Abstract
In this paper we demonstrate in the intact human the possibility of a non-invasive modulation of motor cortex excitability by the application of weak direct current through the scalp.
Excitability changes of up to 40 %, revealed by transcranial magnetic stimulation, were accomplished and lasted for several minutes after the end of current stimulation.
Excitation could be achieved selectively by anodal stimulation, and inhibition by cathodal stimulation.
By varying the current intensity and duration, the strength and duration of the after-effects could be controlled.
The effects were probably induced by modification of membrane polarisation. Functional alterations related to post-tetanic potentiation, short-term potentiation and processes similar to postexcitatory central inhibition are the likely candidates for the excitability changes after the end of stimulation. Transcranial electrical stimulation using weak current may thus be a promising tool to modulate cerebral excitability in a non-invasive, painless, reversible, selective and focal way.
The approach taken in this study to produce localised changes of cerebral excitability in the intact human was modulation of neuronal excitability by weak electric currents applied transcranially. So far, this technique has mainly been used in animal research, primarily through modulation of the resting membrane potential (Terzuolo & Bullock, 1956; Creutzfeld et al. 1962; Eccles et al. 1962; Bindman et al. 1964; Purpura & McMurtry, 1965; Artola et al. 1990; Malenka & Nicoll, 1999). In general, cerebral excitability was diminished by cathodal stimulation, which hyperpolarises neurones. Anodal stimulation caused neuronal depolarisation, leading to an increase in excitability (Bindman et al. 1962; Purpura & McMurtry, 1965), as was shown by spontaneous neuronal discharges and the amplitudes of evoked potentials (Landau et al. 1964; Purpura & McMurtry, 1965; Gorman, 1966). However, in single cortical layers opposite effects were seen (Purpura & McMurtry, 1965), underlining the fact that the effects of DC stimulation depend on the interaction of electric flow direction and neuronal geometry. Enduring effects of 5 h and longer have been described if the stimulation itself lasts sufficiently long, about 10–30 min. These prolonged effects are not simply due to prolonged membrane potential shifts or recurrent excitation, because intermittent complete cancellation of electrical brain activity by hypothermia does not abolish them (Gartside, 1968a,b). Long-term potentiation (LTP) and long-term depression (LTD) have been proposed as the likely candidates for this phenomenon (Hattori et al. 1990; Moriwaki, 1991; Islam et al. 1995; Malenka & Nicoll, 1999).
The concept described here was an attempt to induce neuronal excitability changes in man by application of weak DC stimulation through the intact skull. It has already been demonstrated within invasive presurgical epilepsy diagnostics that intracranial currents of sufficient strength can be achieved in humans by stimulation with surface electrodes at intensities of up to 1.5 mA (Dymond et al. 1975). A suitable candidate for evaluating cortical excitability changes is transcranial magnetic stimulation (TMS), because it allows the quantification of motor-cortical neurone responses in a painless and non-invasive manner. The amplitude of the resulting motor-evoked potential (MEP) represents the excitability of the motor system. In the following, we confirm the principal possibility of altering cortical excitability by applying weak DC. Furthermore we show that systematic DC stimulation with minimum stimulation duration and intensity is necessary for an effective application of weak current in humans. This is of particular importance for inducing effects which outlast the duration of stimulation.
