Abstract
5′-AMP-activated protein kinase (AMPK) has been suggested to play a key role in the regulation of metabolism in skeletal muscle. AMPK is activated in treadmill-exercised and electrically stimulated rodent muscles. Whether AMPK is activated during exercise in humans is unknown.
We investigated the degree of activation and deactivation of α-isoforms of AMPK during and after exercise. Healthy human subjects performed bicycle exercise on two separate occasions at either a low (∼50% maximum rate of O2 uptake (V̇O2,max) for 90 min) or a high (∼75% V̇O2,max for 60 min) intensity. Biopsies from the vastus lateralis muscle were obtained before and immediately after exercise, and after 3 h of recovery.
We observed a 3- to 4-fold activation of the α2-AMPK isoform immediately after high intensity exercise, whereas no activation was observed after low intensity exercise. The activation of α2-AMPK was totally reversed 3 h after exercise. In contrast, α1-AMPK was not activated during either of the two exercise trials.
The in vitro AMP dependency of α2-AMPK was significantly greater than that of α1-AMPK (∼3- vs. ∼2-fold).
We conclude that in humans activation of α2-AMPK during exercise is dependent upon exercise intensity. The stable activation of α2-AMPK, presumably due to the activation of an upstream AMPK kinase, is compatible with a role for this kinase complex in the regulation of skeletal muscle metabolism during exercise, whereas the lack of stable α1-AMPK activation makes this kinase complex a less likely candidate.
5′-AMP-activated protein kinase (AMPK) is activated in skeletal muscle from rats during treadmill exercise and electrical stimulation in situ and in vitro (Winder & Hardie, 1996; Hutber, 1997; Hayashi et al. 1998). The in vivo regulation of AMPK is still unclear, but phosphorylation by an AMPK kinase and allosteric regulation by an increased concentration of AMP and a decreased concentration of creatine phosphate may all lead to increased muscle AMPK activity in response to contractile activity (Hardie et al. 1998; Winder & Hardie, 1999). Studies suggest that the activation of AMPK may lead to the increased fatty acid oxidation observed during and after exercise (Ruderman et al. 1999). However, artificial activation of AMPK by 5-aminoimidazole-4-carboxamide (AICA)-riboside, leading to increased fatty acid oxidation, also increases the rate of glucose uptake in perfused rat muscle (Merrill et al. 1997) and in isolated incubated muscle (Hayashi et al. 1998). Recently, it was confirmed that the effects of AICA-riboside on glucose transport involve recruitment of the glucose transporter GLUT4 to the plasma membrane (Kurth-Kraczek et al. 1999). In addition, two studies have shown a relationship between the activation of glucose transport and AMPK in isolated rat skeletal muscle when subjected to a range of metabolic stressors (e.g. hypoxia and dinitrophenol), electrical stimulation and AICA-riboside (Ihlemann et al. 1999; Hayashi et al. 2000a). Thus, a host of correlative data reported so far support a regulatory role for AMPK in glucose transport in response to contractile activity, but there are also data in slow-twitch rodent muscle showing dissociation between AMPK activation and contraction-induced glucose transport (Derave et al. 2000).
AMPK is a heterotrimeric protein consisting of a catalytic (α) and two non-catalytic (β and γ) subunits (Hardie et al. 1998). Two isoforms of both the α- and β-subunits and several γ-subunit isoforms have been identified (Stapleton et al. 1996; Thornton et al. 1998; Cheung et al. 2000). In rat skeletal muscle both α1- and α2-isoforms are expressed (Gao et al. 1995; Stapleton et al. 1996), and stimulation with contraction or AICA-riboside induces an equal fold increase in the α1- and α2-AMPK activity (Hayashi et al. 2000a). In contrast, when rat hindlimb muscle is stimulated electrically in situ only α2-AMPK is activated (Vavvas et al. 1997).
