Abstract
While it is known that the average firing rate of a population of motoneurones declines with time during a maximal voluntary contraction, at least for many muscles, it is not known how the firing patterns of individual motoneurones adapt with fatigue. To address this issue we used tungsten microelectrodes to record spike trains (mean ± s.e.m., 183 ± 27 spikes per train; range, 100–782 spikes) from 26 single motor units in extensor hallucis longus during sustained (60–180 s) maximal dorsiflexions of the big toe in seven human subjects.
Long spike trains were recorded from 13 units during the first 30 s of a maximal voluntary contraction (mean train duration, 9.6 ± 1.2 s; range, 3.6–21.9 s) and from 13 units after 30 s (mean train duration, 16.6 ± 3.7 s; range, 7.1–58.1 s). Maximal isometric force generated by the big toe declined to 78.3 ± 6.3 % of its control level by 60–90 s and to 39.5 ± 1.4 % of control by 120–150 s. Despite this substantial fatigue, mean firing rates did not change significantly over time, declining only slightly from 15.8 ± 0.7 Hz in the first 30 s to 14.0 ± 0.5 Hz by 60–90 s and 13.6 ± 0.3 Hz by 120–150 s.
To assess fatigue-related adaptation in discharge frequency and variability of individual motor units, each spike train was divided into 2–15 equal segments containing at least 50 interspike intervals. Discharge variability was measured from the coefficient of variation (s.d./mean) in the interspike intervals, with the s.d. being calculated using a floating mean of 19 consecutive intervals. Adaptation was computed as the average change in firing rate or variability that would occur for each 1 s of activity. There were no systematic changes in either firing rate or variability with time.
We conclude that single motoneurones supplying the extensor hallucis longus, a muscle comprised primarily of slow twitch muscle units, show little adaptation in firing with fatigue, suggesting that a progressive reduction in firing rate is not an invariable consequence of the fatigue associated with sustained maximal voluntary contractions.
A sustained maximal voluntary contraction (MVC) ultimately results in loss of the force-generating capacity of the contracting muscle—fatigue sets in. Associated with this fall in force output is a progressive slowing of the muscle's relaxation rate (Bigland-Ritchie et al. 1983b), and of the mean firing rates of motoneurones supplying the muscle (Grimby et al. 1981; Bigland-Ritchie et al. 1983a; Marsden et al. 1983; Woods et al. 1987; Gandevia et al. 1990; Peters & Fuglevand, 1999). Using intramuscular tungsten microelectrodes to sample brief trains of single motor unit action potentials at different times during sustained MVCs, quantitative data on the progressive decline in firing rates has been obtained for three human muscles—adductor pollicis (Bigland-Ritchie et al. 1983b) and first dorsal interosseous (Gandevia et al. 1990) in the hand, and quadriceps femoris in the leg (Woods et al. 1987). Such an approach allows assessment of the temporal change in firing of a population of motoneurones as fatigue develops, but gives little information on how the discharge of individual motoneurones changes with time.
It is important to understand how the discharge patterns of individual motoneurones change during fatigue because it has been argued that a decline in motoneurone firing rates would serve to optimize force output by ‘matching’ the discharge frequency to the contractile slowing of individual muscle units—the ‘muscular wisdom’ hypothesis (Marsden et al. 1983). In the present investigation we have specifically addressed the issue of how individual motoneurones ‘adapt’ to fatigue by using tungsten microelectrodes to follow the discharge of single motor units over the course of a sustained maximal voluntary contraction. We chose to study a muscle of posture and locomotion—the primary extensor of the big toe (extensor hallucis longus)—for which the maximal firing rates of single motor units have not been previously recorded, but for which the force-frequency relationships for single motor units (assessed by intraneural motor-axon microstimulation) are known (Macefield et al. 1996). In addition to measuring peak discharge frequencies, and assessing whether firing rates decline during a fatiguing contraction, we examined the discharge variability of individual motor units to determine whether or not this parameter changes during the course of a fatiguing contraction—discharge variability also has been speculated to play an important role in optimizing force output during fatigue (see Bevan et al. 1992). Some of this work has appeared in abstract form (Fuglevand et al. 1994).
