Abstract
The aim of this study was to estimate the aqueous distributional spaces of the liver as a function of the route of input: portal vein (PV) versus hepatic artery (HA).
Studies were performed in the in situ single (PV) and dual (PV-HA) perfused rat liver (n = 6–10) using Krebs bicarbonate buffer at constant PV (12 ml min−1) and HA (3 ml min−1) flow rates. An impulse input-output response technique was employed, varying the route of input, using non-labelled erythrocytes (intravascular marker), 125I-albumin and [14C]sucrose (extracellular markers), and [14C]urea and 3H2O (total water markers) as the reference indicators.
Distributional spaces were estimated using two different methods, namely standard and specific. The standard method was applied to hepatic outflow data obtained from the single PV perfused liver. The specific method was used when operating in the dual perfused mode to provide an estimate of the excess space perfused solely by the HA input. Specific spaces, interstitial and intracellular volumes, were estimated by difference.
The results were evaluated by means of visual inspection of the outflow profiles and comparison of the distributional spaces. Different hepatic effluent profiles obtained as a function of the route of input indicated that these two inputs did not completely mix within the liver. Estimates of the distributional spaces supported this observation, and further suggested that the arterial input perfuses 9–12 % more hepatic tissue than the venous input.
The knowledge obtained from the existence of a specific arterial space can be extended to help make predictions about the fate of an eliminated compound following arterial administration. Any difference between the HA and PV in terms of hepatic recovery could be attributed to this excess space and its enzyme density.
The dual nature of the afferent blood supply to the liver has prompted various investigators to explore the relative contributions of the hepatic artery (HA) and portal vein (PV) to the events occurring within the liver. One aspect of this is the anatomical and functional relationships between the HA and PV in terms of aqueous distributional spaces perfused by them. The aqueous spaces of the liver have been well characterised under various conditions including altered perfusate flow (Pang et al. 1988b), albumin concentration (Roberts et al. 1990a,b) and when using alternative indicators (e.g. 58Co-EDTA for [14C]sucrose and [14C]urea for 3H2O; Pang et al. 1990, 1991b) in the commonly used single PV perfused liver preparation. However, corresponding data under the more physiologically meaningful condition of dual portal and arterial perfusion are sparse and still controversial. Some investigators (Ahmad et al. 1984; Pang et al. 1994) reported no difference between the aqueous volumes related to HA and PV input, whereas others (Reichen, 1988; Kassissia et al. 1994) observed larger volumes of distribution after arterial administration. In some studies either the arterial (Pang et al. 1988a, 1991a; Chiba et al. 1994) or the venous (Burczynski et al. 1996) distributional spaces were determined, preventing comparison between the HA and PV input.
We report here the determination of the intravascular, extracellular and intracellular distributional spaces as a function of the route of input – PV and HA – in the single and dual perfused rat liver preparation. An impulse input-output response technique was employed using single and dual indicator dilution methods. Normal (non-labelled) erythrocytes were used as the intravascular marker, 125I-albumin and [14C]sucrose as extracellular markers, and [14C]urea and 3H2O as cellular markers. A specific method based on the analysis of outflow profiles using statistical moment theory was proposed for the estimation of the various spaces.
METHODS
Materials
[14C]Urea (7.3 mCi mmol−1; Sigma Chemical Co.), [14C]sucrose (0.1 mCi ml−1), 125I-albumin (1.02 mCi mg−1) and tritiated water (3H2O; 100 mCi ml−1) (all from ICN Biomedicals) were used without further purification.
Perfusion procedure
All experiments were conducted under appropriate Project and Personal Licences issued by the UK Home Office. All animals, which were handled in compliance with Home Office guidelines, had free access to drinking water and standard rat diet. They were kept under a 9–12 h light-dark cycle in a temperature-controlled environment.
Male Sprague-Dawley rats (327.0 ± 7.3 g, n = 10; mean ± s.e.m.) were used as liver donors (13.9 ± 0.6 g, n = 10). The perfusion medium was Krebs bicarbonate buffer (mm: NaCl, 118; NaHCO3, 24.9; CaCl2.6H2O, 2.5; KCl, 4.7; MgSO4.7H2O, 1.2; KH2PO4, 1.2) containing 16.7 mm glucose and 0.01 mm sodium taurocholate. The surgical procedure was the same as that described previously (Sahin & Rowland, 1998a). Anaesthesia was induced with intraperitoneal administration of pentobarbitone (60 mg kg−1; Sagatal), and the depth of anaesthesia was assessed by testing the withdrawal response to toe pinch, and the blink reflex. When there was no reaction to these tests, the surgical procedure was started. The bile duct was cannulated (PE10; o.d., 0.61 mm; i.d., 0.28 mm), and loose ligatures were passed around the PV ensuring exclusion of the HA. The PV was cannulated with a 16GA catheter (Argyle Medicut; o.d., 1.7 mm × 45 mm) and perfusion started at a flow rate of 12 ml min−1, using a peristaltic pump (Minipuls 3, Gilson, Anachem). A tube was inserted into the right atrium to carry the outflow perfusate. This drained all the venous return to the heart causing cessation of respiration and heart beat within 1 min. The HA was cannulated via the coeliac artery using an 18GA (Argyle Medicut; o.d., 1.3 mm × 45 mm) or 20GA catheter (Argyle Medicut; o.d., 1.1 mm × 45 mm), and perfused at a flow rate of 3 ml min−1, using a peristaltic pump (Minipuls 3). All operative procedures were completed within 20–30 min without interruption of flow to the liver. The exposed liver was kept moist with saline and covered with a piece of parafilm, to reduce dehydration. Viability of the liver was assessed from the measurement of bile flow, perfusate recovery, hepatic arterial pressure and from gross appearance. At the end of each experiment a small volume of Evans Blue solution was injected on separate occasions into the injection port of both the PV and HA to visually observe the efficiency of cannulation and to assess the level of stagnation at the injection sites. The perfusate flows were then stopped, and the liver excised and weighed.
