Abstract
Recently we have found that inhibition of bradykinin-induced synovial plasma extravasation by transcutaneous electrical stimulation at strengths which excite unmyelinated afferent axons is mediated by the hypothalamo-pituitary-adrenal axis.
Here we tested whether stimulation of nociceptors in the rat paw by intradermally injected capsaicin inhibits bradykinin-induced synovial plasma extravasation and whether this inhibition is mediated by the hypothalamo-pituitary-adrenal or sympatho-adrenal axis. Furthermore, we tested whether inhibition of bradykinin-induced plasma extravasation generated by intraperitoneally injected capsaicin, which preferentially excites visceral afferents, is mediated by the hypothalamo-pituitary-adrenal or sympatho-adrenal axis. We used normal rats, subdiaphragmatically vagotomized rats, rats with denervated adrenal medullae and rats with acutely transected spinal cords at the segmental levels T1/T2 or T12/L1.
Injection of capsaicin into the plantar or palmar surface of the paws produced a depression of bradykinin-induced plasma extravasation. The inhibition elicited from the forepaw was larger than that from the hindpaw.
The inhibition of bradykinin-induced plasma extravasation elicited from both paws was potentiated by subdiaphragmatic vagotomy.
Denervation of the adrenal medullae abolished the inhibitory effect of intradermal capsaicin in vagus-intact and in vagotomized animals.
After spinalization at the segmental level T1/T2, capsaicin injected into the forepaw did not depress bradykinin-induced plasma extravasation either in vagus-intact or in vagotomized animals. Capsaicin injected into the hindpaw in these spinalized animals produced a small depression. After spinalization at the segmental level T12/L1 no depression was produced by capsaicin injected into the hindpaw.
Depression of bradykinin-induced plasma extravasation generated by intraperitoneal injection of capsaicin in vagus-intact and in vagotomized animals was also abolished or attenuated after denervation of the adrenal medullae. This shows that this depression was also largely dependent on the activation of the sympatho-adrenal system.
We conclude that depression of bradykinin-induced plasma extravasation during stimulation of nociceptors by capsaicin is mediated predominantly by the sympathoadrenal pathway. This finding differs from the inhibitory mechanism of depression of bradykinin-induced plasma extravasation generated by cutaneous electrical stimulation, which is mediated by the hypothalamo-pituitary-adrenal axis.
Bradykinin (BK)-induced synovial plasma extravasation (PE) in the rat knee joint is a neurogenic inflammatory response which is dependent on the sympathetic innervation of the synovia, but not on impulse activity in the sympathetic neurones (Miao et al. 1996a,b). Recently we have shown in the rat that this neurogenic inflammation is depressed in a frequency-dependent manner during activation of afferent C-fibres generated by electrical stimulation of the contralateral hindpaw at ≤ 1–3 Hz. Hypophysectomy, blockade of corticosterone synthesis or removal of the adrenal glands prevented the afferent-induced depression of the BK-induced PE. Removal of the adrenal medullae had no significant effect on the depression, whereas electrical stimulation of the forepaw in animals in which the spinal cord was acutely interrupted at the level of T1T2 elicited the same depression of BK-induced PE as forepaw stimulation in animals with intact spinal cord (Green et al. 1995). PE induced by platelet-activating factor (PAF), which is not dependent on the sympathetic postganglionic terminals, was not depressed by electrical stimulation of cutaneous afferents (Green et al. 1995). It was concluded from these studies that the depression of BK-induced PE is mediated by the hypothalamo-pituitary-adrenal (HPA) axis with little if any contribution from the sympatho-adrenal system and that the HPA axis is activated by the nociceptive system (Green et al. 1995). This conclusion was corroborated by the finding that corticosterone infused intravenously also depressed BK- but not PAF-induced PE and that this depression was also dependent on the sympathetic innervation of the knee joint (Green et al. 1997).
The depression of BK-induced PE generated by transcutaneous electrical stimulation is greater after cutting the abdominal vagus nerves (Miao et al. 1997a). This enhanced depression is thought to be due to removal of inhibition maintained by ongoing activity in abdominal vagal afferents and acting at the central ascending nociceptive system. It was further shown that depression of BK-induced PE during stimulation of visceral afferents generated by intraperitoneal or intravesical injection of capsaicin (CAP) is enhanced after subdiaphragmatic vagotomy and that the vagal afferents responsible project through the coeliac branches of the abdominal vagus nerves (Miao et al. 1997a,b). These afferents probably innervate the small intestine and the proximal colon (Berthoud et al. 1991). In these experiments it was never tested whether the HPA axis mediates the depression.
Transcutaneous electrical stimulation supramaximal for A- and C-fibres activates afferent fibres synchronously. We therefore tested whether more physiological stimulation of afferent nociceptive fibres can also depress the neurogenic inflammation in the synovium and whether this depression is mediated by the same mechanism. We used CAP injected into the plantar or palmar skin in doses of 3–30 μg. This excitatory neurotoxin activates unmyelinated nociceptive afferents and some small-diameter myelinated afferents (Szolcsanyi et al. 1988). In addition we reinvestigated the depression of BK-induced PE during stimulation of visceral afferents generated by intraperitoneal CAP before and after denervation of the adrenal medullae, before and after subdiaphragmatic vagotomy as well as before and after the combination of both interventions. To our surprise, we found (1) that BK-induced PE is depressed more from the forepaw than from the hindpaw stimulation, in animals with intact vagus nerves, (2) that BK-induced PE is much more strongly depressed in vagotomized animals from both paws, (3) that this depression is mediated by activating the sympatho-adrenal system, not by activating the HPA axis, and (4) that depression of BK-induced PE induced by stimulation of spinal visceral afferents is also largely mediated by the sympatho-adrenal system in vagus-intact and in vagotomized animals.