Although it has been shown that acetyl CoA carboxylase, a substrate for AMPK, is deactivated by exercise in humans (Dean et al. 2000), no reports of the effect of exercise on AMPK activity in human skeletal muscle have been published. Therefore, in the present study, the effects of low and high intensity bicycle exercise of equal energy expenditure on isoform-specific AMPK activity in human skeletal muscle were investigated. We demonstrate an exercise intensity-dependent activation of α2-AMPK, whereas α1-AMPK activity was unchanged. In addition, using in vitro kinase assays a marked AMP dependency of α2-AMPK activity was observed, whereas α1-AMPK activity was less AMP dependent.
METHODS
Experimental protocol
Seven healthy men (aged 25 ± 1 years) gave their written informed consent to participate in this study, which was approved by the Copenhagen Ethics Committee and was in agreement with the standards set by the Declaration of Helsinki. The subjects randomly underwent two experimental trials separated by 2–3 weeks. Subjects were instructed to eat a controlled diet (energy sources: carbohydrate, ∼63%; fat, ∼22%; and protein, ∼15%; average, 13.5 MJ day−1) for 3 days before the experiment, and arrived at the laboratory in the morning after an overnight fast. After 45 min of rest a needle biopsy from the vastus lateralis muscle was obtained under local anaesthesia. The subjects then performed bicycle exercise for 90 min at 50% VO2,max (low intensity trial) or for 55 min at 75% VO2,max plus 5 min at 90% V̇O2,max (high intensity trial). Immediately after exercise the subjects were placed in the supine position and another biopsy was obtained from the vastus lateralis muscle. The subjects then rested in the supine or sitting position for 3 h, having free access to water only, before a third biopsy was obtained. The work intensity was applied so that the total energy expenditure was estimated to be similar, and based on oxygen consumption during the experiments, total energy expenditure was similar in the two trials (average, 3800 kJ). The biopsies before and after exercise were always taken in the same leg and the biopsy after rest was always taken in the contralateral leg. Incisions for biopsies were spaced 4–5 cm apart. Biopsies were frozen in liquid nitrogen within 20 s. Body weight, height and body mass index (BMI) were 74 ± 2 kg, 178 ± 2 cm and 23 ± 1 kg m−2, respectively. One to two weeks before the experiments, maximal pulmonary oxygen consumption was determined during an incremental bicycle ergometer test (55 ± 1 ml kg−1 min−1).
Analytical procedures
For determination of muscle glycogen content, muscle biopsies were freeze-dried and dissected free of blood, fat and connective tissue before analysis. Glycogen content was determined as glycosyl units after acid hydrolysis (Lowry & Passonneau, 1972). a-Isoform-specific AMPK activity was measured in immunoprecipitates from 200 μg of muscle lysate protein using an anti-α1-AMPK and an anti-α2-AMPK antibody (kindly provided by Professor D. Grahame Hardie, University of Dundee, UK). The antibodies were raised against peptides predicted from the rat amino acid sequences (Woods et al. 1996). The α1 sequence used is identical in human and rat, wheras the α2 sequence differs by one out of the 15 amino acids. A p81 filter paper assay, using SAMS-peptide (HMRSAMSGLHLVKRR; 200 μmol l−1) as the substrate, was used to measure AMPK activity. Minor modifications were made to the method described in Hayashi et al. (2000a). Thus, biopsies were homogenized 1:20 (wet weight:volume) as described previously (Markuns et al. 1999). After an overnight immunoprecipitation, the immune complexes were washed as described in Hayashi et al. (2000a) followed by a final wash in 400 μl of 120 mmol l−1 Hepes buffer (pH 7.0) containing 240 mmol l−1 NaCl. Each sample was then divided into two; one was used for kinase activity measurements in the absence of AMP and the other for kinase activity measurements in the presence of 0.2 mmol l−1 AMP. The kinase reaction ran for 30 min at 30oC.
Calculations and statistics
Control samples were included in all kinase activity assays, and assay-to-assay variation was accounted for by expressing the data relative to these samples. Data are expressed as means ± s.e.m. Statistical evaluation was done by one- or two-way analysis of variance with repeated measures, as appropriate. When analysis of variance revealed significant differences, a post hoc test was used to correct for multiple comparisons (Student-Newman-Keuls test). Differences between groups were considered statistically significant if P was < 0.05.
