Abstract
Porcine circovirus (PCV) was recently divided into 2 antigenically distinct types that differ (65% amino acid identity) in the protein encoded by open reading frame 2 (ORF2). Porcine circovirus 1 is apparently non-pathogenic and, in contrast, PCV2 is associated with porcine multisystemic wasting syndrome (PMWS). Our objective was to determine the extent of exposure of normal pigs in Canada and Costa Rica to PCV2. Recombinant DNA techniques were used to produce an antigen from ORF2 of PCV2 that was suitable for the detection of antibody in swine sera. The presence of PCV2 nucleotide sequences was detected using polymerase chain reaction (PCR) techniques. Using these tests, specific antibody and nucleotide sequences were demonstrated in sera from a cohort of pigs during a PMWS outbreak. Antibody was detected in normal, healthy hogs slaughtered in Canada (82.4% of 386) and in Costa Rica (14.6% of 322). This is the first report indicating the presence of PCV2 in Latin America. More than 50% of these sera also contained PCV2 nucleotide sequence. Although these hogs were healthy when slaughtered, they were infected with PCV2 and may have previously been ill. The widespread occurrence of PCV2 in swine suggests that this virus is adapted to replication in porcine tissue.
Introduction
Swine viruses are potential human health risks associated with occupational exposure or with the use of pig organs for xenotransplantation in humans. Porcine circovirus (PCV) was initially identified as a contaminant of porcine tissue culture and was not thought to be pathogenic (1). Subsequently, a variant of this virus was isolated from pigs with a clinical syndrome, which has been called postweaning multisystemic wasting syndrome (PMWS) (2).
The most obvious lesions of PMWS at necropsy are enlarged lymph nodes, non-collapsed lungs, ulceration of the gastric pars esophagea, and cranioventral pulmonary consolidation (3). Isolates of PCV from swine in several countries were found to be antigenically and genomically similar to previously reported isolates of PCV from pigs with PMWS (called PCV2), but distinct from the isolate of PCV from continuous PK15 cell cultures (called PCV1) (4).
Exposure to PCV has been detected using polymerase chain reaction (PCR) and serologic and immunohistochemical techniques (3). Furthermore, the detection of PCV2 in a field case dating back to 1994 indicates that this PCV type was circulating in pigs in Quebec (5) at about the same time that clinical PMWS was occurring in Saskatchewan (6). Seroconversion to PCV2, but not to PCV1, mild clinical signs of disease, and lesions of PMWS have been successfully reproduced by experimental inoculation of conventional pigs with a tissue homogenate obtained from pigs affected with PMWS (7). Pigs dually infected with PCV2 and porcine parvovirus (PPV) showed jaundice and hepatomegaly, lesions similar to those seen in recently described field cases of porcine PMWS (8). Piglets infected with PCV2 and PPV grown in cell culture also developed moderate to high titers of antibody to PCV and moderate titers to PPV (9). Porcine circovirus DNA and histopathologic lesions occur in many tissues and transplacental infection with PCV also occurs (10). Hence, this is an infectious pathogen of swine and the extent of exposure to PCV2 and infection with this virus among people and swine should be determined.
Sequence comparison between PCV1 and PCV2 revealed significant differences between the 2 PCV strains, which contain two major open reading frames (ORFs) with ORF2 from the 2 types sharing only 65% amino acid homology (11). The ORF2 is likely to encode the major structural protein of PCV (12). Multiplex PCR has been applied to diagnose samples from individual pigs (13) but it has not generally been applied to cohort or field studies in combination with the PCV2-specific antigen in order to detect specific antibody.
Whether previous studies using the whole virus or infected cell cultures as the enzyme linked immunosorbent assay (ELISA) antigen to detect an antibody to PCV, were able to differentiate between the PCV subtypes that are recognized today, is unclear. The antibody to PCV was generally common in swine herds, however, no correlation was evident between the levels of antibody to PCV1 and reproductive disorders in the herds (14). There is currently very little information concerning the prevalence of an antibody to PCV2 (15), and to our knowledge, this is the first report of a serologic investigation of circovirus in swine from Latin America.
Reported here are the results of a serologic survey of swine sera using the ORF2 antigen from PCV2, produced using recombinant DNA techniques, as an ELISA antigen. These studies were done to determine the proportion of the swine population that had evidence of exposure to PCV2 as a means to judge the potential risk of zoonotic disease transfer from pigs to people.