METHODS
Current stimulation of the motor cortex
Current was induced by a saline-soaked pair of surface sponge electrodes (35 cm2). It was delivered by a specially developed battery-driven stimulator with a maximum output of 1 mA. In the first experiment, different electrode positions were tested to find the optimal positions for DC stimulation. In the subsequent experiments, the optimal electrode arrangement (motor cortex-forehead above the contralateral orbita), which led to significant and reproducible excitability changes, was used; the motor-cortical electrode was fixed over the representational field of the right abductor digiti minimi (ADM) muscle identified by TMS, and the other electrode was fixed contralaterally above the right orbita. In the different experiments, the current flowed continuously for 4 s and for 1–5 min with an intensity of 0.2–1.0 mA. Constant current flow was controlled by a voltmeter. Nearly all subjects were able to feel the current flow as an itching sensation at both the anodal and cathodal electrodes if it exceeded an intensity of 0.4 mA, and/or by perceiving light flashes as the current was turned on and off. The current intensity and duration we used did not exceed the safety limits stated by Agnew & McCreery (1987). Skin temperature below the stimulation electrode during a 5 min stimulation at 1 mA was measured with a Nicolet Viking II and no change was found during and after this period. Also, in view of data obtained in animal experiments in which morphological changes in brain tissue following prolonged DC stimulation were studied (Akimova & Novikova, 1978; Islam et al. 1995), the stimulation protocols used here were regarded as safe. The changes detected by these authors were solely functional in nature; no hint of cell death or destruction of tissue was found.
Measurement of motor system excitability
To detect current-driven changes of excitability, MEPs of the right ADM following stimulation of its motor-cortical representational field were recorded. Magstim Rapid Stimulators (Magstim Inc., Dyfed, UK) and a figure-of-eight coil were used for the magnetic stimulation. The stimulation intensity was adjusted to achieve a baseline MEP of about 2 mV. The MEPs of the ADM were recorded using Ag-AgCl electrodes in a belly tendon arrangement and a laboratory computer, using the Neuroscan system (Neuroscan Inc., Herndon, VA, USA). The mean MEP baseline amplitudes for each experiment are given in Table 1.
Table 1.
Subject characteristics and baseline values for the performed experiments
| Expt no. | n | Mean age (years ± 1 s.d.) | Mean baseline values (mV ± 1 s.e.m.) |
|---|---|---|---|
| 1 | 10 | 29.9 ± 12.3 | 1.64 ± 0.14 |
| 2 | 19 | 26.9 ± 6.5 | 1.94 ± 0.70 |
| 3 | 12 | 24.9 ± 4.0 | 2.01 ± 0.39 |
| 4 | 12 | 24.9 ± 3.7 | 2.02 ± 0.46 |
Expt, experiment; n, number of subjects.
Subjects
Between 10 and 19 healthy subjects were used in each experiment (for details see Table 1). All gave written informed consent and were paid for participating. Those who were ill, pregnant or suffering from drug abuse, or who had metallic implants/implanted electrical devices were excluded by an interview and a short physical examination. Per day, no more than one current stimulation was permitted. The local ethics committee approved the experiments, which conformed to the standards set by the Declaration of Helsinki.
Experimental procedures
Each experiment was conducted according to a repeated-measurement design. The subject was seated in a reclining chair. First, the left motor-cortical representational field of the right ADM was identified by TMS (coil position which led to the largest MEPs of ADM). Then one current stimulation electrode, to which in the following the term cathodal or anodal stimulation refers, was fixed at this position, and the other electrode at the forehead above the contralateral orbita. Additionally, in experiment 1, several other electrode positions were investigated by combining motor-cortical, pre- and postmotor-cortical, occipital and contralateral forehead electrode arrangements.
In experiment 1, a baseline of 10 TMS-evoked MEPs was recorded at 0.25 Hz. This was followed by recording of a randomised series (0.1 Hz) of 12 TMS-evoked MEPs at the end of a 4 s-long current stimulation and another 12 MEPs without preceding current stimulation. Anodal and cathodal stimulation were done in separate sessions.
Experiments 2, 3 and 4 followed the same common pattern of experimental design. First, a baseline of TMS-evoked MEPs (20 stimuli) was recorded at 0.25 Hz. Afterwards the current was switched on. Stimulation duration, current intensity and polarity varied between the experiments (see below). After turning off the current, MEPs were recorded for 5 min at 0.25 Hz. Following a 5 min break, the session ended with the recording of another MEP series of 20 stimuli. Current conditions varied as follows. Experiment 2: in this experiment, the current flowed for 5 min with an intensity of 1 mA. Each subject underwent two measurements, one with cathodal, the other with anodal stimulation of the motor cortex. Experiment 3: current duration varied between 1 and 5 min. Only anodal stimulation of the motor cortex was tested, and the stimulation intensity was held constant at 1 mA. Experiment 4: current intensity varied between 0.2 and 1 mA. Again only anodal stimulation was tested, and the stimulation duration was held constant at 5 min. In experiments 3 and 4, each subject underwent stimulation with five current conditions.