METHODS
The experiments were performed on male Sprague-Dawley rats (300–400 g). All surgical procedures as well as the experiments were carried out under pentobarbital anaesthesia (65 mg kg−1; Abbott Lab, Chicago, IL, USA). Animal care and use conformed to the guidelines of the National Institutes of Health for the care and use of experimental animals. Experimental protocols were approved by the University of California at San Francisco, Committee on Animal Research.
Perfusion of the knee joint
Knee joint perfusion was performed as previously described (Miao et al. 1996b). In brief, following incision of the skin and connective tissue overlying the anterior aspect of the knee and the saphenous vein, Evans blue dye (50 mg kg−1) was administered intravenously in the saphenous vein. Ten minutes after injection of the dye, a 30 gauge needle was inserted into the cavity of the knee joint for the infusion of fluid (250 μl min−1, controlled by a syringe pump from Sage Instruments, Model 351, Cambridge, MA, USA). After infusion of an initial volume of 100–200 μl of vehicle, a second needle (25 gauge) was inserted into the knee joint, approximately 3 mm from the inflow needle. This second needle served as an outflow cannula. Fluid was withdrawn from the joint through the outflow cannula using a second syringe pump. The fluid was infused and withdrawn at a constant rate of 250 μl min−1. Perfusate samples were collected every 5 min for up to 120 min. Samples were analysed for the amount of Evans blue dye by spectrophotometric measurement of absorbance at 620 nm. The absorbance at this wavelength is linearly related to the dye concentration (Carr & Wilhelm, 1964). After a baseline perfusion period of 15 min with vehicle (normal saline), plasma extravasation into the knee joint was stimulated by adding BK (160 ng ml−1, i.e. 0.15 μm) to the perfusion fluid. In a series of control experiments, plasma extravasation into the knee joint was stimulated by adding PAF (52.4 ng ml−1, i.e. 0.1 μm) to the perfusion fluid after a baseline perfusion period of 15 min with vehicle (0.25 % albumin in saline).
Of note, the concentration of BK in various inflamed tissues is in the range 50 nm to 0.1 μm (Swift et al. 1993; Hargreaves et al. 1993) and that of PAF has been measured to be around 1.7 μm (Will et al. 1991; Appleyard & Hiller, 1995).
Noxious stimulation of primary afferents by capsaicin
Spinal afferents from the hindpaw or the forepaw were excited by intraplantar or intrapalmar injection, respectively, of CAP at doses of 3, 10 and 30 μg at intervals of 20 min. Visceral afferents were excited by intraperitoneal injection of CAP at doses of 10−6 to 10−3 mg kg−1 (see Miao et al. 1997a,b).
Surgical procedures
Transection of the spinal cord
The adrenal medullae receive preganglionic sympathetic innervation from the T4 to T12 spinal level (Strack et al. 1988; Kesse et al. 1988; Parker et al. 1990) and the afferent inflow from the plantar skin of the hindpaws occurs to the spinal segments L4 to L6 (Baron et al. 1988). To examine the contribution of spinal pathways to the noxious stimulus-induced inhibition of synovial PE, the spinal cord was exposed by laminectomy and then transected; gelfoam (Upjohn, Kalamazoo, MI, USA) was inserted into the lesion site. To separately examine the contribution of spinal pathways (i.e. to preganglionic neurones innervating the adrenal medullae and lumbar sympathetic outflow) and supraspinal pathways (i.e. to the HPA axis; supraspinal loop to preganglionic neurones innervating the adrenal medulla), spinalization was performed at either of two levels: at T12/L1 where spinal pathways to both the adrenal medullae and supraspinal sites were interrupted and at T1/T2 where spinal pathways to and from the supraspinal sites in brain stem and hypothalamus were transected but the connections from the spinal segments L4 to L6 which receive the afferent input from the hindpaw to the preganglionic neurones of the adrenal medulla were spared.
Denervation of the adrenal medullae
To study the contribution of the adrenal medullae to the effect of intraplantar CAP on BK-induced PE, the adrenal glands were denervated (Celler & Schramm, 1981; Araki et al. 1984; Miao et al. 1997a). Following lateral incisions in the abdominal wall, the suprarenal ganglia and the nerves innervating the adrenal glands were exposed. The nerves connecting to the suprarenal ganglia were cut and the ganglia removed. The removed suprarenal ganglia and the attached nerves to the adrenal medulla, to the major splanchnic nerve and to the minor splanchnic nerve were put on a slide and inspected after the operation; in this way the completeness of denervation of the adrenal medullae was controlled. Perfusion of the knee joints was carried out acutely after adrenal denervation. Bilateral adrenal nerve lesion did not affect the baseline level of BK-induced PE (data not shown).
Vagotomy
Bilateral subdiaphragmatic vagotomy was performed as recently described (Miao et al. 1994, 1997a). Under a dissection microscope, both vagus nerves and their branches were exposed through a lateral incision of the abdominal wall in the left upper quadrant. The vagus nerves were then dissected free from the oesophagus and cut (for a description of the anatomy see Precht & Poley, 1985).
Denervation of the hindlimb
In control experiments the hindpaw in which CAP was injected was denervated by cutting the sciatic nerve proximal to the trifurcation of tibial, common peroneal and sural nerves and by cutting the saphenous nerve.
Experimental procedures and statistics
The results are based on different experimental interventions, each conducted on at least eight knee joints (see Table 1 for data summary). Data are presented as means ±s.e.m.; significant differences between pairs of time-effect curves were determined by two-way (group × time) repeated measures analysis of variance (ANOVA). Differences were considered statistically significant at a P value of < 0.05.
Table 1.