Materials and methods
In vivo passage of PCV2
All experiments involving animals were done with the approval of the University of Saskatchewan Campus Committee on Animal Care. The PCV2 was derived from mesenteric lymph nodes of swine from a Canadian herd (RDC) showing typical signs of PMWS, and passaged once in vivo. Sera collected from these naturally affected pigs were used in Western blots. For in vivo passage, piglets were derived by cesarian-section from a specific pathogen-free sow. At 1 d of age, they were challenged with a combined intraperitoneal and oral injection of the lymph node homogenate. The homogenate was prepared in sterile saline from mesenteric lymph node of 4 pigs from RDC herd that had signs of PMWS. Each of the challenged cesarian-derived piglets received a total of approximately 1 g of tissue (2.5 mL of homogenate). The challenged piglets were housed together, in isolation from other animals, and were fed canned milk and water (50:50) until they self-weaned to high nutrient density, commercially prepared feed.
Sequencing of PCV2 isolated from pigs with PMWS
Viral DNA was extracted from mesenteric lymph nodes of one of the challenged pigs (#412) after in vivo passage. Tissue was homogenized as described above, cell pellets were lysed with proteinase K, and DNA was extracted using phenol/chloroform. A 2-step approach was used for the initial cloning of isolate #412 viral genomic DNA. A primer that hybridized to the conserved loop stem sequences, loop (5′-ACTACAGCAGCGCACTTC-3′) was designed to perform a single primed PCR because of the complementary sequence and the circular nature of the viral DNA. The PCR for the single primed PCR was a 2 stage process. The first stage consisted of 5 cycles of denaturing at 94°C for 1 min, annealing at 37°C for 30 s and extension at 72°C for 2 min. The second stage consisted of 25 cycles of a similar program except that the annealing temperature was increased to 52°C.
The PCR products were cloned into a TA cloning vector according to the manufacturer's suggestion (Invitrogen, Carlsbad, California, USA). Both strands of 3 independent clones were sequenced to ensure sequence fidelity. A set of primers, 1000- (5′-AAAAAAGACTCAGTAATTTATTTCATATGG-3′) and R1F (5′-ATCACTTCGTAATGGTTTTTATT-3′), were designed from the non-coding region of the viral DNA sequences and used to clone the full-length viral genome including the previously omitted loop sequences. Both strands were sequenced by automated DNA sequencing (performed by Plant Biotechnology Institute, National Research Council, Canada) using internal primers.
Multiplex PCR from sera
DNA was extracted from swine serum using the Qiagen blood kit as suggested by the manufacturer (Qiagen, Valencia, California, USA). Two sets of primers were designed to amplify the PCV group specific sequences from primers 1710+/850− and PCV2 strain specific sequences from primers 1100+/1570−. The sequence of 1710+ is 5′-TGCGGTAACGCCTCCTTG-3′ and the sequence of 850- is 5′-CTACAGCTGGGACAGCAGTTG-3′. The sequence of 1100+ is 5′-CATAAATAGTCAGCCTTACCACA-3′ and the sequence of 1570- is 5′-TTCTACAGAATTTGTACTCACCA-3′. The 2 sets of primers have similar annealing temperatures and all 4 were used together at 0.5 μM concentration in a standard hot start PCR with either AmpliTaq Gold (Perkin Elmer, Norwalk, Connecticut, USA) or Plentinum Taq, (Gibco-BRL, Burlington, Ontario) enzyme.
Rabbit sera
For the preparation of polyclonal antibody, rabbits were immunized intramuscularly 3 times at 3-week intervals with PCV1 or PCV2 combined with an oil in water emulsion (Emulsigen; MVP Laboratories, Ralston, Nebraska, USA). The PCV1 antigen was obtained from porcine kidney cells (ATCC CCL-33) as previously described (16). The PCV2 antigen was produced in a similar manner except that #412 was cultivated in PCV-free PK-15 cells.
