates of respiratory neurons studied in REM sleep.
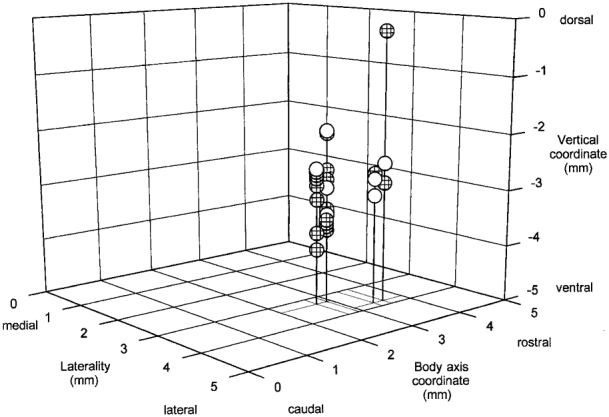
Laterality 0 corresponds to the mid-line; the body axis and vertical coordinates cannot be assigned definite locations in a stereotaxic atlas because of the angle of the brainstem imposed by the animal's position in the apparatus. Hatched symbols indicate the positions of neurons that were more active in REM sleep during mechanical hyperventilation. Open symbols show the positions of neurons that were not activated or whose records could not be interpreted.
Figure 8 shows an example of a respiratory cell that was excited in REM sleep. The cell discharged during late expiration and early inspiration and had an η2 value of 0.9 during spontaneous breathing in NREM sleep (cell I79 in Table 3). It was tonically active during apnoea in NREM sleep (dashed line in Fig. 8). Approximately 1 min after the onset of REM sleep, the activity of the cell increased as indicated by an increase in the slope of the impulse count. With arousal, the activity decreased to pre-REM sleep levels. The excitation in REM sleep occurred below eupnoeic end-tidal CO2 levels in NREM sleep and periods of greater and lesser excitation were unrelated to the level of CO2 (Kendall's tau analysis).
Figure 8. Excitatio in REM sleep of a respiratory neuron during mechanical ventilation.
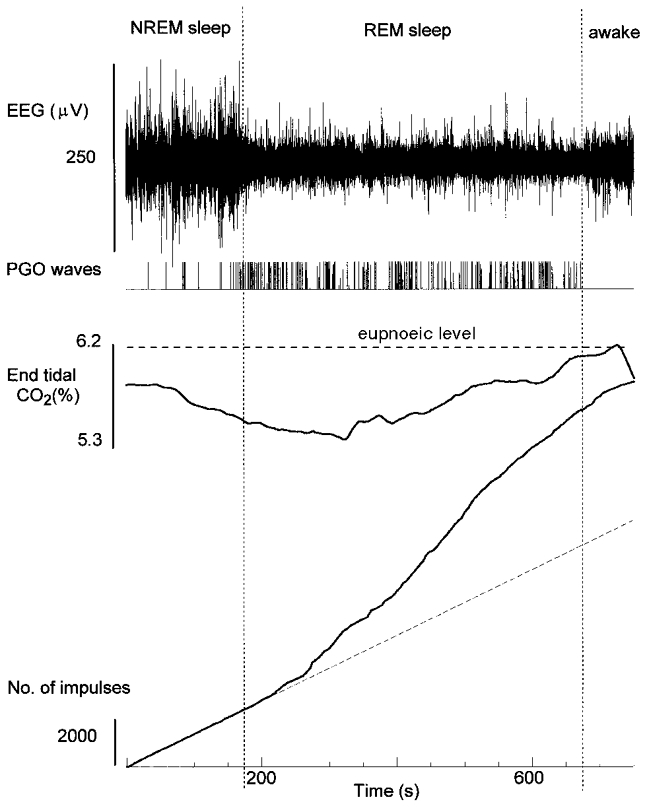
The neuron shown is cell I79 in Table 3. Variables as in Fig. 1. The dashed line is NREM sleep discharge rate of 16.7 impulses s−1.