Effect of surgical intervention on the basal level of BK-induced synovial PE
| Group | Max. BK-induced PEa | Max. inhibition by hindpaw CAPb (%) | Max. inhibition by forepaw CAPb (%) |
|---|---|---|---|
| Control | 0.165 ± 0.01 (16) | 10 ± 3 | 10 ± 3 |
| Sham vagotomy | 0.15 ± 0.01 (16) | 33.3 ± 4.6 (8)* | 54.5 ± 4.3 (8)* |
| Acute vagotomy | 0.233 ± 0.02 (16)† | 71.7 ± 3.4 (8)† | 80.5 ± 1.9 (8)† |
| Adrenal denervation | 0.156 ± 0.01 (8) | 16.8 ± 3.7 (8)‡ | n.d. |
| Acute vagotomy + adrenal denervation | 0.21 ± 0.01 (16)† | 20.2 ± 4.9 (8)§ | 24.6 ± 5.8 (8)§ |
| T1/T2 spinalization | 0.148 ± 0.01 (16) | 24.5 ± 2.7 (8)* | 7.5 ± 5.5 (8)‡ |
| T12/L1 spinalization | 0.116 ± 0.01 (8) | 14.0 ± 4.2(8)‡∥ | n.d. |
| T1/T2 spinalization + acute vagotomy | 0.187 ± 0.01 (16)† | n.d. | 12.2 ± 5.5 (8)‡ |
| Deafferentiationcc | 0.176 ± 0.02 (8) | 11.0 ± 3.4 (8)‡ | n.d. |
Results are means ±s.e.m., with the number of knee joint preparations in parentheses (n.d., not determined).
BK-induced PE was measured as absorbance at 620 nm, which is linearly related to the concentration of circulating Evans blue dye.
Percentage reduction by 30 μg CAP of BK-induced PE with respect to maximal BK-induced PE; values were obtained 10–20 min after intradermal injection of CAP. All statistical values are expressed with respect to BK-induced PE without stimulation 80–90 min after the start of BK infusion (Control); Student's unpaired t test was used for statistical comparisons (P < 0.001).
Deafferentation corresponds to section of the sciatic and saphenous nerves.
Significantly different from control (no CAP)
significantly different from sham vagotomy
not significantly different from control
significantly different from acute vagotomy
not significantly different from T1/T2 spinalization.
Materials
Bradykinin acetate, platelet-activating factor (l-α-phosphatidylcholine, β-acetyl-γ-o-hexadecyl), bovine serum albumin (BSA), capsaicin and Tween 80 were obtained from Sigma Chemical Co. Capsaicin was first dissolved in a solution of ethanol and Tween 80 (1:1 ratio) and then diluted in normal saline (Travenol Laboratories, Inc., Deerfield, IL, USA). Bradykinin acetate and platelet-activating factor were dissolved in normal saline.
RESULTS
Bradykinin (BK) infused into the rat knee joint cavity increased plasma extravasation (PE) by about five times the baseline PE. This increase in PE lasted throughout the BK infusion with a decay by about 10 % by 90 min (Fig. 1, ×). Here we describe the depression of this experimental inflammatory response generated by cutaneous afferent and visceral afferent stimulation under various experimental conditions. Values of maximal BK-induced PE and relative changes of BK-induced PE during intradermal injection of 30 μg capsaicin (CAP) into the hindpaw and the forepaw under these experimental conditions are listed in Table 1.
Figure 1. Capsaicin inhibits BK-induced PE by activating nociceptors.
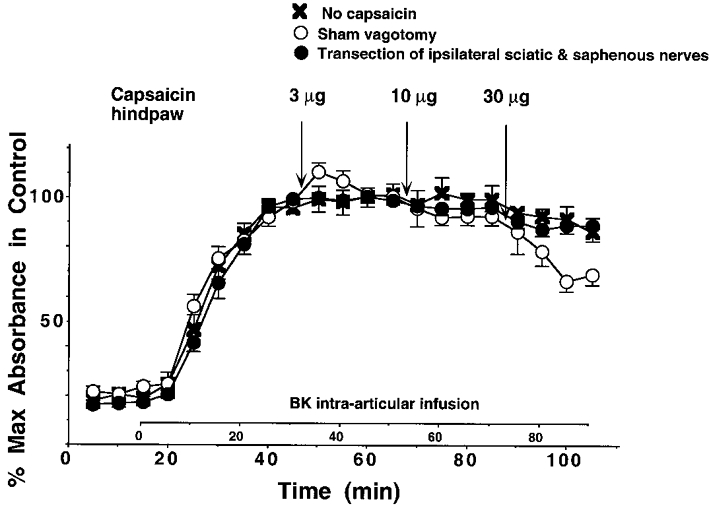
Effect of intradermal injection of CAP (3–30 μg) into the plantar skin of the hindpaw on BK-induced PE in the knee joint in sham-vagotomized rats (○) and in animals with denervated hindpaw (•; sciatic nerve, rostral to the trifurcation of tibial, common peroneal and sural nerve, and saphenous nerve sectioned). ×, control (no CAP injection).
Stimulation of cutaneous nociceptors by CAP
Animals with intact vagus nerves
Stimulation of cutaneous nociceptors in the hindpaw by injection of CAP at doses of 3–30 μg into the plantar skin of the contralateral hindlimb decreased the BK-induced PE only weakly, but significantly at 30 μg (Fig. 1, ○). Intraplantar injection of CAP, at these doses, into the denervated plantar skin (sciatic and saphenous nerves cut) had no effect on BK-induced PE (Fig. 1, •). Furthermore intraplantar injection of CAP had no effect on PE generated by platelet-activating factor (PAF; 10−7m) in the rat knee joint (data not shown).
Stimulation of nociceptors in the forepaw by intrapalmar injection of CAP decreased BK-induced PE significantly at doses of 3–30 μg. This depression was significantly stronger than that elicited from the hindpaw (Fig. 2B, ○).
Figure 2. Inhibition of BK-induced PE by capsaicin is mediated by the sympathoadrenal axis.