Calculations and statistics
MEP amplitudes during and after current stimulation were normalised in each experiment; they are given as current stimulation/pre-current baseline quotient. The experimenter analysing the data was not blind to the purpose of the experiment and the experimental groups. However, the MEP analysis was made automatically by a computer program written in-house; the experimenter just had to look at the original data in order to eliminate artifacts due to insufficient relaxation. Thus the only subjective step of the data analysis was the elimination of artifactual MEPs. However, for experiment 2, an additional data analysis was performed by an experimenter who was blind to the experimental conditions. To compare the results of the first analyser with those of the second, we calculated an inter-rater correlation.
For experiment 1, first a repeated measurement ANOVA was calculated with the independent variables electrode position, current flow and polarity of current stimulation, and the dependent variable MEP amplitude. Then Student's t tests (paired samples, two-tailed, P < 0.05) were performed to test whether the values of the current and non-current conditions differed for each electrode position and current polarity. In this and the following experiments, the statistics were not corrected for multiple comparisons regarding the t tests according to Perneger (1998).
Calculations for experiments 2–4 were done as follows. For the TMS train immediately following the current stimulation, the MEPs were subdivided into successive groups of 15, each covering a time range of 1 min, and the means for each group were calculated. Another mean value was calculated for the 20 stimuli applied 10 min after the end of current stimulation.
For experiment 2, a repeated measurement ANOVA (independent variables, time course after current stimulation and polarity of current stimulation; dependent variable, MEP amplitude) was calculated, then Student's t tests (paired samples, two-tailed, level of significance < 0.05) were performed to compare the baseline MEP amplitudes before current stimulation with those after stimulation. For experiments 3 and 4, 2-factorial repeated measurement ANOVAs were performed; independent variables were duration of current stimulation/intensity of current stimulation, respectively, and time course after current stimulation, with MEP amplitude serving as the dependent variable. In a second step, t values (paired samples, two-tailed, level of significance < 0.05) were calculated for the differences between 1 and 2–5 min current application (experiment 3) and between 0.2 and 0.4–1.0 mA current intensity condition (experiment 4) for each time point after the stimulation.
RESULTS
The first experiment was conducted to find the optimal electrode arrangement to achieve current-driven cerebral excitability changes and to evaluate rapid modifications of excitability during current flow.
We recorded MEPs 50 ms before the end of a 4 s phase of either cathodal or anodal motor-cortical current stimulation at 1 mA, and compared these amplitudes with those obtained without current stimulation. The interval between the individual 4 s stimulation phases was at least 6 s. The ANOVA revealed significant interactions between current flow and stimulation polarity on the one hand and electrode position and stimulation polarity on the other (Table 2). A significant increase of motor-cortical excitability during anodal stimulation and a similar significant decrease during cathodal stimulation of approximately 20 % (two-tailed t test, paired samples) are depicted in Fig. 1A in the case of the motor cortex-forehead arrangement only. All other electrode positions turned out to be inefficient (Fig. 1B), as shown by the results of the t tests.
Table 2.