Expression and purification of GST-ORF2 fusion proteins in E. coli
The complete coding sequence of PCV2-ORF2 (nucleotides 1738 to 1039) was amplified by PCR and cloned into pGEX-5X-3 (Pharmacia Biotech Inc., Baie d'Urfé, Quebec) downstream of, and in-frame with the glutathione S-transferase (GST) sequence, creating plasmid pGEX-II-ORF2. The nucleotide sequence of the construct was verified by DNA sequencing. Expression of the fusion protein was induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a culture at 30°C of BL21 E. coli cells transformed with pGEX-II-ORF2. The GST-ORF2 fusion protein was purified from the bacterial lysate through the use of glutathione affinity column (Amersham-Pharmacia Biotech, Piscataway, New Jersey, USA). The protein concentration in the supernatant was determined using a protein assay (Bio-Rad Protein Assay; Bio-Rad Laboratories, Richmond, California, USA). This supernatant was stored frozen at −20°C until it was used as the antigen for Western blot and enzyme-linked immunosorbent assay (ELISA). Protein antigens for Western blots were boiled for 10 min in sodium dodecyl sulfate (SDS) gel-loading buffer with dithiothreitol (100 mM) (17) and loaded at 5 μg/lane for electrophoresis into 10% polyacrylamide gel. The separated proteins were transferred to nitrocellulose, blocked in 0.1 M phosphate-buffered saline (pH 7.2) with bovine serum albumin (3% w/v; PBS-B) for 1 h. Primary sera were used at 1:50 dilution and secondary conjugated antibodies were used at 1:5000 dilution in PBS-B, unless otherwise indicated. Filters were washed 3 times in PBS-B between each serum and were finally washed in AP buffer (Tris-HCl [100 mM; pH 9.5], NaCl (100 mM), MgCl2 [5 mM]). Immunoreactive protein bands were visualized by reaction with nitroblue tetrazolium (200 μM) and 5-bromo-4-chloro-3-indolyl phosphate (380 μM) in AP buffer.
Antibody assay technique
Antibodies to PCV2 in pig serum were measured by an ELISA modified from Smith et al (18). The antigen (GST-ORF2) was diluted to 100 μg protein per mL with fresh carbonate buffer (pH 9.6). The buffer contained; 2.93 mg of sodium bicarbonate, 1.59 mg of sodium carbonate, and 0.2 mg of sodium azide; per mL. Aliquots (0.1 mL) were added to the wells of the ELISA plates (Immulon II plates; Dynatek Laboratories, Santa Monica, California, USA), and incubated overnight at 4°C. The plates were washed 5 times with distilled water to remove unabsorbed antigen. Each antiserum was diluted 1:100 in Blotto diluent and 0.1 mL was added to the test well. The Blotto diluent contained powdered instant milk (3% w/v), Tween-20 (0.05% v/v), Tris-HCl (20 mM) and sodium chloride (0.5 M). After incubation for 1 h at room temperature (RT), the wells were emptied and washed 3 times with distilled water. An aliquot (0.1 mL) of a 1:1000 dilution in Blotto of affinity-purified, alkaline phosphatase-conjugated, goat anti-pig immunoglobulin G (IgG) (heavy and light chain; Kirkegaard & Perry Laboratories, Gaithersburg, Maryland, USA), was added to each well and the plates were incubated for 1 h at RT. The plates were emptied and washed 5 times in distilled water. The substrate solution (p-nitrophenyl phosphate [3 g/L] in 1 M diethanolamine; 0.5 mM magnesium chloride, pH 9.8) was added in aliquots (0.1 mL) and the reaction proceeded at RT for 1 h. The optical density at wavelength 405 nm (OD 450) was measured in a microplate reader (model 3550; Bio-Rad Laboratories).
Swine sera for test and control were used at a 1:10 dilution. Each plate was tested with the following positive and negative controls: i) antigen, convalescent pig serum, conjugate, and substrate (positive control); ii) antigen, fetal pig serum, conjugate, and substrate (negative control). A satisfactory test occurred when the OD of the positive control was greater than 0.2 and the OD of the negative control was less than 0.1. The convalescent pig serum consisted of antiserum containing antibodies to PCV2 antigens and was obtained from a pig in RDC herd 21 d following diagnosis of PMWS. The fetal pig serum was obtained from piglets that were delivered by cesarian section from sows raised in a herd free of PMWS. A sample of serum was classified as positive for the presence of antibody to PCV2 if the OD was greater than 3 times the OD of the negative control.
Cohort of pigs with naturally occurring PMWS
A cohort of 23 pigs was identified in a herd that was experiencing persistent PMWS. All pigs were clinically normal at 3 wk of age when weaned at the herd of origin. Samples of blood were collected from each pig 5 times; namely, when they were approximately 3, 4, 7, 9, and 11 wk of age. Two of these pigs, with clinical signs of PMWS, were humanely killed at 7 wk of age, and the other 21 pigs remained in the herd as part of commercial swine production.
Seroprevalence of PCV2 in healthy slaughter pigs in Alberta
In May 1996, 795 blood samples were collected from pigs slaughtered in Alberta. Pigs were sampled on a systematic basis, every 5th pig slaughtered during 1 h, each weekday, at different times. Samples were collected as the pigs were exsanguinated, and the pig's ear was notched as the sample was collected. Tattoos were then read from the carcasses of ear-notched pigs. With the assistance of the Alberta Pork Producers' Development Corporation, tattoos were confirmed and the geographic location of the farms was obtained. Tattoos were recorded and any herds with 3 or more samples were included in the analysis. This resulted in 386 samples, from 76 herds in Alberta that were tested to determine the seroprevalence of antibody to PCV2 using GST-ORF2 as the ELISA antigen.