METHODS
General procedures
Thirty-three experiments were performed on 8 male and 2 female subjects, ranging in age from 23 to 54 years (mean age, 33 years). The procedures were approved by the Yale School of Medicine Human Investigations Committee and satisfied the conditions set by the Declaration of Helsinki. All subjects gave their informed written consent. Subjects reclined in a dental chair with the knee flexed to approximately 120deg and the lower leg supported and immobilized by a vacuum pillow. The foot was supported by a stable floor-mounted platform and a Velcro strap secured firmly over the dorsum of the foot to prevent ankle dorsiflexion. The angle between the plantar surface of the foot and the long axis of the lower leg was approximately 115deg. An isometric force transducer, attached to a stable support, was fixed over the nail of the big toe. A Velcro strap provided secure mechanical coupling between the digit and the transducer. Electromyographic activity (EMG) was recorded with 4 mm diameter Ag-AgCl electrodes (In Vivo Metric) placed (interelectrode separation, 2 cm) over the surface of the extensor hallucis longus muscle distal to the tibialis anterior muscle and immediately lateral to the tendon of tibialis anterior. Single-unit EMG was recorded via a lacquer-coated tungsten microelectrode (Frederick Haer, USA), with a shaft diameter of 250 μm and a tip diameter of 1–5 μm, inserted percutaneously into extensor hallucis longus. Post-insertion impedance was 100–300 kΩ. A surface reference electrode was placed over the lateral malleolus. The optimal intramuscular recording site was found whilst observing the tendon responses to intramuscular electrical stimulation through the microelectrode, as well as by recording voluntary EMG as the subject dorsiflexed the big toe.
Data collection
Prior to manual insertion of the intramuscular microelectrode subjects performed a brief, maximal dorsiflexion of the big toe. This was repeated following insertion of the microelectrode. Some subjects reported a deep ache during the second attempt due to the presence of the microelectrode and the maximum dorsiflexion force was reduced; data from these experiments were excluded from the analyses. Subjects then performed a maximal voluntary dorsiflexion of the big toe lasting 1–3 min, during which time the microelectrode was manipulated until a single unit spike train was encountered. Using audio and visual guidance, each unit was tracked for as long as possible during the contraction by the experimenter applying a force to the microelectrode that effectively counteracted the intramuscular force that tended to expel the microelectrode. Only experiments in which the maximal force at the onset of the sustained contraction was comparable to that of the two preceding brief MVCs were included in the analyses. Intramuscular EMG was amplified (× 1000), filtered (0.1–5.0 kHz), digitized at 12.8 kHz and stored on disk via the SC/ZOOM data acquisition and analysis computer system (Department of Physiology, University of Umeå). Surface EMG was digitized at 6400 Hz and force at 400 Hz.
Data analysis
During off-line analysis the morphology of every spike of each spike train was carefully assessed using the spike recognition algorithm (Edin et al. 1988) incorporated in the SC/ZOOM software. The entire waveform of each spike was examined under high temporal resolution and amplitude windows set to provisionally accept spikes above a certain amplitude. Time-window cursors were placed at the start, peak and end of the potential, and the ratio between the amplitudes of the rising and falling phases was calculated. Spikes were accepted if this ratio remained constant, which allows for reductions in spike amplitude with movement of the electrode tip away from the active muscle fibre but does not allow for variations in spike shape, which could reflect changes in the identity of the active unit. This identification process was checked for each spike, facilitated by reference to the instantaneous-frequency profile of the active discriminated unit. Only spike trains composed of at least 100 spikes were used for the statistical analysis of discharge frequency and variability (Andreassen & Rosenfalck, 1980). Each spike train was divided into segments having an equal number of spikes but with no less that 50 interspike intervals per segment. The mean firing rate for each segment was calculated as the inverse of the mean interspike interval. Firing rate adaptation was computed as the average rate of change in firing rate between the first and last segment (in Hz s−1). Discharge variability was calculated as the coefficient of variation (standard deviation divided by the mean interspike interval). To minimize inflation of variability estimates due to long-term trends in the interval data (Wiegner et al. 1993), the standard deviation was calculated using a floating mean of 19 consecutive intervals. The difference between the central interval and the mean interval of the 19 was used as one value in the calculation of the standard deviation (Fig. 1). The 19-interval window was then shifted over one interval and the process repeated until all intervals in a segment were processed. In order to accommodate the 19-interval window, the first and last 9 intervals of a train were not included in the estimate of the standard deviation. Adaptation of discharge variability was expressed for each unit as the average rate of change in coefficient of variation between the first and last segment (in s−1). Values are expressed as means and standard errors, and differences were considered significant at P < 0.05.
Figure 1.
