Abstract
Paired recordings from monosynaptically connected CA3 interneurons and pyramidal cells of rat hippocampal slice cultures were used to compare the modulation of GABA release at synapses from distinct interneurons.
The group II metabotropic glutamate receptor (mGluR) agonist (2S,2′R,3′R)-2-(2′,3′-dicarboxylcyclopropyl) glycine (DCG-IV, 5 μm) reduced the amplitude of IPSPs originating from stratum radiatum but not stratum oriens interneurons. In contrast, the GABAB receptor agonist (-)baclofen (10 μm) reduced the amplitude of unitary IPSPs elicited by all interneurons.
IPSPs mediated by stratum oriens interneurons were unaffected by the N-type calcium channel blocker ω-conotoxin MVIIA (1 μm) but were suppressed by the P/Q-type blocker ω-agatoxin IVA (200 nm). In contrast, IPSPs mediated by stratum radiatum interneurons were abolished by ω-conotoxin MVIIA.
Transmission dynamics were different at synapses from the two groups of interneurons. IPSPs mediated by stratum oriens interneurons showed marked paired-pulse depression (PPD) at intervals of 50–400 ms. IPSPs mediated by stratum radiatum interneurons showed paired-pulse facilitation (PPF) at 50 ms and PPD at longer intervals.
The amplitude of unitary IPSPs from all interneurons was unaffected by the GABAB receptor antagonist CGP52432 (2 μm) as was PPD at both 50 and 400 ms intervals. However, CGP52432 did reduce PPD of extracellularly evoked IPSPs.
Our results show that two groups of inhibitory synapses impinging onto CA3 pyramidal cells can be distinguished according to their dynamic and modulatory properties.
GABAergic interneurons fulfil multiple functions in cortical networks. These include the control of dendritic electrogenesis and spike generation in principal cells (e.g. Miles et al. 1996), the generation or maintenance of rhythms associated with specific behavioural states (see Freund & Buzsáki, 1996), and the development of cortical circuitry (e.g. Allendoerfer & Shatz, 1994). This diversity of functions is paralleled by the heterogeneity of cortical interneurons (Freund & Buzsáki, 1996), which differ in dendritic and axonal arborizations, expression of calcium-binding proteins, firing pattern, and modulation by neurotransmitters released by subcortical afferents (Parra et al. 1998).
The net effect of synaptic inhibition is determined by the firing properties of inhibitory cells as well as the dynamics of GABA release and location of inhibitory synapses. There is considerable evidence that synapses formed by interneurons are themselves heterogeneous. In the hippocampus, perisomatic GABAergic boutons are of a consistently larger size than boutons terminating onto distal dendrites (Miles et al. 1996; Halasy et al. 1996). This difference may be associated with differences in synaptic function, including the probability of transmitter release (Pierce & Lewin, 1994). Distinct subgroups of hippocampal inhibitory synapses express different presynaptic calcium channels (Poncer et al. 1997) and may be differentially modulated by GABAB autoreceptors (Lambert & Wilson, 1993). Finally, synapses from distinct functional groups of cortical interneurons show different temporal patterns of synaptic transmission (Gupta et al. 2000; Maccaferri et al. 2000). Heterogeneity therefore appears to be an essential functional feature of cortical inhibition, allowing populations of synapses with distinct postsynaptic effects to be switched on or off in specific contexts of network activity (Xiang et al. 1998).
Differences in synaptic properties are masked when terminals originating from multiple cell types are stimulated simultaneously. Understanding the diversity of inhibitory synapses therefore requires the study of responses from synapses made by single interneurons onto individual postsynaptic cells. We examined the functional diversity of GABAergic synapses by recording unitary IPSPs from pairs of monosynaptically connected inhibitory and pyramidal cells in area CA3 of rat hippocampus. We compared the sensitivity of synaptic transmission to agonists of presynaptic receptors for glutamate and GABA and antagonists of presynaptic calcium channels, as well as the dynamics of GABA release upon repetitive discharge. Two groups of synapses from morphologically identified interneurons could be distinguished based on such properties. Coexpression of various synaptic characteristics may reflect a functional interdependence.