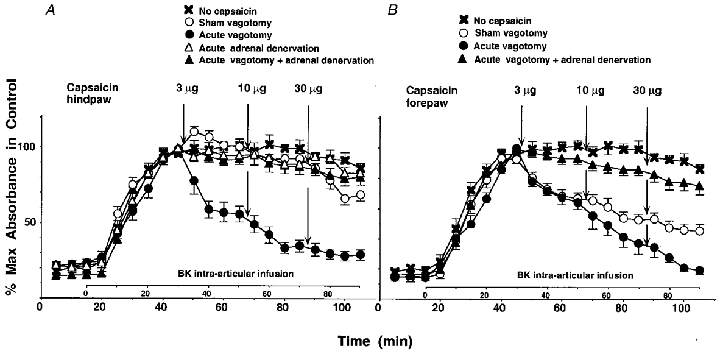
Effect of intradermal injection of CAP (3–30 μg) into the plantar skin of the hindpaw (A) or into the palmar skin of the forepaw (B) on BK-induced PE in the knee joint in sham-vagotomized rats (○), in vagotomized rats (•), in rats with intact vagus nerves and denervated adrenal medullae (▵) and in vagotomized rats with denervated adrenal medullae (▴). ×, control (no CAP injection). ○ and × in A, same data as in Fig. 1.
After denervation of the adrenal medullae, intraplantar injection of CAP no longer significantly inhibited BK-induced PE at 30 μg (Fig. 2A, ▵).
Subdiaphragmatically vagotomized animals
After acute subdiaphragmatic vagotomy, BK-induced PE was strongly inhibited by intraplantar CAP. This inhibition was already significant at 3 μg and decreased to 20–30 % of the maximal BK-induced PE with the 30 μg dose (Fig. 2A, •). For the forepaw stimulation, the potentiating effect of vagotomy was smaller than for the hindpaw stimulation, being most notable at 30 μg (Fig. 2B, •).
In vagotomized animals with denervated adrenal medullae, stimulation of nociceptors by CAP injected in the hind- or forepaw did not significantly depress BK-induced PE (Fig. 2A and B, ▴). The degree of BK-induced PE in these animals was not significantly different from that in animals with denervated adrenal medullae but intact vagus nerves (Fig. 2A, compare ▵ and ▴; changes of BK-induced PE in response to intrapalmar CAP in animals with denervated adrenal medullae were not measured) and was slightly reduced when compared to the controls (no CAP; Fig. 2A and B, compare × with ▴).
Animals with acutely transected spinal cord
Modulation of BK-induced PE in the rat knee joint during activation of nociceptive afferents by injection of CAP into the hindpaw or the forepaw was investigated in animals in which the spinal cord was acutely transected at the level T1/T2 or T12/L1 in order to test whether the spinal cord, isolated from supraspinal centres, can mediate the depression and whether the HPA axis is involved. When CAP was injected into the palmar skin of the forepaw of animals spinalized at T1/T2 (i.e. caudal to the afferent inflow from the forepaw to the spinal cord), no depression of BK-induced PE was observed either in animals with intact vagus nerves (Fig. 3B, □) or in subdiaphragmatically vagotomized animals (Fig. 3B, ▪).
Figure 3. Spinal pathways mediate the inhibition of BK-induced PE by capsaicin.
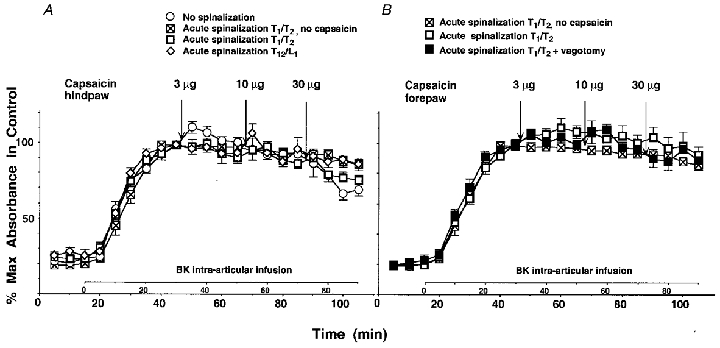
A, effect of intradermal injection of CAP (3–30 μg) into the plantar skin of the hindpaw on BK-induced PE in the knee joint in rats acutely spinalized at the level T1/T2 (□) or T12/L1 (⋄). ○, effect of intradermal injection of CAP in the hindpaw on BK-induced PE in normal sham-vagotomized rats (same data as in Fig. 1). ⊠, control (no CAP injection) in animals spinalized at the level T1/T2 (same data as in B). B, effect of intradermal injection of CAP (3–30 μg) into the palmar skin of the forepaw on BK-induced PE in the knee joint in animals acutely spinalized at the level T1/T2. The vagus nerves were intact (□) or cut (▪). ⊠, control (no CAP injection) in spinal animals.
When CAP was injected into the plantar skin of the hindpaw of animals spinalized at T1/T2, BK-induced PE was weakly depressed at 30 μg CAP. This depression was not significantly different from the depression observed in non-spinalized animals with intact vagus nerves and intact innervation of the adrenal medullae (Fig. 3A, compare □ with ○). When CAP was injected into the plantar skin of the hindpaw of animals spinalized at T12/L1 (i.e. caudal to the preganglionic outflow to the adrenal medulla but rostral to the afferent inflow from the hindpaw to the spinal cord) BK-induced PE was not depressed and not significantly different from that in control animals (Fig. 3A, compare ⋄ with ○).
Stimulation of visceral afferents by CAP
Stimulation of visceral afferents, and afferents from the parietal peritoneum, using intraperitoneal CAP or intravesical CAP also depressed BK-induced PE; this depression was strongly enhanced in vagotomized animals as already reported by Miao et al. (1997a,b) (Fig. 4, compare ○ and •). The depression of BK-induced PE in animals with intact vagus nerves and in vagotomized animals was proposed to have been mediated by the HPA axis as is the case for the depression of BK-induced PE generated by electrical stimulation of cutaneous afferents (see Green et al. 1995). We further investigated the depression of BK-induced PE during stimulation of visceral afferents by intraperitoneal injection of CAP after denervation of the adrenal medullae. In the animals with denervated adrenal medullae but intact vagus nerves, BK-induced PE was not significantly depressed by intraperitoneal CAP at doses of 10−6 to 10−3 mg kg−1 (Fig. 4, compare ▵ with ×). In vagotomized animals with denervated adrenal medullae the depression was significantly attenuated when compared to animals which were vagotomized alone (Fig. 4, compare ▴ with •); however, some depression was still left at doses of 10−4 and 10−3 mg kg−1 when compared to the control animals (Fig. 4, compare ▴ with ×).
