Abstract
Sarco-endoplasmic reticulum Ca2+-ATPase from fast skeletal (SERCA1) or cardiac muscle (SERCA2a) was expressed in embryonic chicken and neonatal rat cardiac myocytes by adenovirus vectors, with c-myc tags on both constructs to compare expression and distinguish exogenous from endogenous SERCA2a in myocytes.
Expression of the two isoforms was similar (approximately 3-fold higher than endogenous SERCA). However, SERCA1 activity was 2-fold greater than SERCA2a activity, due to intrinsic differences in turnover rates. Activation of both exogenous SERCA isoforms by Ca2+ was displaced to slightly lower [Ca2+], suggesting that the overexpressed isoforms were independent of phospholamban. In fact, phospholamban and calsequestrin expression were unchanged.
Decay time constants of cytosolic Ca2+ transients from cells overexpressing SERCA1 were reduced by 30–40 % and half-widths by 10–15 % compared to controls. SERCA2a overexpression produced much less acceleration of transients in chick than in rat, and less acceleration than SERCA1 overexpression in either species. There was no significant change in resting [Ca2+], peak amplitudes, or in the amount of Ca2+ releasable by caffeine from overexpression of either SERCA isoform. However, the amplitudes of the transients increased with SERCA1 overexpression when pacing frequency limited refilling of the sarcoplasmic reticulum.
It is concluded that total SERCA transport velocity has a primary effect on the decay phase of transients. Transport velocity is affected by SERCA isoform turnover rate, temperature, and/or SERCA copy number.
Active transport of cytosolic calcium into intracellular stores by sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) is important in regulating calcium signalling. In skeletal and cardiac muscle, SERCA is especially critical, as transport of Ca2+ into the sarcoplasmic reticulum (SR) is required to terminate transient rises in cytosolic Ca2+ that couple membrane excitation to contractile activation, as well as to replenish emptied Ca2+ stores (Carsten, 1964; Fanburg et al. 1964; Inesi et al. 1964). Consistent with this role, specific inhibition of SERCA by thapsigargin drastically reduces cytosolic Ca2+ transients, tension development and kinetics of relaxation in cardiac myocytes, in the absence of changes in action potential (Kirby et al. 1992). Furthermore, mice heterozygous for a null mutation in the cardiac isoform of SERCA have impaired cardiac function (Periasamy et al. 1999). Relevant to these findings is the observation that SERCA mRNA and protein levels decrease in end-stage heart failure (Gwathmey et al. 1987; Mercadier et al. 1990; Takahashi et al. 1992; Hasenfuss et al. 1994), and the suggestion that defective expression of the ATPase may play a pathogenic role (Morgan et al. 1990; Hasenfuss et al. 1994, 1997; Schmidt et al. 1998).
To date, there are three known SERCA genes with several splice variants. SERCA1 is the endogenous isoform of fast-twitch skeletal muscle, while SERCA2a is found in slow-twitch skeletal and cardiac muscle (Brandl et al. 1986; Kaprielian & Fambrough, 1987; Zarain-Herzberg et al. 1990). SERCA3 is mainly expressed in epithelial and endothelial cells (Anger et al. 1994), and platelets (Bokkala et al. 1995). Expression of exogenous SERCA1 or SERCA2a genes in cardiac myocytes has been recently achieved by transfecting cultured cells (Inesi et al. 1997, 1998; Hajjar et al. 1997; Giordano et al. 1997; Sumbilla et al. 1999), or by developing transgenic mice (He et al. 1997; Loukianov et al. 1998; Baker et al. 1998). Although the amounts of exogenous gene expression varied among studies, it was reported that increases in SERCA levels produced accelerated decay phases in cytosolic calcium transients. Presently, however, it is not clear whether expression of exogenous SERCA in cardiac myocytes affects cytosolic Ca2+ transients by increasing the SR calcium load or by accelerating the velocity of the transients by SR ATPase activity. It is also uncertain whether the Ca2+ transients may be affected in a different way by expression of exogenous SERCA1 or SERCA2a, since the two isoforms were shown to have different Ca2+ transport turnover rates (Sumbilla et al. 1999).
We have now carried out a systematic study of SERCA isoforms expressed in cultured embryonic chicken and neonatal rat cardiac myocytes. Viral vectors enabled us to target 100 % of cultured cells with controlled, reproducible SERCA expression 3- to 4-fold greater than controls, and higher than previously obtained by other laboratories (1·2-fold, He et al. 1997; 2-fold, Hajjar et al. 1997; 2·5-fold, Loukianov et al. 1998; 1·5-fold, Baker et al. 1998). We studied Ca2+ transients using the fluorescent indicators fluo-4 and fura-2, under conditions of single or repetitive stimulation. We also measured total Ca2+ released by caffeine from intracellular compartments, and determined the time course of store refilling during restitution protocols. Finally, we discuss variables which might account for differences in the effects of SERCA overexpression observed by ourselves and others.
METHODS
Adenovirus vectors
Adenovirus vectors were constructed by pJM17 (Graham & Prevec, 1992) or Cre-lox recombination (Hardy et al. 1997). Viruses Ad.Sr1 and Ad.Sr2a expressed chicken SERCA1 (Karin et al. 1989) or SERCA2a (Campbell et al. 1991), driven by a cytomegalovirus promoter and containing a c-myc tag (Evan et al. 1985). Plaque purification and amplification were as described by Strock et al. (1998) and Sumbilla et al. (1999).
Primary cell culture and infection
Chicken embryonic cardiac myocytes were prepared from 8-day embryos and cultured in medium 199 with 5 % fetal calf serum, at 37°C and 5 % CO2 (Inesi et al. 1998). For fluorescence studies, 650 cells mm−2 were plated on collagen-coated coverslips. To obtain rat myocytes, 1-day-old rats were decapitated and the hearts were excised. Harvesting of cardiac tissue was carried out using protocols approved by the University of Maryland Institutional Animal Care and Use Committee. Myocytes were dissociated as described by Simpson et al. (1989) and 250 cells mm−2 were plated on collagen-coated dishes or coverslips. Cells were cultured in 1 % CO2 in minimal essential medium with Hanks’ salts, 5 % calf serum, 1·5 mm vitamin B12, and 0·1 mm bromodeoxyuridine.
Myocytes were infected 24 h after plating by treatment with 2–10 pfu (plaque-forming units) of adenovirus per seeded cell in serum-free medium for 1 h. Control plates were mock infected, or infected with empty virus. Afterwards, the infection medium was diluted × 4 with complete medium. Cells were ready for measurements after 2–3 days.