Injection preparation
Tracer injection preparation
Two tracer markers, one extracellular and the other total water (i.e. 125I-albumin and [14C]urea; [14C]sucrose and 3H2O), were injected simultaneously as a bolus (50 μl) in saline; doses (means ± s.e.m.) used were: 125I-albumin, 0.033 ± 0.003 μCi; [14C]sucrose, 0.059 ± 0.002 μCi; [14C]urea, 0.068 ± 0.005 μCi; and 3H2O, 0.29 ± 0.015 μCi.
Erythrocyte injection preparation
The samples of unlabelled red blood cells (RBCs) for injection were freshly prepared as needed from untreated Sprague-Dawley rats. The rats were deeply anaesthetised by inhalation of halothane (2% Fluothane, Zeneca Pharmaceuticals); blood (10–12 ml) was collected by terminal cardiac puncture, transferred to a heparinised tube, and then centrifuged at 2000 r.p.m. for 20 min at 4°C. The plasma and buffy coat were removed and the packed erythrocytes were washed three times with cold saline. After the final wash the supernatant was removed, and the packed red cells were diluted to 10 ml (40–50 % v/v) for use in the determination of the intravascular volume of the liver. Before each injection, the RBC suspension was gently mixed by inversion to ensure homogeneity.
Experimental procedure
During the stabilisation period (20–30 min), the liver was monitored for leakage, total perfusate flow, bile flow, arterial perfusate pressure and for physical appearance, and then the preparation was allocated into one of two groups, A and B.
In group A (n = 5) the dual perfusion mode was employed.
In group B (n = 5), dual and single perfusion modes were utilised. Initially, the liver was perfused through both the PV and HA. Then the HA flow was stopped while maintaining the PV perfusion.
In each preparation, a bolus dose of reference markers was administered randomly into the injection port of either the PV or HA cannula, followed, approximately 5–10 min after a washout period, by injection into the alternate vessel. In the case of RBCs, the washout period (about 2–3 min) was shorter than that with the labelled markers. In group B, after the HA flow was stopped, the liver was stabilised for 10–15 min before the reference markers were administered, in a randomised order, into the PV cannula. Immediately after an injection, the total effluent was automatically collected using a locally made motor-driven carousel (Pharmacy workshop, University of Manchester, UK); the total collection period for RBCs was 45 s whereas the other markers (albumin, sucrose, urea, water) were collected initially at 1–1.5 s intervals for 1–1.5 min and thereafter at increasing time intervals for a further 2 min.
The activities of 3H and 14C, and 125I and 14C, in 200 μl of outflow perfusate were determined simultaneously by liquid scintillation (LKB Wallac 1409) after the addition of 5 ml scintillation fluid (Optiphase ‘Hisafe’ II, Wallac) with results expressed as disintegrations per minute (d.p.m.). In the case of the RBCs, an aliquot (100–150 μl) of the outflow perfusate was haemolysed with distilled water and the absorbance at 415 nm was determined with a Ultraspec II spectrophotometer (LKB Biochrom).
Data analysis
The outflow concentration of the injected radiolabelled material at the midpoint time of the sampling interval (C(t)) was transformed to the frequency outflow (f(t)) using the following equation:
| (1) |
where Q is the total perfusate flow (ml s−1). The fractional recovery of the injected dose (F) and mean transit time (MTT) were estimated from the statistical moments of the hepatic outflow profiles (Roberts et al. 1988):
| (2) |
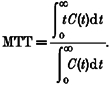 |
(3) |
The statistical moments were estimated by numerical integration after subtracting the mean transit delay caused by the non-hepatic region (input catheter and outflow tubing) of the experimental system (PV: 2.5 1 ± 0.04 s, n = 9; HA: 2.73 ± 0.12 s; n = 12; means ± s.e.m.). The time delay in this region caused by the connecting catheters and outflow tubing was determined separately (Sahin & Rowland, 1998b). Briefly, the system was perfused with the perfusion medium at flow rates of 3 ml min−1 for the HA system and 12 ml min−1 for the PV system. A bolus dose of all reference markers was injected separately into either the HA or PV system and then into the alternate system. The outflow perfusate was collected automatically every second for 30 s and the MTT corresponding to each system was calculated using eqn (3).