Eighteen cells were analysed quantitatively. Across the population of cells, impulse rates in REM sleep averaged approximately 2.6 times those in NREM sleep (Table 3). However, this varied from cell to cell (Table 3). The delay to an increased rate of discharge varied from 0 s, i.e. the increase began at the onset of the REM sleep period, to more than a minute and averaged approximately 40 s.
When studying respiratory neurons CO2 level was systematically varied to determine if it had an effect on emergent activity. The activity of the cell in Fig. 8 did not vary with changes in end-tidal CO2 percentage except at high levels that caused rhythmic breathing (not shown). Figure 9 illustrates the transition of activity of a respiratory cell from tonic to bursting and finally rhythmic activity as end-tidal CO2 was increased from 5.0 to 5.3 %. During this recording session eupnoeic end-tidal CO2 at the end of NREM sleep and the beginning of REM sleep was 5.6 % (Fig. 9). At the transition to REM sleep the animal was mechanically hyperventilated, and end-tidal CO2 was increased from 5.0 to 5.3 %. Rhythmic breathing occurred when end-tidal CO2 was 5.3 %.
Figure 9. Effect of CO2 on the activity of an inspiratory neuron during mechanical ventilation in REM sleep.
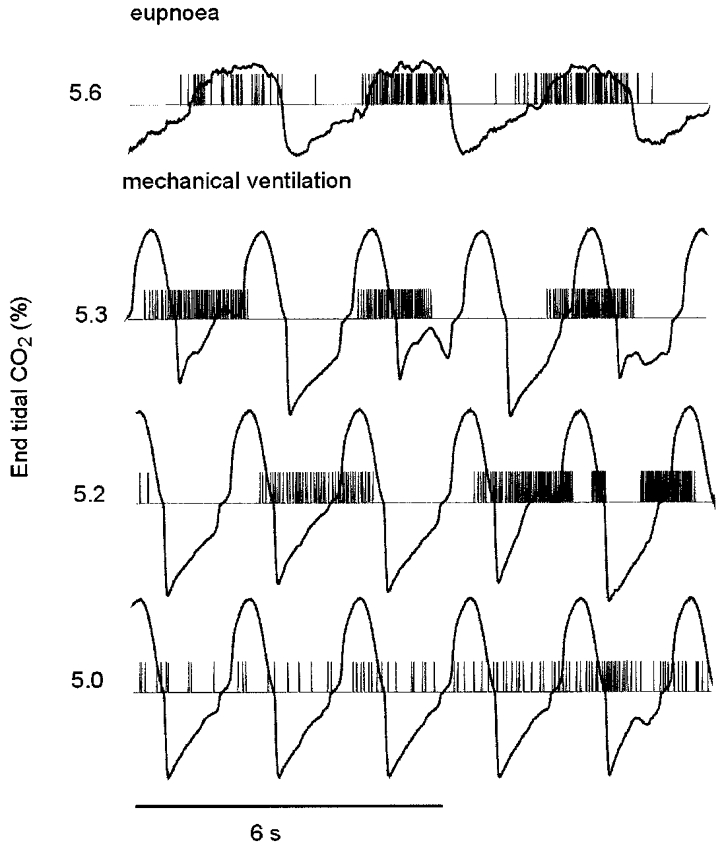
The neuron is the cell shown in Fig. 1. The tracings are computer reconstructed impulses and airflow. The CO2 percentage is shown at the left.
The threshold for rhythmic activity in REM sleep expressed as the maximal percentage of CO2 in the expired gas, θREM) was determined for 12 neurons and averaged 0.33 ± 0.18 % (s.d.) less than the eupnoeic end-tidal CO2 percentage in relaxed wakefulness or NREM sleep (θW/NREM). That is, θREM = θW/NREM − 0.33. θREM was approximately equal to end-tidal CO2 levels during spontaneous breathing in REM sleep.