Functional assays
Cell lawns were rinsed with phosphate-buffered saline (PBS) and cells were harvested by scraping in a resuspension medium (10 ml per 100 mm dish) with 50 mm Mops (pH 7·0), 10 mm NaF, 1 mm EDTA, 0·3 m sucrose and protease inhibitors (0·4 mm Pefabloc SC (Roche), 0·5 mm dithiothreitol, 10 μg ml−1 aprotinin, 2 μg ml−1 leupeptin, 1 μg ml−1 pepstatin A). Suspensions were centrifuged for 5 min at 2000 g and the cell pellets frozen and stored at −70°C.
Assays of 45Ca2+ uptake and Ca2+-dependent ATPase activity were performed in myocyte lysates or purified SR vesicles as described by Sumbilla et al. (1999). Mitochondrial uptake and ATPase activity were inhibited by 1 μm ruthenium red and 5 mm NaN3 in the reactions. Control assays in 1 μm thapsigargin were carried out to test whether measured activity was specifically due to SERCA.
Western blotting and immunofluorescence
Total protein was measured by the BCA assay kit (Pierce) after sonication of the cell pellets. Proteins were separated in 7·5 % polyacrylamide gels (Laemmli, 1970), transferred onto nitrocellulose paper, and stained with primary and secondary antibodies. Reactive bands were visualized by an ECL Western blotting detection kit (Amersham Pharmacia Biotech) and densitometry was done by a NucleoVision workstation (Nucleotech) with Gel Expert software. Primary monoclonal antibodies were: CaF3-5C3 for SERCA1 (Karin et al. 1989), CaS-3H2 for SERCA2a (Kaprielian & Fambrough, 1987), Myc1-9E10 for the c-myc tag (Evan et al. 1985), MA3-922 for phospholamban and MA3-913 for calsequestrin (Affinity Bioreagents).
In situ immunofluorescence staining of SERCA was performed as previously described (Inesi et al. 1998). Secondary antibodies used for fluorescence imaging were biotinylated horse anti-mouse IgG (Vector Labs) followed by incubation with fluorescein-streptavidin (Amersham), or Texas red-labelled goat anti-mouse IgG (Kirkegaard & Perry Laboratories). Fluorescence images were taken using a digital CCD camera (SpectraSource Instruments).
Cytosolic calcium measurements
Cytosolic Ca2+ was monitored by loading cells with membrane-permeant forms of fura-2 or fluo-4 (Molecular Probes). Cytoplasmic loading was favoured by incubating cells at 22°C with 1 μm dye and 0·2 % Pluronic F-127 (Molecular Probes) in pH 7·4 Ringer solution containing (mm): 10 Hepes, 135 NaCl, 4·0 KCl, 1·0 MgCl2, 1·8 CaCl2 and 10 glucose. Cells were loaded for 20 min in fura-2 or 5 min in fluo-4, then washed with dye-free Ringer solution. Coverslips were then placed in a circulating bath with Ringer solution held at 30 ± 1°C. Cells exhibiting visible store loading of dye, unresponsiveness, or ectopic beats during 1 Hz pulsing were excluded from the experiment (< 10 % of cells).
Fluorescence emission from single cells was measured using 380 or 358 nm excitation for fura-2, or 488 nm for fluo-4. Data were sampled at 10 pts ms−1 by an A/D converter (Computer Boards). Field stimulation of 10 V and 20 ms duration was delivered by platinum wire electrodes. Background fluorescence at the relevant wavelengths was collected for every plate and used to correct the measured signals prior to analysis.
Total releasable Ca2+ was estimated by loading cells with fura-2 and sampling emission at 380 and 358 nm excitation every 500 ms while exchanging the external Ringer solution with medium containing 10 mm caffeine and 1 μm thapsigargin. Li+ replaced Na+ to inhibit Na+-Ca2+ exchange (Bers, 1987). Cells were stimulated at 1·0 Hz for at least 1 min prior to a change of medium.
Dye calibration and processing
Fluo-4 data were converted to fractional fluorescence change by dividing by the resting cell fluorescence (ΔF/F0). Fura-2 data were converted to the ratio R of fluorescence at 380 and 358 nm excitation and [Ca2+] was calculated by the equation used by Klein et al. (1988):
For slow-sampling fluorescence measurements while emptying intracellular stores, the steady-state approximation of the above equation was used, where dR/dt ≈ 0.
Values for Rmax at saturating [Ca2+] and Rmin at 0 Ca2+ (Grynkiewicz et al. 1985) were measured by exposing cells to Ringer solution with 10 μm ionomycin and 5 mm Ca2+ for Rmax, or 0 mm Ca2+ and 5 mm EGTA for Rmin (Klein et al. 1988). These values depend on cell type and optical apparatus, and were found to be 0·45 for Rmax and 2·1 for Rmin. Values of the on (kon) and off (koff) rate constants were set to 2·00 × 108 M−1 s−1 and 30 s−1, respectively (Klein et al. 1988; Sipido & Wier, 1991).
The Ca2+-insensitive fraction of the total fura-2 fluorescence signal (from organellar dye) was estimated by quenching the cytoplasmic dye with Mn2+ and monitoring the fluorescence at 358 nm excitation (Miyata et al. 1991). Cells were bathed in Ringer solution with 0 Ca2+ (no EGTA) and 2 mm Mn2+ and stimulated to allow Mn2+ entry via Ca2+ channels. Only 5–10 % of the total cell fluorescence remained after this treatment.
Data processing and statistical analysis
Data were processed and analysed with customised software programmed in IDL (Research Systems). Data are shown as means ± s.e.m. where n > 15. The threshold for statistical significance was set as P < 0·05 following a Student’s unpaired t test.
RESULTS
Exogenous SERCA isoform expression
Within the first 3 days following seeding of embryonic chicken cardiac myocytes, at least 85 % of cells in culture could be identified as cardiac myocytes by specific staining of endogenous SERCA2a with a monoclonal antibody (Fig. 1A and B). Transfer of SERCA cDNA by adenovirus occurs very efficiently, and under appropriate conditions (see Methods), 100 % of cells express exogenous SERCA, as shown by infecting cells with Ad.Sr1 and staining with a SERCA1-specific monoclonal antibody (Fig. 1D), or with an antibody to the c-myc tag subcloned into the cDNA (not shown). No SERCA1 was detected in uninfected cells (Fig. 1C), or in cells infected with Ad.Sr2a or empty virus (not shown). Exogenous SERCA was appropriately targeted to intracellular membranes with a pattern similar to that of endogenous SERCA2a in the SR membranes (Fig. 1E and F). Similar reticular patterns of expression were obtained in neonatal rat cardiac myocytes.
