Abstract
We previously described an ultrarapid delayed rectifier current in dog atrial myocytes (IKur,d) with properties resembling currents reported for Kv3.1 channels in neural tissue; however, there was no direct molecular evidence for Shaw subfamily (Kv3) subunit expression in the heart. To identify the molecular basis of IKur,d, we cloned a full-length cDNA (dKv3.1) from canine atrium with homology-based reverse transcription (RT)- polymerase chain reaction (PCR) cloning techniques.
A 1755 bp full-length cDNA (dKv3.1) was obtained, with 94.2 % homology to rat brain Kv3.1 (rbKv3.1). The deduced amino acid sequence had 99.3 % homology with rbKv3.1.
Heterologous expression of dKv3.1 in Xenopus oocytes produced currents with activation voltage dependence, rectification, and activation and deactivation kinetics that strongly resemble native IKur,d. Like IKur,d, dKv3.1 was found to be highly sensitive to extracellular 4-aminopyridine (4-AP) and tetraethylammonium (TEA).
RNase protection assays, Western blots and immunohistochemical studies demonstrated the presence of dKv3.1 transcripts and proteins in dog atrial preparations and isolated canine atrial myocytes. Protein corresponding to the Kv1.5 subunit, which can also carry ultrarapid delayed rectifier current, was absent. Unlike neural tissues, which express two splice variants (Kv3.1a and Kv3.1b), canine atrium showed only Kv3.1b transcripts.
Whole-cell patch-clamp studies showed that IKur,d is absent in canine ventricular myocytes, and immunohistochemical and Western blot analysis demonstrated the absence of dKv3.1 protein in canine ventricle.
We conclude that the Shaw-type channel dKv3.1 is present in dog atrium, but not ventricle, and is the likely molecular basis of canine atrial IKur,d.
K+ channels are the most diverse class of ion channels and play a vital role in the functioning of diverse cell types (Hille, 1992; Rudy et al. 1999). A wide range of K+ currents, including transient outward (Ito), inward rectifier (IK1) and slow (IKs), rapid (IKr) and ultrarapid (IKur) delayed rectifier currents, are of key importance in shaping the cardiac action potential (Barry & Nerbonne, 1996; Deal et al. 1996; Sanguinetti & Zou, 1997; Nattel et al. 1999). IKur is a recently described family of delayed rectifier currents which share very rapid activation and slow inactivation but have diverse pharmacology and molecular biology (Nattel et al. 1999).
A number of Shaker family genes encoding pore-forming (α) subunits, including Kv1.1, Kv1.2, Kv1.4, Kv1.5, Kv2.1 and Kv4.2, have been cloned from the heart (Barry & Nerbonne, 1996; Deal et al. 1996; Nattel et al. 1999). Kv1.4 (Shaker subfamily), Kv4.2 and Kv4.3 (Shal subfamily) appear to underlie Ito (Dixon et al. 1996; Wang et al. 1999; Xu et al. 1999b), while Kv1.2, Kv1.5 (Shaker) and Kv2.1 (Shab) are believed to encode IKur in human, mouse and rat hearts (Barry & Nerbonne, 1996; Deal et al. 1996; Dixon et al. 1996; Feng et al. 1997; Sanguinetti & Zou, 1997; Nattel et al. 1999; Wang et al. 1999; Xu et al. 1999a). Co-assembly of minK with KvLQT1 underlies IKs and the human ether {kern 0 0}ago-go-related gene product (HERG) carries IKr (Sanguinetti & Zou, 1997), probably in association with the recently cloned subunit MiRP1 (Abbott et al. 1999).
Canine atrial myocytes possess an ultrarapid delayed rectifier current (IKur,d) that plays a significant role in canine atrial repolarization and has a variety of unusual properties, including inward rectification at positive potentials and great sensitivity to 4-AP and TEA (Yue et al. 1996). These properties distinguish IKur,d from other native IKurs and from all previously described cardiac K+ channel clones (Barry & Nerbonne, 1996; Deal et al. 1996; Sanguinetti & Zou, 1997; Nattel et al. 1999). Many of these unusual properties are characteristic of Kv3.1 channels cloned from brain and lymphocytes (Grissmer et al. 1992; Rettig et al. 1992). Kv3.1 transcripts of unknown physiological significance have been detected in ferret heart (Brahmajothi et al. 1996), but to date no Kv3 cDNAs have been cloned from cardiac tissues, nor has corresponding protein been detected in cardiac tissues. The present study was designed to determine whether a clone related to Kv3.1 that may underlie IKur,d is expressed in the canine heart.
METHODS
Mongrel dogs of either sex were anaesthetized with intravenous pentobarbitone (30 mg kg−1) and the hearts removed via a right thoracotomy. Tissues for cell isolation were placed in pre-oxygenated Tyrode sulution. Tissues for molecular sudy were fast-frozen.
Human atrial tissue samples were obtained from the atria of tissues undergoing coronary artery bypass surgery, were snap-frozen in liquid nitrogen and stored at −80°C for subsequent use. The human tissue samples were from patients without any known atrial disease and were grossly normal at the time of excision. All procedures for tissue procurement were approved as appropriate by Human or Animal Research Ethics Committees of the Montreal Heart Institute; this included informed patient consent.
Preparation of RNA
RNA samples were prepared as previously described (Yue et al. 1999b). In brief, total RNA was isolated from frozen atrial muscle tissue which was free of blood, or from cultured atrial myocytes, and subsequently incubated with DNase I (0.1 U μl−1, Ambion) for 15 min to eliminate DNA. Phenol-chloroform extraction and isopropyl alcohol precipitation was followed by mRNA purification with the Poly(A) Quik mRNA isolation system (Stratagene). The concentration of RNA was quantified by spectrophotometric analysis (Spectronic Genesys) at a wavelength of 260 nm, and the integrity of each RNA sample was confirmed by analysis on a denaturing agarose gel.
Cloning dKv3.1 from canine atrium
Full-length Kv3.1 cDNA clones were isolated from canine atrial mRNA using standard RT-PCR procedures. A 723 bp fragment was amplified from the 3′ end with the use of the primer pair complementary to the corresponding cDNA sequences:
CTGCTGCTTATCATCTTCCTG (nucleotides 1036–1056)
and GGTCAAGTCACTCTCACAGCCTC (nucleotides 1738–1760)
based on a sequence that is highly conserved across various Kv3.1 clones and shows little homology with other genes. Similarly, a 1362 bp fragment was amplified from the 5′ end with the use of the primer pair:
ATGGGCCAAGGGGACGAG (nucleotides 1–18)
and CTTTGGTAGTTTCTGC TTAGC (nucleotides 1342–1362).