METHODS
Hippocampal slice cultures were prepared from 6-day-old rats killed by decapitation following a protocol approved by the Veterinary Department of the Canton of Zurich, and maintained as described previously (Gähwiler et al. 1998). After 2–4 weeks, slices were placed in a chamber continuously superfused with (mm): Na+, 149; Cl−, 152; K+, 2.7; Ca2+, 2.8; Mg2+, 2.0; HCO3−, 11.6; H2PO4−, 0.4; and glucose, 5.6; with 10 μg l−1 Phenol Red (pH 7.4, 31°C).
Whole-cell recordings were obtained from interneurons with cell bodies in stratum oriens or radiatum using an Axopatch 200A amplifier. Pipettes (3–5 MΩ) pulled from borosilicate glass contained (mm): potassium gluconate, 120; KCl, 10; Hepes, 10; EGTA, 1; Mg-ATP, 4; Na-GTP, 0.4; CaCl2, 0.1; and MgCl2, 0.5. Cells were voltage clamped at −60 mV. A 5 ms voltage step to 0 mV elicited an ‘action current’ in the interneuron. Series resistance and cell capacitance were regularly checked (14.1 ± 1.3 MΩ and 18.0 ± 2.0 pF, respectively, n = 33). A pyramidal cell was then recorded in current-clamp mode with an Axoclamp-2A amplifier using sharp microelectrodes filled with KCl (1 m) and Hepes (10 mm) and held at approximately −70 mV. Unitary IPSPs were recorded in the presence of 6-nitro-7-sulphamoylbenzo[f]quinoxaline-2,3-dione (NBQX, 20 μm) and (±)3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP, 10 μm).
When paired stimuli were delivered, the second IPSP was measured after the waveform of the first had been fitted to a double exponential using a Simplex algorithm and then subtracted. IPSP amplitudes were measured on a 5 ms window around the peak.
Biocytin (1%) was sometimes included in the interneuron recording solution. Cells were filled for 20–45 min and slices were then fixed overnight in 0.1 m phosphate buffer containing 4% paraformaldehyde, 2.5% gluteraldehyde and 15% picric acid. Samples were subsequently washed in phosphate buffer, incubated in sucrose, freeze-thawed and processed with avidin-horseradish peroxidase (ABC kit, Vector Laboratories, Burlingame, USA) using diaminobenzidine as the chromogen and nickel intensification. Camera lucida drawings were made with a w100 objective using Neurolucida software (MicroBrightField, Colchester, USA). The apparent diameter of axonal varicosities was measured using a w100 objective with addtional w2 physical zoom on a Zeiss LSM410, from 4–13 randomly selected sections of axons.
Drugs were purchased from the following sources. NBQX, DCG-IV and CPP were from Tocris Cookson (Bristol, UK); ω-conotoxin MVIIA was from Latoxan (Rosans, France); CGP52432 and (-)baclofen were gifts from Novartis (Basel, Switzerland); and ω-agatoxin IVA was a gift from Pfizer (Groton, USA).
RESULTS
Inhibition of GABA release at unitary synapses by presynaptic receptor agonists
Hippocampal inhibitory synapses are subject to presynaptic modulation by both GABA and glutamate (Thompson & Géhwiler, 1989; Poncer et al. 1995). However, it is unclear whether these neurotransmitters exert their effects similarly at all synapses (Lambert & Wilson, 1993). We examined this question by recording pairs of visually identified inhibitory and pyramidal cells in area CA3 of rat hippocampal slice cultures. The mean amplitude of unitary IPSPs was 3.6 ± 0.5 mV (n = 33) and was not correlated with the location of the presynaptic interneuron. In 14 cell pairs in which the soma of the interneuron was located in stratum oriens, application of the group II mGluR agonist DCG-IV (5 μm) for 5–10 min produced no significant change in unitary IPSP amplitude (+2.7 ± 2.7%, P = 0.5, paired t test, Fig. 1A). In contrast, in nine cell pairs with the interneuron located in stratum radiatum, application of the same concentration of DCG-IV reduced IPSP amplitude by 37.8 ± 6.6% (P < 0.05, Fig. 1B). This effect was reversible and was not accompanied by any significant change in either the input resistance or holding current of the pyramidal cell (cf. Poncer et al. 1995).