Two different methods were used for the calculation of the volume of distribution (VH), namely standard and specific. The standard method was applied to hepatic outflow data obtained with the single portal perfused liver.
Single portal perfusion:
| (4) |
where VPV,s is the volume of distribution following venous administration, MTTPV,s is the MTT after the PV injection, and QPV,s is the PV flow rate.
In the specific method, the liver is divided into two spaces: a specific arterial space, volume VSA, receiving a fraction θ (= 0.17; Sahin & Rowland, 1998b) of the arterial flow (QHA) and a common space, volume VC, which receives all the portal flow and the remaining fraction (1 – θ = 0.83) of the arterial flow. The volumes of distribution associated with each input were estimated as follows (Sahin & Rowland, 1998b, 1999; Appendix).
Dual perfusion:
Following the venous injection:
| (5) |
where VPV,d is the volume of distribution following venous administration and MTTPV,d is the MTT after the venous injection into the dual perfused liver.
Following the arterial injection:
| (6) |
where VHA is the volume of distribution and MTTHA is the total transit time of solute through the liver following HA administration.
The interstitial (IS) and intracellular (IC) distributional spaces were calculated as:
| (7) |
| (8) |
where the subscripts VS, EC and TW refer to the vascular (RBC), extracellular (albumin, sucrose) and total water (urea, water) spaces, respectively. In the calculation of VIC, the reference VEC chosen was that for sucrose.
Regardless of the reference marker, HA and PV dual (PVd) results obtained from groups A and B were pooled whereas PV single (PVs) results were obtained from group B only. All tabulated results were expressed as means ± s.e.m., and were compared using Student's paired or unpaired t test. A P value of less then 0.05 was considered significant.
RESULTS
The liver was free from blotches throughout the experiment and stable bile production was maintained (6.9 ± 0.31 μl min−1, n = 10). Arterial perfusate pressure (55 ± 2 mmHg, n = 10) was relatively constant throughout the experiments and remained stable during the injections. Regardless of the reference marker, fractional recovery following administration into the liver was over 90 %.
Outflow profiles and transit times
Regardless of the perfusion mode (single or dual), as the volume accessed by the reference marker increased, the outflow profiles became broader and flatter, and the fractional output at the peak decreased from RBCs to water for venous administration (Fig. 1A and B). For all except the total water markers urea (P < 0.05) and tritiated water (P < 0.01), the corresponding MTTs were very similar for the two venous perfusion modes (Table 1). A similar progression in outflow profiles was seen after arterial injection (Fig. 1C), except that for all reference markers the output profiles were even flatter and broader (Fig. 2), and hence the MTTs were significantly longer than those following PV injection (P < 0.001).
Figure 1.
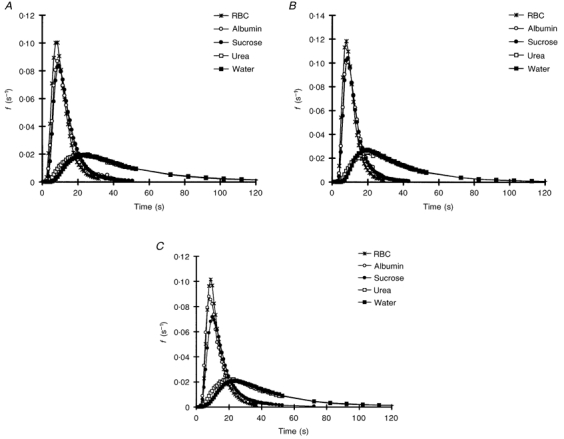
Fractional rate of efflux (f) of RBCs, albumin, sucrose, urea and water following bolus administrations into the single portal vein (A) and dual perfused (portal vein (B) and hepatic artery (C)) rat liver preparations. The liver was perfused at constant portal venous (12 ml min−1) and hepatic arterial (3 ml min−1) flow rates.
Table 1.
Distributional parameters obtained following bolus injection of reference markers into the hepatic artery (HA) and portal vein of the dual perfusion system (PVd) and into the portal vein of the single perfusion system (PVs)
| Marker | Route | MTT (s) | VH (ml g−1) |
|---|---|---|---|
| RBC | HA | 12.92 ± 0.45 | 0.173 ± 0.008 |
| PVd | 8.83 ± 0.46 | 0.153 ± 0.009 | |
| PVs | 9.93 ± 0.46 | 0.149 ± 0.011 | |
| Albumin | HA | 15.41 ± 0.55 | 0.221 ± 0.012 |
| PVd | 11.62 ± 0.61 | 0.201 ± 0.013 | |
| PVs | 13.26 ± 0.90 | 0.201 ± 0.022 | |
| Sucrose | HA | 18.06 ± 0.63 | 0.243 ± 0.010 |
| PVd | 12.31 ± 0.87 | 0.216 ± 0.012 | |
| PVs | 14.26 ± 0.80 | 0.215 ± 0.021 | |
| Urea | HA | 47.11 ± 1.31 | 0.662 ± 0.029 |
| PVd | 34.64 ± 1.85 | 0.598 ± 0.034 | |
| PVs | 41.36 ± 1.46 | 0.620 ± 0.044 | |
| Water | HA | 50.37 ± 1.70 | 0.690 ± 0.027 |
| PVd | 34.82 ± 2.12 | 0.617 ± 0.030 | |
| PVs | 46.60 ± 2.01 | 0.698 ± 0.051 |
Values are means ±s.e.m. (n = 5−10). MTT, mean transit time; Vh, volume of distribution.