Discussion
Mechanical hyperventilation produces apnoea. In this state diaphragmatic muscle units are generally silent and most central respiratory neurons are neither excited nor inhibited (Orem & Vidruk, 1998). Accordingly, the apnoeic state is ideal for studying state-specific influences on respiratory neurons and diaphragmatic muscle units without obscuration by an overlying drive from the respiratory oscillator. The mechanically hyperventilated state is ideal also because ventilation can be held constant across wakefulness and sleep, and the possible effects of changes in inputs from feedback mechanisms are eliminated. For example, upper airway resistance is irrelevant because the animals are intubated, and changes in mechanics, e.g. chestwall compliance, affect only the work of the ventilator and not the animal. In addition, CO2 levels can be controlled and held constant across sleep and wakefulness.
Our results show REM sleep-specific effects on respiratory neurons and motoneurons. The respiratory neurons were located within a small volume in the medulla, and the heterogeneity of respiratory cell types observed within this small volume indicates that they were most likely within the ventral respiratory group. Furthermore, we conclude that they were not at the caudal end of this group, which is below the obex, nor at the rostral end at the level of the retrofacial nucleus because both ends contain augmenting expiratory cells (Von Euler, 1986). Since these were not observed, it is likely to be that the cells studied here were in the ventral respiratory group between the retrofacial nucleus and the obex. The exception was one augmenting inspiratory cell located dorsal to the others. Both the location and discharge profile indicate that this cell was located in the dorsal respiratory group (Von Euler, 1986).
There is little diaphragmatic activity and respiratory neuronal activity is, depending on the cell, absent, tonic, non-reciprocal or, in some cases, weakly entrained to the ventilator during mechanical hyperventilation in NREM sleep and relaxed wakefulness (Orem & Vidruk, 1998). However in REM sleep, muscle and neuronal activity either emerge out of silence or increase dramatically. This activity is non-rhythmic at low end-tidal CO2 percentages and becomes rhythmic as end-tidal CO2 levels are increased. The CO2 level at which rhythmic breathing develops in REM sleep is less than end-tidal CO2 levels during spontaneous breathing in relaxed wakefulness and NREM sleep but equal to those during spontaneous breathing in REM sleep. Emergent activity is poorly related to pontogeniculo-occipital waves and has a time course indicating that it develops slowly after the onset of REM sleep and continues in episodes of variable length. There may be one or several episodes within the REM sleep period.
Effects of endogenous excitatory drive on breathing
Irregularities in breathing in REM sleep persist during hypercapnia (Phillipson et al. 1977), metabolic alkalosis (Sullivan et al. 1978), hypoxia (Phillipson et al. 1978) and hyperoxia (Sullivan et al. 1978) and after extensive deafferentation of the brain (Netick & Foutz, 1980). Thus it seems that the pattern of breathing in REM sleep is determined in part by internal neural events (Orem, 1980; Sullivan, 1980). There have been two views on the nature of the internal events. According to some authors they are related to behavioural mechanisms and are the dreamer reacting to the dream (Aserinsky & Kleitman, 1953; Phillipson & Sullivan, 1978), whereas for others they are the result of fundamental neurophysiological processes of REM sleep (Orem, 1980; Sullivan, 1980). In the present experiments, effects of these internal events on respiratory neurons and muscles were seen without interference from the oscillator. They appeared as variable activity with no evident pattern. Furthermore, they were related to the density of pontogeniculo-occipital waves, but this relation was so weak that it does not support the idea that they arise from phasic REM-sleep processes. Thus, it is not clear if endogenous excitation originates from behavioural controllers that are creating obscure motor patterns or from unknown endogenous mechanisms. It is clear however that the emergent activity observed in this study is a form of excitation that could cause the characteristic irregularities of breathing in REM sleep.
Rhythmic breathing occurred in REM sleep at CO2 levels below those during spontaneous breathing in relaxed wakefulness and NREM sleep. In experiments in which end-tidal CO2 was maintained at a constant percentage just below eucapnoeic levels in NREM sleep and wakefulness, emergent rhythmic breathing occurred in REM sleep. This finding of a lower CO2 set-point in REM sleep explains the observation in this and other studies (Netick et al. 1984) of lower end-tidal CO2 levels in cats during spontaneous breathing in that state. It may explain also the failure to obtain apnoea in REM sleep following hypoxia-induced hyperventilation in dogs (Xi et al. 1993) and the absence of periodic breathing at high altitudes during REM sleep (Reite et al. 1975; Normand et al. 1990). We propose that the lower set-point is the result of summation of inputs from chemoreceptors with endogenous excitatory drive at the level of the respiratory oscillator.