Figure 1.
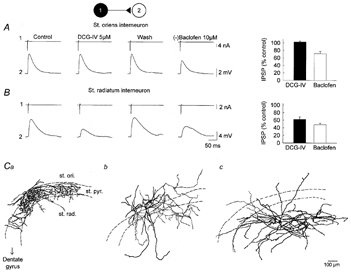
mGlu and GABAB receptor-mediated depression of GABA release at synapses from distinct interneurons
Unitary IPSPs recorded in CA3 pyramidal cells (2) in response to ‘action currents’ evoked in interneurons (1) located either in stratum oriens (A) or in stratum radiatum (B). Right, the mGluR2/3 agonist DCG-IV (5 μm) had no effect on the amplitude of IPSPs from a stratum oriens interneuron whereas the IPSP from a stratum radiatum interneuron was reversibly suppressed by ∼60%. IPSPs from both stratum oriens and stratum radiatum interneurons were reduced by ∼33 and 45%, respectively, by the GABAB receptor agonist (-)baclofen (10 μm). Average and s.e.m. of 20–30 consecutive IPSPs. Left, average effect of DCG-IV and (-)baclofen (n = 5–14 pairs). C, camera lucida reconstruction of the interneurons recorded in stratum oriens (st. ori.) and radiatum (st. rad.). Stratum oriens interneurons were of two types, with an axonal arbor innervating either pyramidal cell somata (st. pyr., Ca) or proximal apical and basal dendrites (Cb). All interneurons recorded in stratum radiatum had similar morphology, with an axonal arbor extending largely within stratum radiatum and sometimes in stratum lacunosum moleculare (Cc).
In contrast to DCG-IV, subsequent application of the GABAB receptor agonist (-)baclofen (10 μm) depressed unitary IPSPs in all cell pairs (Fig. 1A and B). Unitary IPSP amplitude was reduced by 28.5 ± 6.1% in eight pairs with the interneuron located in stratum oriens, and 52.0 ± 3.9% in five pairs with stratum radiatum interneurons (ANOVA, P < 0.02). This effect was reversible (not shown) and sometimes accompanied by a hyperpolarization of the pyramidal cell by a few millivolts that was compensated by DC current injection, with little change in input resistance (−7.2 ± 6.0%, n = 14).
We conclude that, whereas presynaptic GABAB receptors are present and functional at both classes of inhibitory synapse, group II mGluRs may be restricted to a sub-population of synapses formed by some interneurons located in the stratum radiatum.
In order to identify the types of interneuron forming the two groups of synapses, cells were filled with 1% biocytin and subsequently reconstructed. Stratum radiatum interneurons making DCG-IV-sensitive synapses (n = 7, Fig. 1Cc) formed a rather homogeneous group with a radial dendritic arbor and a thin axon spreading mostly within stratum radiatum. Interneurons in stratum oriens making DCG-IV-insensitive synapses fell into two morphologically distinct groups (Fig. 1Ca and Cb). Some showed a bipolar dendritic arbor parallel to stratum pyramidale with a profuse axonal arbor extending within stratum pyramidale and proximal stratum oriens (n = 4). Two other interneurons had a dendritic arbor that resembled that of a pyramidal cell, extending in stratum oriens and up to stratum lacunosum moleculare, but a more widespread axon, ramifying within stratum oriens, pyramidale and lucidum. In addition, stratum oriens interneurons showed ∼63% larger axonal varicosities than stratum radiatum interneurons (1.56 ± 0.02 and 0.96 ± 0.02 μm, respectively, P < 0.001; n = 4 and 7 cells, 546 and 928 boutons, respectively).