Seroprevalence of PCV2 in healthy slaughter pigs in Costa Rica
Sera from 322 healthy slaughter pigs in Costa Rica were collected between February 15th, 1996 and April 15th, 1996. The samples were collected from pigs raised at 14 different locations. The sample size at each location was calculated based on the proportion of hogs produced at each of the sites enrolled in the National Herd Health Project representing the geographical areas with major swine production in the country. Individual pigs were selected for inclusion in the sampling using a random numbers table.
Results
In vivo passage of PCV2
In vivo passage of strain #412 confirmed this to be a virulent strain capable of producing lesions consistent with PMWS in 2 of 2 piglets sacrificed at 3 wk of age and 3 of 3 pigs sacrificed at 8 wk of age. Clinical evaluation showed an average weight gain of less than 100 g/d until 3 wk of age and dyspnea with puffing and mouth breathing. Postmortem examination showed good body condition with mild enlargement and brown-red discoloration of all body lymph nodes. The lungs were diffusely voluminous with prominent interlobular and subpleural edema. The lung of one pig was submitted for immunohistochemical examination to the Western College of Veterinary Medicine, Saskatoon, Saskatchewan, and showed lung lesions typical of PMWS with scattered cells that stained positively for PCV2 using hyperimmune porcine antisera.
Sequencing of PCV2 isolated from pigs with PMWS
The nucleotide sequence of PCV2 strain #412 (GenBank Locus AF085695) was similar (96% to 99% identity) to other sequences reported for PCV2.
Multiplex PCR
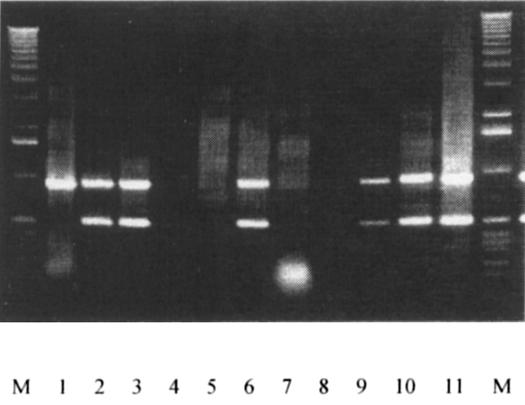
A multiplex PCR assay was shown to differentiate between PCV1 and PCV2. The DNA products with unique sizes characteristic of each type of PCV were obtained and sequenced to confirm that type-specific sequences were amplified. The primer pair 1710+/850− is PCV group-specific and 1100+/1570− is PCV2 strain-specific, and can be used to differentiate between PCV1 and PCV2 (Figure 1).
Figure 1. PCR test. Multiplex PCR of field samples using primer pairs of 1710+/850− and 1100+/1570−. The PCV group-specific pair 1710+/850− generates products of approximately 976 bp, and the PCV2-specific pair 1100+/1570− produces products of approximately 470 bp. The flanking lanes are the molecular mass markers (1 kb DNA ladder); the reaction products are from DNA extracted from CCL-33 (lane 1), PCV2 #412 (lane 2), and swine sera from a herd with naturally occurring PMWS disease (lanes 3 to 11).
GST-ORF2 fusion protein
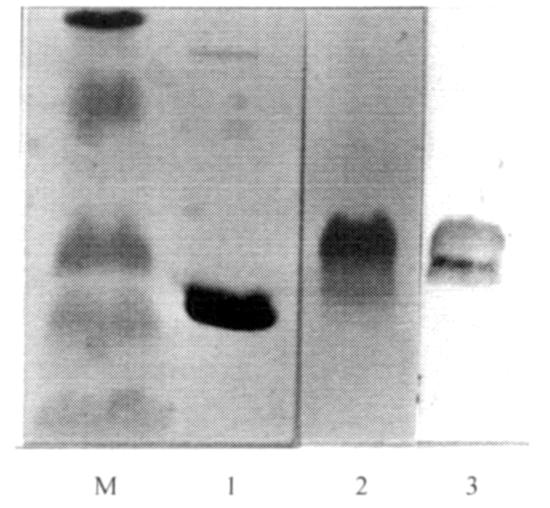
In order to avoid the problem of mixed infection in tissue culture by both types of PCV, resulting in a nonspecific antigen, we produced a fusion protein of GST and ORF2 from PCV2. The anti-GST antibody (Amersham-Pharmacia Biotech) detected one major protein band with an apparent molecular mass of 35 kDa (Figure 2, lane 2) in contrast to the predicted molecular weight of 52 kDa, indicating that proteolysis of the fusion protein had occurred. As shown in Figure 2, lane 3; the GST-ORF2 fusion protein reacted with swine serum collected from the RDC herd which was experiencing naturally-occurring PMWS. The GST-ORF2 fusion protein reacted with polyclonal rabbit sera against PCV2, but did not react with rabbit serum raised against PCV1, when each was used at 1:50 dilution in a Western blot (Figure 3).