Figure 1.
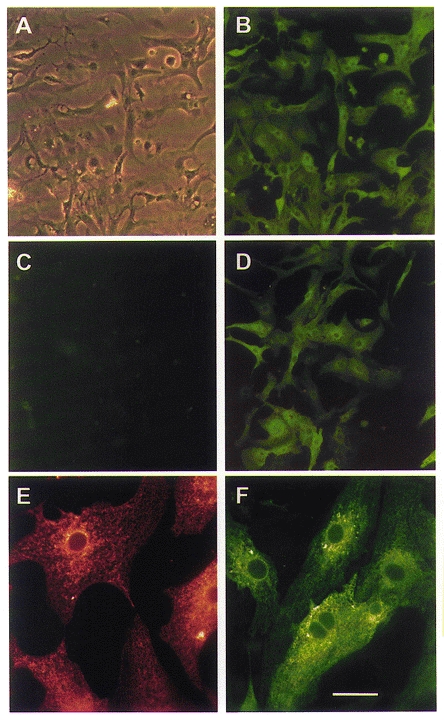
Immunostaining of SERCA in embryonic chicken cardiac myocytes
A and B, cultured embryonic chicken cardiac myocytes stained with monoclonal antibodies specific for endogenous SERCA2. A, phase-contrast image. B, fluorescence image of the same field. C and D, fluorescence images of myocytes stained with antibodies specific for exogenous SERCA1. C, uninfected myocytes. D, cells infected with Ad.Sr1. E and F, higher-magnification images showing targeting of exogenous SERCA to the SR. E, uninfected myocytes stained with a monoclonal antibody specific for endogenous SERCA2 and Texas red-conjugated secondary antibody. F, cells infected with Ad.Sr1 and stained with a monoclonal antibody specific for exogenous SERCA1 and fluorescein-conjugated secondary antibody. Scale bar in F corresponds to 60 μm in A-D and to 20 μm in E and F.
Expression levels of endogenous and exogenous SERCA were compared by Western blotting using isoform-specific antibodies and/or an antibody to the c-myc tag common to both cDNA constructs. We found that both SERCA1 and SERCA2a could be expressed to maximum levels corresponding to approximately 3 times the endogenous SERCA2a level in control myocytes (Fig. 2A). However, it should be noted that with maximal exogenous gene expression, endogenous SERCA2a expression is reduced by 30–60 % (Inesi et al. 1998).
Figure 2.
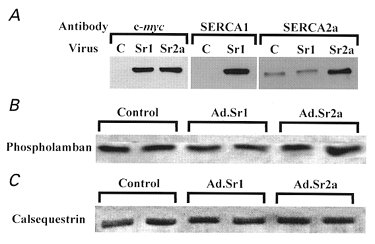
Protein expression in embryonic chicken cardiac myocytes
Embryonic chicken cardiac myocytes were infected with Ad.Sr1 (expressing SERCA1) or Ad.Sr2a (expressing SERCA2a), and cell lysates were probed with various monoclonal antibodies. A, right, antibody to the c-myc tag found only in exogenous SERCA1 and SERCA2a; middle, antibody to SERCA1; left, antibody to endogenous and exogenous SERCA2a. Lane headings: C, control; Sr1, Ad.Sr1 infected; Sr2a, Ad.Sr2a infected. B, antibody to phospholamban. C, antibody to calsequestrin. For B and C, each lane was run in duplicate.
Phospholamban and calsequestrin expression are not significantly affected by exogenous SERCA expression as shown by representative Western blots in Fig. 2B and C. Densitometry results from four experiments indicated that phospholamban levels under conditions of SERCA1 and SERCA2a overexpression were, respectively, 1·02 ± 0·13 and 1·01 ± 0·11 times the level of controls (P > 0·8), while calsequestrin levels were 0·96 ± 0·07 and 1·01 ± 0·08 times that of controls (P > 0·3, > 0·9).
ATPase activity
The ability to control exogenous SERCA expression by manipulating adenovirus titres permitted us to achieve nearly identical levels of exogenous SERCA1 and SERCA2a, as demonstrated by Western blots (Fig. 3A, inset) obtained with the same antibody to c-myc. Nevertheless, the ATPase Ca2+ uptake rate of the SERCA2a isoform was approximately half that of SERCA1 (Fig. 3A). This difference is due to the lower SERCA2a turnover rate that we have previously demonstrated in detail (Sumbilla et al. 1999).
Figure 3.
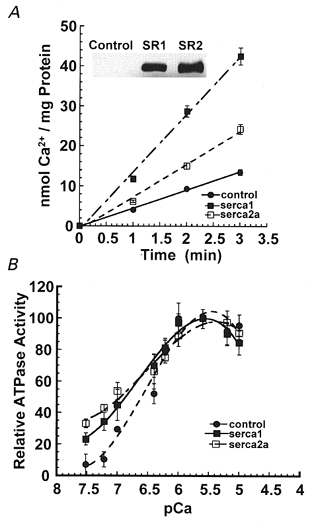
SERCA activity in embryonic chicken cardiac myocytes
Embryonic chicken cardiac myocytes were infected with Ad.Sr1 or Ad.Sr2a. A, rates of 45Ca2+ uptake by SERCA (nmol Ca2+ per mg protein) in cell lysates. Inset, Western blot shows similar expression levels of SERCA1 and SERCA2a detected by the same anti-c-myc antibody. B, Ca2+-dependent ATPase activity (%) measured at varying pCa. •, endogenous activity in control cells; ▪, activity in cells overexpressing SERCA1; □, activity in cells overexpressing SERCA2a.
Figure 3B displays the Ca2+ dependence of relative ATPase activity in control cells expressing endogenous SERCA2a only, and cells overexpressing either SERCA1 or SERCA2a. The activity is shown as a percentage of the maximum for each group, as a function of [Ca2+]. It is apparent that the activation curve of cells overexpressing either exogenous SERCA isoform is slightly displaced towards lower Ca2+ concentrations, as compared with cells expressing only endogenous SERCA2a. Since the native isoforms have a similar Ca2+ sensitivity (Sumbilla et al. 1999), this suggests that levels of endogenous SERCA2a are sufficient to fully bind all available phospholamban in the myocytes, and is consistent with the finding of identical phospholamban levels under conditions of endogenous or exogenous SERCA expression (Fig. 2B).