Figure 4. Effect of intraperitoneal capsaicin is enhanced by vagotomy and is dependent on the adrenal medulla.
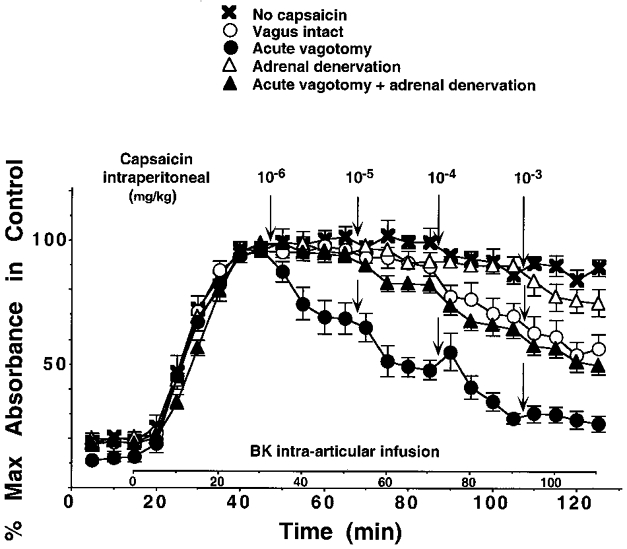
Effect of intraperitoneal injection of CAP (10−6 to 10−3 mg kg−1) on BK-induced PE in animals with intact vagus nerves (○), in vagotomized animals (•), in animals with intact vagus nerves but denervated adrenal medullae (▵) and in vagotomized animals with denervated adrenal medullae (▴). ×, no intraperitoneal CAP applied.
DISCUSSION
We have recently shown that activation of cutaneous afferents by transcutaneous electrical stimulation or intrathecal application of nicotine to the lumbar spinal cord depresses BK-induced PE. This depression was mediated by the HPA axis since spinalization, hypophysectomy and blockade of corticosterone synthesis in the adrenal cortex abolished the depression, whereas removal of the adrenal medullae or their denervation did not affect the depression (Miao et al. 1994, 1996b; Green et al. 1995). Furthermore, depression of the BK-induced PE generated by electrical stimulation of cutaneous afferents or by intrathecal nicotine was enhanced after subdiaphragmatic vagotomy (Miao et al. 1994, 1997a). The vagal afferents involved project through the coeliac branches of the abdominal vagus nerves because interruption of these branches had the same effect as complete subdiaphragmatic vagotomy (Miao et al. 1997b). Afferents which project through the coeliac branches innervate the small intestine and the proximal part of the large intestine (Berthoud et al. 1991). We concluded from these experiments that both intrathecal nicotine as well as electrical stimulation of unmyelinated afferents activate an ascending pathway to the hypothalamus which in turn activates the HPA axis and that this ascending pathway is under powerful inhibitory control from the abdominal viscera mediated by activity in vagal afferents. Interruption of these vagal afferents removes impulse activity to the nucleus of the solitary tract and therefore the ongoing inhibition acting at the ascending pathway to the HPA axis, leading to an enhanced activation of the HPA axis and therefore to an enhanced depression of BK-induced PE.
Both types of activation of the ascending pathway to the hypothalamus are quite non-physiological. Therefore, in the present study we used intraplantar and intrapalmar (i.e. the sites of prior electrical stimulation) injection of CAP, which excites nociceptive afferents asynchronously. Using this type of afferent stimulation we could show that BK-induced PE was depressed in normal animals, in particular from the forepaw, and that this depression was enhanced after subdiaphragmatic vagotomy, the enhancement being significantly stronger for the hindpaw stimulation than for the forepaw stimulation. However, the HPA axis was not involved in this inhibition of BK-induced PE because it was abolished by denervation of the adrenal medullae both in vagotomized and in vagus-intact animals and because stimulation of nociceptive afferents of the forepaw in animals which had been spinalized at the segmental level T1/T2 (i.e. caudal to the segmental spinal afferent inflow from the forepaw) had no effect on BK-induced PE. These data show that the sympatho-adrenal system is involved in the depression. Furthermore, we confirmed previous results showing that the sympathetic outflow to the rat knee joint is not involved in this depression, because removal of the suprarenal ganglion and cutting the branches from the greater and minor splanchnic nerves to the adrenal gland (Araki et al. 1984) leaves the sympathetic chain to the hind-limb unaffected.
By the same token we have additionally shown in the present study that depression of BK-induced PE during stimulation of visceral afferents and of afferents from the parietal peritoneum by intraperitoneal CAP is also largely mediated by the sympatho-adrenal system, in normal and in vagotomized animals. Recently, we concluded that this depression is mediated by the HPA axis (Miao et al. 1997a,b). This conclusion was erroneously deduced from the experimental observation that depression of BK-induced PE generated by transcutaneous electrical stimulation at strengths that excite afferent C-fibres is mediated by the HPA axis (Green et al. 1995, 1997). We do not know whether intraperitoneally injected CAP excites vagal afferents. It has been shown in neonatal rats that most vagal afferents that project through the coeliac branches of the vagus nerves are sensitive to capsaicin (Berthoud & Neuhuber, 1994; Berthoud et al. 1997); however, this does not prove that these afferents are excited by capsaicin injected intraperitoneally. We can at least say that the depression of BK-induced PE in subdiaphragmatically vagotomized animals is induced by activation of spinal afferents.