We previously showed that GABA release at synapses from stratum oriens or stratum radiatum interneurons was mediated by P- and N-type Ca2+ channels, respectively (Poncer et al. 1997). We asked whether expression of presynaptic group II mGluRs was correlated with a particular presynaptic calcium channel subtype. In six cell pairs with stratum oriens interneurons, DCG-IV (5 μm) had no effect on the amplitude of unitary IPSPs (Fig. 2A; 0.5 ± 2.6%, P = 0.5) and the N-type calcium channel antagonist ω-conotoxin MVIIA (1 μm) had no significant effect on synaptic transmission (−4.4 ± 2.9%, P = 0.4). However, transmission was suppressed by 97.9 ± 1.6% (n = 4) upon subsequent application of the P/Q-type antagonist ω-agatoxin IVA (200 nm). In four experiments where ω-agatoxin IVA was applied alone after washout of DCG-IV, unitary IPSPs were reduced to 1.7 ± 0.6% of their control amplitude. In contrast, in four cell pairs with stratum radiatum interneurons, DCG-IV reversibly reduced the amplitude of unitary IPSPs by 51.5 ± 9.4% (Fig. 2B) and subsequent application of ω-conotoxin MVIIA suppressed transmission by 97.9 ± 1.4%. Expression of N-type calcium channels and of group II presynaptic mGluRs were therefore correlated in all cell pairs (n = 14).
Figure 2.

DCG-IV suppresses GABA release at inhibitory synapses expressing N-type calcium channels
In a stratum oriens interneuron and pyramidal cell pair (A), DCG-IV (5 μm) produced no change in IPSP amplitude. IPSPs were also unaffected by the N-type calcium channel antagonist ω-conotoxin MVIIA (CmTx, 1 μm), but were blocked by the P/Q-type calcium channel antagonist ω-agatoxin IVA (Aga IV, 200 nm). The IPSP generated in another pyramidal cell by a stratum radiatum interneuron (B) was reduced by ∼45% upon DCG-IV application. Subsequent application of ω-conotoxin MVIIA (1 μm) abolished synaptic transmission. Aa and Ba, average of 20–30 consecutive IPSPs. Ab and Bb, each point represents the mean amplitude of 3 consecutive IPSPs normalized to control.
Temporal dynamics of GABA release at unitary synapses
GABA release may be affected not only by neuromodulators acting on presynaptic receptors but also as a result of prior activity. Extracellularly evoked IPSPs have often been shown to exhibit short-term depression (e.g. Davies & Collingridge, 1993). However, synapse-specific dynamics may be overlooked when fibres of unknown origin are simultaneously stimulated.
Activity-dependent, short-term plasticity at synapses formed by a presynaptic cortical neuron depends on the identity of its postsynaptic target (e.g. Reyes et al. 1998; Scanziani et al. 1998). We asked whether unitary IPSPs elicited by an interneuron in several pyramidal cells showed distinct or similar patterns of short-term plasticity. Pairs of stimuli separated by 50 ms were delivered to an interneuron and IPSPs were recorded sequentially in three pyramidal cells (Fig. 3A). Although the IPSP varied in amplitude and time course among the three cells, the paired-pulse ratio (PPR) of the response remained constant within 5%.
Figure 3.