Cytosolic calcium transients
The dye fluo-4 was used to monitor cytosolic Ca2+ transients in cardiac myocytes infected with Ad.Sr1 and Ad.Sr2a (Fig. 4A–D). These data were normalized to control for differences in dye loading among cells. Each trace in Fig. 4 represents data from 15–30 cells from at least four cell preparations. Transients were aligned according to the time of application of the stimulus and averaged. Individual cell transients were characterized by determining (i) the time constant of a least-squares fit of a single exponential plus constant to the falling phase, and (ii) the full width at half-maximum (half-width, w½). Figure 4A (chicken) and B (rat) show that transients from cells overexpressing SERCA1 had smaller exponential decay time constants (τ) and narrower half-widths compared to uninfected myocytes. For example, in embryonic chicken cells overexpressing SERCA1, τ and w½ were decreased by 38 % (P < 0·001) and 11 % (P < 0·01), respectively. On the other hand, the effects of exogenous SERCA2a expression (Fig. 4C and D) on τ and w½ were more modest, with reductions of 6 % (P > 0·2) and 8 % (P > 0·1), respectively, following SERCA2a overexpression in chicken myocytes. However, the effect of SERCA2a overexpression on τ and w½ was significant in rat myocytes (30 and 29 % reduction, respectively, P < 0·001), although smaller in magnitude compared with SERCA1 overexpression (43 and 52 %, respectively, P < 0·001). The parameters of Ca2+ transients measured from chicken and rat cardiac myocytes are summarized in Table 1. It should be emphasized that, in spite of the different functional effects, Western blots demonstrated equal levels of exogenous SERCA1 and SERCA2a protein expression in both chicken (Fig. 2A, left) and rat (not shown).
Figure 4.
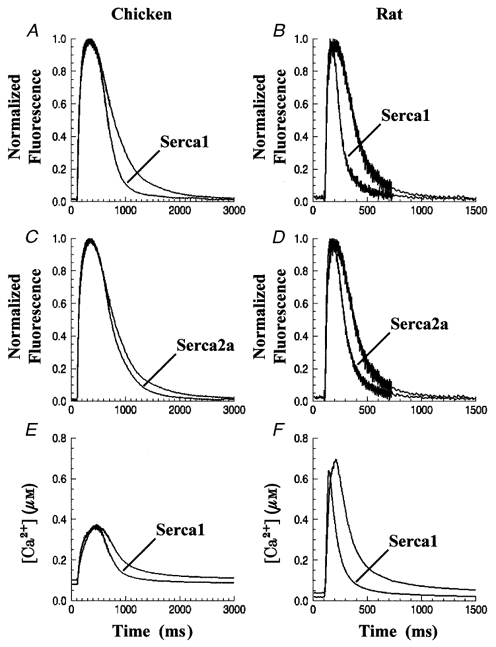
Cytosolic Ca2+ transients in embryonic chicken and neonatal rat cardiac myocytes
Embryonic chicken and neonatal rat cardiac myocytes were infected with Ad.Sr1 or Ad.Sr2. Cytosolic Ca2+ transients were measured in single cells using fluo-4 (A-D) or fura-2 (E and F). Each trace represents the average of transients from 15–30 cells over 3–5 preparations. Note the different time scales of the displayed transients from embyronic chicken (left) and neonatal rat (right). Ca2+ concentrations in E and F were calculated from the fura-2 fluorescence at 380 and 358 nm excitation (see Methods).
Table 1.
Calcium transient parameters
| Species | Infection group | Time to peak(ms) | Half-width (ms) | Decay constant(ms) |
|---|---|---|---|---|
| Chicken | Control (39) | 279 ± 21 | 611 ± 34 | 229 ± 12 |
| SERCA1 (24) | 228 ± 23* | 544 ± 30* | 141 ± 10* | |
| SERCA2a (24) | 284 ± 23 | 562 ± 34 | 216 ± 19 | |
| Rat | Control (28) | 93 ± 6.2 | 219 ± 11 | 129 ± 4.3 |
| SERCA1 (17) | 62 ± 5.0* | 106 ± 7.2* | 73 ± 4.0* | |
| SERCA2a (17) | 75 ± 6.1* | 155 ± 9.7* | 90 ± 4.4* |
Cytosolic Ca2+ transients in single cells loaded with fluo-4 were measured and all parameters averaged (values shown are means ± s.e.m.). Numbers in parentheses are the number of cells per group.
Significantly different (P < 0.05) from control cells.
Transients were also studied using fura-2 as the Ca2+ indicator. Displayed in Fig. 4E and F are Ca2+ transients from controls and cells overexpressing SERCA1, averaged from data collected over four experiments. As seen with fluo-4, transients from cells overexpressing SERCA1 yielded smaller decay constants and half-widths than control cells, showing a 33 % reduction (P < 0·001) for τ and a 12 % reduction (P < 0·02) for w½ in chicken myocytes overexpressing SERCA1. In rat, there was a 41 % reduction (P < 0·001) in τ and a 54 % reduction in w½ (P < 0·001). Most importantly, these differences were similar to the ones observed using fluo-4 as the indicator.
The estimates of [Ca2+] provided by fura-2 revealed that peak and resting cytoplasmic Ca2+ concentrations were relatively unchanged as a consequence of gene transfer. The mean resting [Ca2+] in embryonic chicken myocytes was 101 ± 45 and 80 ± 18 nm (P > 0·5) for controls and cells overexpressing SERCA1, respectively, while the peak amplitude of the Ca2+ transients was 376 ± 44 and 374 ± 56 nm (P > 0·7). The resting [Ca2+] in neonatal rat myocytes was 42 ± 7 and 24 ± 5 nm (P < 0·02) for controls and cells overexpressing SERCA1, respectively, and peak [Ca2+] was 795 ± 96 and 686 ± 76 nm (P > 0·3). Thus, in both chicken and rat cardiac myocytes, there were no significant differences in cytosolic Ca2+ peak concentrations between controls and cells overexpressing exogenous SERCA, and modest differences in resting [Ca2+]. It should be noted that in general, rat myocytes had much larger transient amplitudes, faster decay rates and smaller half-widths when compared to chicken myocytes, both under control conditions and when overexpressing exogenous SERCA (Table 1).