The reflex activation of the adrenal medullae by noxious stimulation and its inhibitory control from the abdominal viscera via vagal afferents could be mediated by the following circuits (Fig. 5). A spino-bulbo-spinal pathway is activated together with a spinal pathway by the noxious input, leading to the reflex activation of the preganglionic neurones innervating the adrenal medullae. Such spinal and spino-bulbo-spinal somato-sympathetic reflex pathways to sympathetic preganglionic neurones innervating the adrenal medullae, which are activated by noxious stimulation, have been shown to exist in experiments in which activity in the adrenal nerve and release of catecholamines from the adrenal medullae have been measured. For example, stimulation of cutaneous nociceptive afferents activates the preganglionic neurones to the adrenal medullae and leads to release of catecholamines from the adrenal medullae in rats with intact spinal cord and in spinalized rats (Araki et al. 1984; Ito et al. 1984; Kurosawa et al. 1985; for review see Sato, 1987; Sato et al. 1997). From our studies reported here we also postulate, for the hindpaw-induced reflexes, a spinal pathway from the lumbar segments L4 and L5 (which receive the afferent input from the plantar skin of the hindpaw) to the preganglionic neurones in the segments T4 to T12, which innervate the adrenal medullae (Strack et al. 1988): spinalization at the segmental level T12/L1 (which separates the afferent inflow from the hindpaw and the efferent outflow to the adrenal medullae) entirely abolished the depression of BK-induced PE which is normally generated by noxious hindpaw stimulation; in animals spinalized at the segmental level T1/T2 some depression was present which was similar in degree to that in normal animals with intact vagus nerves.
Figure 5. Schematic diagram showing the proposed neural circuits in spinal cord and brain stem that modulate synovial BK-induced PE via the adrenal medullae.
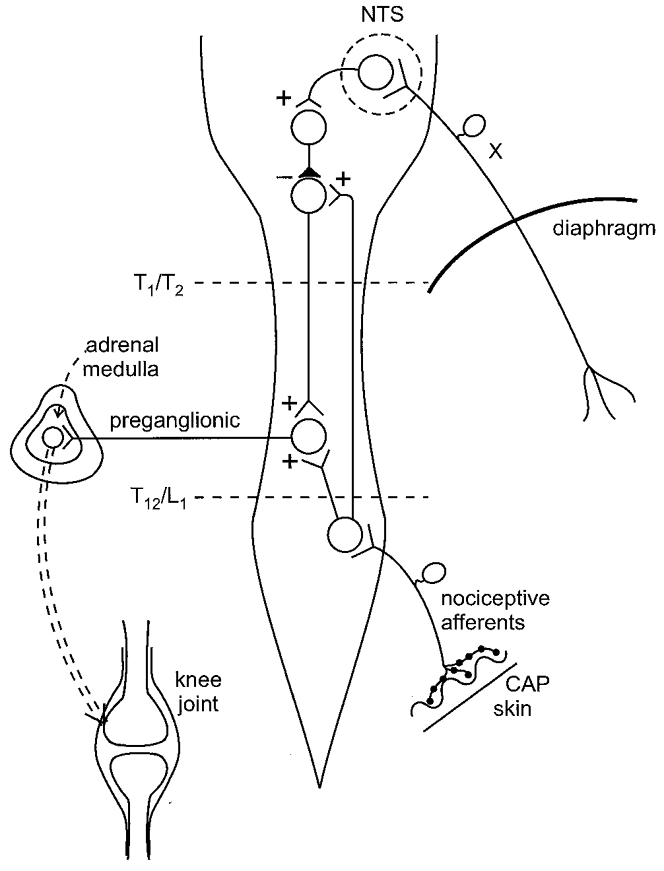
Noxious stimulation of skin by CAP (only the situation for the nociceptive afferent input from the hindlimb is shown) leads to activation of preganglionic neurones, innervating the adrenal medullae, via a spinal and a supraspinal circuit. These circuits are under inhibitory control maintained by activity in abdominal vagal afferents. Removal of this inhibition by subdiaphragmatic vagotomy (X) enhances the reflex activation of the preganglionic neurones and therefore the activation of the adrenal medullae. This leads to greater depression of BK-induced PE by a signal from the adrenal medullae which may be adrenaline. Spinalization at the level T12/L1 interrupts spinal and supraspinal reflex pathways activated by nociceptive input from the hindlimb and abolishes any reflex depression of BK-induced PE. Spinalization at the level T1/T2 leaves the spinal pathway from the hindlimb to the preganglionic neurones intact but interrupts the supraspinal loop; now vagotomy is not followed by a greater depression of BK-induced PE generated by hindpaw stimulation and forepaw stimulation has no effect on BK-induced PE in animals with intact vagus nerves and in vagotomized animals (because the afferent input from the forepaw is rostral to the segmental level T1/T2). Neural inhibition maintained by activity in abdominal vagal afferents acts at the supraspinal circuit in the lower brain stem and/or at the level of the spinal cord (not shown in the figure). +, excitation;–, inhibition; NTS, nucleus of the solitary tract.
The spino-bulbo-spinal pathway is normally inhibited by activity in abdominal vagal afferents. This inhibition could occur at the supraspinal level as shown in Fig. 5. After spinalization at a high thoracic level, only the spinal pathway to the preganglionic neurones would function (as if the spino-bulbo-spinal reflex loop were entirely inhibited by vagal activity). Inhibition maintained by activity in vagal afferents may occur additionally at the spinal level (i.e. at the interneurone between second-order neurones and preganglionic neurones) and be exerted by a separate descending pathway which is activated by vagal activity (not shown in Fig. 5). This idea is supported by preliminary experiments showing that intrathecal application of the α-adrenoceptor antagonist phentolamine and/or of the opioid receptor antagonist naloxone at the lumbar spinal cord, which blocks inhibition of nociceptive impulse transmission exerted by descending pathways from the lower brain stem, enhances the depression of BK-induced PE generated by noxious stimulation (F. J.-P. Miao, W. Jänig & J. D. Levine, unpublished observations). However, direct evidence that the inhibitory vagal effect is mediated by these monoaminergic and opioidergic mechanisms acting at the spinal cord level has yet to be obtained.