RESULTS
Bicycle exercise activates α2-AMPK
Intense bicycle exercise for 60 min activated muscle α2-AMPK by 3- to 4-fold (P < 0.05) whereas low intensity exercise for 90 min did not (P > 0.72, Fig. 1A). The enhanced α2-AMPK activity reversed to basal levels after 3 h of recovery. In contrast, neither low nor high intensity exercise affected α1-AMPK activity in the working muscle (Fig. 1B).
Figure 1.
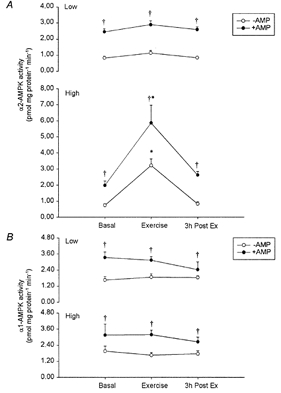
Effects of exercise on isoform-specific AMPK activity
Muscle α2-AMPK (A) and α1-AMPK (B) activity in the low intensity exercise trial (top panels) and in the high intensity exercise trial (bottom panels). Activities were measured in vitro in the absence (○) or presence (•) of AMP. Significant differences (P < 0.05) from Basal as well as 3h Post Ex (*) and from the same measurement in the absence of AMP (†) are indicated. Data are presented as means ±s.e.m., n = 6–7.
Isoform-specific AMP-dependent AMPK activity
α2-AMPK activity measured in vitro after intensive washing of the immune complexes was dependent on the presence of 200 μmol l−1 AMP in the assay mixture. The effect of AMP was independent of the activation level of α2-AMPK and raised the activity of the kinase by on average 3.0 (± 0.1)-fold (n = 40, Fig. 1A). Compared to α2-AMPK, α1-AMPK activity was significantly less AMP dependent (1.89 (± 0.1)-fold increase, n = 40, P < 0.001, Fig. 1B). The absolute activity in immunoprecipitates from resting non-stimulated human skeletal muscle was higher in α1- compared to α2-AMPK complexes (1.7 ± 0.2 vs. 0.78 ± 0.08 pmol (mg protein)−1 min−1, n = 14, P < 0.01).
Effects of exercise on glycogen metabolism
Muscle glycogen content decreased from 530 ± 57 to 285 ± 54 mmol (kg dry weight)−1 in the high intensity exercise trial (P < 0.05) and from 462 ± 32 to 320 ± 43 mmol (kg dry weight)−1 in the low intensity trial (P < 0.05). Due to the shorter exercise time, the rate of glycogen degradation during exercise was considerably greater in the high compared to the low intensity trial (4.5 ± 0.7 vs. 1.6 ± 0.3 mmol min−1 (kg dry weight)−1, P < 0.05). Glycogen content was not significantly increased in the 3 h following exercise.
DISCUSSION
Previous studies in rodent skeletal muscles, in which total AMPK activity was measured, showed an activation of AMPK in relation to the intensity of treadmill exercise (Rasmussen & Winder, 1997). Furthermore, a relationship between total AMPK activity and force development during electrical stimulation of isolated skeletal muscle from rats has been demonstrated (Ihlemann et al. 1999). In the present study, in which a-isoform-specific AMPK activity was measured in human skeletal muscle, we found a significant α2-AMPK activation after high but not low intensity exercise, whereas no activation of α1-AMPK was seen. In agreement with the present findings, a preliminary study in rodents showed an α2-AMPK activation that was dependent on the number of tetanic contractions performed during incubation in vitro as well as on the treadmill running speed (Hayashi et al. 2000b). Increasing the AMP:ATP ratio may cause the stable activation of AMPK by phosphorylation due to activation of the upstream AMPK kinase (Hardie et al. 1998). Since the intracellular AMP:ATP ratio changes in relation to bicycle exercise intensity (Howlett et al. 1998), the observed intensity dependency of α2-AMPK activation is not unexpected. At 50% of V̇O2,max the AMP:ATP ratio does not change appreciably (Howlett et al. 1998), explaining the lack of stable α2-AMPK activation at this intensity. Even though creatine phosphate is expected to decrease somewhat at 50% of V̇O2,max, this only leads to allosteric AMPK activation and not phosphorylation by AMPK kinase (Winder & Hardie, 1999). A further consideration is that during submaximal exercise not all muscle fibres are recruited, and changes in the activated fibres will be diluted by the presence of such non-recruited fibres in the biopsy. Thus, during low intensity exercise changes in AMPK activity in activated fibres may be missed due to dilution by non-recruited fibres.