Relative size of calcium stores
The amplitude and time course of Ca2+ transients is dependent on the Ca2+ load in the SR. The SERCA ATPases are the main route for refilling the SR after Ca2+ release, so it was critical to examine the SR Ca2+ content before and after overexpression of the SERCA isoforms. We monitored cytoplasmic [Ca2+] while exposing the cells to a modified Ringer solution (see Methods) containing 10 mm caffeine and 1 μm thapsigargin. Cells were paced at 1·0 Hz for at least 1 min before a delay of about 6 s, after which the medium was rapidly exchanged with the modified Ringer solution. Recordings of fura-2 fluorescence showed that upon addition of caffeine, cytosolic [Ca2+] rose quickly and remained relatively stable at the elevated level (Fig. 5). Data derived from 19 cells per group, over five separate experiments, yielded an average rise in cytosolic [Ca2+] to 480 ± 65 nm in control cells (Fig. 5A), and 496 ± 76 nm in chicken myocytes expressing SERCA1 (Fig. 5B; P > 0·8). Assuming that the concentration of myoplasmic Ca2+ buffers is unchanged by expression of SERCA isoforms (cf. Fig. 2B and C), under the conditions of these experiments, the steady-state level of cytosolic Ca2+ is proportional to the SR Ca2+ content. Note that even though SERCA overexpression accounts for a significant increase in the number of Ca2+ binding sites, the presence of thapsigargin inhibits Ca2+ binding to these sites (Sagara & Inesi, 1991). These experiments show that the maximal Ca2+ load of the SR was not significantly different in controls and in myocytes overexpressing SERCA.
Figure 5.
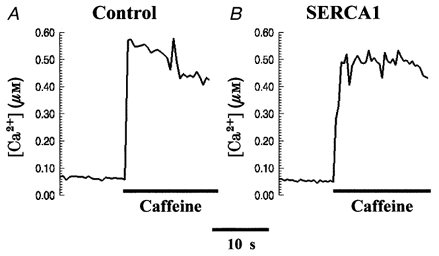
SR Ca2+ content in embryonic chicken cardiac myocytes
Embryonic chicken cardiac myocytes loaded with fura-2 were exposed to a modified Ringer solution containing 10 mm caffeine and 1 μm thapsigargin while fluorescence was sampled at 358 and 380 nm excitation every 500 ms. A, control cell. B, cell overexpressing SERCA1.
Time course of refilling of calcium stores
Expression of exogenous SERCA causes an acceleration of the removal of Ca2+ from the cytosol during and after a Ca2+ transient. However, these observations provide no information concerning the rate at which Ca2+ becomes available again for release from the SR during a subsequent stimulation. We used a paired-pulse protocol to estimate the rate of refilling of the SR Ca2+ stores, in which a conditioning pulse was followed after a variable recovery period by a test pulse of identical amplitude and duration. Prior to the application of the conditioning pulse, the cells were paced at 1·0 Hz for 10 s. Figure 6A shows fluo-4 fluorescence transients obtained with this protocol in a control cell (upper traces) and in a cell overexpressing SERCA1 (lower traces). Five or six transients are shown superimposed, corresponding to different recovery intervals, with the individual traces aligned at the time of application of the conditioning stimulus. The control transients recovered gradually as the recovery interval was increased, seen most easily as an increase in the peak amplitude of the transients arising from stimulation by the test pulses. In cells overexpressing SERCA1, however, the recovery was virtually complete after 600 ms. Both sets of traces show that the test transient recorded after 1000 ms was of similar amplitude to the conditioning transient and, more significantly, that an additional recovery interval (1200 ms) resulted in no further increase of the test transient amplitude. The latter observation is consistent with the finding of Fig. 5 that SR Ca2+ content was unchanged by exogenous SERCA expression. Figure 6B plots the time course of refilling of the SR Ca2+ stores. Results from 20 (control) or 16 (SERCA1-overexpressing) chicken myocytes were averaged. The points represent the difference between the peak fluorescence during the test transient minus the baseline fluorescence just prior to the test stimulation. The quantity is proportional to the SR Ca2+ content if myoplasmic Ca2+ buffers are in the linear range of binding, a condition which probably holds during the relatively small Ca2+ transients in these chicken myocytes. The data show that releasable Ca2+ recovers at a rate 95 % greater in SERCA1-overexpressing cells than in control cells. In contrast, and in agreement with the absence of a significant effect of expression of SERCA2a on Ca2+ transients, there was no significant difference between control myocytes and myocytes expressing SERCA2a in the rate of refilling of the SR after a conditioning pulse (not shown).
Figure 6.
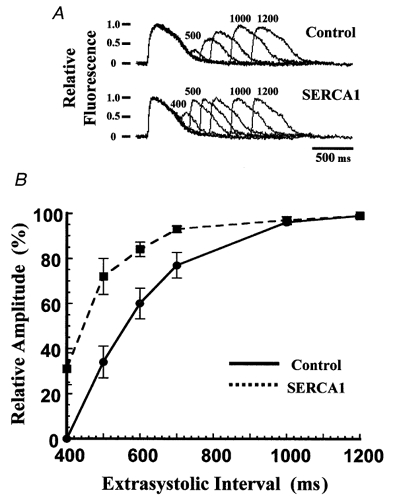
Time course of refilling of Ca2+ stores in embryonic chicken cardiac myocytes
A, chicken cardiac myocytes loaded with fluo-4 were stimulated with a two-pulse protocol after pacing at 1·0 Hz. The last paced transient and the extrasystolic transient were recorded while varying the extrasystolic interval. Each record was normalized to its conditioning transient and superimposed as a series. Upper series, control myocyte. Lower series, myocyte overexpressing SERCA1. Time intervals are indicated above the traces (in ms). B, relative amplitudes of the Ca2+ transients as a function of the extrasystolic interval. Amplitude was calculated as the difference between the peak of the transient and the baseline fluorescence just prior to the stimulus pulse. Points are means ± s.e.m. •, control cells (n = 20); ▪, cells overexpressing SERCA1 (n = 16).
Temperature dependence of Ca2+ transport and Ca2+ transient decay constants
The experiments described above reveal a kinetic relation between Ca2+ transport by SERCA and the decay phase of Ca2+ transients. In order to obtain further evidence for such a relationship, we performed comparative measurements of Ca2+ transport and Ca2+ transients at two different temperatures, in uninfected rat myocytes expressing only endogenous SERCA. We found that the velocity of Ca2+ transport and the decay phase of the Ca2+ transients were highly temperature dependent (Fig. 7), with Q10 values of 2·3 for Ca2+ transport, and 2·4 for the decay time constant of the Ca2+ transients.