Figure 2.

Representative fractional rate of efflux of sucrose as a function of route of input (HA and PV) and perfusion mode (single and dual).
Volume of distribution
Regardless of the marker (RBCs, albumin, sucrose, urea, water), the corresponding distributional volumes estimated following arterial administration were larger than those following venous administration (P < 0.001; Table 1), except for water following single mode PV perfusion. Also, this excess space was similar, irrespective of the marker, being 9 % for albumin, 10 % for urea, 11 % for sucrose and water, and 12 % for RBCs. In the case of venous administration, the volumes of distribution of vascular and extracellular markers were not influenced by the perfusion mode (dual or single). Although the distribution volumes of the total water space markers (urea, tritiated water) tended to be dependent on the perfusion mode, the difference was not statistically significant (Table 1).
The estimated volumes of the three specific spaces (intravascular (IV), IS, IC) are listed in Table 2. Operating in the dual perfused mode, all spaces, except the IS albumin space, estimated following the HA input were significantly larger than those following PV input (P < 0.001). The IS and IC spaces estimated following venous administration tended to be marginally lower in the dual perfusion mode; however, for a given space, the difference was not significant
Table 2.
Intravascular, interstitial and intracellular spaces of distribution (ml g−1) estimated in the single and dual perfused rat liver preparations
| Route of administration | ||||
|---|---|---|---|---|
| Reference space | Marker | PVs | PVd | HA |
| Intravascular space | RBC | 0.149 ± 0.011 | 0.153 ± 0.009 | 0.173 ± 0.008 |
| Interstitial space | Albumin | 0.052 ± 0.018 | 0.038 ± 0.007 | 0.041 ± 0.007 |
| Sucrose | 0.067 ± 0.015 | 0.051 ± 0.014 | 0.059 ± 0.013 | |
| Intracellular space | Urea | 0.403 ± 0.026 | 0.384 ± 0.035 | 0.422 ± 0.030 |
| Water | 0.482 ± 0.034 | 0.400 ± 0.021 | 0.450 ± 0.019 | |
Values are means ±s.e.m. (n = 5−10).
DISCUSSION
Outflow profiles and transit times
It is generally believed that the majority of the sinusoids are common channels for both the HA and PV streams and receive a mixed blood. If this is so, regardless of the route of administration, very similar hepatic outflow profiles are expected (Hollenberg & Dougherty, 1966). However, different hepatic outflow profiles (i.e. slightly delayed and diminished peaks after arterial administration compared to PV input), and hence MTTs, do not favour this idea. Different profiles as a function of route of input were reported earlier, using 125I-albumin in dog liver (Cohn & Pinkerson, 1969), and more recently, using erythrocytes, albumin, sucrose (Kassissia et al. 1994), urea (Sahin & Rowland, 1998b) and water (Sahin & Rowland, 1999) in rat liver. Pang et al. (1994) have attributed the profiles with slightly delayed and attenuated peaks after HA injection to a much greater delay in the arterial catheter. Although the non-hepatic region (e.g. catheters) of the experimental system is known to have an effect on the outflow profile of a compound, especially for vascular and extracellular markers (Goresky & Silverman, 1964), this was not the case in our experiments because the MTTs of the reference markers (Table 1) were much greater than the MTTs of the non-hepatic regions of both the HA (2.73 ± 0.12 s, n = 12) and PV systems (2.51 ± 0.04 s, n = 9). Furthermore, rapid washout of Evans Blue dye from the injection site ruled out the possibility of stagnation in the catheter. All these observations suggest that the arterial outflow profiles are more likely to be distorted within the liver rather than within the non-hepatic region of the experimental system.
The PV and HA branches run parallel within the liver and their terminal branches supply blood to the sinusoids. In the rat, unlike the PV, the HA flow drains into the sinusoids via various pathways including arteriovenous anastomosis, arteriosinusoidal twigs and peribiliary capillary plexus (Mitra, 1966; Bloch, 1970; Grisham & Nopanitaya, 1981). Of these, the peribiliary capillary plexus, which is a complex capillary vessel that surrounds the bile duct and receives the majority of its afferents from the HA, may provide a more tortuous path for arterial blood resulting in distortion in the arterial profiles. Recently, Kassissia et al. (1994) suggested that the distortion caused by the peribiliary capillary plexus cannot be corrected for readily as the transfer function of these capillaries cannot be estimated.