A third PCR was performed with the use of the primer pair:
ATGGGCCAAGGGGACGAG (sense primer)
and GGTCAAGTCACTCTCACAGCCTC (antisense primer)
and the two previously amplified fragments as the templates, resulting in a single full-length cDNA (1761 bp), including the start codon (nucleotides 1–3), the rest of the coding region until nucleotides 1755, the stop codon (nucleotides 1756–1758) and three additional untranslated nucleotides (1759–1761). The PCR product was cloned to pGEM-T vector (Promega) and the DNA sequence was analysed with Sequenase V2.0 (Amersham) in both directions.
RNase protection assay (RPA)
cDNA fragments corresponding to the 3′-teminal segments 1451–1758 and 1163–1758 of Kv3.1 were cloned to PGEM-T easy vector for preparation of RNA probes. The cDNA template was linearized with Sal I restriction enzyme to ensure that a significant amount of non-hybridizing sequence (∼80 bp) was included in the probe to allow easy distinction between the probe and the specific protected band. RNA probes were synthesized using in vitro transcription kits (Ambion).
RNase protection assays (RPAs) were performed according to the HybSpeed RPA (Ambion) protocol. For each sample point, 10 or 20 μg total RNA was used in the assay. Yeast tRNA (20 μg) was used as a negative control to test for the presence of probe self-protection bands.
Western blot analysis
Membrane preparations were prepared from canine atrial tissue, human atrial tissue, canine ventricle, or isolated canine atrial myocytes as previously described (Yue et al. 1999b). Membrane proteins (20 μg) were fractionated on 8 % SDS-polyacrylamide gels and transferred to polyscreen PVDF membranes, and subsequently probed with commercially available antibodies directed against Kv3.1b or Kv1.5 (Alomone Labs). The Kv3.1 antibody was raised against an amino acid sequence with 100 % homology to the deduced amino acid sequence of dKv3.1, corresponding to residues 567–585 of rat brain Kv3.1b. Bound antibodies were detected using the CPSD chemiluminescent phosphatase substrate.
Immunocytochemistry
Freshly isolated canine cardiac myocytes were plated on laminin-coated coverslips and maintained in serum-free medium 199 for 1 h at 37°C in a 95 % air-5 % CO2 incubator. Cells were then washed with PBS (phosphate-buffered saline, containing (mm): 136.9 NaCl, 2.7 KCl, 10.1 Na2HPO4 and 1.76 KH2PO4) and fixed in 1.5 % formaldehyde for 10 min. After another wash with PBS, cells were incubated in 1 % Triton X-100 for 10 min to permeabilize membranes. Cells were then washed with PBS 3 times, and were incubated in blocking buffer (1 % bovine serum albumin (BSA) in PBS) overnight at 4°C to block non-specific sites. Cells were then incubated with antibody against Kv3.1b (Alomone, 1:50 dilution), or antibody pre-incubated with the peptide sequence against which it had been raised, for 2 h at room temperature. After washing with PBS, cells were incubated with the secondary antibody, anti-rabbit IgG conjugated with fluorescein isothiocyanate (FITC; 1:200, Santa Cruz Biotechnology) at room temperature for 1 h. Fluorescence microscopy was performed with a BioRad MicroRadiance confocal microscope. Secondary antibodies were excited with an argon laser at an excitation wavelength of 488 nm and fluorescent emission imaged at a wavelength of 525 nm.
Immunohistochemistry
Dog atria and ventricles were frozen in isopentane (precooled in liquid nitrogen), embedded in Cryomatrix (Shandon), and cut with a cryostat into 8–15 μm thick tissue sections. The sections were laid out on gelatin-coated slides, fixed for 30 min in 4 % paraformaldehyde, and incubated in 1 % Triton X-100 for 30 min. After three washes in PBS, the sections were incubated overnight with Kv3.1 antibody at 4°C, washed with PBS, and incubated with secondary antibodies conjugated with FITC for 1 h. Fluorescent imaging was performed in the same fashion as for isolated cells.
Electrophysiological methods
dKv3.1 cDNA was sub-cloned into Psp64 vector for expresssion. 5′-Capped and polyadenylated cRNA was transcribed from dKv3.1 Eco RI-linearized cDNA templates using in vitro Transcription Kits (Ambion). Xenopus laevis oocytes were injected with 46 μl cRNA (1–20 pg μl−1) and incubated for 20 h at 16°C. Whole-cell macroscopic currents were recorded with conventional two-electrode voltage-clamp techniques as described previously (Feng et al. 1998). Electrodes with resistances of approximately 1.0–1.5 MΩ were filled with 3 mol l−1 KCl and connected to a GeneClamp-500 amplifier (Axon Instruments). The bath solution contained (mmol l−1): 100 NaCl, 5 KCl, 0.3 CaCl, 2 MgCl2 and 10 Hepes (pH 7.4). Experiments were conducted at room temperature (22 −23°C).
Canine cardiomyocytes were isolated by previously described coronary artery perfusion methods (Yue et al. 1996). In brief, dogs were anaesthetized with pentobarbital (30 mg kg−1i.v.) and their hearts removed by a right thoracotomy. The left circumflex coronary artery was cannulated and right atrial and ventricular tissues perfused at 12 ml min−1 with Tyrode solution (37°C) aerated with 100 % O2. Any leaking arterial branches were ligated with silk thread. The tissue was then perfused with Ca2+-free Tyrode solution for 20 min, followed by the same solution containing collagenase (100 unit ml−1; CLSII, Worthington Biochemical Corp., Freehold, NJ, USA) and 1 % BSA for 40 min. A small sample of tissue was removed at 5 min intervals, minced and examined by microscopy to assess cell yield. Cells were stored at room temperature in a high-K+ storage solution until needed.
The whole-cell patch-clamp technique was used to record ionic currents in the voltage-clamp mode. Borosilicate glass electrodes (outer diameter, 1.0 mm) were filled with pipette solution and connected to a patch-clamp amplifier (Axopatch 1-D, Axon Instruments). Electrodes with a tip resistance of 1–2 MΩ were used to record whole-cell currents. Recordings were low-pass filtered at 10 kHz, and series resistance was compensated. The pipette solution contained (mm): 0.1 GTP, 110 potassium aspartate, 20 KCl, 1 MgCl2, 5 Mg2ATP, 10 Hepes, 5 sodium phosphocreatine and 10 EGTA (pH 7.3, KOH). The extracellular (Tyrode) solution contained (mm): 126 NaCl, 2 CaCl2, 5.4 KCl, 0.8 MgCl2, 0.33 NaH2PO4, 0.2 CdCl2, 10 dextrose and 10 Hepes; pH 7.4 (NaOH). The high-K+ storage solution contained (mm): 20 KCl, 10 KH2PO4, 10 dextrose, 70 glutamic acid, 10 β-hydroxybutyric acid, 10 taurine, 10 mannitol and 5 EGTA; and 1 % albumin; pH 7.4 (KOH).