Figure 2. Western blot with swine serum. Molecular mass markers (81, 51.2, 33.6, 28.6, and 21.1 kDa) are in the far left lane. Lanes 1; glutathione S-transferase (GST) and 2 (GST-ORF2) show bound goat anti-pig GST to GST in lane 1 and to a broad band of proteins of approximately 35 kDa molecular mass in lane 2. Lane 3 shows antibody in swine serum from RDC herd with naturally-occurring PMWS bound to GST-ORF2.
Figure 3. Western blot with rabbit sera. There are 5 lanes in this figure; molecular mass markers (207, 121, 81, 51.2, 33.6, 28.6, and 21.1 kDa) are in the middle. Lanes 1 (GST) and 2 (GST-ORF2) were reacted with rabbit anti-PCV1 and show no reaction with the antigens. Lanes 3 (GST) and 4 (GST-ORF2) were reacted with rabbit anti-PCV2 and show a distinct band of approximately 35 kDa.
Cohort of pigs with naturally occurring PMWS
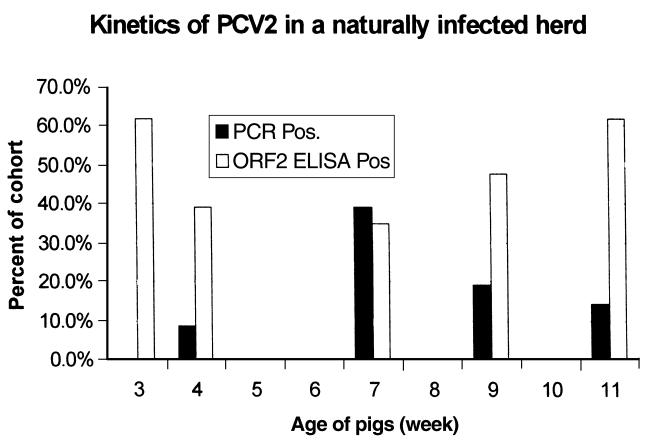
The antibody response of pigs that were experiencing a natural outbreak of PMWS was measured using GST-ORF2 in an ELISA format to evaluate the association of antibody response and disease. The proportion of the cohort of 23 pigs that was seropositive to GST-ORF2 showed an initial decline, consistent with waning passively transferred antibody. Figure 4 shows that this was followed by an increase in the proportion of the cohort that was seropositive beyond 7 wk of age, when 2 pigs with PMWS were humanely killed. The multiplex PCR done on these sera showed that PCV2-specific nucleotide sequences were also present preceding or coincident with clinical signs of PMWS disease. There is a temporal relationship between PCV2 nucleotide sequences in serum and antibody, as shown in Figure 4; however, as expected, the detection of specific nucleotide sequences was not a good predictor of the amount of antibody in an individual sample.
Figure 4. Kinetics of PCV2. Blood samples taken from a cohort of 23 pigs at 3, 4, 7, 9, and 11 wk of age were tested for PCV2 viral DNA by using PCR and for antibodies to GST-ORF2 fusion protein of PCV2 by using ELISA. Peak prevalence of circulating viral DNA occurred prior to, or concurrent with, clinical signs of PMWS. Two pigs with PMWS (254 and 294) that had circulating viral DNA but did not have a positive antibody response were humanely killed at 7 wk of age.
Seroprevalence of PCV2 in healthy slaughter pigs in Alberta
Overall, 386 serum samples were tested and 318 of these (82.4%) demonstrated antibody to PCV2-ORF2. Although these sera were obtained from clinically healthy pigs without discernable lesions, PMWS had been observed in swine raised in the area. Eleven herds had mean antibody levels greater than the grand mean plus one standard deviation. These herds were located near Edmonton, Alberta (mean distance of 101 km). In contrast, herds with antibody levels less than the grand mean minus 1 standard deviation were located farther away. This is also the region with the greatest concentration of swine in Alberta.