Results of the 2factorial repeated measurement ANOVAs conducted for experiments 1–4
| Expt no. | Variables | d.f. | F values | P values |
|---|---|---|---|---|
| 1 | Current flow | 1 | 2.427 | 0.1579 |
| Electrode position | 5 | 0.560 | 0.7299 | |
| Polarity of current stimulation | 1 | 1.502 | 0.2552 | |
| Current flow × electrode position | 5 | 0.827 | 0.5383 | |
| Current flow × polarity of current stimulation | 1 | 7.744 | 0.0238 * | |
| Electrode position × polarity of current stimulation | 5 | 2.613 | 0.0389 * | |
| Current flow × electrode position × polarity of current stimulation | 5 | 0.650 | 0.6633 | |
| 2 | Time course | 10 | 1.442 | 0.1658 |
| Polarity of current stimulation | 1 | 48.875 | < 0.0001 * | |
| Polarity of current stimulation × time course | 10 | 10.289 | < 0.0001 * | |
| 3 | Duration of current stimulation | 4 | 5.295 | 0.0011 * |
| Time course | 5 | 14.790 | < 0.0001 * | |
| Duration of current stimulation × time course | 20 | 2.768 | 0.0001 * | |
| 4 | Intensity of current stimulation | 4 | 6.011 | 0.004 * |
| Time course | 5 | 23.798 | < 0.0001 * | |
| Intensity of current stimulation × time course | 20 | 3.736 | < 0.0001 * |
The results of experiment 1 show that the effect of current flow depends on electrode position and polarity of current flow. For experiment 2, the ANOVA reveals a dependency of the aftereffect on polarity of current stimulation and an interaction of polarity and time course. As shown by the results of experiments 3 and 4, the independent variables – current duration, stimulation intensity and time course after current stimulation – determine the MEP size. Furthermore, the interactions between time course, stimulation duration and intensity indicate prolonged effects of longer and more intense stimulation on MEP size. d.f., degrees of freedom. *P < 0.05.
Figure 1. Cortical excitability change during current flow.
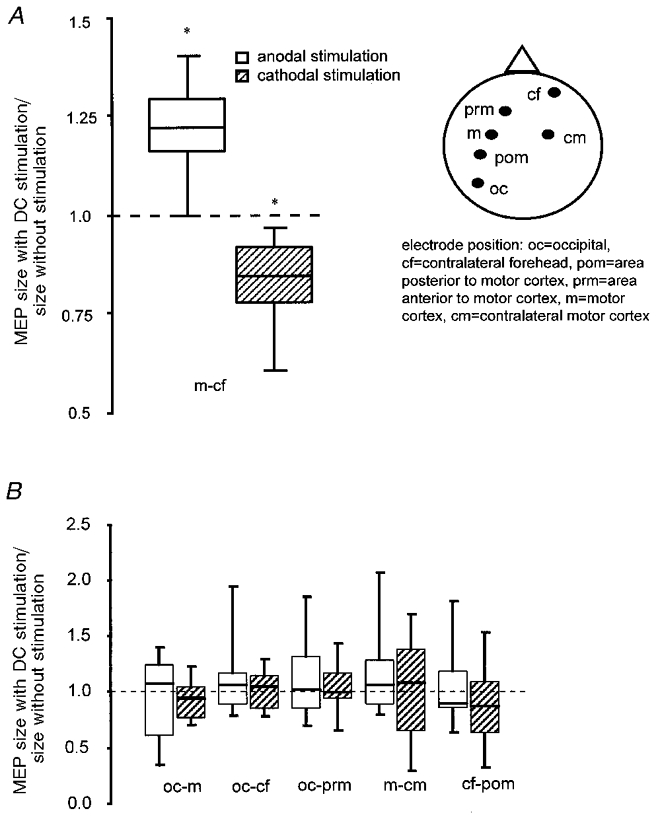
Rapidly induced effects of weak DC stimulation on the size of the motor-evoked potential (MEP) in the right abductor digiti minimi (ADM) muscle, revealed by transcranial magnetic stimulation (TMS), using the motor cortex-contralateral forehead arrangement (A), and the lack of effect using other diverse electrode positions (B). Normalised MEP amplitudes during stimulation are divided by normalised MEP amplitudes without stimulation. During DC stimulation, the MEP amplitude increased with anodal and decreased with cathodal current stimulation. Asterisks indicate significant differences between the values with and without stimulation (two-tailed t test, paired samples, P < 0.05). The boxes cover the range 25th to 75th percentiles, the error bars the 10th to 90th percentiles; the horizontal lines in the boxes indicate the median. Stimulation polarity always refers to the motor cortical electrode, respectively pre- and postmotor cortical electrode, except for the occipital-contralateral forehead condition, where it refers to the contralateral forehead electrode.