Data analysis
Group data are presented as the mean ±s.e.m. A non-linear least-squares curve-fitting program (Clampfit in pCLAMP) was used to fit current activation, deactivation and inactivation kinetics. Boltzmann distributions and concentration-response curves were fitted to experimental data as indicated with the use of Prism software.
RESULTS
Cloning of dKv3.1
A full-length Kv3.1 cDNA (dKv3.1) was cloned from dog atria. Of 10 positive clones, 7 were sequenced, and all had identical sequences. The nucleotide sequence of dKv3.1 (Genbank accession no. AF 153198) shows 94.2 % homology to that of Kv3.1 cloned from rat brain (Genbank accession no. X62840). The 585 deduced amino acid sequence has 99.3 % homology with rat brain Kv3.1 (Fig. 1).
Figure 1. Deduced amino acid sequence of dKv3.1 compared with that of rat brain Kv3.1 (RBKv3.1).

Dots indicate identical units. The transmembrane domains and pore regions are indicated by boxes.
Expression of dKv3.1 in dog atrium
Figure 2A shows the results of RPA with the nucleotide 1451–1758 probe, which protected 308 bp bands as predicted, in RNA from dog atria (lanes 3 and 4), isolated atrial myocytes (lane 5) and rat brain (positive control, lane 6). Note the expected size difference from the riboprobe (lane 1) and the absence of a protected band in the tRNA control (lane 2). Similar bands were observed in all atrial samples tested (5 RNA extracts from one dog atrium each, and extracts from each of two atrial cell isolates).
Figure 2. Evaluation of the presence of dKv3.1 mRNA in canine atrium.
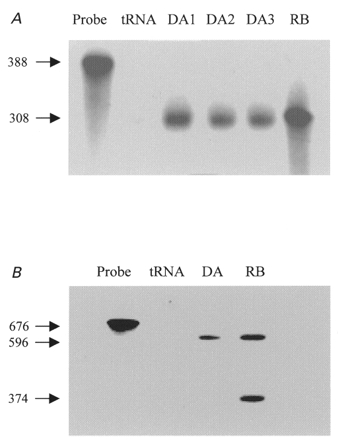
A, RPA with a probe for Kv3.1b. Lane 1, probe. Lane 2, negative control (tRNA). Lanes 3 and 4, RNA from one dog atrium in each lane (DA1, DA2). Lane 5, RNA extracted from isolated canine atrial myocytes (DA3). Lane 6, RNA from rat brain (RB). B, RPA with a probe for Kv3.1a and Kv3.1b. Lane 1, probe. Lane 2, tRNA. Lane 3, RNA from dog atrium. Lane 4, rat brain sample.
To determine whether the alternative splice variant (Kv3.1a) is expressed, we used a larger probe (nucleotides 1163–1758) with 596 nucleotides complementary to Kv3.1b and 374 nucleotides complementary to Kv3.1a. As shown in Fig. 2B, two protected bands with appropriate sizes (596 and 374 bp) were observed in rat brain samples. Canine atrial tissue showed only one band, corresponding to Kv3.1b, in four experiments.
In order to establish the expression of ion channel subunit proteins in dog atrium, we performed the Western blot studies illustrated in Fig. 3. Anti-Kv3.1b antibody identified a single protein band at 97 kDa in preparations from dog atrial tissue (Fig. 3A, lane 1) and isolated atrial myocytes (lane 2). The band was absent when antibody was pre-exposed to Kv3.1 peptide antigen (lane 3). Kv3.1 protein was detected in all five atrial tissues and two isolated myocyte samples studied. Figure 3B shows Western blots for human (lane 1) and canine (lane 2) atrial preparations probed with an antibody directed against the Kv1.5 subunit protein. A clear signal at the molecular mass expected (75 kDa) is present in human atrium, consistent with the established role of Kv1.5 subunits in human atrial IKur. In contrast, Kv1.5 protein was not detected in canine atrium. Similar results were obtained in five experiments.
Figure 3. Protein studies in dog atrium.
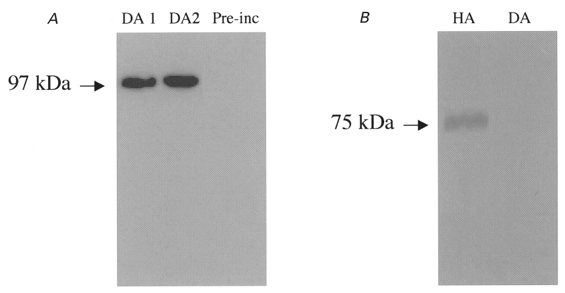
A, Western blots of membrane proteins prepared from dog atrium (lane 1, DA1) and isolated dog atrial myocytes (lane 2, DA2) probed with a Kv3.1 antibody. Lane 3, dog atrial membrane preparation after pre-incubation of antibody with Kv3.1 peptide (Pre-inc). B, Western blots with Kv1.5 antibody obtained with human atrial (HA) and dog atrial (DA) tissues.
Figure 4A shows results of immunocytochemical studies. No fluorescent staining was observed when the antibody was pre-incubated with Kv3.1 peptide (a), but clear staining is observed in three cells incubated in antibody not pre-exposed to antigen (b, c and d), particularly at cell junctions and along the membrane. Similar results were obtained in myocytes isolated from each of four dogs. Figure 4B shows fluorescence in atrial tissue sections (left panels) and corresponding bright-field images (right panels). Figure 4Ba shows clear fluorescent staining corresponding in location to the cell membranes in Fig. 4B b. The section in Fig. 4B c was treated in the same fashion as that shown in Fig. 4B a, except that the Kv3.1 antibody had been pre-incubated with the peptide against which the antibody had been raised. Pre-incubation prevented completely the detection of membrane-related fluorescence. Similar results were obtained from atrial tissues of each of six dogs.
Figure 4. Immunohistochemical evidence for the presence of dKv3.1 in dog atrial myocytes.
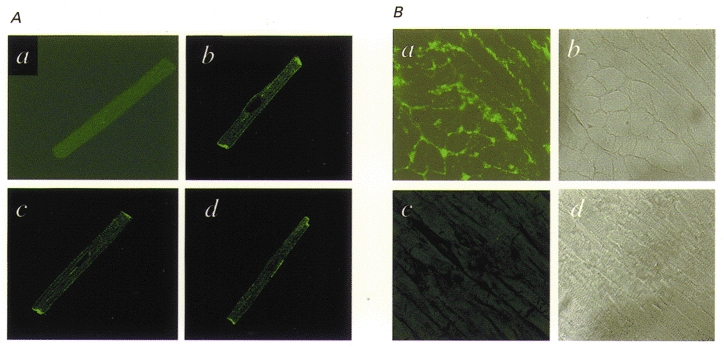
A, immunocytochemical images of canine atrial myocytes prepared with primary antibody pre-incubated (a) and not pre-incubated (b-d) with the Kv3.1 peptide against which the antibody had been raised. B, tissue sections of dog atrium: a is a typical immunohistochemical image prepared with Kv3.1 antibody and b is a bright-field micrograph of the same section; c was prepared in the same fashion as a, but with the Kv3.1 antibody pre-incubated with Kv3.1 peptide, and d is a bright-field image of the section shown in c.