In situ electrical stimulation of rat hindlimb muscle activates α2-AMPK but not α1-AMPK (Vavvas et al. 1997). A preliminary report also suggests a-isoform-specific AMPK activation in response to treadmill exercise (Hayashi et al. 2000b). Here we extend this notion to human skeletal muscle as a lack of α1-AMPK activation was observed in response to both low and high intensity exercise. Only during intense electrical stimulation of isolated muscle has an activation of α1-AMPK been reported (Hayashi et al. 2000a). This finding may relate to the extreme stimulatory conditions adopted in vitro, which probably introduce hypoxia in the muscle cells. In fact, hypoxia per se was found to be the most potent stimulus for activation of both α1-AMPK and α2-AMPK in isolated skeletal muscle (Hayashi et al. 2000a).
The a-AMPK isoform-specific activation during exercise indicates that the upstream AMPK kinase selectively phosphorylates AMPK complexes containing the α2- rather than the α1-isoform. Since both a-isoforms can be readily activated by AMPK kinase (Salt et al. 1998) it might be speculated that different subcellular co-localization patterns of AMPK kinase and the different a-isoform-containing AMPK complexes are responsible for this selectivity.
AMPK has been suggested to be a key factor in increasing glucose uptake in contracting skeletal muscle (Merrill et al. 1997). Although several studies support such a connection, it has also been reported that AMPK activity and glucose transport may be completely dissociated in contracting perfused slow-twitch rat muscle (Derave et al. 2000). Thus, the role of AMPK in muscle glucose transport during exercise remains to be defined. It must be remembered that the allosteric regulation of AMPK is lost during immunoprecipitation, and thus in vitro kinase activity only reflects stable changes, such as phosphorylation. Therefore, the lack of stable α2-AMPK activation during low intensity exercise does not necessarily mean that α2-AMPK is not activated during exercise as the true degree of activation in vivo (allosteric and phosphorylation) cannot be determined from the present study. Several findings support this view, for example: (1) in electrically stimulated rat muscle the AMPK substrate acetyl-CoA carboxylase (ACC) is inactivated at short treatment times with almost no change in apparent AMPK activity (Hutber et al. 1997); (2) in AICA-riboside-treated rat muscle ACC is maximally inactivated at low concentrations that only cause partial activation of AMPK (Merrill et al. 1997); (3) recently, it was reported that muscle ACC activity decreased and phosphorylation increased even at low exercise intensities during one-legged exercise in humans (Dean et al. 2000). These studies suggest some degree of allosteric activation of muscle AMPK that causes effects on downstream targets without any apparent stable activation of AMPK. In this respect it is worth noting that muscle glucose uptake is enhanced during both low and high intensity bicycle exercise (Wahren et al. 1971). Thus, the present data are not incompatible with a role for α2-AMPK in increasing glucose uptake in muscle during exercise. On the other hand, the lack of stable activation of α1-AMPK during exercise, even during relatively high intensity exercise, makes it a less likely candidate for a physiological regulator of glucose transport in human skeletal muscle during exercise.
The AMP dependency of AMPK in vitro was evident in the present study, although α2-AMPK was more dependent on AMP than α1-AMPK. This is in accordance with findings in rodent skeletal muscle (Salt et al. 1998; Cheung et al. 2000). If the different AMP dependency is also valid in vivo, it further supports the idea that α2-AMPK is preferentially activated during intense exercise when AMP levels in the muscle increase (Howlett et al. 1998).