After-effects of the weak current stimulation are represented in Fig. 2. Here, current was delivered for 5 min at an intensity of 1 mA, again separately with anodal and cathodal polarities. The results of the ANOVA show a significant effect of the polarity of current stimulation and a significant interaction of polarity and time course (Table 2).
Figure 2. Polarity-specific after-effect of DC stimulation.
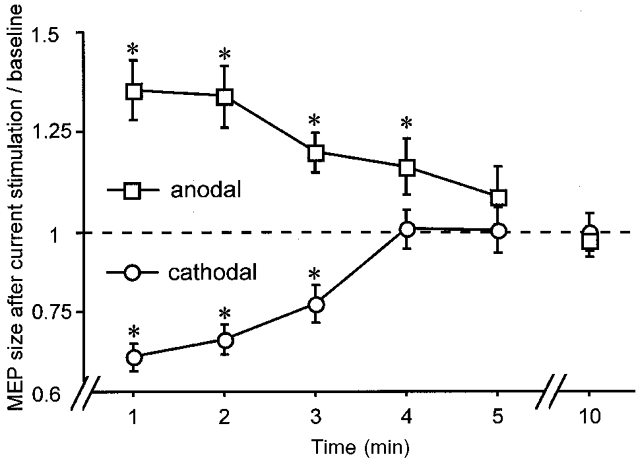
Time course of polarity-specific motor cortex excitability changes outlasting stimulation duration, shown after 5 min DC stimulation at 1 mA. MEP amplitudes returned to baseline within 5 min. Asterisks indicate significant differences between MEP amplitudes after stimulation and at baseline (two-tailed t test, paired samples, P < 0.05).
As revealed by the results of the t tests, within the first 5 min after anodal stimulation a significant increase in MEP amplitude of about 40 % could be induced initially, diminishing nearly linearly during the subsequent minutes. In contrast, cathodal stimulation resulted in a decrease of MEP amplitude compared to baseline values of about 30 % initially. Ten minutes after the offset of current flow, control stimulation results revealed a return of the MEP responses to the baseline values. On re-analysis of the data from this experiment by a second subject, blind to the stimulation conditions, an inter-rater correlation of 0.96 was obtained.
Experiments 3 and 4 rendered more precisely the clear dependency of the after-effect on stimulation duration (Fig. 3A), which was varied between 1 and 5 min, and intensity (Fig. 3B), which was modulated between 0.2 and 1 mA.
Figure 3. Size and endurance of the DC stimulation after-effect depends on stimulation duration and current intensity.
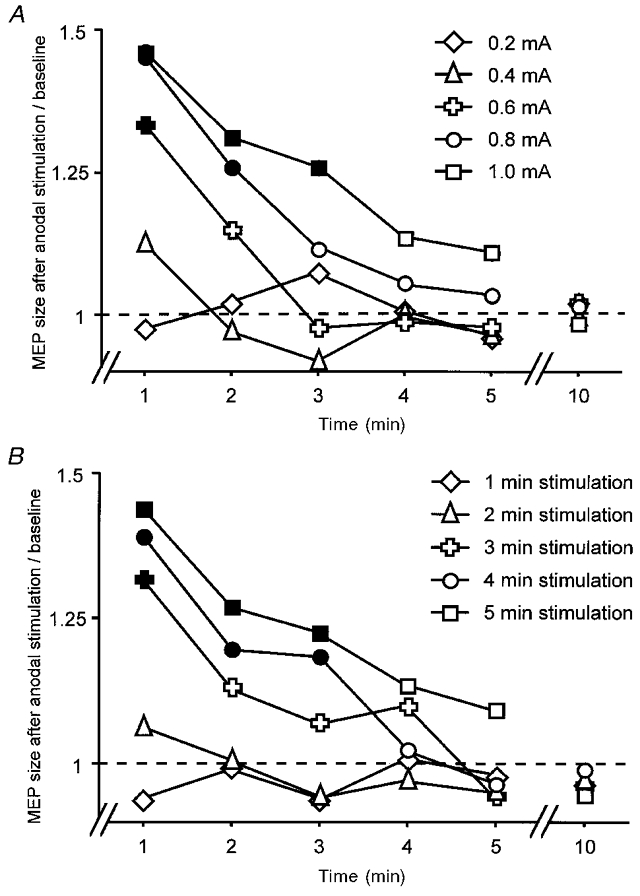
Dependency of the size of prolonged motor cortex excitability changes after anodal DC stimulation on current intensity (A) and stimulation duration (B). The MEP amplitudes relative to baseline are plotted against the time course. Filled symbols indicate significant differences (two-tailed t test, paired samples, P < 0.05) from the lowest stimulation intensity of 0.2 mA (A) or the shortest stimulation duration of 1 min (B). A minimum of 0.6 mA current intensity stimulation or a minimum stimulation duration of 3 min was needed to induce stimulation after-effects. Increasing either current intensity or stimulation duration led to prolonged and larger after-effects.