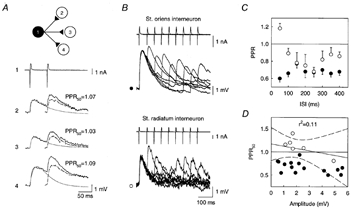
Transmission dynamics at synapses from distinct CA3 interneurons
A, synaptic responses of 3 postsynaptic pyramidal cells to paired stimuli delivered to a stratum radiatum interneuron. The mean amplitude of the first response varied from 1.6 mV (cell 3) to 2.5 mV (cell 4) but PPF was similar in all postsynaptic cells. Dashed trace, second IPSP isolated after digital subtraction of the first IPSP fitted with two exponentials (smooth trace). B, pairs of stimuli at intervals of 50–400 ms delivered to either stratum oriens or stratum radiatum interneurons. A and B show the mean of 25–30 consecutive responses. C, IPSPs induced by stratum oriens interneurons (•) showed marked PPD at all interstimulus intervals (ISIs). IPSPs from stratum radiatum interneurons (○) showed PPF at short intervals (< 100 ms) and PPD at longer intervals. Each point represents the mean and s.e.m. from 8 (stratum oriens) and 5 (stratum radiatum) pairs. D, PPRs were not correlated with the amplitude of the first IPSP in 21 cell pairs. Continuous line, linear regression. Dashed lines, 95% confidence intervals.
PPRs of IPSPs from distinct interneurons were then examined for interstimulus intervals ranging from 50 to 400 ms. In eight cell pairs with stratum oriens interneurons, PPD was observed for all interstimulus intervals tested (Fig. 3B and C). Depression was maximal at the shortest interval (−39.3 ± 3.6% at 50 ms). At intervals above 4 s, the amplitude of the second IPSP was not significantly different from that of the first (101.7 ± 2.1%, P = 0.5). In contrast, in five cell pairs with stratum radiatum interneurons, PPF was observed at an interstimulus interval of 50 ms (18.5 ± 0.6%), whereas PPD was apparent at longer intervals (Fig. 3B and C). Depression was maximal at 250 ms (−31.5 ± 4.9%). Again, the amplitude of the second IPSP was not different from control at interstimulus intervals > 4 s (103.4 ± 1.4%, P = 0.5).
When two IPSPs occur in short succession, the decrease in membrane resistance associated with the first may reduce the amplitude of the second. This shunt is likely to depend on the amplitude of the first IPSP. However, in 22 cell pairs with either stratum oriens (n = 15) or stratum radiatum (n = 7) interneurons, no significant correlation was found between the PPR at an interstimulus interval of 50 ms (PPR50) and the IPSP amplitude (Fig. 3D, P = 0.5). The difference in PPRs at short intervals between the two groups of interneurons therefore cannot be accounted for by a shunt of the postsynaptic membrane resistance.
One of the most commonly invoked mechanisms to account for PPD at GABAergic synapses is activation of presynaptic GABAB autoreceptors (e.g. Davies & Collingridge, 1993; Lambert & Wilson, 1994). However, several examples of GABAB-independent PPD have been reported, usually in conditions where fewer synapses were activated (e.g. Wilcox & Dichter, 1994). We therefore compared the effects of the potent GABAB receptor antagonist CGP52432 (Pozza et al. 1999) on the temporal dynamics of unitary and extracellularly evoked IPSPs in CA3 pyramidal cells. In paired recordings with the interneuron located in either stratum oriens (n = 4) or stratum radiatum (n = 5), no significant change in the amplitude of the first IPSP was detected upon application of 2 μm CGP52432 (99.2 ± 0.9% of control amplitude, P = 0.5; Fig. 4A). PPRs at interstimulus intervals of either 50 or 400 ms were also unaffected by CGP52432 (PPR50 and PPR400 were 100.4 ± 2.6 and 104.2 ± 14.1 % of control, respectively), independent of the location of the presynaptic interneuron, and data were therefore pooled (Fig. 4Ab). These experiments were repeated with extracellularly evoked IPSPs of similar amplitude (Fig. 4B). In such conditions, IPSPs were also unaffected in amplitude by CGP52432. However, the PPR was significantly increased by CGP52432 at both 50 and 400 ms interstimulus intervals (122.7 ± 9.0 and 129.2 ± 8.2% of control, respectively), suggesting that presynaptic GABAB receptor activation is not induced by GABA release at terminals from a single axon but requires simultaneous release from several inhibitory afferents.
Figure 4.