Systematic lesion studies of spinal ascending and descending tracts will have to show which pathways between nociceptive spinal afferent input, vagal afferent input to the nucleus of the solitary tract and preganglionic neurones to the adrenal medullae mediate the activation of the preganglionic neurones and its inhibitory control.
The results reported in this paper are intriguing from two points of view. Firstly, depression of BK-induced PE during noxious stimulation of plantar skin by CAP is mediated by the adrenal medullae, but depression of BK-induced PE during electrical stimulation of plantar skin at a strength that excites unmyelinated afferents is mediated by the HPA axis. Both depressions are enhanced after subdiaphragmatic vagotomy. Electrical stimulation of afferents (at up to 3 Hz, see Green et al. 1995; Miao et al. 1997a) leads to synchronous activation of A- and C-fibres; this is undoubtedly an artificial afferent input and not a biological one. However, this electrical stimulation activates the HPA axis; therefore, an ascending pathway to the hypothalamus which leads to this activation must exist. This raises the question, which remains to be answered, of which physiological stimuli acting in the body or on the body surface lead to the selective activation of the HPA axis? What are the essential differences between the two ways for activation of afferents innervating the paw? CAP activates many C-afferents and some Aδ-afferents (Szolcsanyi et al. 1988) asynchronously, but not Aβ-afferents and not most Aδ-afferents. Transcutaneous electrical stimulation indiscriminately activates all afferents synchronously. Is it the synchronous activation of all afferent fibres that leads to the selective activation of the HPA axis but not to the activation of the sympatho-adrenal system?
Secondly, Khasar et al. (1998a,b) have shown that subdiaphragmatic vagotomy decreases the baseline paw-withdrawal threshold and enhances BK-induced hyperalgesic behaviour in response to mechanical stimulation of the dorsal hindpaw skin in rats. These changes do not occur in animals with denervated adrenal medullae or are reversed when the adrenal medullae are denervated after subdiaphragmatic vagotomy has been performed. These changes have a slow time course; they develop and are reversed in about 7–14 days following the respective interventions (vagotomy, denervation of the adrenal medullae). These experiments imply that after vagotomy a hormonal signal is released from the adrenal medullae and leads to the sensitization of the nociceptor population in the skin. The mechanism of sensitization has a long time course, taking about 1 week to reach a maximum. How do these experiments connect with the experiments reported here? Depression of BK-induced PE following activation of the sympatho-adrenal system in rats that were acutely vagotomized occurs immediately.
In conclusion, we have shown that stimulation of nociceptive afferents in the paws by CAP leads to depression of an inflammatory response, BK-induced PE, in the synovia of the knee joint. This depression is mediated by activation of the sympatho-adrenal system and is markedly enhanced after section of the abdominal vagus nerves. The reflex pathways mediating the activation of the preganglionic neurones innervating the adrenal medullae are spinal and supraspinal; these reflex pathways are under inhibitory control maintained by activity in vagal afferents. Future investigations will have to show the functional types of vagal afferents involved, the central inhibitory systems involved, whether adrenaline from the adrenal medulla mediates the depression of the synovial inflammatory response, and which stimulation parameters at the primary afferents lead to selective activation of the sympatho-adrenal system (as occurs with intradermal CAP) and which to activation of the HPA axis (as occurs with transdermal electrical stimulation).
Acknowledgments
This work was supported by grants from TRDRP (8RT-0032) and NIH (AR-32634).
References
- Appleyard CB, Hiller K. Biosynthesis of platelet-activating factor in normal and inflamed human colon mucosa: evidence for the involvement of the pathway of platelet-activating factor synthesis de novo in inflammatory bowel disease. Clinical Science. 1995;88:713–717. doi: 10.1042/cs0880713. [DOI] [PubMed] [Google Scholar]
- Araki T, Ito K, Kurosawa M, Sato A. Responses of adrenal sympathetic nerve activity and catecholamine secretion to cutaneous stimulation in anesthetized rats. Neuroscience. 1984;12:289–299. doi: 10.1016/0306-4522(84)90154-4. [DOI] [PubMed] [Google Scholar]
- Baron R, Jänig W, Kollmann W. Sympathetic and afferent somata projecting in hindlimb nerves and the anatomical organization of the lumbar sympathetic nervous system in the rat. Journal of Comparative Neurology. 1988;275:460–468. doi: 10.1002/cne.902750310. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. American Journal of Physiology. 1991;260:R200–207. doi: 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R, Neuhuber WL. Distribution and morphology of vagal afferents and efferents supplying the digestive system. In: Taché Y, Wingate DL, Burks TF, editors. Innervation of the Gut. Pathophysiological Implications. Boca Raton: CRC Press; 1994. pp. 43–66. [Google Scholar]
- Berthoud H-R, Patterson LM, Willing AE, Mueller K, Neuhuber WL. Capsaicin-resistant vagal afferent fibres in the rat gastrointestinal tract: anatomical identification and functional integrity. Brain Research. 1997;746:195–206. doi: 10.1016/s0006-8993(96)01222-x. [DOI] [PubMed] [Google Scholar]