The DNA was extracted from the sera of these pigs and PCV2 specific nucleotide sequences were detected in 203 pigs (52.6%). Contingency table analysis and Fisher's exact test did not show significant association between the categorical data of presence of detectable antibody and PCV2-specific nucleotide sequences (P > 0.3).
Seroprevalence of PCV2 in healthy slaughter pigs in Costa Rica
Overall, 322 serum samples from 14 herds were tested and 47 of these (14.6%) demonstrated antibody to PCV2-ORF2. Contingency table analysis and Fisher's exact test confirmed that the prevalence of detectable antibody in hogs from throughout Costa Rica was significantly less than in hogs from Alberta (P < 0.001). The distribution of PCV2 is not uniform since, in 1 herd, 52.9% of pigs that were sampled had detectable antibody levels. The DNA was extracted from sera of 64 pigs in this herd and PCV2 specific nucleotides were detected in 12 (18.7%). Six herds (from which a total of 99 pigs were sampled) had no samples with detectable antibody.
Discussion
Open reading frame 2 from PCV2 was cloned downstream and in-frame with GST for use as an antigen in an ELISA format to detect antibody to PCV2. In parallel, we used multiplex PCR to determine whether antibody to PCV2 and viral DNA were detected concurrently. Serum samples were collected from young swine during a natural outbreak of PMWS and from healthy slaughter hogs from Canada and Costa Rica. We detected antibody in a cohort of swine during PMWS disease and in a high proportion of healthy hogs slaughtered for human consumption. PCV2-specific nucleotide sequences were also detected in about half of the swine sera and demonstrated that people were inevitably exposed to PCV2 as consumers or workers in the swine industry. The widespread occurrence of PCV2 in swine suggests that this virus is adapted to replication in porcine tissue.
There is a lot of interest in PMWS, a relatively new disease of swine, and in detecting the presence of PCV2. The virus has been detected indirectly by its ability to induce antibodies in infected swine. Clearly PCV1 and PCV2 differ in virulence for swine, and there is an antigenic difference between them (7). Previous serology studies have used PCV1 as an antigen to detect antibodies to porcine circoviruses (14,19) even though PCV1 infection of swine has not been shown to result in disease (10). In contrast, PCV2 is detected in lesions of PMWS, a severe wasting disease of swine (2). As may be expected, PCV2 is seldom the only infectious agent present in pigs with PMWS (8,9). However, PCV2 may be the infectious agent that is primarily responsible for disease (20).
During passage of PCV2 strain #412 in vivo, we found that injection of pigs with tissue homogenate from pigs affected with PMWS results in infection with PCV2 and histopathologic lesions similar to those seen in natural cases of PMWS. This is similar to results using other strains of PCV2 (7). Pigs developed an antibody response to PCV2 antigen.
Antibody was detected prior to, or concurrent with, the appearance of clinical signs of PMWS disease in a cohort of naturally-infected pigs. The fact that PMWS disease developed in some individuals despite a pre-existing antibody response suggests that antibody response to natural infection is not protective. When applied on a cohort basis, both tests (detection of specific nucleotide sequences and increases in the proportion of seropositive pigs) were associated with PMWS clinical signs.
Determining which test is “better” for diagnosis depends on whether one wants to find evidence of herd exposure or the presence of contagious virus. Pigs with clinical signs of PMWS generally have a high level of circulating PCV2 DNA (5), and are likely able to spread PMWS disease. However, pigs experimentally infected with PCV2, harbor detectable nucleotide sequences in many tissues, but do not produce a detectable signal in blood using multiplex PCR (data not shown). We did not determine the minimum amount of template DNA required for a positive result from the multiplex PCR; however a quantitative PCR has been described elsewhere (21). Pigs that have both circulating PCV2 and antibody are likely to be infectious, since macrophages can take up PCV2 and support viral replication (11,22). Thus, macrophages could phagocytose viral-antibody complexes, which could then replicate in macrophages, and under these circumstances antibody is not able to neutralize infectivity.
A high proportion of swine in Alberta; a region from which PMWS has been reported (2), had a high level of antibody to PCV2 (82.4%) or specific DNA sequences (52.6%). A similar pattern occurred in serum collected from swine in Costa Rica in 1996; and to our knowledge, this is the first report of the presence of PCV2 in Latin America. Although the prevalence of seropositive samples was significantly lower than in Canada, the high prevalence in swine populations in diverse countries means that the restriction of swine movement will not reduce spread of PCV2.
Since more than half of the swine were PCR positive and still reached market weight, the presence of PCV2 alone does not appear to be sufficient to cause debilitating disease. Nevertheless, we do not know if these carrier animals experienced suboptimal health or production. Serologic evidence of past infection may indicate previous episodes of disease since PCV2 is associated with disease at least in some cases (3,8).