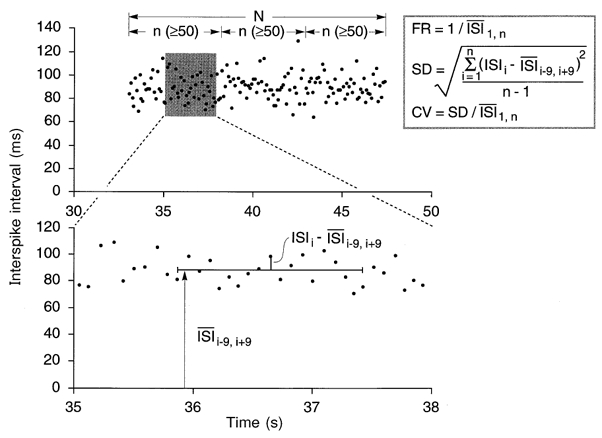
The analyses of motor unit activity were restricted to discharge trains of >100 spikes (26 units). Each train was divided into segments of equal numbers of spikes but with no less than 50 interspike intervals per segment. The upper panel shows a sample plot of interspike intervals (ISIs) versus time. The mean firing rate (FR) for each segment was determined as the inverse of the mean ISI. Discharge variability was calculated as the coefficient of variation (CV, s.d./mean) of the ISIs. To minimize inflation of variability estimates due to long-term trends in the interval data, the s.d. was calculated over a floating mean of 19 consecutive intervals (lower panel).
RESULTS
Unit sample
Successful unitary recordings of long spike trains, using highly selective intramuscular microelectrodes, were made from 26 single motor units in extensor hallucis longus during sustained maximal dorsiflexions of the big toe in seven of the ten subjects. Each spike train was composed of at least 100 spikes (mean ± s.e.m., 183 ± 27; range, 100–782). Thirteen units were sampled during the first 30 s of a maximal voluntary contraction, with a mean train duration of 9.6 ± 1.2 s (range, 3.6–21.9 s), and another 13 units were sampled after 30 s, with a mean train duration of 16.6 ± 3.7 s (range, 7.1–58.1 s). All units were recorded until the intramuscular recording site was lost: this usually occurred because of a progressive fall in spike amplitude that ultimately prevented the unit's discharge from being followed. A representative unitary recording is shown in Fig. 2. The activity of this single motor unit was first detected 91 s after the start of the maximal voluntary contraction, when force had declined to some 30 % of its initial level. On average, by 60–90 s the maximal isometric force generated by the big toe was 78.3 ± 6.3 % of its control maximal level (measured at the onset of the contraction), declining to 39.5 ± 1.4 % by 120–150 s.
Figure 2.
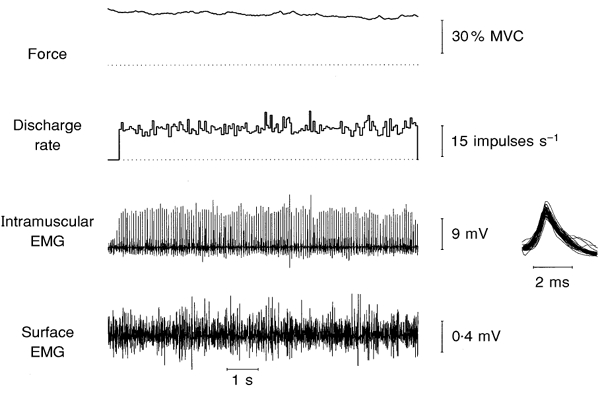
Train of action potentials recorded from a single motor unit in EHL during a sustained maximum voluntary contraction. This unit was first detected 91 s after the start of the contraction. The inset to the right shows 50 consecutive action potential waveforms superimposed.
Changes in discharge frequency during fatigue
Although the force generating capacity of the muscle was significantly lower than control after 1 min of contraction (P = 0.004, Student's t test), there were no significant changes in motoneurone firing rates. The mean discharge frequency (pooled data) recorded within the first 10 s of the contraction was 16.7 ± 1.7 Hz. Measured over the initial 30 s of the contraction this value was 15.8 ± 0.7 Hz, declining slightly to 14.0 ± 0.5 Hz by 60–90 s and to 13.6 ± 0.3 Hz by 120–150 s. In addition to this lack of significant change in the firing rates of a population of motoneurones, individual motoneurones failed to change their firing rates. This was assessed by dividing each long spike train into equal segments comprised of at least 50 interspike intervals, and calculating the mean firing rate for each segment. Firing rate adaptation was then computed, for each unit, as the average rate of change in firing frequency across the segments. Mean firing rate adaptation for those units recorded during the first 30 s of a contraction and those recorded after 30 s are presented in Table 1. Negative values indicate a decrease in firing rate with time. While there was a tendency for the firing rates of individual motoneurones to decline during the initial 30 s, there was no significant difference between the two groups of data (unpaired t test). Mean firing rates generated by the 13 units recorded during the initial 30 s of the contraction are shown in Fig. 3.