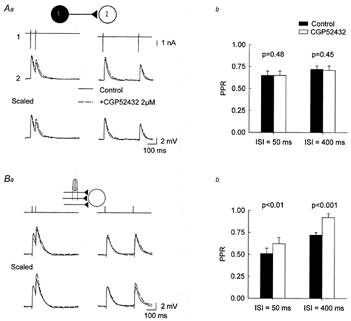
GABAB autoreceptors do not contribute to PPD of unitary IPSPs
Aa, pairs of IPSPs generated by a stratum oriens interneuron in a postsynaptic pyramidal cell. The selective GABAB receptor antagonist CGP52432 (2 μm, dashed trace) had no significant effect on either the amplitude or the PPD of the IPSP. Average of 30 consecutive responses. Ab, summary data from 9 independent cell pairs with the interneuron located in either stratum oriens or stratum radiatum. Ba, similar experiment using extracellular synaptic stimulation. The intensity of stimulation was set so that IPSP amplitude was similar to that in the above experiments. CGP52432 did not affect the amplitude of evoked IPSPs but significantly reduced PPD at both 50 and 400 ms interstimulus intervals. Bb, summary data from 10 independent experiments.
DISCUSSION
The analysis of several presynaptic properties in 33 cell pairs revealed two classes of inhibitory synapses impinging on CA3 pyramidal cells. Synapses formed by some stratum radiatum interneurons were depressed by GABAB and group II mGlu receptor agonists, expressed N-type calcium channels and showed synaptic facilitation at short intervals and depression at longer intervals. Synapses formed by two morphologically distinct populations of stratum oriens interneurons were depressed to a lesser extent by GABAB receptor agonists and were unaffected by group II mGluR agonists. They expressed P-type calcium channels and showed synaptic depression at all interstimulus intervals between 50 and 400 ms. In addition, our results show that transmission dynamics at synapses from a single interneuron were independent of GABAB autoreceptor activation.
In the neocortex, where the anatomy and physiology of inhibitory cells are highly heterogeneous, a taxonomy based purely on transmission dynamics reduced the number of subtypes of GABAergic synapses to three (Gupta et al. 2000). Similarly, our results show that some inhibitory synapses made onto CA3 pyramidal cells may fall into only two groups based on four modulatory and dynamic properties. However, only presynaptic cells that could easily be identified as interneurons were examined in this study. Interneurons with their somata located within the pyramidal cell layer or cells of very small size were excluded as well as cells that failed to produce a detectable IPSP (< 250 μV) in neighbouring pyramidal cells. Several morphological subtypes described in other studies (e.g. Gulyas et al. 1993; Maccaferri et al. 2000) were not examined in our experiments, such as O-LM interneurons projecting specifically to stratum lacunosum moleculare. Functional properties of synapses made by these interneurons therefore remain to be characterized.
Four presynaptic properties were compared and appeared to be strongly correlated: the sensitivity of GABA release to agonists of GABAB and group II mGlu receptors, the pharmacology of presynaptic calcium influx and PPF or PPD. As described previously, unitary IPSPs were entirely suppressed by blocking either N- or P-type calcium channels (Poncer et al. 1997), suggesting that the release of GABA, unlike that of glutamate, is mediated by only one type of presynaptic channel. Expression of one or the other subtype may affect the function and modulation of inhibitory synapses. We suggest that coexpression of specific presynaptic calcium channels and receptors for neurotransmitters is not coincidental but rather reflects a functional interdependence. We found that only synapses expressing N-type presynaptic calcium channels were affected by group II mGluR agonists. Activation of group II mGluRs was shown to suppress transmitter release by a specific G-protein-mediated inhibition of N-type calcium channels at the retinotectal synapse of the goldfish (Zhang & Schmidt, 1999), whereas P-type presynaptic channels at the calyx of Held are inhibited by group III mGluRs (Takahashi et al. 1996). In contrast, GABAB receptor activation inhibits both channel types, although N-type channels are blocked to a larger extent (Mintz & Bean, 1993; Doze et al. 1995). Accordingly, we observed that suppression of GABA release by (-)baclofen was more pronounced at synapses expressing N- rather than P-type channels.