Postweaning multisystemic wasting syndrome is apparently transmissible to other pigs by an aerosol of swine tissue (7). In this study PCV2 was detected in healthy swine slaughtered for human consumption using a conventional process that results in the generation of numerous aerosols. Abattoir workers and production personnel who are exposed to pig tissue are at risk of zoonotic disease caused by organisms such as Streptococcus suis (23) and Salmonella enterica (24). Confinement swine production, with people and swine in the same airspace, is increasing and there will be a continuing potential to transfer infectious agents from pigs to people and to expose people to swine-related antigens. Porcine circovirus 2 is a very small, non-enveloped, stable virus that is resistant to inactivation (25). Exposure to PCV2 may be more prevalent than exposure to other agents that are clearly zoonotic (such as S. suis) since there is low prevalence of S. suis antibody in people with occupational exposure to pigs (26).
Although DNA alone does not necessarily mean that infectious virus is present, PCV2-specific nucleotide sequences were amplified from DNA extracted from boiled pork liver, but were not found in cooked pork muscle tissue. Porcine circovirus 2 DNA is infectious to swine when injected (27). There is no evidence that humans have been infected with PCV during normal contact with pork products. However, individuals that are immunocompromised and receive xenotransplantation might be at greater risk of infection. Other sources of immunosuppression, including co-infection with other viral agents, could lead to disease since porcine respiratory and reproductive syndrome virus (PRRSV) and parvovirus seem to potentiate PMWS in swine (14). Likewise, PCV2 might potentiate pathogenic effects of other organisms such as Chlamydia sp. (28), which are related to important human pathogens. Hence, swine intended for future use in xenotransplantation should be screened for the presence of PCV2 as well as other zoonotic agents. The widespread occurrence of PCV2 in swine suggests that this virus is adapted to replication in porcine tissue and human exposure may result in an immune response without infection.
Footnotes
Acknowledgments
The authors thank Boehringer-Ingelheim and the Natural Sciences and Engineering Research Council of Canada for supporting the research. Also the technical assistance of Betty Chow, Elaine VanMoorlehem, and Tammy Karkut was greatly appreciated.
Address correspondence and reprint requests to Dr. Philip J. Willson, telephone: (306) 966-7465, fax: (306) 966-7478, e-mail: willson@sask.usask.ca
Received November 3, 2002. Accepted May 27, 2002.
Published with the permission of the Director of VIDO as journal series # 322.
References
- 1.Meehan BM, McNeilly F, Todd D, et al. Characterization of novel circovirus DNAs associated with wasting syndromes in pigs. J Gen Virol 1998;79:2171–2179. [DOI] [PubMed]
- 2.Ellis J, Hassard L, Clark E, et al. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can Vet J 1998;39:44–51. [PMC free article] [PubMed]
- 3.Rosell C, Segales J, Plana-Duran J, et al. Pathological, immunohistochemical, and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J Comp Pathol 1999;120:59–78. [DOI] [PubMed]
- 4.Allan GM, McNeilly F, Meehan BM, et al. Isolation and characterisation of circoviruses from pigs with wasting syndromes in Spain, Denmark and Northern Ireland. Vet Microbiol 1999; 66:115–123. [DOI] [PubMed]
- 5.Larochelle R, Antaya M, Morin M, Magar R. Typing of porcine circovirus in clinical specimens by multiplex PCR. J Virol Methods 1999;80:69–75. [DOI] [PubMed]
- 6.Harding J, Clark E, Strokappe J, Willson P, Ellis J. Postweaning multisystemic wasting syndrome: Epidemiology and clinical presentation. Swine Health Prod 1998;6:249–254.