Table 1.
Mean (±s.e.m.) discharge parameters for motor units detected within and after the initial 30 s of a sustained maximal voluntary isometric contraction of extensor hallucis longus
| n | Train onset (s) | Train duration (s) | No. of spikes | Initial firing rate (s) | Change in firing rate (Hz s−1) | Initial CV | Change in CV (s−1) | |
|---|---|---|---|---|---|---|---|---|
| Units recorded < 30 s | 13 | 9.8 ± 2.4 | 9.6 ± 1.2 | 144 ± 21 | 15.8 ± 1.4 | −0.18 ± 0.169 | 0.18 ± 0.07 | 1.7 × 10−4± 3.9 × 10−3 |
| Units recorded > 30 s | 13 | 63.5 ± 7.2 | 16.6 ± 3.7 | 222 ± 49 | 13.6 ± 0.7 | 0.05 ± 0.05 | 0.17 ± 0.08 | −1.9 × 10−3± 2.2 × 10−3 |
Initial firing rate, mean firing rate for initial segment (at least 50 interspike intervals). Initial CV, coefficient of variation for initial segment (at least 50 interspike intervals).
Figure 3.
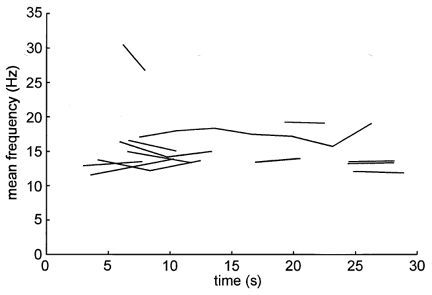
Mean firing rates of the 13 motor units recorded during the first 30 s of a maximal voluntary contraction. Each line is defined by the mean data points from each segment of 50 spikes, such that those trains composed of just 100 spikes would be represented by a line starting with the mean frequency calculated over data segment 1 and finishing with the mean frequency calculated over segment 2. Inflections in the longer trains represent the mean values calculated over each 50 spike segment.
Changes in discharge variability during fatigue
Discharge variability, the coefficient of variation in firing rate, likewise remained relatively constant during the course of a maximal voluntary contraction. In the first 30 s, discharge variability (measured from the pooled data and expressed as a percentage) was 18.8 ± 1.4 %. This was similar when measured at 60–90 s (17.7 ± 1.4 %) but was slightly but insignificantly lower when measured between 120–150 s (11.3 ± 1.1 %). This tendency for discharge variability to decline with the development of fatigue may be accounted for by the positive correlation between discharge frequency and coefficient of variation (r = 0.68)—as noted above there was a slight tendency for firing rate to decline with time. Table 1 shows the mean changes in coefficient of variation for individual motor units recorded before and after the initial 30 s of the sustained maximal contraction: there was no significant change in the variability of long spike trains with fatigue.
DISCUSSION
This study attempted to follow the discharge of single human motor units during a sustained fatiguing contraction of extensor hallucis longus, the prime dorsiflexor of the big toe. Until now, only brief trains of single-unit action potentials, recorded with high-impedance intramuscular tungsten microelectrodes, have been sampled during maximal voluntary contractions of human muscles (Bigland-Ritchie et al. 1983a,b, 1992a; Bellemare et al. 1983; Woods et al. 1987; Gandevia et al. 1990). While such recordings have provided valuable information on the population discharge of human motoneurones during maximal efforts, and have documented a decline in firing rate with the development of fatigue, such studies cannot address the issue as to how individual motoneurones adapt to fatigue. Moreover, because brief trains of spikes are so short they do not contain enough information to allow measurement of discharge variability (Andreassen & Rosenfalck, 1980). Three new observations have been made in the present study, we have (i) measured the maximal volitional firing rates of another human postural and locomotor muscle (extensor hallucis longus), (ii) shown that the maximal firing rates of single motor units in this muscle do not decline with the development of fatigue and, (iii) shown that the regularity of firing for individual units does not change with the fatigue associated with a maximal voluntary contraction, at least for this muscle.