For a non-eliminated compound, the MTT is a function of both the size of the space, V, into which a compound can distribute and flow rate, Q (MTT = V/Q). Therefore, for a given flow rate, with an increase in size of the space, an increase in the MTT is expected. Although the MTT after arterial administration was always longer than that after PV administration, independent of the reference marker employed, for a given marker, the MTT after venous injection was minimally affected by the perfusion mode, indicating that the HA flow has a very small or no effect on the PV input. The current results confirm the earlier data of a longer MTT after arterial than portal administration (Gascon-Barre et al. 1988; Kassissia et al. 1994). However, this is in contrast to the study of Cohn & Pinkerson (1969) who observed a slightly longer MTT following portal venous administration to the dog, and to that of Ahmad et al. (1984) who observed no effect on MTT as a result of route of input in the rat. Nevertheless, the latter observation could be an artefact as the results were obtained from single perfusion of the liver via either the PV or HA, each at the same flow rate.
Volume of distribution
Various approaches to HA perfusion in the rat liver preparation have been published (Ahmad et al. 1984; Reichen, 1988; Kassissia et al. 1994; Pang et al. 1994) and the conclusions drawn, as to the volumes of distribution accessible to markers following HA and PV administration, have differed considerably. In these studies, the volume of distribution was estimated as the product of total flow rate and MTT. However, application of this method requires a closed and well-mixed system (Meier & Zierler, 1954). As these requirements are violated in the dual perfused liver, because of its dual input and partial intrahepatic mixing, the use of this method is inappropriate. This problem was partly addressed by Reichen (1988) who proposed the concept of ‘equivalent’ space to reflect the fact that the requirements for the volume calculations are not strictly met in the dual perfused rat liver; however, no solution was proposed. Previously, we have compared the standard and specific methods by means of volume estimates (Sahin & Rowland, 1999), and observed that HA estimates using the standard method are consistently larger than PV estimates by about 40 %. However, when aspects of the specific arterial space and HA flow segregation were considered in the estimation of distributional volumes, the difference was only 9–15 % in favour of the HA input. Furthermore, using the water content of the liver obtained by desiccation as the reference (i.e. 0.72 ± 0.01 ml g−1; Sahin & Rowland, 1998b), the specific method proved superior to the standard method especially following arterial administration (0.79 ± 0.04 vs. 1.10 ± 0.07 ml g−1 for labelled water; Sahin & Rowland, 1999). All these findings support the idea that the conclusions deduced from the volume estimates are dependent upon the method used and could be misleading. Although the PV estimates were minimally affected by the choice of method, the effect was dramatic for the HA estimates. Therefore, in the present study only the specific method was applied to the hepatic outflow data obtained from the dual perfused liver. For each reference marker, we calculated the contribution of specific arterial space to the total and found that about 9–12 % of the total space is irrigated by the HA flow only. These results are in good agreement with our previous estimates of 9.7 % using labelled urea (Sahin & Rowland, 1998b) and also with the literature estimates of 10–11 % using labelled RBCs (Field & Andrews, 1968; Ahmad et al. 1984).
Intravascular space
Different methods have been employed for determination of the vascular space using labelled (e.g. 51Cr) RBCs: these include measurement of radioactivity either in the outflow perfusate (Ahmad et al. 1984; Reichen, 1988; Kassissia et al. 1994) or in the tissue samples (Gonzalez & Bassingthwaighte, 1990). To avoid exposure to the radioactivity during injection and sampling procedures of 51Cr, we adopted a spectrophotometric approach for measuring erythrocytes in hepatic outflow samples.
Literature estimates of the vascular space, especially following HA administration, vary widely (12–36 % of liver weight with a mean value of 27 %); estimates following PV administration are smaller (11–21 % with a mean value of 17 %). Conclusions drawn from these studies differed: some authors (Ahmad et al. 1984; Pang et al. 1994) claimed that the route of input had no effect on the vascular volume, whilst others (Reichen, 1988) concluded that there was a significantly larger vascular volume following HA input. Furthermore, ineffectiveness of HA flow on the volume estimates following venous administration was reported by Reichen (1988), Pang et al. (1994), and by Burczynski et al. (1996). The results of the present study confirm this last reported observation. Additionally, close agreement between the current study and mean literature estimates following the PV administration (e.g. 15 vs. 17 %) supports the use of the spectrophotometric method. On the other hand, the difference between the values of HA estimates of this study and the literature values could be attributed to the different methods of calculation. However, it should be noted that the use of RBCs may underestimate the total vascular space. This is because axial migration of red cells will result in them having a shorter MTT than that for the plasma. This error will, of course, give rise to a slight overestimate of the tissue spaces.