Functional expression of dKv3.1 in Xenopus oocytes
Figure 5A shows currents elicited in Xenopus oocytes expressing dKv3.1. Depolarization induced rapidly activating and slowing inactivating currents, with tail currents evident upon repolarization to −30 mV. Positive to +30 mV, the step current failed to increase despite an increased driving force, pointing to inward rectification. Figure 5B shows IKur,d recordings in dog atrial myocytes. Currents were obtained with a holding potential of −50 mV and 140 ms test pulses to various voltages. The test pulses were preceded by an 80 ms pulse to +30 mV ending 10 ms before the test pulse, in order to suppress Ito. Step and tail current-voltage (I-V) relations of dKv3.1 (in 8 oocytes, Fig. 5C) and IKur,d (in 8 myocytes, Fig. 5D) have similar forms.
Figure 5. Comparison between currents carried by dKv3.1 and IKur,d.
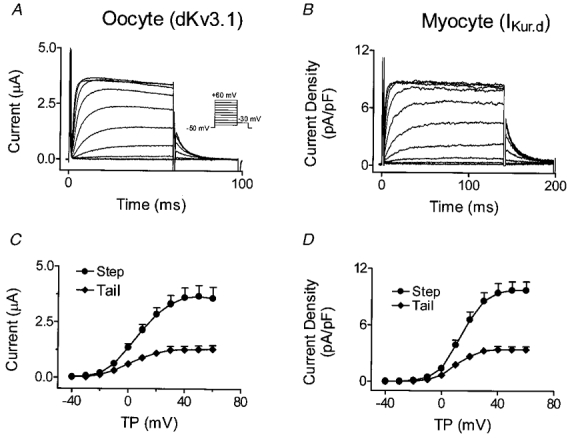
A, currents recorded from an oocyte 20 h after dKv3.1 cRNA injection. B, currents in a dog atrial myocyte. C, mean (±s.e.m.) current-voltage relation of step and tail currents recorded from oocytes (n = 8). D, mean current density-voltage relation of step and tail currents recorded from myocytes (n = 8). TP, test potential.
Analysis of dKv3.1 tail currents (recorded as illustrated in Fig. 6A) provided the Boltzmann relation shown in Fig. 6B. Half-maximal activation voltages (V1/2) and slope factors (k) in eight cells averaged 1.8 ± 0.6 and 7.3 ± 0.5 mV, respectively. Figure 6C illustrates voltage-dependent inactivation produced in an oocyte by 60 s conditioning pulses to various potentials. Mean (±s.e.m.) inactivation voltage dependence with 1, 10 and 60 s prepulses (n = 8 each) is shown in Fig. 6D. V1/2 averaged −0.7 ± 0.3, −2.3 ± 1.5 and −26.6 ± 0.9 mV and slope factor −10.1 ± 0.2, −7.9 ± 0.4 and −5.2 ± 0.3 mV with 1, 10 and 60 s conditioning pulses, respectively. In eight dog atrial myocytes subjected to 1 s prepulses with the voltage protocol shown in Fig. 6C and D, V1/2, k and maximum inactivation averaged 0.5 ± 0.1 mV, −9.1 ± 1.0 mV and 24.3 ± 4.1 %, similar to dKv3.1 results with 1 s prepulses (−0.7 ± 0.3 mV, −10.1 ± 0.2 mV and 25.1 ± 1.2 %, respectively).
Figure 6. Voltage- and time-dependent properties of dKv3.1 currents in oocytes.
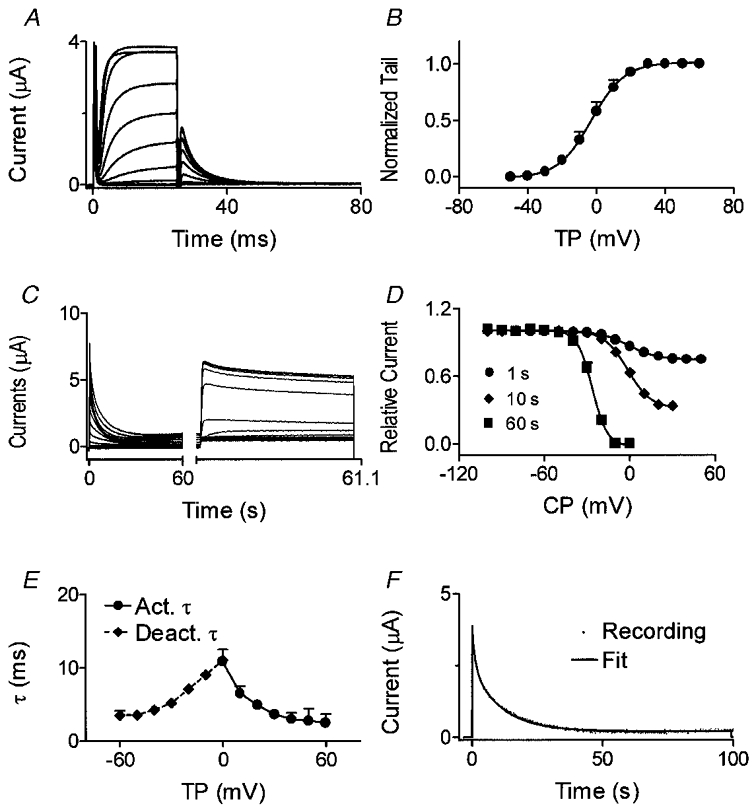
A, currents elicited by 20 ms pulses from a Vh of −80 mV. Tail currents were recorded upon repolarization to −30 mV. B, normalized tail currents fitted by a Boltzmann distribution (n = 8). C, currents elicited by 60 s conditioning pulses to conditioning potentials (CP) between −100 and +50 mV followed by a 1100 ms test pulse to +50 mV. D, inactivation curve with conditioning pulses of 1, 10 and 60 s (n = 8 for each). E, voltage-dependent activation and deactivation time constants (n = 8). F, time-dependent inactivation of dKv3.1 during a 100 s pulse to +40 mV and biexponential fit.
Activation time constants (τ) ranged from 19.1 ± 2.8 ms at −10 mV to 2.7 ± 0.6 ms at +50 mV, and deactivation τ ranged from 3.5 ± 0.6 ms (−60 mV) to 13.3 ± 0.7 ms (−10 mV) (Fig. 6E). The development of inactivation during a pulse to +40 mV was biexponential (Fig. 6F). During a 20 s pulse, dKv3.1 decreased by 72.2 ± 2.7 % (n = 8), and a 100 s pulse induced complete inactivation (Fig. 6F). Inactivation τ averaged 568 ± 74 ms and 8.1 ± 0.6 s during 20 s pulses, and 2.0 ± 0.4 s and 11.6 ± 0.8 s during 100 s pulses (n = 8).