Methodological considerations
A major challenge for this study was to follow the action potentials of single motor units during a maximal voluntary contraction for more than just a few spikes. This proved difficult, despite the use of highly selective tungsten microelectrodes. Although these microelectrodes allow one to move through a muscle and sample the activity of single units or muscle fibres, the fact that the muscle is contracting isometrically and maximally means that the intramuscular pressures are high. To maintain a unitary recording required the experimenter to apply an equivalent force to the microelectrode that counteracted its tendency to be ejected by the muscle, while monitoring the action potentials on the computer monitor and loudspeaker. This naturally resulted in some variation in spike amplitude during a recording sequence, but the signature shape and hence identity of a motor unit or muscle fibre action potential was preserved until the recording site was lost. All candidate unitary recordings were carefully checked off-line using the spike recognition facility of SC/ZOOM. Some spike trains were short, but we excluded those with fewer than 100 spikes. On average, the train duration was 9.5 s for those sampled during the initial 30 s and 16.6 s for those sampled after 30 s. All successful recordings were made in subjects who had previously practised generating a maximal dorsiflexion of the big toe. Transmission of dorsiflexion torque at the ankle to the force transducer on the nail of the hallux was minimized by strapping the foot to a baseplate. The peak force produced at the onset of the sustained maximal contraction was comparable to that produced during a brief maximal contraction—trials were excluded in which this condition was not met. In addition, trials in which subjects felt a deep ache during the contraction due to the presence of the microelectrode were also excluded from the analyses. We therefore are confident that we have succeeded in recording from single motor units during sustained maximal voluntary contractions of extensor hallucis longus in well-motivated subjects under minimal discomfort.
Maximal firing rates in extensor hallucis longus
The maximal firing rates of single motor units in extensor hallucis longus, measured during the first 10 s of a sustained maximal contraction, were 16.7 ± 5.9 Hz (mean ± s.d.). These are lower than those for the intrinsic hand muscles—29.9 ± 8.6 Hz for adductor pollicis (Bellemare et al. 1983) and 30.4 ± 6.2 Hz for first dorsal interosseus (Gandevia et al. 1990)—as well for extensor digitorum (27.4 ± 7.0 Hz, Peters & Fuglevand, 1999) and biceps brachii (31.1 ± 10.1 Hz; Bellemare et al. 1983). In the lower limb, peak firing rates are similar for quadriceps femoris (25.6 ± 5.8 Hz; Woods et al. 1987) and tibialis anterior (28.2 ± 9.9 Hz; Bigland-Ritchie et al. 1992a), while those of soleus are much lower (10.7 ± 2.9 Hz; Bellemare et al. 1983). The lower firing rates of soleus motor units seem consistent with the contractile profile of this muscle, which is composed primarily of slow-twitch muscle fibres (see Bellemare et al. 1983). The present data indicate that the maximal volitional firing rates of extensor hallucis longus are closer to those of a slow postural muscle, although perhaps not as slow as soleus. Moreover, the relatively low firing rates in extensor digitorum is consonant with the known contractile properties of human toe extensor motor units (Macefield et al. 1996), which are slow and require lower stimulation frequencies to generate the same relative force compared with motor units in muscles that control the digits of the hand (Thomas et al. 1991; Fuglevand et al. 1999). In addition, the slope of the steep region of the sigmoidal force-frequency curve is lower for the toe extensor muscles, due to their larger twitch-tetanus ratios, such that the frequency range over which force can be increased is narrower for the toe extensor muscles than for the faster thenar muscles (Macefield et al. 1996). For toe extensor motor units, the steep range of force modulation extends, on average, from 5.5 to 16.3 Hz (Macefield et al. 1996), with half-maximal force being produced at 9.6 Hz. The present maximal firing rates (16.7 Hz), therefore, coincide with the upper limit of the steep part of the force-frequency relationship. Because firing rates higher than these would not translate into much higher forces in the plateau region, this provides support for the idea that the peak firing rates generated during a maximal voluntary contraction are somehow ‘matched’ to the contractile properties of the muscle units. However, this needs to be reconciled with the observations that changes in the mechanical properties of muscle, brought about by changes in either temperature or length (Bigland-Ritchie et al. 1992a,b), do not influence the discharge frequencies of individual motor units.