Interstitial space
Albumin and sucrose are the most commonly used markers for evaluation of the liver IS space. Although there is a debate about the use of sucrose for assessment of the extracellular space, because of possible hepatocyte uptake (Pierson et al. 1978), this was not confirmed by others (Alpini et al. 1986). Albumin (3.6- 7.5 nm) and sucrose (0.5 nm) differ with respect to molecular size (Garlick & Renkin, 1970; Barrowman et al. 1982) and hence accessible IS space: sucrose essentially enters the entire IS space whereas albumin is excluded from a portion of this space. A survey of the literature estimates revealed that the size of the IS space accessed by albumin is 70–75 % of the IS sucrose space (Goresky, 1963; Reichen, 1988; Kassissia et al. 1994) following venous administration whereas albumin has access to only 35–57 % of the IS sucrose space, after arterial administration (Pang et al. 1988a; Reichen, 1988; Kassissia et al. 1994). This discrepancy according to route of entry was attributed to the poor permeability characteristics of the peribiliary capillary plexus; sucrose, but not albumin, can diffuse through the tight capillaries and enter the IS space of the peribiliary capillary plexus (Pang et al. 1988a; Reichen, 1988). However, this discrepancy was not observed in the present study. Regardless of the route of input, the accessible IS albumin space was 70–75 % (HA vs. PV), in good agreement with the previously reported data. We attribute the reported discrepancy for HA input to the differing methods of calculation, as the estimate of IS albumin space for HA is reduced from 70 to 46 % by the application of the standard method for the volume calculation.
Intracellular space
In the estimation of the IC space, the value of the extracellular space (VEC) was taken as that for sucrose and not albumin, as unlike sucrose and the total water space markers, tritiated water and urea, albumin does not access the entire IS space. Available data including physicochemical properties, in vitro hepatocyte permeability (Alpini et al. 1986) and in situ rat liver (Goresky, 1963; Pang et al. 1990) studies suggest that urea is a suitable alternative to tritiated water for the estimation of the total water space of the liver. Although urea has been widely used in the single PV perfused liver preparation, data for the dual perfusion are lacking. This study thus extends knowledge to the dual perfused rat liver preparation. The total aqueous space accessible to urea was 94 and 96 % of that of the tritiated water space after HA and PV administrations, respectively. These results suggest that urea can be used as an alternative to labelled water in the dual perfused liver. Furthermore, regardless of the route of administration, the estimate of the IC space obtained in the current study (40–48 %) agrees well with the previously reported values of 35–41 % (Greenway & Stark, 1971; Reichen, 1988).
This study highlights two major points.
Estimation of aqueous distributional spaces as the product of flow rate and mean transit time, especially for the arterial input, can be misleading as arterial blood segregation occurs within the liver. Therefore, an alternative method has been used for estimating the volume of distribution of compounds in the dual perfused liver preparation. Previously, this method proved to be superior to the standard method (Sahin & Rowland, 1999), and also provides the additional advantage of allowing an estimate of the specific arterial space.
Arterial input irrigates 9–12 % more hepatic tissue than that following the venous input. The existence of such a specific space may have relevance to the systemic exposure of compounds after HA administration, as sometimes arises during the treatment of the hepatic carcinomas, many of which reside predominantly on the arterioles. Furthermore, unlike PV only administration (which occurs for example following oral administration, as all splanchnic blood drains into the PV), hepatic extraction of compounds following arterial administration will be governed by events in both the common and specific spaces. If there is any difference in extraction as a function of route of input, this difference may be attributed to the specific space and its enzyme content, as extraction across the common space will be the same regardless of route of hepatic input.
In addition, a spectrophotometric method worked well for the determination of the vascular space and can be used as an alternative to the radiochemical determination of the vascular space.
Acknowledgments
Selma Sahin was supported by a scholarship from the Turkish Government.
APPENDIX
In the following analysis the dual perfused liver is considered to consist of two parallel spaces: a common space (VC) which is perfused by the PV flow and a fraction of the HA flow (1 – θ = 0.83), and a specific space (VSA) which receives the remaining fraction of the HA flow (θ = 0.17; Sahin & Rowland, 1998b). Therefore, by definition the total volume of the liver (VT) is given by:
| (A1) |
The common and specific spaces are defined as follows.
Common space:
| (A2) |
where
| (A3) |
QC is the blood (perfusion) flow to the common space, MTTC is the MTT following the portal venous administration, and QPV and QHA are the PV and HA flow rates, respectively.
Specific space:?
total MTT (MTTHA) and VSA following the arterial administration are given by (Sahin & Rowland, 1998b):
| (A4) |
| (A5) |
so that:
| (A6) |
By rearranging eqn (A6) one obtains:
| (A7) |
which by appropriate substitution then yields:
| (A8) |
Substituting eqn (A3) for QC into eqn (A8) and collecting terms yields:
| (A9) |
Equation (A9) is basically the same as eqn (A10), used by Field & Andrews (1968) for the estimation of total distributional volume of the liver, VH:
| (A10) |
Finally, eqn (A8) is seen to be the same as eqn (6), recognising that, in the context of the model, VT and MTTC are equivalent to the experimental terms VHA and MTTPV,d respectively, and QC MTTC = VPV,d.