- Carr J, Wilhelm DL. The evaluation of increased vascular permeability in the skin of guinea pigs. Australian Journal of Experimental Biology and Medical Sciences. 1964;42:511–522. doi: 10.1038/icb.1964.48. [DOI] [PubMed] [Google Scholar]
- Celler BG, Schramm LP. Pre- and postganglionic sympathetic activity in splanchnic nerves of rats. American Journal of Physiology. 1981;241:R55–61. doi: 10.1152/ajpregu.1981.241.1.R55. [DOI] [PubMed] [Google Scholar]
- Green PG, Jänig W, Levine JD. Negative feedback neuroendocrine control of inflammatory response in the rat is dependent on the sympathetic postganglionic neuron. Journal of Neuroscience. 1997;17:3234–3238. doi: 10.1523/JNEUROSCI.17-09-03234.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PG, Miao FJ-P, Jänig W, Levine JD. Negative feedback neuroendocrine control of the inflammatory response in rats. Journal of Neuroscience. 1995;15:4678–4686. doi: 10.1523/JNEUROSCI.15-06-04678.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves KM, Roszkowski MT, Swift JD. Bradykinin and inflammatory pain. Agents and Actions. 1993;41(suppl.):65–73. [PubMed] [Google Scholar]
- Ito K, Sato A, Shimamura K, Swenson RS. Convergence of noxious and non-noxious cutaneous afferents and baroreceptor afferents onto single adrenal sympathetic neurons in anesthetized rats. Neuroscience Research. 1984;1:105–116. doi: 10.1016/s0168-0102(84)80008-5. [DOI] [PubMed] [Google Scholar]
- Kesse WK, Parker TL, Coupland RE. The innervation of the adrenal gland. I. The source of pre- and postganglionic nerve fibres to the rat adrenal gland. Journal of Anatomy. 1988;157:33–41. [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Miao FJ-P, Jänig W, Levine JD. Modulation of bradykinin-induced mechanical hyperalgesia in the rat skin by activity in the abdominal vagal afferents. European Journal of Neuroscience. 1998a;10:435–444. doi: 10.1046/j.1460-9568.1998.00030.x. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Miao FJ-P, Jänig W, Levine JD. Vagotomy-induced enhancement of mechanical hyperalgesia in the rat is sympathoadrenal-mediated. Journal of Neuroscience. 1998b;18:3043–3049. doi: 10.1523/JNEUROSCI.18-08-03043.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa M, Saito H, Sato A, Tsuchiya T. Reflex changes in sympatho-adrenal medullary functions in response to various thermal cutaneous stimulations in anaesthetized rats. Neuroscience Letters. 1985;56:149–154. doi: 10.1016/0304-3940(85)90121-1. [DOI] [PubMed] [Google Scholar]
- Miao F, Jänig W, Dallman MF, Benowitz NL, Heller PH, Basbaum AI, Levine JD. Role of vagal afferents and spinal pathways in modulating inhibition of bradykinin-induced plasma extravasation by intrathecal nicotine. Journal of Neurophysiology. 1994;72:1199–1207. doi: 10.1152/jn.1994.72.3.1199. [DOI] [PubMed] [Google Scholar]
- Miao FJ-P, Green PG, Coderre TJ, Jänig W, Levine JD. Sympathetic-dependence in bradykinin-induced synovial plasma extravasation is dose-related. Neuroscience Letters. 1996a;205:165–168. doi: 10.1016/0304-3940(96)12403-4. [DOI] [PubMed] [Google Scholar]
- Miao FJ-P, Jänig W, Green PG, Levine JD. Inhibition of bradykinin-induced synovial plasma extravasation produced by intrathecal nicotine is mediated by the hypothalamo-pituitary adrenal axis. Journal of Neurophysiology. 1997a;78:1285–1292. doi: 10.1152/jn.1996.76.5.2813. [DOI] [PubMed] [Google Scholar]
- Miao FJ-P, Jänig W, Levine JD. Role of sympathetic postganglionic neurones in synovial plasma extravasation induced by bradykinin. Journal of Neurophysiology. 1996b;75:715–724. doi: 10.1152/jn.1996.75.2.715. [DOI] [PubMed] [Google Scholar]
- Miao FJ-P, Jänig W, Levine JD. Vagal branches involved in inhibition of bradykinin-induced synovial plasma extravasation by intrathecal nicotine and noxious stimulation in the rat. The Journal of Physiology. 1997b;498:473–481. doi: 10.1113/jphysiol.1997.sp021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker TL, Mohamed AA, Coupland RE. The innervation of the adrenal gland. IV. The source of pre- and postganglionic nerve fibres to the guinea-pig adrenal gland. Journal of Anatomy. 1990;172:17–24. [PMC free article] [PubMed] [Google Scholar]
- Precht JC, Poley TL. Organization and distribution of the rat subdiaphragmatic vagus and associated paraganglia. Journal of Comparative Neurology. 1985;235:182–195. doi: 10.1002/cne.902350204. [DOI] [PubMed] [Google Scholar]
- Sato A. Neural mechanisms of somatic sensory regulation of catecholamine secretion from the adrenal gland. Advances in Biophysics. 1987;23:39–80. doi: 10.1016/0065-227x(87)90004-9. [DOI] [PubMed] [Google Scholar]
- Sato A, Sato Y, Schmidt RF. The impact of somatosensory input on autonomic functions. Reviews of Physiology, Biochemistry and Pharmacology. 1997;130:1–328. [PubMed] [Google Scholar]
- Strack AN, Sawyer WB, Marubio LM, Loewy AD. Spinal origin of sympathetic preganglionic neurones in the brain. Brain Research. 1988;455:187–191. doi: 10.1016/0006-8993(88)90132-1. [DOI] [PubMed] [Google Scholar]
- Swift JQ, Garry MG, Roszkowski MT, Hargreaves KM. Effect of flurbiprofen on tissue levels of immunoreactive bradykinin and acute postoperative pain. Journal of Oral and Maxillofacial Surgery. 1993;51:112–117. doi: 10.1016/s0278-2391(10)80002-3. [DOI] [PubMed] [Google Scholar]
- Szolcsanyi J, Anton F, Reeh PW, Handwerker HO. Selective excitation by capsaicin of mechano-heat sensitive nociceptors in rat skin. Brain Research. 1988;446:262–268. doi: 10.1016/0006-8993(88)90885-2. [DOI] [PubMed] [Google Scholar]
- Will PC, Thomas TK, Iverson L, Buckman D, Weis W, Wilson C, Srivastava A. Platelet activating factor as a proinflammatory mediator in acetic-induced colitis in the rat. Agents and Actions. 1991;34:181–184. doi: 10.1007/BF01993272. [DOI] [PubMed] [Google Scholar]