Changes in firing rate with fatigue
Unlike previous studies of motor unit activity during sustained maximal voluntary contraction in other muscles (Grimby et al. 1981; Bigland-Ritchie et al. 1983a; Marsden et al. 1983; Woods et al. 1987; Gandevia et al. 1990; Peters & Fuglevand, 1999), no systematic change in discharge rate was observed for individual motor units of extensor hallucis longus (EHL). One possible explanation may be the relatively small sample of motor units whose discharge behaviour we were able to follow from the outset of the maximal contraction. Typically, most of the decline in motor unit firing rate is observed within the initial 10–20 s of a maximal contraction (Bigland-Ritchie et al. 1983a; Marsden et al. 1983). Nevertheless, when we calculated the average firing rate for the population of units recorded within the initial 10 s, the firing rate (∼17 Hz) was slightly, but not significantly different from the average population rate determined over 60–90 s of the contraction (∼14 Hz). Therefore, despite a relatively small sample, there appeared to be little evidence of a decline in firing rate during a sustained maximal contraction in EHL.
Several factors have been proposed to explain the fall in discharge rate normally observed in other muscles (for reviews see Windhorst & Boorman, 1995; Fuglevand, 1996). For example, accumulation of metabolites associated with prolonged contraction is thought to activate small-diameter muscle afferents (Kniffki et al. 1978; Kaufman et al. 1983; Sinoway et al. 1993) that inhibit homonymous motoneurones (Bigland-Ritchie et al. 1986; Woods et al. 1987). However, human EHL, like human tibialis anterior (Johnson et al. 1973), is probably composed of a high proportion of oxidative fibres, and therefore might endure a sustained contraction without a major disturbance in the extracellular environment—thereby minimizing the activation of metabolite-sensitive receptors.
Decline in motoneurone firing rate also occurs as a consequence of intrinsic adaptation in the responsiveness of motoneurones to excitatory input (Kernell & Monster, 1982a,b; Spielman et al. 1993; Sawczuk et al. 1995). The degree of adaptation, however, varies for different types of motoneurones. Motoneurones that innervate slow twitch muscle units exhibit little reduction in firing rate in response to sustained depolarizing current (Kernell & Monster, 1982b; Spielman et al. 1993). Because motor units of human EHL are predominantly slow (Macefield et al. 1996), their motoneurones may also display little adaptation during prolonged activity. Also, it seems unlikely that loss of force in EHL was due to de-recruitment of motor units because de-recruitment is invariably preceded by a substantial decline in firing rate (Peters & Fuglevand, 1999). It appears that little fatigue-related adaptation in neural activity occurs in a postural muscle like EHL; loss of force, therefore, may be primarily related to changes in contractile function of the muscle fibres.
Changes in discharge variability with fatigue
It has been suggested that generation of less-regular patterns of motor unit discharge might aid in maintaining force output during prolonged muscle activity (Laouris et al. 1991; Bevan et al. 1992). Greater variability in interspike intervals would probably lead to an increased probability of occurrence of short interval or ‘doublet’ discharges. Doublet discharges are known to induce prolonged enhancement of force in some types of motor units (compare Burke et al. 1976 with Macefield et al. 1996). Moreover, the inclusion of doublets during prolonged stimulation of whole muscle (Binder-Macleod & Barker, 1992) or single motor units (Bevan et al. 1992) has been shown to reduce the magnitude of fatigue. Therefore, an increase in discharge variability during sustained voluntary effort might capitalize on doublet-induced enhancement of motor unit force and help optimize force output during fatigue.
The regularity in the timing of motor-unit discharges in extensor hallucis longus during sustained maximal voluntary contractions, however, did not change significantly with the development of fatigue. This contrasts with the behaviour of motor units in the human masseter muscle during a prolonged submaximal isometric contraction, for which an increase in discharge variability was observed as fatigue set in (Nordstrom & Miles, 1991). An increase in variability has also been reported during fatigue of first dorsal interosseous motor units during intermittent, submaximal ramp-and-hold contractions (Enoka et al. 1989). It is possible that this discrepancy in discharge variability may reflect differences in fatigue-related responses in different muscles, or differences associated with tasks that vary in intensity (submaximal vs. maximal) or duration (Enoka & Stuart, 1992). Additional work is required to characterize the fatigue conditions that provoke alteration in discharge variability and the neural mechanisms responsible for such changes.