References
- Ahmad AB, Bennett PN, Rowland M. Influence of route of hepatic administration on drug availability. Journal of Pharmacology and Experimental Therapeutics. 1984;230:718–725. [PubMed] [Google Scholar]
- Alpini G, Garrick RA, Jones MJT, Nunes R, Tavoloni N. Water and nonelectrolyte permeability of isolated rat hepatocytes. American Journal of Physiology. 1986;251:C872–882. doi: 10.1152/ajpcell.1986.251.6.C872. [DOI] [PubMed] [Google Scholar]
- Barrowman JA, Perry MA, Kvietys PR, Granger DN. Exclusion phenomenon in the liver interstitium. American Journal of Physiology. 1982;243:G410–414. doi: 10.1152/ajpgi.1982.243.5.G410. [DOI] [PubMed] [Google Scholar]
- Bloch EH. The termination of hepatic arterioles and the functional unit of the liver as determined by microscopy of the living organ. Annals of the New York Academy of Sciences. 1970;170:78–87. [Google Scholar]
- Burczynski FJ, Luxon BA, Weisiger RA. Intrahepatic blood flow distribution in the perfused rat liver: effect of hepatic artery perfusion. American Journal of Physiology. 1996;271:G561–567. doi: 10.1152/ajpgi.1996.271.4.G561. [DOI] [PubMed] [Google Scholar]
- Chiba M, Poon K, Hollands J, Pang KS. Glycine conjugation activity of benzoic acid and its acinar localisation in the perfused rat liver. Journal of Pharmacology and Experimental Therapuetics. 1994;268:409–416. [PubMed] [Google Scholar]
- Cohn JN, Pinkerson AL. Intrahepatic distribution of hepatic arterial and portal venous flows in the dog. American Journal of Physiology. 1969;216:285–289. doi: 10.1152/ajplegacy.1969.216.2.285. [DOI] [PubMed] [Google Scholar]
- Field CD, Andrews WH. Investigation of the hepatic arterial ‘space’ under various conditions of flow in the isolated perfused dog liver. Circulation Research. 1968;23:611–622. doi: 10.1161/01.res.23.5.611. [DOI] [PubMed] [Google Scholar]
- Garlick DG, Renkin EM. Transport of large molecules from plasma to interstitial fluid and lymph in dogs. American Journal of Physiology. 1970;219:1595–1605. doi: 10.1152/ajplegacy.1970.219.6.1595. [DOI] [PubMed] [Google Scholar]
- Gascon-Barre M, Huet PM, St-Onge-Brault G, Brault A, Kassissia I. Liver extraction of vitamin D3 is independent of its hepatic venous or arterial route of delivery. Studies in isolated-perfused rat liver preparations. Journal of Pharmacology and Experimental Therapeutics. 1988;245:975–981. [PubMed] [Google Scholar]
- Gonzalez F, Bassingthwaighte JB. Heterogeneities in regional volumes of distribution and flows in rabbit heart. American Journal of Physiology. 1990;258:H1012–1024. doi: 10.1152/ajpheart.1990.258.4.H1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goresky CA. A linear method of determining liver sinusoidal and extravascular volumes. American Journal of Physiology. 1963;204:626–640. doi: 10.1152/ajplegacy.1963.204.4.626. [DOI] [PubMed] [Google Scholar]
- Goresky CA, Silverman M. Effect of correction of catheter distortion on calculated liver sinusoidal volumes. American Journal of Physiology. 1964;207:883–892. doi: 10.1152/ajplegacy.1964.207.4.883. [DOI] [PubMed] [Google Scholar]
- Greenway CV, Stark RD. Hepatic vascular bed. Physiological Reviews. 1971;51:23–65. doi: 10.1152/physrev.1971.51.1.23. [DOI] [PubMed] [Google Scholar]
- Grisham JW, Nopanitaya W. Scanning electron microscopy of casts of hepatic microvessels: Review of methods and results. In: Lautt WW, editor. Hepatic Circulation in Health and Disease. New York: Raven Press; 1981. pp. 87–107. [Google Scholar]
- Hollenberg M, Dougherty J. Liver blood flow measured by portal venous and hepatic arterial routes with Kr85. American Journal of Physiology. 1966;210:926–932. doi: 10.1152/ajplegacy.1966.210.5.926. [DOI] [PubMed] [Google Scholar]
- Kassissia I, Brault A, Huet PM. Hepatic artery and portal vein vascularization of normal and cirrhotic rat liver. Hepatology. 1994;19:1189–1197. [PubMed] [Google Scholar]
- Meier P, Zierler KL. On the theory of the indicator-dilution method for measurement of blood flow and volume. Journal of Applied Physiology. 1954;6:731–744. doi: 10.1152/jappl.1954.6.12.731. [DOI] [PubMed] [Google Scholar]
- Mitra SK. The terminal distribution of the hepatic artery with special reference to arterio-portal anastomosis. Journal of Anatomy. 1966;100:651–663. [PMC free article] [PubMed] [Google Scholar]