Effects of K+ channel blockers
The response of IKurs to K+ channel blockers is often a signature of their molecular basis (Nattel et al. 1999). The response of dKv3.1 in one oocyte to 4-AP is illustrated in Fig. 7A, which shows potent inhibition and no major change in kinetics. Activation voltage dependence (as assessed by tail currents) was not affected by 4-AP. For example, V1/2 in the presence of 10 μm 4-AP, which reduced the current by about 50 %, averaged 2.5 ± 0.4 mV in eight cells, not significantly different from the average value (3.2 ± 0.5 mV) in the same cells before 4-AP infusion. Concentration- response data (Fig. 7B) were fitted by a Hill equation (B = 100/[1 +{IC50/D}nH], where B is the percentage of maximal current block at concentration D, and nHis the Hill coefficient). The 4-AP IC50 averaged 14.4 ± 1.5 μm with a Hill coefficient of 0.53 ± 0.05 (n = 8). TEA inhibited dKv3.1 in a concentration-dependent manner (Fig. 7C), without altering dKv3.1 activation voltage dependence (n = 8, V1/2 = 4.0 ± 0.6 and 4.0 ± 0.5 mV before and after 100 μm TEA, respectively). The TEA IC50 averaged 133 ± 14 μm and the Hill coefficient 0.86 ± 0.09 (n = 6,Fig. 7D). Dendrotoxin (DTX; 100 nm) had no effect on dKv3.1. For example, tail current amplitude following a pulse to +30 mV averaged 1.2 ± 0.1 and 1.1 ± 0.1 μA before and after 100 nm DTX, respectively (n = 8,P = n.s.).
Figure 7. Response of dKv3.1 to 4-AP and TEA.
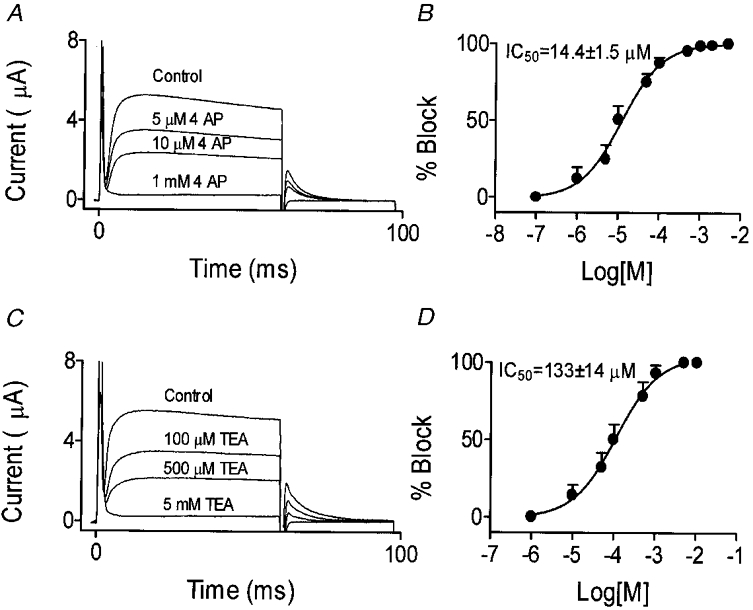
A, original recordings from one oocyte under control conditions and after exposure to varying concentrations of 4-AP. B, dose-response curve for 4-AP effects on tail currents following an activating pulse to +30 mV (n = 8). C, original recordings from another oocyte under control conditions and after exposure to varying concentrations of TEA. D, dose-response curve for TEA inhibition of dKv3.1 tail currents following an activating pulse to +30 mV (n = 6).
Evaluation of dKv3.1 expression and corresponding currents in canine ventricle
In order to evaluate further the correspondence between dKv3.1 expression and IKur,d, we performed Western blot, immunohistochemical and voltage clamp studies on canine ventricle. Figure 8A shows canine atrial (lane 1) and ventricular (lane 2) membranes probed with Kv3.1 antibody. Whereas a clear signal at the expected size (97 kDa) is present in atrium, no signal is detectable in ventricle. Similar results were obtained in three experiments. Figure 8B (left) shows an immunohistochemical stain of dog ventricular tissue probed with Kv3.1 antibody and a corresponding bright-field image (right). The immunohistochemical image resembles that for atrial sections stained with antibody pre-incubated with Kv3.1 peptide (e.g. see Fig. 4B c), showing no specific staining. Similar results were obtained in three experiments with canine ventricular tissue. Figure 8C shows currents from a canine ventricular myocyte recorded with 180 ms test pulses from a holding potential of −50 mV, preceded by an 80 ms prepulse to +30 mV (10 ms before the test pulse) to suppress Ito. No current is present under control conditions (Fig. 8C, left) and the addition of 50 μm 4-AP did not alter the recordings (Fig. 8C, right). Similar results were obtained for all eight canine ventricular myocytes studied. The absence of ultrarapid delayed rectifier current, and in fact of any current with the 4-AP sensitivity typical of IKur,d, excludes the functional expression of IKur,d in canine ventricular cells. Thus the expression (or lack of it) of dKv3.1 in canine cardiac tissues is concordant with the presence (or absence) of IKur,d.
Figure 8. Evaluation of dKv3.1 protein and IKur,d current expression in dog ventricle.
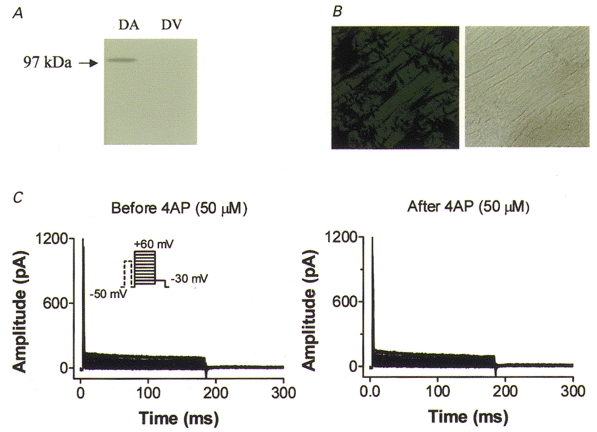
A, Western blots of dog atrial (DA) and ventricular (DV) tissues with Kv3.1 antibody. B, immunohistochemical image obtained with Kv3.1 antibody (left) and bright-field image (right) on a dog ventricular tissue section. C, currents recorded from a dog ventricular myocyte with the same voltage protocol and conditions as used to record IKur,d in atrium (Fig. 5B). Currents were recorded before (left) and after (right) superfusion with 50 μm 4-AP.