- 7.Balasch M, Segales J, Rosell C, et al. Experimental inoculation of conventional pigs with tissue homogenates from pigs with post-weaning multisystemic wasting syndrome. J Comp Pathol 1999;121:139–148. [DOI] [PubMed]
- 8.Allan GM, Kennedy S, McNeilly F, et al. Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus. J Comp Pathol 1999;121:1–11. [DOI] [PubMed]
- 9.Ellis J, Krakowka S, Lairmore M, et al. Reproduction of lesions of postweaning multisystemic wasting syndrome in gnotobiotic piglets. J Vet Diagn Invest 1999;11:3–14. [DOI] [PubMed]
- 10.Allan GM, McNeilly F, Cassidy JP, et al. Pathogenesis of porcine circovirus; experimental infections of colostrum deprived piglets and examination of pig foetal material. Vet Microbiol 1995;44:49–64. [DOI] [PubMed]
- 11.Morozov I, Sirinarumitr T, Sorden SD, et al. Detection of a novel strain of porcine circovirus in pigs with postweaning multisystemic wasting syndrome. J Clin Microbiol 1998;36: 2535–2541. [DOI] [PMC free article] [PubMed]
- 12.Mankertz A, Mankertz J, Wolf K, Buhk HJ. Identification of a protein essential for replication of porcine circovirus. J Gen Virol 1998;79:381–384. [DOI] [PubMed]
- 13.Miyata H, Tsunoda H, Kazi A, et al. Identification of a novel GC-rich 113-nucleotide region to complete the circular, single-stranded DNA genome of TT virus, the first human circovirus. J Virol 1999;73:3582–3586. [DOI] [PMC free article] [PubMed]
- 14.Tischer I, Bode L, Peters D, Pociuli S, Germann B. Distribution of antibodies to porcine circovirus in swine populations of different breeding farms. Arch Virol 1995;140:737–743. [DOI] [PubMed]
- 15.Allan GM, Ellis JA. Porcine circoviruses: a review. J Vet Diagn Invest 2000;12:3–14. [DOI] [PubMed]
- 16.Dulac GC, Afshar A. Porcine circovirus antigens in PK-15 cell line (ATCC CCL-33) and evidence of antibodies to circovirus in Canadian pigs. Can J Vet Res 1989;53:431–433. [PMC free article] [PubMed]
- 17.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor: Laboratory Press, New York, 1989.
- 18.Smith PH, Ziola B, Stockdale PH. Solid-phase enzyme immunoassay of bovine antibody responses following immunization against and natural infection with Pasteurella haemolytica. Vet Immunol Immunopathol 1983;5:33–45. [DOI] [PubMed]
- 19.Tischer I, Bode L, Apodaca J, et al. Presence of antibodies reacting with porcine circovirus in sera of humans, mice, and cattle. Arch Virol 1995;140:1427–1439. [DOI] [PubMed]
- 20.Kennedy S, Moffett D, McNeilly F, et al. Reproduction of lesions of postweaning multisystemic wasting syndrome by infection of conventional pigs with porcine circovirus type 2 alone or in combination with porcine parvovirus. J Comp Pathol 2000;122:9–24. [DOI] [PubMed]
- 21.Liu Q, Wang L, Willson P, Babiuk LA. Quantitative, competitive PCR analysis of porcine circovirus DNA in serum from pigs with postweaning multisystemic wasting syndrome. J Clin Microbiol 2000;38:3474–3477. [DOI] [PMC free article] [PubMed]
- 22.Kiupel M, Stevenson GW, Mittal SK, Clark EG, Haines DM. Circovirus-like viral associated disease in weaned pigs in Indiana. Vet Pathol 1998;35:303–307. [DOI] [PubMed]
- 23.Tambyah PA, Kumarasinghe G, Chan HL, Lee KO. Streptococcus suis infection complicated by purpura fulminans and rhabdomyolysis: case report and review. Clin Infect Dis 1997;24: 710–712. [DOI] [PubMed]
- 24.Wegener HC, Baggesen DL. Investigation of an outbreak of human salmonellosis caused by Salmonella enterica subsp. enterica serovar Infantis by use of pulsed field gel electrophoresis. Int J Food Microbiol 1996;32:125–131. [DOI] [PubMed]
- 25.Allan GM, Phenix KV, Todd D, McNulty MS. Some biological and physico-chemical properties of porcine circovirus. Zentralbl Veterinarmed B 1994;41:17–26. [DOI] [PubMed]
- 26.Elbers AR, Vecht U, Osterhaus AD, et al. Low prevalence of antibodies against the zoonotic agents Brucella abortus, Leptospira spp., Streptococcus suis serotype II, hantavirus, and lymphocytic choriomeningitis virus among veterinarians and pig farmers in the southern part of The Netherlands. Vet Q 1999;21:50–54. [DOI] [PubMed]
- 27.Fenaux M, Halbur PG, Haqshenas G, et al. Cloned genomic DNA of type 2 porcine circovirus is infectious when injected directly into the liver and lymph nodes of pigs: characterization of clinical disease, virus distribution, and pathologic lesions. J Virol 2002;76:541–551. [DOI] [PMC free article] [PubMed]
- 28.Carrasco L, Segales J, Bautista MJ, et al. Intestinal chlamydial infection concurrent with postweaning multisystemic wasting syndrome in pigs. Vet Rec 2000;146:21–23. [DOI] [PubMed]