It has been suggested that the prolonged activation of AMPK observed after treadmill exercise (Rasmussen et al. 1998) and after in situ electrical stimulation (Vavvas et al. 1997) could mediate the enhanced free fatty acid oxidation observed after contractile activity and thereby decrease the rate of glucose oxidation. Together with the suggested enhancement of glucose uptake this would create conditions favourable for glycogen resynthesis following exercise. Whereas in the study of treadmill-exercised rodent muscles a close relationship between AMPK activation and glycogen resynthesis was evident (Rasmussen et al. 1998), the present study does not support such an association. This is because α2-AMPK activity 3 h after exercise was not different from the basal level whereas the muscles were still glycogen depleted.
In conclusion, bicycle exercise of a moderately high intensity leads to a stable activation of α2-AMPK in the working muscles, indicating activation of an AMPK kinase. Our data are compatible with a role for α2-AMPK in metabolic regulation during exercise. In addition, α2-AMPK, isolated from human skeletal muscle, displays a greater dependency on AMP in vitro than does α1-AMPK. Taken together with the total lack of α1-AMPK activation during even moderately high intensity exercise this may indicate that α1-containing AMPK complexes do not play an important physiological role in the regulation of metabolism in human skeletal muscle during exercise.
Acknowledgments
This study was supported by grant no. 504–14 from the Danish National Research Foundation. J.F.P.W. was supported by a postdoctoral fellowship from the Danish Medical Research Council. Professor D. G. Hardie is acknowledged for his kind donation of the a-AMPK isoform-specific antibodies. Betina Bolmgren and Irene B. Nielsen are acknowledged for superior technical assistance.
Note added in proof
Since this paper was submitted for publication, N. Fujii, T. Hayashi, M. F. Hirshman, J. T. Smith, S. A. Habinowski, L. Kaijser, J. Mu, O. Ljungqvist, M. J. Birnbaum, L. A. Witters, A. Thorell & L. J. Goodyear have published data also showing isoform-specific activation of AMPK in human skeletal muscle during exercise (Biochemical and Biophysical Research Communications273, 1150–1155 (2000)).
References
- Cheung CF, Salt IP, Davies A, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma subunit isoforms and their role in AMP binding. Biochemical Journal. 2000;346:659–669. [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Daugaard JR, Young ME, Saha A, Vavvas D, Asp S, Kiens B, Kim K-H, Witters LA, Richter EA, Ruderman NB. Exercise disminishes the activity of acetyl CoA carboxylase in human muscle. Diabetes. 2000;49:1295–1300. doi: 10.2337/diabetes.49.8.1295. [DOI] [PubMed] [Google Scholar]
- Derave W, Hua AI, Ihleman J, Witters LA, Kristiansen S, Richter EA, Ploug T. Dissociation of AMP-activated protein kinase activation and glucose transport in contracting slow-twitch muscle. Diabetes. 2000;49:1281–1287. doi: 10.2337/diabetes.49.8.1281. [DOI] [PubMed] [Google Scholar]
- Gao G, Widmer J, Stapleton D, Teh T, Cox T, Kemp BE, Witters LA. Catalytic subunits of the porcine and rat 5′-AMP-activated protein kinase are members of the SNF1 protein kinase family. Biochimica et Biophysica Acta. 1995;1266:73–82. doi: 10.1016/0167-4889(94)00222-z. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annual Reviews of Biochemistry. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport. Diabetes. 2000a;49:527–531. doi: 10.2337/diabetes.49.4.527. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hirshman MF, Habinowski SA, Witters LA, Goodyear LJ. The alpha2 isoform of the 5′ AMP-activated protein kinase may mediate contraction stimulated glucose transport in skeletal muscle. Diabetes. 2000b;48(suppl. 1):A13. [Google Scholar]
- Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- Howlett RA, Parolin ML, Dyck DJ, Hultman E, Jones NL, Heigenhauser GJ, Spriet LL. Regulation of skeletal muscle glycogen phosphorylase and PDH at varying exercise power outputs. American Journal of Physiology. 1998;275:R418–425. doi: 10.1152/ajpregu.1998.275.2.R418. [DOI] [PubMed] [Google Scholar]
- Hutber CA, Hardie DG, Winder WW. Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase. American Journal of Physiology. 1997;272:E262–266. doi: 10.1152/ajpendo.1997.272.2.E262. [DOI] [PubMed] [Google Scholar]
- Ihlemann J, Ploug T, Hellsten Y, Galbo H. Effect of tension on contraction-induced glucose transport in rat skeletal muscle. American Journal of Physiology. 1999;277:E208–214. doi: 10.1152/ajpendo.1999.277.2.E208. [DOI] [PubMed] [Google Scholar]
- Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW. 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. 1999;48:1667–1671. doi: 10.2337/diabetes.48.8.1667. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. London: Academic Press, Inc.; 1972. pp. 1–291. [Google Scholar]
- Markuns JF, Wojtaszewski JFP, Goodyear LJ. Insulin and exercise decrease glycogen synthase kinase-3 activity by different mechanisms in rat skeletal muscle. Journal of Biological Chemistry. 1999;274:24896–24900. doi: 10.1074/jbc.274.35.24896. [DOI] [PubMed] [Google Scholar]
- Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinasefatty acid oxidation, and glucose uptake in rat muscle. American Journal of Physiology. 1997;273:E1107–1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Hancock CR, Winder WW. Postexercise recovery of skeletal muscle malonyl-CoAacetyl-CoA carboxylase, and AMP-activated protein kinase. Journal of Applied Physiology. 1998;85:1629–1634. doi: 10.1152/jappl.1998.85.5.1629. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Winder WW. Effect of exercise intensity on skeletal muscle malonyl-CoA and acetyl-CoA carboxylase. Journal of Applied Physiology. 1997;83:1104–1109. doi: 10.1152/jappl.1997.83.4.1104. [DOI] [PubMed] [Google Scholar]
- Ruderman NB, Saha AK, Vavvas D, Witters LA. Malonyl-CoAfuel sensing, and insulin resistance. American Journal of Physiology. 1999;276:E1–18. doi: 10.1152/ajpendo.1999.276.1.E1. [DOI] [PubMed] [Google Scholar]
- Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG. AMP-activated protein kinase: greater AMP dependenceand preferential nuclear localization, of complexes containing the alpha2 isoform. Biochemical Journal. 1998;334:177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, Kemp BE. Mammalian AMP-activated protein kinase subfamily. Journal of Biological Chemistry. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- Thornton C, Snowden MA, Carling D. Identification of a novel AMP-activated protein kinase beta subunit isoform that is highly expressed in skeletal muscle. Journal of Biological Chemistry. 1998;273:12443–12450. doi: 10.1074/jbc.273.20.12443. [DOI] [PubMed] [Google Scholar]
- Vavvas D, Apazidis A, Saha AK, Gamble J, Patel A, Kemp BE, Witters LA, Ruderman NB. Contraction-induced changes in acetyl-CoA carboxylase and 5′-AMP- activated kinase in skeletal muscle. Journal of Biological Chemistry. 1997;272:13255–13261. doi: 10.1074/jbc.272.20.13255. [DOI] [PubMed] [Google Scholar]
- Wahren J, Felig P, Ahlborg G, Jorfeldt L. Glucose metabolism during leg exercise in man. Journal of Clinical Investigation. 1971;50:2715–2725. doi: 10.1172/JCI106772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. American Journal of Physiology. 1996;270:E299–304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. AMP-activated protein kinasea metabolic master switch: possible roles in type 2 diabetes. American Journal of Physiology. 1999;277:E1–10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- Woods A, Salt I, Scott J, Hardie DG, Carling D. The alpha1 and alpha2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Letters. 1996;397:347–351. doi: 10.1016/s0014-5793(96)01209-4. [DOI] [PubMed] [Google Scholar]