DISCUSSION
We have cloned a Shaw subfamily K+ channel subunit-encoding cDNA, dKv3.1, from canine atrium. The presence of both dKv3.1 mRNA and protein was demonstrated in canine atrial tissue and isolated atrial myocytes. Heterologous expression of dKv3.1 in Xenopus oocytes generated currents very similar to native atrial IKur,d. Both dKv3.1 protein and IKur,d were absent in canine ventricle, further supporting their inter-relationship.
Expression of Kv3.1 in the heart and the molecular basis of IKur,d
Previous work evaluating the presence of Kv3 transcripts in cardiac tissue has been limited. Rettig et al. (1992) were unable to detect Kv3.1 mRNA in the rat heart by Northern blot with a probe directed to the 3′ untranslated region, and RPA showed no expression of Kv3.1 mRNA in dog ventricle (Dixon et al. 1996). Brahmajothi et al. (1996) applied in situ hybridization to ferret hearts, and found Kv3.1 mRNA of unknown functional significance in 10–39 % of ferret cardiac myocytes. Our study demonstrates the presence of definable Kv3.1 cDNA in canine atrium, shows that its expression produces a current very similar to native IKur,d, and demonstrates corresponding mRNA and protein in native tissues.
The Kv1.5 gene encodes the ultrarapid delayed rectifier (IKur) in human atrium, and Kv1.2 and/or Kv2.1 genes encode IKur in rat hearts (Barry & Nerbonne, 1996; Deal et al. 1996; Bou-Abboud & Nerbonne, 1999; Nattel et al. 1999; Xu et al. 1999a). Table 1 shows a comparison of the properties of dKv3.1 currents with native IKur,d and with currents carried by other cloned cardiac K+ channel subunits with similar kinetics (Rettig et al. 1992; Grissmer et al. 1994; Barry & Nerbonne, 1996; Deal et al. 1996; Castellano et al. 1997; Harvey, 1997; Murakoshi et al. 1997; Feng et al. 1998; Klemic et al. 1998; Mathie et al. 1998; Nattel et al. 1999). The pharmacological properties of IKur,d differ clearly from those of Kv1.5, Kv1.2 and Kv2.1. The sensitivity of IKur,d to 4-AP is substantially greater than that of Kv1.2 and Kv2.1, and its sensitivity to TEA is much greater than that of Kv1.5 and Kv2.1. The insensitivity of IKur,d to DTX contrasts with the great sensitivity of Kv1.2. Results for previously reported Kv3.1 clones are generally similar to dKv3.1, with the exception of 4-AP sensitivity, which appears slightly greater for dKv3.1 and IKur,d compared with Kv3.1 from other tissues. The > 99 % amino acid homology between dKv3.1 and Kv3.1 cloned from brain is consistent with previous findings that neural and lymphocyte Kv3.1 gene products in mouse, rat and man are highly conserved (Luneau et al. 1991; Grissmer et al. 1992; Rettig et al. 1992; Ried et al. 1993). Unlike other Shaker subfamily genes, Shaw-related genes can exhibit alternative splicing within the coding region, producing transcripts (Kv3.1a and Kv3.1b) which encode functionally indistinguishable K+ channels (Luneau et al. 1991; Rettig et al. 1992). We found that, unlike neural tissue, canine atrium expresses only the Kv3.1b variant.
Table 1.
Comparison of properties of dKv3.1 with native IKur,d and with other cardiac clones having similar kinetics
| IKur,d | dKv3.1 | Kv1.5 | Kv1.2 | Kv2.1 | Kv3.1 | |
|---|---|---|---|---|---|---|
| Activation | ||||||
| V½ (mV) | 6.9 ± 1.9 | 1.8 ± 0.6 | −14 ± 4 | −27 ± 6 | 2.1 ± 4.2 | 16 ± 1 |
| k (mV) | 9.2 ± 2.2 | 7.8 ± 0.5 | 5.9 ± 0.9 | 13 ± 2 | 17.4 ± 1.3 | 9 ± 4 |
| τ (ms, −10 to +50 mV) | 19.2–1.7 | 19.1–2.7 | 10–2 | 6.3 | 39–11 | 17.7–1.9 |
| Deactivation | ||||||
| τ (ms at −60 mV) | 3.8 | 3.5 | 23 | 23 | 4.3 | 1.4 |
| Inactivation | ||||||
| τ | τ1 = 0.6 s | τ1 = 0.7 s | τ1 = 1.32 s | NR | τ = 4 s (0 mV) | NR |
| τ2 = 5.6 s | τ2 = 8.9 s | τ2 = 16.77 s | — | τ = 7 s (+80 mV) | — | |
| Rectification | Inward | Inward | Outward | NR | NR | Inward |
| Pharmacological properties | ||||||
| 4AP (IC50μmol l−1) | 5.3 | 14.4 | 181 | 590 | 500 | 49 |
| TEA (IC50 mmol l−1) | 0.3 | 0.13 | 330 | 0.56 | 5.6–10 | 0.2 |
| DTX (IC50 nmol l−1) | NS | NS | > 1000 | 0.4–17 | NR | NS |
NR, not reported; NS, not sensitive. Results for IKur,d are from Yue et al. 1996; results for dKv3.1 are from the present study; and results for Kv1.5, Kv1.2, Kv2.1 and Kv3.1 are from: Grissmer et al. 1992, 1994; Rettig et al. 1992; Castellano et al. 1997; Harvey, 1997; Murakoshi et al. 1997; Feng et al. 1998; Klemic et al. 1998; Mathie et al. 1998.
We obtained a full-length clone of dKv3.1 by the PCR cloning method. We found that the deduced amino acid sequence of dKv3.1 has four amino acids that differ from previously published Kv3.1 sequences from other tissues (Rettig et al. 1992; Grissmer et al. 1992). Two of the amino acid substitutions were quite conservative, involving valine replacement by isoleucine or alanine. The others, serine to proline at position 160 and glycine to aspartic acid at position 230, are more significant, but do not appear to have major effects based on the similarity in biophysical properties between dKv3.1 currents and those described for previous Kv3.1 clones.
Potential significance
Since the first voltage-gated K+ channel was cloned from Drosophila melanogaster in 1987 (Tempel et al. 1987), nine Shaker-related gene subclasses (Kv1 to Kv9) have been cloned from mammalian tissues (Jan & Jan, 1992; Hugnot et al. 1996; Salinas et al. 1997). Shaker-related voltage-gated channel subunits previously cloned from heart include Shaker (Kv1.1, 1.2, 1.4 and 1.5), Shab (Kv2.1) and Shal (Kv4.2 and Kv4.3) subfamilies (Deal et al. 1996; Barry & Nerbonne, 1996). The molecular basis of native currents has been a subject of much interest, allowing for new insights into fundamental mechanisms of cardiac electrophysiology and pharmacology. To our knowledge, this is the first report of the cloning of a Shaw subfamily cDNA from the heart. This finding is significant, not only because it establishes the molecular basis of a physiologically important current in the dog which is functionally similar to currents in other species (including man), but also because it points for the first time in a definitive way to a role for Kv3 K+-channel subunits in the heart.