Figure 7.
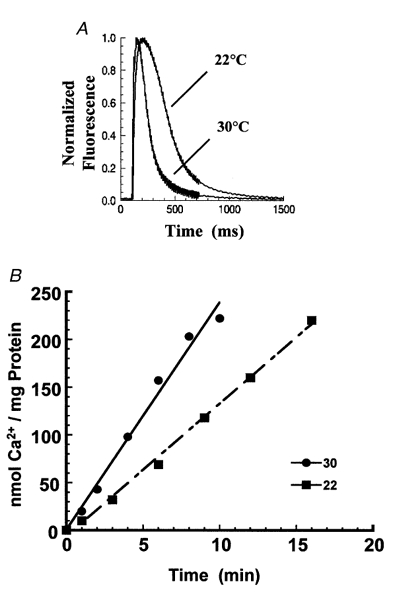
Effects of temperature on Ca2+ uptake and Ca2+ transients
A, cytosolic Ca2+ transients recorded at 22 and 30 °C in control rat cardiac myocytes. B, rates of 45Ca2+ uptake by endogenous SERCA2a (nmol Ca2+ per mg protein) in rat cardiac myocyte lysates. ▪, uptake at 22 °C; •, uptake at 30 °C.
DISCUSSION
The transfer of exogenous SERCA genes into cardiac myocytes can be obtained very efficiently by the use of recombinant adenovirus vectors (Inesi et al. 1997, 1998; Hajjar et al. 1997; Giordano et al. 1997). Furthermore, levels of SERCA expression can be adjusted by manipulating viral titre, up to a maximum that is 3–4 times higher than that of endogenous SERCA in uninfected myocytes (Sumbilla et al. 1999). Here we demonstrate that following transfection of either SERCA1 or SERCA2a genes, nearly identical expression levels of the two isoforms can be obtained, as revealed by Western blots. The 3- to 4-fold increase in SERCA protein expression in our experiments is considerably higher than previously obtained in transgenic mice (He et al. 1997; Loukianov et al. 1998; Baker et al. 1998), or in cultured rat myocytes transfected with exogenous SERCA2a cDNA using viral vectors (Giordano et al. 1997; Hajjar et al. 1997). Furthermore, we demonstrate that the exogenous SERCA gene products are properly targeted to the SR, since in situ immunostaining of endogenous SERCA2a and exogenous SERCA1 protein showed a similar reticular pattern with minimal nuclear involvement (Fig. 1).
Under conditions of identical expression levels, we found that the velocity of Ca2+ transport in myocytes expressing the SERCA2a isoform was approximately half that in myocytes expressing the SERCA1 isoform (Fig. 3). This difference is due to the lower turnover of the SERCA2a isoform (Sumbilla et al. 1999). It is noteworthy that both exogenous SERCA isoforms appeared to be free of control by phospholamban, as revealed by the pattern of Ca2+ activation (Fig. 3B). This is consistent with the lack of phospholamban overexpression in parallel with exogenous SERCA gene expression (Fig. 2B), and suggests that endogenous phospholamban is already saturated by interaction with endogenous SERCA2a.
The main effect of overexpression of SERCA1 in chicken or rat myocytes was a 1·5- to 1·75-fold increase in the rate of decline, and a decrease in the half-width of 0·11- to 0·54-fold, in Ca2+ transients elicited by stimulation, compared to controls (Fig. 4, Table 1). Both of these effects are directly attributable to the increased rate of removal of Ca2+ from the cytoplasm due to the exogenously expressed SERCA. However, this increased removal rate was related more to the expression of a SERCA isoform with a faster turnover rate than to an overall increase in the copy number of SERCA pumps. This is evidenced by the observations that (i) overexpression of SERCA2a had a relatively smaller effect on the decline and half-width of Ca2+ transients in both chicken and rat than overexpression of SERCA1, and (ii) elevation of temperature, which increases the turnover rate of SERCA pumps but not their copy number, increased the rate of removal of cytosolic Ca2+ in a manner qualitatively similar to overexpression of the faster SERCA isoform.
The observed changes in the waveform of Ca2+ transients caused by overexpression of SERCA isoforms were not associated with changes in the amplitude of the Ca2+ transients (Fig. 4E and F), or in the SR Ca2+ content (Fig. 5). It should be understood that in the steady state, the maximal lumenal concentration of free Ca2+ is determined by the dissociation constant of the Ca2+ pump in the low affinity state (Inesi, 1994), and not by the number of pump units. In turn, the total Ca2+content (bound plus free) is determined by the volume of the SR lumen and by the buffering capacity of calsequestrin, the predominant Ca2+ buffer within the SR. We have demonstrated that calsequestrin expression is unchanged in our experiments (Fig. 2B), although we have no evidence for a change in SR volume. Therefore, in our experiments, the observation that the amplitudes of Ca2+ transients are unchanged by SERCA overexpression is consistent with the idea that the ‘extra’ pump served only to restore cytosolic Ca2+ to the SR more rapidly, without altering its capacity to store or release Ca2+.
The lack of change of the Ca2+ transient peak amplitude in myocytes expressing exogenous SERCA isoforms as observed by us is in agreement with the results of Yao et al. (1998) and Giordano et al. (1997). On the other hand, increased amplitudes have been observed by Baker et al. (1998), Loukianov et al. (1998) and Hajjar et al. (1997). It is possible that this difference may be due to varying experimental conditions. For instance, the results of the recovery experiments (Fig. 6) demonstrate that the observed amplitude of Ca2+ transients is also partially dependent on recovery interval. Therefore, cells overexpressing SERCA isoforms might exhibit an increase in the amplitude of Ca2+ transients under conditions producing a kinetic limit in control cells. Effects of exogenous SERCA overexpression on frequency- response patterns have also been observed in human cardiac myocytes (del Monte et al. 1999). It is also possible that in longer-term overexpression models, such as transgenic mice, adaptive responses may produce an increase in SR volume and thereby permit higher Ca2+ loads.