In order to induce after-effects, a stimulus duration of at least 3 min at 1 mA or an intensity of 0.6 mA for 5 min was required. In addition, a clear increase of MEP amplitude and endurance of the effect with rising stimulus duration and amplitude can be seen (Fig. 3A and B; Table 2).
DISCUSSION
In summary, we show here in the intact human the possibility of selectively enhancing or reducing cerebral excitability by the use of weak anodal and cathodal electrical currents, and the prolongation of these effects for some minutes after the end of stimulation. In general accordance with basic neurophysiology, anodal stimulation of the motor cortex enhanced excitability, whilst it was diminished by cathodal stimulation. The reason for this is most probably that anodal stimulation–as so far shown in animals–results in neuronal depolarisation and increasing neuronal excitability while cathodal stimulation has opposite results. However, a contribution of a hyperpolarisation of inhibitory interneurones of superficial layers in the case of anodal polarisation and a reverse contribution of these neurones in the case of cathodal stimulation cannot be ruled out. Electrode position is critical for achieving this effect. Only the motor cortex-contralateral forehead arrangement resulted in significant excitability changes. This probably reflects the well-known fact that electrical field interactions with neuronal geometry are important for the influence of DC flow on neuronal excitability modifications. Amplitude and endurance beyond the end of stimulation are current-intensity and stimulation-duration dependent. In the animal, too, these currents have to flow for a few minutes to produce effects that last beyond the time of stimulation (Bindman et al. 1964). While the effects during current flow are probably due to shifts in neuronal resting membrane potential, as has been shown in the animal during DC stimulation, their endurance for minutes beyond the end of stimulation must be explained by other mechanisms, which may be induced by the changes in the spontaneous discharge rate (Bindman et al. 1964). The time course of these effects is similar to post-tetanic potentiation or short-term potentiation for anodal stimulation, and a post-exercise central inhibition for cathodal stimulation (Samii et al. 1996). However, the cellular and molecular mechanisms responsible for these current-driven cortical excitability changes are largely unknown as yet, so it remains unclear whether the after-effects also fulfill the biochemical criteria for these processes.
Currently, we have no hint of a LTP or LTD effect of the performed DC stimulation in humans, which so far can be achieved by transient or permanent deafferentation or as associative sensory and motor stimulation (Ziemann et al. 1998a,b; Hamdy et al. 1998; Stefan et al. 2000). However, in the animal LTP- and LTD-like effects can be achieved by a further prolongation of stimulation duration (Bindman et al. 1964; Weiss et al. 1998)
Mainly based on the available animal data, we assume the cortex to be the most likely substrate for this effect. Additional spinal excitation changes, however, cannot be excluded for certain, particularly for anodal stimulation in which spontaneous pyramidal cell discharges could possibly influence spinal excitability. However, in the completely relaxed muscle as investigated here we would expect no further inhibition of the pyramidal tract with cathodal stimulation. Thus particularly the cathodal results support the cortex as the most likely location of the effect. Additionally, in a parallel study with magnetic resonance imaging (fMRI) we present motor cortical activation changes after DC stimulation, which further argues in favour of a cortical location in humans (J. Baudewig, M. A. Nitsche, W. Paulus and J. Frahm, manuscript in preparation).