Different studies have found variously that the ventilatory response to CO2 in REM sleep is absent (Phillipson et al. 1977; Cohen et al. 1991; Praud et al. 1991), slightly reduced (Sullivan, 1980; Netick et al. 1984), or, inasmuch as it reflects chemosensitivity, intact (Parisi et al. 1992; Meza et al. 1998). These studies differ in the species studied, the maturity of the subjects and the methods that were used. The results of the present study indicate that CO2 sensitivity, as indicated by the CO2 set-point, is increased in REM sleep. However, it is also likely that a ventilatory response to CO2 would be disorganized by the intermittent endogenous excitatory drive demonstrated here.
Origins of endogenous excitatory drive
There may be multiple sources of excitation to the respiratory system in REM sleep. It is certain that excitation of the brain in REM sleep is widespread and that many areas of the brain have projections to respiratory neurons and motoneurons. The absence of a strong relationship between endogenous excitatory drive to the respiratory system and phasic REM phenomena casts doubt on the idea that the source of excitation is the phasic REM generator in the pons (Datta, 1995). The pneumotaxic centre is located in the dorsolateral pons, but in spite of the suggestion that this region is part of the dorsal pontine REM centre (Datta, 1995), a role for it in the control of breathing in REM sleep has not been established. Nevertheless, there are projections from the parabrachial pons to the ventral medullary respiratory group (Ellenberger & Feldman, 1990; Nunez-Abades et al. 1993; Dobbins & Feldman, 1994), and they may be responsible for some or all of the endogenous excitatory drive. Other brainstem regions may be involved also. There are neurons in the medullary reticular formation that are active specifically in REM sleep and that are in an area known to project to the ventral respiratory group (Smith et al. 1989). Some of these neurons have activity related to the rate of breathing in REM sleep (Netick et al. 1977).
Significance
The excitatory drive demonstrated in these studies may be a major determinant of the pattern of breathing in REM sleep. It can account for the irregularities in rate and depth of breathing, the decreased CO2 set-point, and variability in the response to chemical and perhaps other respiratory stimuli. The physiological significance of an excitatory drive is not known. REM sleep accounts for half of the 16 h of sleep in the newborn human and even more in the premature infant (Roffwarg et al. 1966). Respiratory movements in utero in lambs occur in a REM sleep-like state (Dawes et al. 1972), and it has been suggested that an endogenous excitatory drive may cause these movements (Sullivan, 1980). The drive demonstrated in this paper, like the drive that produces respiratory movements in utero, does not depend on chemical stimulation, and it may be that it is a vestigial process that is important during development but that has no physiological purpose in adulthood.
Note added in proof
Histological analysis of the locations of respiratory neurons recorded in this study was performed after final submission of the manuscript. This revealed that the microelectrode penetrations were within the ventral respiratory group between the retrofacial nucleus and the obex caudally (stereotaxic coordinates, posterior 12–14).
Acknowledgments
Becky Tilton cared for the animals and participated in all surgeries. Dr Margaret Hamilton participated in some of the diaphragmatic recordings and assisted in some surgeries. Dr Thomas E. Dick acted as a consultant. Supported by grants HL21257, HL62589 and a grant from the U.S. Department of Education for Graduate Assistance in Areas of National Need.