Transmission dynamics at synapses formed by a given interneuron were very similar in different postsynaptic pyramidal cells (Fig. 3A), as described in neocortex (Gupta et al. 2000), allowing paired-pulse profiles at synapses from distinct interneurons to be compared. IPSPs induced by stratum radiatum interneurons showed facilitation at intervals < 100 ms whereas IPSPs from stratum oriens interneurons showed depression at all intervals. The lack of effect of CGP52432 on PPD of unitary IPSPs indicates that GABAB autoreceptors were not activated during paired stimuli in our recording conditions. However, CGP52432 reduced PPD of extracellularly evoked IPSPs of similar amplitude. Such extracellular stimulation is likely to activate more axons than those terminating onto the recorded pyramidal cell. Synchronous GABA release from multiple afferents therefore appears to be required to activate presynaptic, as well as postsynaptic (Isaacson et al. 1993; Scanziani, 2000), GABAB receptors. Differences in paired-pulse profiles may thus reflect differential inactivation of GABAA receptors (Pearce et al. 1995), release probability (Wilcox & Dichter, 1994), presynaptic calcium channels (e.g. Brody & Yue, 2000) or presynaptic expression of calcium-binding proteins (Chard et al. 1995).
Although the physiological conditions for activation of presynaptic receptors for glutamate and GABA remain to be established, differential expression of these receptors may permit a selective tuning of distinct sources of inhibition by hippocampal activity. This selectivity may be further enhanced by the diversity of firing patterns of hippocampal interneurons (Parra et al. 1998) as well as by the dynamic properties of GABA release at distinct inhibitory synapses.
Acknowledgments
We thank L. Heeb and L. Rietschin for expert technical assistance, Natasa Savic for performing some of the experiments of this work and Dr Richard Miles for critical reading of the manuscript. This work was supported by the Swiss National Science Foundation (3100-41829.94; 7UNPJ48530), Dr Eric Slack-Gyr Foundation and a Marie Curie fellowship from the European Commission (J.C.P.).
References
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annual Review of Neuroscience. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- Brody DL, Yue DT. Relief of G-protein inhibition of calcium channels and short-term synaptic facilitation in cultured hippocampal neurones. Journal of Neuroscience. 2000;20:889–898. doi: 10.1523/JNEUROSCI.20-03-00889.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard PS, Jordan J, Marcuccilli CJ, Miller RJ, Leiden JM, Roos RP, Ghadge GD. Regulation of excitatory transmission at hippocampal synapses by calbindin D28k. Proceedings of the National Academy of Sciences of the USA. 1995;92:5144–5148. doi: 10.1073/pnas.92.11.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Collingridge GL. The physiological regulation of synaptic inhibition by GABAB autoreceptors in rat hippocampus. Journal of Physiology. 1993;472:245–265. doi: 10.1113/jphysiol.1993.sp019945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doze VA, Cohen GA, Madison DV. Calcium channel involvement in GABAB receptor-mediated inhibition of GABA release in area CA1 of the rat hippocampus. Journal of Neurophysiology. 1995;74:43–53. doi: 10.1152/jn.1995.74.1.43. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsçki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Thompson SM, McKinney RA, Debanne D, Robertson RT. Organotypic slice cultures of neural tissue. In: Banker G, Goslin K, editors. Culturing Nerve Cells. 2. Cambridge, MA, USA: MIT Press; 1998. pp. 461–498. chap. III. [Google Scholar]