Conclusions
We conclude that single motoneurones supplying the extensor hallucis longus muscle show no significant adaptation in firing rate or variability during the fatigue associated with a maximal voluntary contraction, suggesting that progressive adaptation in firing behaviour is not an invariable consequence of sustained maximal voluntary contractions.
Acknowledgments
This work was supported by the National Institutes of Health, Grants NS 14576 and HL 30062 to BBR.
References
- Andreassen S, Rosenfalck A. Regulation of the firing pattern of single motor units. Journal of Neurology, Neurosurgery, and Psychiatry. 1980;43:897–906. doi: 10.1136/jnnp.43.10.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemare F, Woods JJ, Johansson R, Bigland-Ritchie B. Motor-unit discharge rates in maximal voluntary contractions of three human muscles. Journal of Neurophysiology. 1983;50:1380–1392. doi: 10.1152/jn.1983.50.6.1380. [DOI] [PubMed] [Google Scholar]
- Bevan L, Laouris Y, Reinking RM, Stuart DG. The effect of the stimulation pattern on the fatigue of single motor units in adult cats. The Journal of Physiology. 1992;449:85–108. doi: 10.1113/jphysiol.1992.sp019076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Dawson NJ, Johansson RS, Lippold OCJ. Reflex origin for the slowing of motoneuron firing rates in fatigue of human voluntary contractions. The Journal of Physiology. 1986;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Furbush FF, Gandevia SC, Thomas CK. Voluntary discharge frequencies of human motoneurons at different muscle lengths. Muscle and Nerve. 1992a;15:130–137. doi: 10.1002/mus.880150203. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Johansson R, Lippold OJC, Smith S, Woods JJ. Changes in motoneurone firing rates during sustained maximal voluntary contractions. The Journal of Physiology. 1983a;340:335–346. doi: 10.1113/jphysiol.1983.sp014765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Johansson RS, Lippold OJC, Woods JJ. Contractile speed and EMG changes during fatigue of sustained maximal voluntary contractions. The Journal of Physiology. 1983b;340:335–346. doi: 10.1152/jn.1983.50.1.313. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Thomas CK, Rice CL, Howarth JV, Woods JJ. Muscle temperature, contractile speed, and motoneuron firing rates during human voluntary contractions. Journal of Applied Physiology. 1992b;73:2457–2461. doi: 10.1152/jappl.1992.73.6.2457. [DOI] [PubMed] [Google Scholar]
- Binder-MacLeod SA, Barker CB. Use of a catchlike property of human skeletal muscle to reduce fatigue. Muscle and Nerve. 1991;14:850–875. doi: 10.1002/mus.880140909. [DOI] [PubMed] [Google Scholar]
- Burke RE, Rudomin P, Zajac FE. The effect of activation history on tension production by individual muscle units. Brain Research. 1976;109:515–529. doi: 10.1016/0006-8993(76)90031-7. [DOI] [PubMed] [Google Scholar]
- Edin BB, Bäckström PA, Bäckström LO. Single unit retrieval in microneurography: a microprocessor-based device controlled by an operator. Journal of Neuroscience Methods. 1988;24:137–144. doi: 10.1016/0165-0270(88)90057-x. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Robinson GA, Kossev AR. Task and fatigue effects on low-threshold motor units in human hand muscle. Journal of Neurophysiology. 1989;62:1344–1359. doi: 10.1152/jn.1989.62.6.1344. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Stuart DG. Neurobiology of muscle fatigue. Journal of Applied Physiology. 1992;72:1631–1648. doi: 10.1152/jappl.1992.72.5.1631. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ. Neural aspects of fatigue. Neuroscientist. 1996;2:203–206. [Google Scholar]
- Fuglevand AJ, Macefield VG, Bigland-Ritchie B. Force-frequency and fatigue properties of motor units in muscles that control digits of the human hand. Journal of Neurophysiology. 1999;81:1718–1729. doi: 10.1152/jn.1999.81.4.1718. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Macefield VG, Howell JN, Bigland-Ritchie B. Adaptation of motor unit discharge rate and variability during sustained maximal voluntary contractions. Society for Neuroscience Abstracts. 1994;20:1759. [Google Scholar]