- Pang KS, Barker F, III, Cherry WF, Goresky CA. Esterases for enalapril hydrolysis are concentrated in the periphepatic venous region of the rat liver. Journal of Pharmacology and Experimental Therapeutics. 1991a;257:294–301. [PubMed] [Google Scholar]
- Pang KS, Barker F, III, Schwab AJ, Goresky CA. (14C)Urea and 58Co-EDTA as reference indicators in hepatic multiple indicator dilution studies. American Journal of Physiology. 1990;259:G32–40. doi: 10.1152/ajpgi.1990.259.1.G32. [DOI] [PubMed] [Google Scholar]
- Pang KS, Cherry WF, Accaputo J, Schwab AJ, Goresky CA. Combined hepatic arterial-portal venous and hepatic arterial-hepatic venous perfusions to probe the abundance of drug metabolising activities: perihepatic venous O-deethylation activity for phenacetin and periportal sulfation activity for acetaminophen in the once-through rat liver preparation. Journal of Pharmacology and Experimental Therapeutics. 1988a;247:690–700. [PubMed] [Google Scholar]
- Pang KS, Lee WF, Cherry WF, Yuen V, Accaputo J, Fayz S, Schwab AJ, Goresky CA. Effects of perfusate flow rate on measured blood volume, Disse space, intracellular water space, and drug extraction in the perfused rat liver preparation: characterization by the multiple indicator dilution technique. Journal of Pharmacokinetics and Biopharmaceutics. 1988b;16:595–632. doi: 10.1007/BF01062014. [DOI] [PubMed] [Google Scholar]
- Pang KS, Sherman IA, Schwab AJ, Geng W, Barker F, III, Dlugosz JA, Cuerrier G, Goresky CA. Role of the hepatic artery in the metabolism of phenacetin and acetaminophen: An intravital microscopic and multiple-indicator dilution study in perfused rat liver. Hepatology. 1994;20:672–683. [PubMed] [Google Scholar]
- Pang KS, Xu N, Goresky CA. D2O as a substitute for 3H2O, as a reference indicator in liver multiple-indicator dilution studies. American Journal of Physiology. 1991b;261:G929–936. doi: 10.1152/ajpgi.1991.261.6.G929. [DOI] [PubMed] [Google Scholar]
- Pierson RN Jr, Price DC, Wang J, Rakesh KJ. Extracellular water measurements: organ tracer kinetics of bromide and sucrose in rats and man. American Journal of Physiology. 1978;235:F254–264. doi: 10.1152/ajprenal.1978.235.3.F254. [DOI] [PubMed] [Google Scholar]
- Reichen J. Role of the hepatic artery in canalicular bile formation by the perfused rat liver. A multiple indicator dilution study. Journal of Clinical Investigation. 1988;81:1462–1469. doi: 10.1172/JCI113477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MS, Donaldson JD, Rowland M. Models of hepatic elimination: Comparison of stochastic models to describe residence time distributions and to predict the influence of drug distribution, enzyme heterogeneity, and systemic recycling on hepatic elimination. Journal of Pharmacokinetics and Biopharmaceutics. 1988;16:41–83. doi: 10.1007/BF01061862. [DOI] [PubMed] [Google Scholar]
- Roberts MS, Fraser S, Wagner A, McLeod L. Residence time distributions of solutes in the perfused rat liver using a dispersion model of hepatic elimination: 1. Effect of changes in perfusate flow and albumin concentration on sucrose and taurocholate. Journal of Pharmacokinetics and Biopharmaceutics. 1990a;18:209–234. doi: 10.1007/BF01062200. [DOI] [PubMed] [Google Scholar]
- Roberts MS, Fraser S, Wagner A, McLeod L. Residence time distributions of solutes in the perfused rat liver using a dispersion model of hepatic elimination: 2. Effect of pharmacological agents, retrograde perfusions, and enzyme inhibition on Evans blue, sucrose, water, and taurocholate. Journal of Pharmacokinetics and Biopharmaceutics. 1990b;18:235–258. doi: 10.1007/BF01062201. [DOI] [PubMed] [Google Scholar]
- Sahin S, Rowland M. Development of an optimal method for the dual perfusion of the isolated rat liver. Journal of Pharmacological and Toxicological Methods. 1998a;39:35–43. doi: 10.1016/s1056-8719(97)00100-7. [DOI] [PubMed] [Google Scholar]
- Sahin S, Rowland M. Estimation of specific hepatic arterial water space. American Journal of Physiology. 1998b;275:G228–236. doi: 10.1152/ajpgi.1998.275.2.G228. [DOI] [PubMed] [Google Scholar]
- Sahin S, Rowland M. Distribution kinetics of salicylic acid in the dual perfused rat liver preparation. Drug Metabolism and Disposition. 1999;27:373–378. [PubMed] [Google Scholar]