References
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–274. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- Bizzi E, Brooks DC. Functional connections between pontine reticular formation and lateral geniculate nucleus during deep sleep. Archives italiennes de Biologie. 1963;101:666–680. [PubMed] [Google Scholar]
- Cohen G, Xu C, Henderson-Smart D. Ventilatory response of the sleeping newborn to CO2 during normoxic rebreathing. Journal of Applied Physiology. 1991;71:168–174. doi: 10.1152/jappl.1991.71.1.168. [DOI] [PubMed] [Google Scholar]
- Datta S. Neuronal activity in the peribrachial area: relationship to behavioral state control. Neuroscience and Biobehavioral Reviews. 1995;19:67–84. doi: 10.1016/0149-7634(94)00043-z. [DOI] [PubMed] [Google Scholar]
- Dawes GS, Fox HE, Leduc BM, Liggins GC, Richards RT. Respiratory movements and rapid eye movement sleep in the foetal lamb. The Journal of Physiology. 1972;220:119–143. doi: 10.1113/jphysiol.1972.sp009698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. Journal of Comparative Neurology. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Research. 1990;513:35–42. doi: 10.1016/0006-8993(90)91086-v. [DOI] [PubMed] [Google Scholar]
- Horner RL, Kozar LF, Kimoff RJ, Phillipson EA. Effects of sleep on the tonic drive to respiratory muscle and the threshold for rhythm generation in the dog. The Journal of Physiology. 1994;474:525–537. doi: 10.1113/jphysiol.1994.sp020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L, Kimura H, Tojima H, Davies RO, Pack AI. Suppression of hypoglossal motoneurons during the carbachol-induced atonia of REM sleep is not caused by fast synaptic inhibition. Brain Research. 1993;611:300–312. doi: 10.1016/0006-8993(93)90517-q. [DOI] [PubMed] [Google Scholar]
- Kubin L, Reignier C, Tojima H, Taguchi O, Pack AI, Davies RO. Changes in serotonin level in the hypoglossal nucleus region during carbachol-induced atonia. Brain Research. 1994;645:291–302. doi: 10.1016/0006-8993(94)91663-2. [DOI] [PubMed] [Google Scholar]
- Kubin L, Tojima H, Reignier C, Pack AI, Davies RO. Interaction of serotonergic excitatory drive to hypoglossal motoneurons with carbachol-induced, REM sleep-like atonia. Sleep. 1996;19:187–195. [PubMed] [Google Scholar]
- Meza S, Giannouli E, Younes M. Control of breathing during sleep assessed by proportional assist ventilation. Journal of Applied Physiology. 1998;84:3–12. doi: 10.1152/jappl.1998.84.1.3. [DOI] [PubMed] [Google Scholar]
- Netick A, Dugger WJ, Symmons RA. Ventilatory response to hypercapnia during sleep and wakefulness in cats. Journal of Applied Physiology. 1984;56:1347–1354. doi: 10.1152/jappl.1984.56.5.1347. [DOI] [PubMed] [Google Scholar]
- Netick A, Foutz AS. Respiratory activity and sleep-wakefulness in the deafferented paralyzed cat. Sleep. 1980;3:1–12. doi: 10.1093/sleep/3.1.1. [DOI] [PubMed] [Google Scholar]
- Netick A, Orem J, Dement W. Neuronal activity specific to REM sleep and its relationship to breathing. Brain Research. 1977;120:197–207. doi: 10.1016/0006-8993(77)90900-3. [DOI] [PubMed] [Google Scholar]
- Normand H, Barragan M, Benoit O, Bailliart O, Raynaud J. Periodic breathing and O2 saturation in relation to sleep stages at high altitude. Aviation Space and Environmental Medicine. 1990;61:229–235. [PubMed] [Google Scholar]
- Nunez-Abades PA, Morillo AM, Pasaro R. Brainstem connections of the rat ventral respiratory subgroups: afferent projections. Journal of the Autonomic Nervous System. 1993;42:99–118. doi: 10.1016/0165-1838(93)90042-s. [DOI] [PubMed] [Google Scholar]
- Orem J. Medullary respiratory neuron activity: relationship to tonic and phasic REM sleep. Journal of Applied Physiology. 1980;48:54–65. doi: 10.1152/jappl.1980.48.1.54. [DOI] [PubMed] [Google Scholar]