- Gandevia SC, Macefield G, Burke D, McKenzie DK. Voluntary activation of human motor axons in the absence of muscle afferent feedback. Brain. 1990;113:1563–1581. doi: 10.1093/brain/113.5.1563. [DOI] [PubMed] [Google Scholar]
- Grimby L, Hannerz J, Hedman B. The fatigue and voluntary discharge properties of single motor units in man. The Journal of Physiology. 1981;316:545–554. doi: 10.1113/jphysiol.1981.sp013805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles an autopsy study. Journal of the Neurological Sciences. 1973;18:111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. Journal of Applied Physiology. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Time course and properties of late adaptation in spinal motoneurons of the cat. Experimental Brain Research. 1982a;46:191–196. doi: 10.1007/BF00237176. [DOI] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Motoneurone properties and motor fatigue an intracellular study of gastrocnemius motoneurones of the cat. Experimental Brain Research. 1982b;46:197–204. doi: 10.1007/BF00237177. [DOI] [PubMed] [Google Scholar]
- Kniffki KD, Mense S, Schmidt RF. Responses of group IV afferent units from muscle to stretch, contraction and chemical stimulation. Experimental Brain Research. 1978;31:511–522. doi: 10.1007/BF00239809. [DOI] [PubMed] [Google Scholar]
- Laouris Y, Bevan L, Spielmann JM, Nordstrom MA, Reinking RM, Stuart DG. Changes in motoneuron discharge variability during prolonged activation with extracellular stimulation: possible implications for fatigue. In: Atlan G, Beliveau L, Bouissou P, editors. Muscle Fatigue: Biochemical and Physiological Aspects. Paris: Masson; 1991. p. 242. [Google Scholar]
- Macefield VG, Fuglevand AJ, Bigland-Ritchie B. Contractile properties of single motor units in human toe extensors assessed by intraneural motor axon stimulation. Journal of Neurophysiology. 1996;75:2509–2519. doi: 10.1152/jn.1996.75.6.2509. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Meadows JC, Merton PA. ‘Muscular wisdom’ that minimizes fatigue during prolonged effort in man: peak rates of motoneuron discharge and slowing of discharge during fatigue. In: Desmedt JE, editor. Motor Control Mechanisms in Health and Disease. New York: Raven Press; 1983. pp. 169–211. [PubMed] [Google Scholar]
- Nordstrom MA, Miles TS. Discharge variability and physiological properties of human masseter motor units. Brain Research. 1991;541:50–56. doi: 10.1016/0006-8993(91)91072-9. [DOI] [PubMed] [Google Scholar]
- Peters EJD, Fuglevand AJ. Cessation of human motor unit discharge during sustained maximal voluntary contraction. Neuroscience Letters. 1999;274:66–70. doi: 10.1016/s0304-3940(99)00666-7. [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Powers RK, Binder MD. Spike frequency adaptation studied in hypoglossal motoneurons of the rat. Journal of Neurophysiology. 1995;73:1799–1810. doi: 10.1152/jn.1995.73.5.1799. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Hill JM, Pickar JG, Kaufman MP. Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. Journal of Neurophysiology. 1993;69:1053–1059. doi: 10.1152/jn.1993.69.4.1053. [DOI] [PubMed] [Google Scholar]
- Spielmann JM, Laouris Y, Nordstrom MA, Robinson GA, Reinking RM, Stuart DG. Adaptation of cat motoneurons to sustained and intermittent extracellular activation. The Journal of Physiology. 1993;464:75–120. doi: 10.1113/jphysiol.1993.sp019625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CK, Bigland-Ritchie B, Johansson RS. Force-frequency relationships of human thenar motor units. Journal of Neurophysiology. 1991;65:1509–1516. doi: 10.1152/jn.1991.65.6.1509. [DOI] [PubMed] [Google Scholar]
- Wiegner AW, Wierzbicka MM, Davies L, Young RR. Discharge properties of single motor units in patients with spinal cord injuries. Muscle and Nerve. 1993;16:661–671. doi: 10.1002/mus.880160613. [DOI] [PubMed] [Google Scholar]
- Windhorst U, Boorman G. Overview: Potential role of segmental motor circuits in muscle fatigue. In: Gandevia SC, Enoka RM, McComas AJ, Stuart DG, Thomas CK, editors. Fatigue: Neural and Muscular Mechanisms. New York: Plenum; 1995. pp. 241–258. [Google Scholar]
- Woods JJ, Furbush F, Bigland-Ritchie B. Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. Journal of Neurophysiology. 1987;58:125–137. doi: 10.1152/jn.1987.58.1.125. [DOI] [PubMed] [Google Scholar]