- Gulyas AI, Miles R, Hajos N, Freund TF. Precision and variability in postsynaptic target selection of inhibitory cells in the hippocampal CA3 region. European Journal of Neuroscience. 1993;5:1729–1751. doi: 10.1111/j.1460-9568.1993.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- Halasy K, Buhl EH, Lorinczi Z, Tamas G, Somogyi P. Synaptic target selectivity and input of GABAergic basket and bistratified interneurons in the CA1 area of the rat hippocampus. Hippocampus. 1996;6:306–329. doi: 10.1002/(SICI)1098-1063(1996)6:3<306::AID-HIPO8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Lambert NA, Wilson WA. Heterogeneity in presynaptic regulation of GABA release from hippocampal inhibitory neurons. Neuron. 1993;11:1057–1067. doi: 10.1016/0896-6273(93)90219-h. [DOI] [PubMed] [Google Scholar]
- Lambert NA, Wilson WA. Temporally distinct mechanisms of use-dependent depression at inhibitory synapses in the rat hippocampus in vitro. Journal of Neurophysiology. 1994;72:121–130. doi: 10.1152/jn.1994.72.1.121. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Roberts JDB, Szucs P, Cottingham CA, Somogyi P. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. Journal of Physiology. 2000;524:91–116. doi: 10.1111/j.1469-7793.2000.t01-3-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Bean BP. GABAB receptor inhibition of P-type Ca2+ channels in central neurons. Neuron. 1993;10:889–898. doi: 10.1016/0896-6273(93)90204-5. [DOI] [PubMed] [Google Scholar]
- Parra P, Gulyas AI, Miles R. How many subtypes of inhibitory cells in the hippocampus? Neuron. 1998;20:983–993. doi: 10.1016/s0896-6273(00)80479-1. [DOI] [PubMed] [Google Scholar]
- Pearce RA, Grunder SD, Faucher LD. Different mechanisms for use-dependent depression of two GABAA-mediated IPSCs in rat hippocampus. Journal of Physiology. 1995;484:425–435. doi: 10.1113/jphysiol.1995.sp020675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Lewin GR. An ultrastructural size principle. Neuroscience. 1994;58:441–446. doi: 10.1016/0306-4522(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Poncer JC, McKinney RA, Gähwiler BH, Thompson SM. Either N- or P-type calcium channels mediate GABA release at distinct hippocampal inhibitory synapses. Neuron. 1997;18:463–472. doi: 10.1016/s0896-6273(00)81246-5. [DOI] [PubMed] [Google Scholar]
- Poncer JC, Shinozaki H, Miles R. Dual modulation of synaptic inhibition by distinct metabotropic glutamate receptors in the rat hippocampus. Journal of Physiology. 1995;485:121–134. doi: 10.1113/jphysiol.1995.sp020717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozza MF, Manuel NA, Steinmann M, Froestl W, Davies CH. Comparison of antagonist potencies at pre- and post-synaptic GABAB receptors at inhibitory synapses in the CA1 region of the rat hippocampus. British Journal of Pharmacology. 1999;127:211–219. doi: 10.1038/sj.bjp.0702498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nature Neuroscience. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Scanziani M. GABA spillover activates postsynaptic GABAB receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Gähwiler BH, Charpak S. Target cell-specific modulation of transmitter release at terminals from a single axon. Proceedings of the National Academy of Sciences of the USA. 1998;95:12004–12009. doi: 10.1073/pnas.95.20.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Forsythe ID, Tsujimoto T, Barnes-Davies M, Onodera K. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science. 1996;274:594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Gähwiler BH. Activity-dependent disinhibition. III. Desensitization and GABAB receptor-mediated presynaptic inhibition in the hippocampus in vitro. Journal of Neurophysiology. 1989;61:524–533. doi: 10.1152/jn.1989.61.3.524. [DOI] [PubMed] [Google Scholar]
- Wilcox KS, Dichter MA. Paired pulse depression in cultured hippocampal neurons is due to a presynaptic mechanism independent of GABAB autoreceptor activation. Journal of Neuroscience. 1994;14:1775–1788. doi: 10.1523/JNEUROSCI.14-03-01775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–988. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- Zhang C, Schmidt JT. Adenosine A1 and class II metabotropic glutamate receptors mediate shared presynaptic inhibition of retinotectal transmission. Journal of Neurophysiology. 1999;82:2947–2955. doi: 10.1152/jn.1999.82.6.2947. [DOI] [PubMed] [Google Scholar]