Our results may be important in understanding some species-related differences in ionic remodelling caused by sustained atrial tachycardia. Ionic changes in a dog model of chronic atrial tachycardia-induced susceptibility to atrial fibrillation strongly resemble those in atrial cells from patients with atrial fibrillation, except that IKur,d is unaltered in the dog model (Yue et al. 1997) whereas the corresponding human current, IKur, is downregulated in the clinical samples (Van Wagoner et al. 1997). The demonstration of a different molecular basis for IKur,d (Kv3.1) compared with IKur (Kv1.5) suggests that the specific genes encoding these two currents, which are similar in a variety of functional ways, may be subject to different transcriptional regulation mechanisms. The difference in molecular bases may also be important for understanding differences in adrenergic regulation, with IKur being inhibited by an α-adrenergic, protein kinase C-mediated pathway (Li et al. 1996) and IKur,d being enhanced by the same signal transduction mechanism (Yue et al. 1999a).
Potential limitations
Kv3.1 channels are found in rapidly firing central nervous system neurons (Gan et al. 1996; Ho et al. 1997), and in certain peripheral populations of T lymphocytes (Chandy et al. 1993). In order to exclude the possibility that dKv3.1 transcripts and proteins were being detected from non-myocyte elements in the heart, we performed RNase protection assays and Western blots with the use of isolated myocytes. The similarity of these results to findings with whole myocardial preparations supports the notion that the dKv3.1 detected was from cardiac myocytes and not non-myocyte elements.
A demonstration of decreased IKur,d in response to dKv3.1 knockdown by antisense oligodeoxynucleotide exposure would have provided an incontrovertible demonstration of the role of dKv3.1 subunits in IKur,d channels. We attempted such experiments, with the use of lipofectamine-promoted transfection of cultured canine atrial myocytes. A covalently linked fluorescent label was used to identify uptake of the antisense construct. Unfortunately, canine atrial myocytes remained well-differentiated in culture (unlike human or rabbit atrial cells (Feng et al. 1997; Wang et al. 1999)), failed to take up antisense, and showed no change in IKur,d. The evidence that dKv3.1 underlies IKur,d thus remains the cloning of dKv3.1 from dog atrium, the demonstration of dKv3.1 mRNA and protein in dog atrium, the fact that expression of dKv3.1 in Xenopus oocytes produces currents with properties very similar to those of IKur,d and different from cardiac IKur in other species or corresponding clones, and the concordant absence of dKv3.1 protein and IKur,d in dog ventricle.
Acknowledgments
Financial support was from the Medical Research Council of Canada and the Quebec Heart Foundation. Drs Wang and Rindt are research scholars of the Heart and Stroke Foundation of Canada (HSFC), and Dr Yue was supported by a HSFC Research Studentship. The authors thank Xiaofan Yang for technical assistance, Dr Hong Han, Dr Martin G. Sirois and Kevin Petrecca for help with immunochemistry, and Annie Laprade, Christiane Calvé and Luce Bégin for typing the manuscript.
References
- Abbott GW, Sesti F, Spawski I, Buck M E, Lehmann MH, Timothy KW, Keating MT, Goldstein SA. MiRP1 forms Ikr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- Barry DM, Nerbonne JM. Myocardial potassium channels: electrophysiological and molecular diversity. Annual Review of Physiology. 1996;58:363–394. doi: 10.1146/annurev.ph.58.030196.002051. [DOI] [PubMed] [Google Scholar]
- Bou-Abboud E, Nerbonne JM. Molecular correlates of the calcium-independent, depolarization-activated K+ currents in rat atrial myocytes. The Journal of Physiology. 1999;517:407–420. doi: 10.1111/j.1469-7793.1999.0407t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmajothi MV, Morales MJ, Liu S, Rasmusson RL, Campbell DL, Strauss HC. In situ hybridization reveals extensive diversity of K+ channel mRNA in isolated ferret cardiac myocytes. Circulation Research. 1996;78:1083–1089. doi: 10.1161/01.res.78.6.1083. [DOI] [PubMed] [Google Scholar]
- Castellano A, Chiara MD, Mellstrom B, Molina A, Monje F, Naranjo JR, Lopez-Barneo J. Identification and functional characterization of a K+ channel alpha-subunit with regulatory properties specific to brain. Journal of Neuroscience. 1997;17:4652–4661. doi: 10.1523/JNEUROSCI.17-12-04652.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandy K, Gutman G, Grissmer S. Physiological role, molecular structure and evolutionary relationships of voltage-gated potassium channels in T lymphocytes. Neuroscience. 1993;5:125–134. [Google Scholar]
- Deal KK, England SK, Tamkun MM. Molecular physiology of cardiac potassium channels. Physiological Reviews. 1996;76:49–67. doi: 10.1152/physrev.1996.76.1.49. [DOI] [PubMed] [Google Scholar]
- Dixon JE, Shi W, Wang HS, McDonald C, Yu H, Wymore RS, Cohen IS, McKinnon D. Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circulation Research. 1996;79:659–668. doi: 10.1161/01.res.79.4.659. [DOI] [PubMed] [Google Scholar]
- Feng J, Wible B, Li GR, Wang Z, Nattel S. Antisense oligodeoxynucleotides directed against Kv1.5 mRNA specifically inhibit ultrarapid delayed rectifier K+ current in cultured adult human atrial myocytes. Circulation Research. 1997;80:572–579. doi: 10.1161/01.res.80.4.572. [DOI] [PubMed] [Google Scholar]
- Feng J, Xu D, Wang Z, Nattel S. Ultrarapid delayed rectifier current inactivation in human atrial myocytes: properties and consequences. American Journal of Physiology. 1998;275:H1717–1725. doi: 10.1152/ajpheart.1998.275.5.H1717. [DOI] [PubMed] [Google Scholar]
- Gan L, Perney TM, Kaczmarek LK. Cloning and characterization of the promoter for a potassium channel expressed in high frequency firing neurons. Journal of Biological Chemistry. 1996;271:5859–5865. doi: 10.1074/jbc.271.10.5859. [DOI] [PubMed] [Google Scholar]
- Grissmer S, Ghanshani S, Dethlefs B, McPherson JD, Wasmuth JJ, Gutman GA, Cahalan MD, Chandy KG. The Shaw-related potassium channel gene, Kv3.1, on human chromosome 11, encodes the type 1 K+ channel in T cells. Journal of Biological Chemistry. 1992;267:20971–20979. [PubMed] [Google Scholar]
- Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, Karmilowicz MJ, Auperin DD, Chandy KG. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Molecular Pharmacology. 1994;45:1227–1234. [PubMed] [Google Scholar]