- Orem J. Central respiratory activity in rapid eye movement sleep: augmenting and late inspiratory cells. Sleep. 1994;17:665–673. doi: 10.1093/sleep/17.8.665. (published erratum appears in Sleep 18, 137) [DOI] [PubMed] [Google Scholar]
- Orem J. Excitatory drive to the respiratory system in REM sleep. Sleep. 1996;19:S154–S156. doi: 10.1093/sleep/19.suppl_10.154. [DOI] [PubMed] [Google Scholar]
- Orem J. Augmenting expiratory neuronal activity in sleep and wakefulness and in relation to duration of expiration. Journal of Applied Physiology. 1998;85:1260–1266. doi: 10.1152/jappl.1998.85.4.1260. [DOI] [PubMed] [Google Scholar]
- Orem J, Dick T. Consistency and signal strength of respiratory neuronal activity. Journal of Neurophysiology. 1983;50:1098–1107. doi: 10.1152/jn.1983.50.5.1098. [DOI] [PubMed] [Google Scholar]
- Orem J, Netick A, Dement WC. Breathing during sleep and wakefulness in the cat. Respiration Physiology. 1977;30:265–289. doi: 10.1016/0034-5687(77)90035-4. [DOI] [PubMed] [Google Scholar]
- Orem J, Vidruk EH. Activity of medullary respiratory neurons during ventilator-induced apnea in sleep and wakefulness. Journal of Applied Physiology. 1998;84:922–932. doi: 10.1152/jappl.1998.84.3.922. [DOI] [PubMed] [Google Scholar]
- Parisi RA, Edelman NH, Santiago TV. Central respiratory carbon dioxide chemosensitivity does not decrease during sleep. American Review of Respiratory Diseases. 1992;145:832–836. doi: 10.1164/ajrccm/145.4_Pt_1.832. [DOI] [PubMed] [Google Scholar]
- Phillipson EA, Kozar LF, Rebuck AS, Murphy E. Ventilatory and waking responses to CO2 in sleeping dogs. American Review of Respiratory Diseases. 1977;115:251–259. doi: 10.1164/arrd.1977.115.2.251. [DOI] [PubMed] [Google Scholar]
- Phillipson EA, Sullivan CE. Respiratory control mechanisms during NREM and REM sleep. In: Guilleminault C, Dement WC, editors. Sleep Apnea Syndromes. New York: Liss; 1978. pp. 47–64. [Google Scholar]
- Phillipson EA, Sullivan CE, Read DJC, Murphy E, Kozar LF. Ventilatory and waking responses to hypoxia in sleeping dogs. Journal of Applied Physiology. 1978;44:512–520. doi: 10.1152/jappl.1978.44.4.512. [DOI] [PubMed] [Google Scholar]
- Praud JP, Egreteau L, Benlabed M, Curzi-Dascalova L, Nedelcoux H, Gaultier C. Abdominal muscle activity during CO2 rebreathing in sleeping neonates. Journal of Applied Physiology. 1991;70:1344–1350. doi: 10.1152/jappl.1991.70.3.1344. [DOI] [PubMed] [Google Scholar]
- Reite M, Jackson D, Cahoon RL, Weil JV. Sleep physiology at high altitude. Electroencephalography and Clinical Neurophysiology. 1975;38:463–471. doi: 10.1016/0013-4694(75)90188-1. [DOI] [PubMed] [Google Scholar]
- Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. Journal of Comparative Neurology. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Sullivan CE. Breathing in sleep. In: Orem J, Barnes CD, editors. Physiology in Sleep. New York: Academic Press; 1980. pp. 213–272. [Google Scholar]
- Sullivan CE, Kozar LF, Murphy E, Phillipson EA. Primary role of respiratory afferents in sustaining breathing rhythm. Journal of Applied Physiology. 1978;45:11–17. doi: 10.1152/jappl.1978.45.1.11. [DOI] [PubMed] [Google Scholar]
- Von Euler C. Brain stem mechanisms for generation and control of breathing pattern. In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology, section 3, The Respiratory System, Control of Breathing. part 1. II. Bethesda, MD, USA: American Physiological Society; 1986. pp. 1–67. [Google Scholar]
- Xi L, Smith CA, Saupe KW, Henderson KS, Dempsey JA. Effects of rapid-eye-movement sleep on the apneic threshold in dogs. Journal of Applied Physiology. 1993;75:1129–1139. doi: 10.1152/jappl.1993.75.3.1129. [DOI] [PubMed] [Google Scholar]