An interesting aspect of our results is the lesser effect of SERCA2a overexpression on the cytosolic Ca2+ transients, as compared with overexpression of SERCA1 (Fig. 4). The effect of SERCA2a overexpression is very evident in rat myocytes, but is less clear in chicken myocytes due to the lower sensitivity of this system to SERCA overexpression. It should be pointed out that Giordano et al. (1997) and Hajjar et al. (1997) have previously reported effects of SERCA2a overexpression in cultured rat myocytes, and He et al. (1997) and Baker et al. (1998) have observed faster cytosolic Ca2+ transient kinetics in transgenic mice overexpressing SERCA2a than in wild-type. Thus, some of the differences between our results and those of others could be due to species differences, whereas we attribute the lesser effects of SERCA2a overexpression to the different turnover rates of the SERCA1 and SERCA2a isoforms (Sumbilla et al. 1999). In fact, the interdependence of SERCA kinetics and cytosolic Ca2+ decay can be simply demonstrated by varying the SERCA turnover rate with temperature (Fig. 7). It is clear that the decay phase of the Ca2+ transients is greatly affected by the velocity of Ca2+ transport (i.e. resequestration into the SR) by SERCA. This velocity, and by extension the Ca2+ transient decay, can be influenced by the SERCA turnover rate as determined by isoform and/or temperature, and to a lesser extent by the SERCA copy number. The apparent Ca2+ load in the SR is then dependent on the velocity of transport and the time allowed for transport between stimuli.
Acknowledgments
The authors are grateful to Dr Douglas Fambrough (Johns Hopkins University) for the kind gift of molecular reagents, and Jerry Domanico for editing the manuscript. This research was supported by the National Institutes of Health (NIH PO1-HL27867) and the University of Maryland Interdisciplinary Training Program in Muscle Biology (NIH 5T32-AR07592).
References
- Anger M, Samuel JL, Marotte F, Wuytack F, Rappaport L, Lompre AM. In situ mRNA distribution of sarco(endo)plasmic reticulum Ca(2+)-ATPase isoforms during ontogeny in the rat. Journal of Molecular and Cellular Cardiology. 1994;26:539–550. doi: 10.1006/jmcc.1994.1064. [DOI] [PubMed] [Google Scholar]
- Baker DL, Hashimoto K, Grupp IL, Ji Y, Reed T, Loukianov E, Grupp G, Bhagwhat A, Hoit B, Walsh R, Marban E, Periasamy M. Targeted overexpression of the sarcoplasmic reticulum Ca2+-ATPase increases cardiac contractility in transgenic mouse hearts. Circulation Research. 1998;83:1205–1214. doi: 10.1161/01.res.83.12.1205. [DOI] [PubMed] [Google Scholar]
- Bers DM. Ryanodine and the calcium content of cardiac SR assessed by caffeine and rapid cooling contractures. American Journal of Physiology. 1987;253:C408–415. doi: 10.1152/ajpcell.1987.253.3.C408. [DOI] [PubMed] [Google Scholar]
- Bokkala S, el Daher SS, Kakkar VV, Wuytack F, Authi KS. Localization and identification of Ca2+ATPases in highly purified human platelet plasma and intracellular membranes. Evidence that the monoclonal antibody PL/IM 430 recognizes the SERCA 3 Ca2+ATPase in human platelets. Biochemical Journal. 1995;306:837–842. doi: 10.1042/bj3060837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl CJ, Green NM, Korczak B, MacLennan DH. Two Ca2+-ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986;44:597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- Campbell AM, Kessler PD, Sagara Y, Inesi G, Fambrough DM. Nucleotide sequences of avian cardiac and brain SR/ER Ca2+-ATPases and functional comparisons with fast twitch Ca2+-ATPase. Calcium affinities and inhibitor effects. Journal of Biological Chemistry. 1991;266:16050–16055. [PubMed] [Google Scholar]
- Carsten ME. Cardiac calcium pump. Proceedings of the National Academy of Sciences of the USA. 1964;52:1456–1462. doi: 10.1073/pnas.52.6.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Monte F, Harding SE, Schmidt U, Matsui T, Kang ZB, Dec GW, Gwathmey JK, Rosenzweig A, Hajjar RJ. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100:2308–2311. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Molecular and Cellular Biology. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. Journal of General Physiology. 1985;85:247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanburg B, Finkel RM, Martonosi A. Role of calcium in the mechanism of relaxation of cardiac muscle. Journal of Biological Chemistry. 1964;239:2298–2306. [PubMed] [Google Scholar]
- Giordano FJ, He HP, McDonough P, Meyer M, Sayen MR, Dillmann WH. Adenovirus-mediated gene transfer reconstitutes depressed sarcoplasmic reticulum Ca2+-ATPase levels and shortens prolonged cardiac myocyte Ca2+ transients. Circulation. 1997;96:400–403. doi: 10.1161/01.cir.96.2.400. [DOI] [PubMed] [Google Scholar]
- Graham FL, Prevec L. Adenovirus-based expression vectors and recombinant vaccines. In: Ellis RW, editor. Vaccines: New Approaches to Immunological Problems. Woburn, MA, USA: Butterworth-Heinemann; 1992. pp. 363–390. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, Morgan JP. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circulation Research. 1987;61:70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- Hajjar RJ, Kang JX, Gwathmey JK, Rosenzweig A. Physiological effects of adenoviral gene transfer of sarcoplasmic reticulum calcium ATPase in isolated rat myocytes. Circulation. 1997;95:423–429. doi: 10.1161/01.cir.95.2.423. [DOI] [PubMed] [Google Scholar]
- Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps ML. Construction of adenovirus vectors through Cre-lox recombination. Journal of Virology. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenfuss G, Meyer M, Schillinger W, Preuss M, Pieske B, Just H. Calcium handling proteins in the failing heart. Basic Research in Cardiology. 1997;92:87–93. doi: 10.1007/BF00794072. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, Holubarsch C, Posival H, Just H, Drexler H. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circulation Research. 1994;75:434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- He H, Giordano FJ, Hial-Dandan R, Choi D-J, Rockman HA, McDonough PM, Bluhm WF, Meyer M, Sayen MR, Swanson E, Dillmann WH. Overexpression of the rat sarcoplasmic reticulum Ca2+ ATPase gene in the heart of transgenic mice accelerates calcium transients and cardiac relaxation. Journal of Clinical Investigation. 1997;100:380–389. doi: 10.1172/JCI119544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inesi G. Teaching active transport at the turn of the twenty-first century: recent discoveries and conceptual changes. Biophysical Journal. 1994;66:554–560. doi: 10.1016/s0006-3495(94)80872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inesi G, Ebashi S, Watanabe S. Preparation of vesicular relaxing factor from bovine heart tissue. American Journal of Physiology. 1964;207:1339–1344. doi: 10.1152/ajplegacy.1964.207.6.1339. [DOI] [PubMed] [Google Scholar]