- Harvey AL. Recent studies on dendrotoxins and potassium ion channels. General Pharmacology. 1997;28:7–12. doi: 10.1016/s0306-3623(96)00173-5. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer Association Inc.; 1992. pp. 115–139. [Google Scholar]
- Ho CS, Grange RW, Joho RH. Pleiotropic effects of a disrupted K+ channel gene: reduced body weight, impaired motor skill and muscle contraction, but no seizures. Proceedings of the National Academy of Sciences of the USA. 1997;94:1533–1538. doi: 10.1073/pnas.94.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugnot JP, Salinas M, Lesage F, Guillemare E, de Weille J, Heurteaux C, Mattei MG, Lazdunski M. Kv8.1, a new neuronal potassium channel subunit with specific inhibitory properties towards Shab and Shaw channels. EMBO Journal. 1996;15:3322–3331. [PMC free article] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Structural elements involved in specific K+ channel functions. Annual Review of Physiology. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- Klemic KG, Shieh CC, Kirsch GE, Jones SW. Inactivation of Kv2.1 potassium channels. Biophysical Journal. 1998;74:1779–1789. doi: 10.1016/S0006-3495(98)77888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GR, Feng J, Wang Z, Fermini B, Nattel S. Adrenergic modulation of ultrarapid delayed rectifier K+ current in human atrial myocytes. Circulation Research. 1996;78:903–915. doi: 10.1161/01.res.78.5.903. [DOI] [PubMed] [Google Scholar]
- Luneau CJ, Williams JB, Marshall J, Levitan ES, Oliva C, Smith JS, Antanavage J, Folander K, Stein RB, Swanson R. Alternative splicing contributes to K+ channel diversity in the mammalian central nervous system. Proceedings of the National Academy of Sciences of the USA. 1991;88:3932–3936. doi: 10.1073/pnas.88.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A, Wooltorton JR, Watkins CS. Voltage-activated potassium channels in mammalian neurons and their block by novel pharmacological agents. General Pharmacology. 1998;30:13–24. doi: 10.1016/s0306-3623(97)00034-7. [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Shi G, Scannevin RH, Trimmer JS. Phosphorylation of the Kv2.1 K+ channels alters voltage-dependent activation. Molecular Pharmacology. 1997;52:821–828. doi: 10.1124/mol.52.5.821. [DOI] [PubMed] [Google Scholar]
- Nattel S, Yue L, Wang Z. Cardiac ultrarapid delayed rectifiers: a novel potassium current family of functional similarity and molecular diversity. Cellular Physiology and Biochemistry. 1999;9:217–226. doi: 10.1159/000016318. [DOI] [PubMed] [Google Scholar]
- Rettig J, Wunder F, Stocker M, Lichtinghagen R, Mastiaux F, Beckh S, Kues W, Pedarzani P, Schroter KH, Ruppersberg JP. Characterization of a Shaw-related potassium channel family in rat brain. EMBO Journal. 1992;11:2473–2486. doi: 10.1002/j.1460-2075.1992.tb05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried T, Rudy B, Vega-Saenz DM, Lau D, Ward DC, Sen K. Localization of a highly conserved human potassium channel gene (NGK2-KV4; KCNC1) to chromosome 11p15. Genomics. 1993;15:405–411. doi: 10.1006/geno.1993.1075. [DOI] [PubMed] [Google Scholar]
- Rudy B, Chow A, Lau D, Amarillo Y, Ozaita A, Saganich M, Moreno H, Nadal MS, Hernandez-Pineda R, Hernandez-Cruz A, Erisir A, Leonard C, Vega-Saenz de Miera E. Contribution of Kv3 channels to neuronal excitability. Annals of the New York Academy of Sciences. 1999;868:304–343. doi: 10.1111/j.1749-6632.1999.tb11295.x. [DOI] [PubMed] [Google Scholar]
- Salinas M, Duprat F, Heurteaux C, Hugnot JP, Lazdunski M. New modulatory alpha subunits for mammalian Shab K+ channels. Journal of Biological Chemistry. 1997;272:24371–24379. doi: 10.1074/jbc.272.39.24371. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Zou A. Molecular physiology of cardiac delayed rectifier K+ channels. Heart and Vessels. 1997;12:170–172. [PubMed] [Google Scholar]
- Tempel BL, Papazian DM, Schwarz TL, Jan YN, Jan LY. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila. Science. 1987;237:770–775. doi: 10.1126/science.2441471. [DOI] [PubMed] [Google Scholar]
- Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circulation Research. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- Wang Z, Feng J, Shi H, Pond A, Nerbonne J, Nattel S. Potential molecular basis of different physiological properties of the transient outward K+ current in rabbit and human atrial myocytes. Circulation Research. 1999;84:551–561. doi: 10.1161/01.res.84.5.551. [DOI] [PubMed] [Google Scholar]
- Xu H, Barry DM, Li H, Brunet S, Guo W, Nerbonne JM. Attenuation of the slow component of delayed rectification, action potential prolongation, and triggered activity in mice expressing a dominant-negative Kv2 alpha subunit. Circulation Research. 1999a;85:623–633. doi: 10.1161/01.res.85.7.623. [DOI] [PubMed] [Google Scholar]
- Xu H, Li H, Nerbonne JM. Elimination of the transient outward current and action potential prolongation in mouse atrial myocytes expressing a dominant negative Kv4 α subunit. The Journal of Physiology. 1999b;519:11–21. doi: 10.1111/j.1469-7793.1999.0011o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S, Imoto K, Kawamura T, Higashida H, Iwabe N, Miyata T, Numa S. Potassium channels from NG108–15 neuroblastoma-glioma hybrid cells. Primary structure and functional expression from cDNAs. FEBS Letters. 1989;259:37–42. doi: 10.1016/0014-5793(89)81488-7. [DOI] [PubMed] [Google Scholar]
- Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circulation Research. 1997;81:512–525. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]
- Yue L, Feng J, Li GR, Nattel S. Characterization of an ultrarapid delayed rectifier potassium channel involved in canine atrial repolarization. The Journal of Physiology. 1996;496:647–662. doi: 10.1113/jphysiol.1996.sp021716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L, Feng J, Wang Z, Nattel S. Adrenergic control of the ultrarapid delayed rectifier current in canine atrial myocytes. The Journal of Physiology. 1999a;516:385–398. doi: 10.1111/j.1469-7793.1999.0385v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L, Melnyk P, Gaspo R, Wang Z, Nattel S. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circulation Research. 1999b;84:776–784. doi: 10.1161/01.res.84.7.776. [DOI] [PubMed] [Google Scholar]