Why has this rather simple technique, although readily available for decades, not gained more attention in human research? Most groups applied current intensities and stimulation durations that were hardly sufficient to change cerebral excitability profoundly, although for example celerity in simple reaction time protocols was improved (Elbert et al. 1981; Jaeger et al. 1987). Consequently, the results of these studies were not regarded as very promising (Lolas, 1977; Elbert et al. 1981; Jaeger et al. 1987).
In the only study in which TMS was used as an objective method to measure cerebral excitability (Priori et al. 1998), low stimulation intensities (< 0.5 mA) and durations (7 s) were applied. The authors reported diminished MEP amplitude during anodal stimulation preceded by cathodal stimulation; all other tested combinations of DC stimulation polarities did not change MEP amplitude. At first glance the diminished MEP amplitude after anodal stimulation appears to be in contrast to the findings of our study. However, as shown in Fig. 1B, the electrode position is critical. Priori et al. (1998) used a motor cortex-chin electrode arrangement, which results in a current flow different from the one applied in our study. Also, Priori et al. (1998) found excitability changes only after anodal stimulation preceded by cathodal stimulation. We did not test this condition. However, at the current intensity used by Priori et al. (1998) we found no after-effect following DC stimulation, which is in accordance with their results. Because the direction of current flow relative to neuronal geometry determines the direction of the polarising effect (Landau et al. 1964), this and the different stimulation conditions (switching from cathodal to anodal stimulation and vice versa in the experiments of Priori et al. (1998)) are the likely explanations for the different results.
In conclusion, the transcranial application of weak current appears to be a promising tool for clinical neuroplasticity research, for it allows a painless, selective, focal, non-invasive and reversible excitability modulation of the cortex. However, important research still has to be done, mainly in uncovering the mode of function and in finding a way to prolong the effects of weak current application further, as has already successfully been done in animal research (Bindman et al. 1964; Weiss et al. 1998).
Acknowledgments
We wish to thank Dr F. Tergau and S. Wischer for help with the experiments and P. Wenig for building the electrical stimulation device. This project was supported by DFG grant PA 419/9–1.
References
- Agnew WF, McCreery DB. Considerations for safety in the use of extracranial stimulation for motor evoked potentials. Neurosurgery. 1987;20:143–147. doi: 10.1097/00006123-198701000-00030. [DOI] [PubMed] [Google Scholar]
- Akimova IM, Novikova TA. Ultrastructural changes in the cerebral cortex following transcranial micropolarization. Biulleten Eksperimentalnoi Biologii i Meditsiny. 1978;86:737–739. [PubMed] [Google Scholar]
- Artola A, Brocher S, Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990;347:69–72. doi: 10.1038/347069a0. [DOI] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OCJ, Redfearn JWT. Long-lasting changes in the level of the electrical activity of the cerebral cortex produced by polarizing currents. Nature. 1962;196:584–585. doi: 10.1038/196584a0. [DOI] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OCJ, Redfearn JWT. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. The Journal of Physiology. 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeld OD, Fromm GH, Kapp H. Influence of transcortical dc-currents on cortical neuronal activity. Experimental Neurology. 1962;5:436–452. doi: 10.1016/0014-4886(62)90056-0. [DOI] [PubMed] [Google Scholar]
- Dymond AM, Coger RW, Serafetinides EA. Intracerebral current levels in man during electrosleep therapy. Biological Psychiatry. 1975;10:101–104. [PubMed] [Google Scholar]