- Inesi G, Lewis D, Sumbilla C, Nandi A, Kirtley M, Ordahl CP. ATPase gene transfer and mutational analysis of the cation translocation mechanism. Annals of the New York Academy of Sciences. 1997;834:207–220. doi: 10.1111/j.1749-6632.1997.tb52252.x. [DOI] [PubMed] [Google Scholar]
- Inesi G, Lewis D, Sumbilla C, Nandi A, Strock C, Huff KW, Rogers TB, Johns DC, Kessler PD, Ordahl CP. Cell-specific promoter in adenovirus vector for transgenic expression of SERCA1 ATPase in cardiac myocytes. American Journal of Physiology. 1998;274:C645–653. doi: 10.1152/ajpcell.1998.274.3.C645. [DOI] [PubMed] [Google Scholar]
- Kaprielian Z, Fambrough DM. Expression of fast and slow isoforms of the Ca2+-ATPase in developing chick skeletal muscle. Developmental Biology. 1987;124:490–503. doi: 10.1016/0012-1606(87)90502-1. [DOI] [PubMed] [Google Scholar]
- Karin NJ, Kaprielian Z, Fambrough DM. Expression of avian Ca2+-ATPase in cultured mouse myogenic cells. Molecular and Cellular Biology. 1989;9:1978–1986. doi: 10.1128/mcb.9.5.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby MS, Sagara Y, Gaa ST, Inesi G, Lederer WJ, Rogers TB. Thapsigargin inhibits contraction and Ca2+ transient in cardiac cells by specific inhibition of the sarcoplasmic reticulum Ca2+ pump. Journal of Biological Chemistry. 1992;267:12545–12551. [PubMed] [Google Scholar]
- Klein MG, Simon BJ, Szucs G, Schneider MF. Simultaneous recording of calcium transients in skeletal muscle using high- and low-affinity calcium indicators. Biophysical Journal. 1988;53:971–988. doi: 10.1016/S0006-3495(88)83178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loukianov E, Ji Y, Grupp IL, Kirkpatrick DL, Baker DL, Loukianova T, Grupp G, Lytton J, Walsh RA, Periasamy M. Enhanced myocardial contractility and increased Ca2+ transport function in transgenic hearts expressing the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. Circulation Research. 1998;83:889–897. doi: 10.1161/01.res.83.9.889. [DOI] [PubMed] [Google Scholar]
- Mercadier J-J, Lompre AM, Duc P, Boheler KR, Fraysse JB, Wisnewsky C, Allen PD, Komajda M, Schwartz K. Altered sarcoplasmic reticulum Ca2+ ATPase gene expression in the human ventricle during end-stage heart failure. Journal of Clinical Investigation. 1990;85:305–309. doi: 10.1172/JCI114429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata H, Silverman HS, Sollott SJ, Lakatta EG, Stern MD, Hansford RG. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. American Journal of Physiology. 1991;261:H1123–1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- Morgan JP, Erny RE, Allen PD, Grossman W, Gwathmey JK. Abnormal intracellular calcium handling, a major cause of systolic and diastolic dysfunction in ventricular myocardium from patients with heart failure. Circulation. 1990;81:III21–32. [PubMed] [Google Scholar]
- Periasamy M, Reed TD, Liu LH, Ji Y, Loukianov E, Paul RJ, Nieman ML, Riddle T, Duffy JJ, Doetschman T, Lorenz JN, Shull GE. Impaired cardiac performance in heterozygous mice with a null mutation in the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 (SERCA2) gene. Journal of Biological Chemistry. 1999;274:2556–2562. doi: 10.1074/jbc.274.4.2556. [DOI] [PubMed] [Google Scholar]
- Sagara Y, Inesi G. Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations. Journal of Biological Chemistry. 1991;266:13503–13506. [PubMed] [Google Scholar]
- Schmidt U, Hajjar RJ, Helm PA, Kim CS, Doye AA, Gwathmey JK. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. Journal of Molecular and Cellular Cardiology. 1998;30:1929–1937. doi: 10.1006/jmcc.1998.0748. [DOI] [PubMed] [Google Scholar]
- Simpson PC, Long CS, Waspe LE, Henrich CJ, Ordahl CP. Transcription of early developmental isogenes in cardiac myocyte hypertrophy. Journal of Molecular and Cellular Cardiology. 1989;21:79–89. doi: 10.1016/0022-2828(89)90774-8. [DOI] [PubMed] [Google Scholar]
- Sipido KR, Wier WG. Flux of Ca2+ across the sarcoplasmic reticulum of guinea-pig cardiac cells during excitation-contraction coupling. The Journal of Physiology. 1991;435:605–630. doi: 10.1113/jphysiol.1991.sp018528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strock C, Cavagna M, Peiffer WE, Sumbilla C, Lewis D, Inesi G. Direct demonstration of Ca2+ binding defects in sarco-endoplasmic reticulum Ca2+ ATPase mutants overexpressed in COS-1 cells transfected with adenovirus vectors. Journal of Biological Chemistry. 1998;273:15104–15109. doi: 10.1074/jbc.273.24.15104. [DOI] [PubMed] [Google Scholar]
- Sumbilla C, Cavagna M, Zhong L, Ma H, Lewis D, Farrance I, Inesi G. Comparison of SERCA1 and SERCA2a expressed in COS-1 cells and cardiac myocytes. American Journal of Physiology. 1999;277:H2381–2391. doi: 10.1152/ajpheart.1999.277.6.H2381. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Allen PD, Izumo S. Expression of A-, B-, and C-type natriuretic peptide genes in failing and developing human ventricles: correlation with expression of Ca2+ ATPase gene. Circulation Research. 1992;71:9–17. doi: 10.1161/01.res.71.1.9. [DOI] [PubMed] [Google Scholar]
- Yao A, Su Z, Dillmann WH, Barry WH. Sarcoplasmic reticulum function in murine ventricular myocytes overexpressing SR CaATPase. Journal of Molecular and Cellular Cardiology. 1998;30:2711–2718. doi: 10.1006/jmcc.1998.0834. [DOI] [PubMed] [Google Scholar]
- Zarain-Herzberg A, MacLennan DH, Periasamy M. Characterization of rabbit cardiac sarco(endo)plasmic reticulum Ca2+ ATPase gene. Journal of Biological Chemistry. 1990;265:4670–4677. [PubMed] [Google Scholar]