- Eccles JC, Kostyuk PG, Schmidt RF. The effect of electric polarization of the spinal cord on central afferent fibres and on their excitatory synaptic action. The Journal of Physiology. 1962;162:138–150. doi: 10.1113/jphysiol.1962.sp006920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert T, Lutzenberger W, Rockstroh B, Birbaumer N. The influence of low-level transcortical DC-currents on response speed in humans. International Journal of Neuroscience. 1981;14:101–114. doi: 10.3109/00207458108985821. [DOI] [PubMed] [Google Scholar]
- Gartside IB. Mechanisms of sustained increases of firing rate of neurones in the rat cerebral cortex after polarization: reverberating circuits or modification of synaptic conductance? Nature. 1968a;220:382–383. doi: 10.1038/220382a0. [DOI] [PubMed] [Google Scholar]
- Gartside IB. Mechanisms of sustained increases of firing rate of neurones in the rat cerebral cortex after polarization: role of protein synthesis. Nature. 1968b;220:383–384. doi: 10.1038/220383a0. [DOI] [PubMed] [Google Scholar]
- Gorman AL. Differential patterns of activation of the pyramidal system elicited by surface anodal and cathodal cortical stimulation. Journal of Neurophysiology. 1966;29:547–564. doi: 10.1152/jn.1966.29.4.547. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Rothwell JC, Aziz Q, Singh KD, Thompson DG. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nature Neuroscience. 1998;1:64–68. doi: 10.1038/264. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Moriwaki A, Hori Y. Biphasic effects of polarizing current on adenosine-sensitive generation of cyclic AMP in rat cerebral cortex. Neuroscience Letters. 1990;116:320–324. doi: 10.1016/0304-3940(90)90094-p. [DOI] [PubMed] [Google Scholar]
- Islam N, Aftabuddin M, Moriwaki A, Hattori Y, Hori Y. Increase in the calcium level following anodal polarization in the rat brain. Brain Research. 1995;684:206–208. doi: 10.1016/0006-8993(95)00434-r. [DOI] [PubMed] [Google Scholar]
- Jaeger DET, Lutzenberger W, Birbaumer N. The effects of externally applied transcephalic weak direct currents on lateralization in choice reaction tasks. Journal of Psychophysiology. 1987;1:127–133. [Google Scholar]
- Landau WM, Bishop GH, Clare MH. Analysis of the form and distribution of evoked cortical potentials under the influence of polarizing currents. Journal of Neurophysiology. 1964;27:788–813. doi: 10.1152/jn.1964.27.5.788. [DOI] [PubMed] [Google Scholar]
- Lolas F. Brain polarization: Behavioral and therapeutic effects. Biological Psychiatry. 1977;12:37–47. [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation – a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Moriwaki A. Polarizing currents increase noradrenaline-elicited accumulation of cyclic AMP in rat cerebral cortex. Brain Research. 1991;544:248–252. doi: 10.1016/0006-8993(91)90061-y. [DOI] [PubMed] [Google Scholar]
- Perneger TV. What's wrong with Bonferroni adjustments. British Medical Journal. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor cortex through the scalp. NeuroReport. 1998;9:2257–2260. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. Journal of Neurophysiology. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Samii A, Wassermann EM, Ikoma K, Mercuri B, Hallett M. Characterization of postexercise facilitation and depression of motor evoked potentials to transcranial magnetic stimulation. Neurology. 1996;46:1376–1382. doi: 10.1212/wnl.46.5.1376. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen L, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Terzuolo CA, Bullock TH. Measurement of imposed voltage gradient adequate to modulate neuronal firing. Proceedings of the National Academy of Sciences of the USA. 1956;42:687–694. doi: 10.1073/pnas.42.9.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SR, Eidsath A, Li XL, Heynen T, Post RM. Quenching revisited: low level direct current inhibits amygdala-kindled seizures. Experimental Neurology. 1998;154:185–192. doi: 10.1006/exnr.1998.6932. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. Journal of Neuroscience. 1998a;18:1115–1123. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Hallett M, Cohen LG. Mechanisms of deafferentation-induced plasticity in human motor cortex. Journal of Neuroscience. 1998b;18:7000–7007. doi: 10.1523/JNEUROSCI.18-17-07000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


