Abstract
Permeabilities of the luminal and basolateral membranes of pancreatic duct cells to CO2 and HCO3− were examined in interlobular duct segments isolated from guinea-pig pancreas. Intracellular pH (pHi) was measured by microfluorometry in unstimulated, microperfused ducts loaded with the pH-sensitive fluoroprobe 2′7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF).
When HCO3−/CO2 was admitted to the bath, pHi decreased transiently as a result of CO2 diffusion and then increased to a higher value as a result of HCO3− uptake across the basolateral membrane by Na+-HCO3− cotransport.
When HCO3−/CO2 was admitted to the lumen, pHi again decreased but no subsequent increase was observed, indicating that the luminal membrane was permeable to CO2 but did not allow HCO3− entry to the cells from the lumen. Only when the luminal HCO3− concentration was raised above 125 mm was HCO3− entry detected. The same was true of duct cells stimulated with forskolin.
Recovery of pHi from an acid load, induced by exposure to an NH4+ pulse, was dependent on basolateral but not luminal Na+ and could be blocked by basolateral application of methylisobutylamiloride and H2DIDS. This indicates that the Na+-H+ exchangers and Na+-HCO3− cotransporters are located exclusively at the basolateral membrane.
In the presence of HCO3−/CO2, substitution of basolateral Cl− with glucuronate caused larger increases in pHi than substitution of luminal Cl−. This suggests that the anion exchanger activity in the basolateral membrane is greater than that in the luminal membrane.
We conclude that the luminal and basolateral membranes are both freely permeable to CO2, but while the basolateral membrane has both uptake and efflux pathways for HCO3−, the luminal membrane presents a significant barrier to the re-entry of secreted HCO3−, largely through the inhibition of the luminal anion exchanger by high luminal HCO3− concentrations.
The ductal system of the exocrine pancreas produces a bicarbonate-rich fluid secretion in response to secretin and some other stimuli. However, there are significant species-dependent variations in the pattern of pancreatic HCO3− secretion in vivo. In the guinea-pig (Padfield et al. 1989) and in several other species, including dog, cat and human (Case & Argent, 1993), the HCO3− concentration of the juice may reach 140–150 mm during maximal stimulation with secretin. In the rat, however, the highest HCO3− concentration is about 70 mm (Sewell & Young, 1975). We have recently reported that interlobular duct segments isolated from guinea-pig pancreas secrete a HCO3−-rich fluid (> 130 mm) during stimulation with 10 nm secretin (Ishiguro et al. 1998), which indicates that the in vivo behaviour of pancreatic ducts is well preserved in this in vitro preparation.
In order to maintain such a high concentration of HCO3− in pancreatic juice during sustained secretion, pancreatic duct cells must possess the following characteristics. (1) HCO3− must be actively accumulated in the cell by transport across the basolateral membrane. We have previously shown that Na+-HCO3− cotransport plays a dominant role in this process (Ishiguro et al. 1996a). (2) The luminal membrane must possess a mechanism which specifically transports HCO3− into the lumen. In a previous paper (Ishiguro et al. 1996b), we demonstrated a secretin-stimulated HCO3− efflux pathway in the luminal membrane that has yet to be identified. (3) The luminal membrane must not contain transporters that would allow HCO3− secreted into the lumen to re-enter the cell.
To compare the passive permeability characteristics of the basolateral and luminal membranes of guinea-pig pancreatic duct cells to extracellular CO2 and HCO3−, we have examined the changes in intracellular pH that occur in unstimulated, microperfused, interlobular duct segments when either the basolateral or the luminal membrane is exposed to changes in HCO3− or CO2 concentration.
METHODS
Isolation and culture of interlobular ducts
Female Hartley guinea-pigs (350–450 g) were killed by cervical dislocation, in accordance with national guidelines. As described previously (Ishiguro et al. 1996a), the pancreas was removed and interlobular ducts (diameter 100–150 μm, length 800–1200 μm) were isolated and cultured overnight, during which time the ends of the duct segments sealed spontaneously.
Solutions
The standard Hepes-buffered solution contained (mm): 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 D-glucose and 10 Hepes; and was equilibrated with 100 % O2. The Na+-free Hepes-buffered solution contained N-methyl-D-glucamine (NMDG+) in place of Na+. The standard HCO3−-buffered solution contained (mm): 115 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 D-glucose and 25 NaHCO3; and was equilibrated with 95 % O2-5 % CO2. The 25 mm HCO3−-0 % CO2 solution was gassed with 100 % O2. High-HCO3− solutions contained 125 or 145 mm NaHCO3 and the concentration of NaCl was reduced accordingly to maintain osmolarity. The Cl− concentrations of these solutions were 24 and 4 mm, respectively, compared with 124 mm in the standard HCO3−-buffered solution. The Na+-free HCO3−-buffered solution contained NMDG-Cl in place of NaCl, choline bicarbonate in place of NaHCO3 and 10 μm atropine to avoid any muscarinic effects resulting from the high concentration of choline. Cl−-free solutions were made by replacing Cl− with glucuronate. In solutions containing NH4+, the concentration of Na+ was reduced to maintain osmolarity. All solutions, except for the high-HCO3− solutions and the 25 mm HCO3−-0 % CO2 solution, were adjusted to pH 7.4 at 37°C. The pH values of the high-HCO3− solutions equilibrated with 5 % CO2 were in the range 8.2–8.3.
Microperfusion of the isolated ducts
The lumen of the interlobular duct segment was microperfused as described previously (Ishiguro et al. 1999). Both ends of the duct were cut open using sharpened needles and one end was cannulated with concentric holding and perfusion pipettes. The duct lumen was perfused at a rate of approximately 10–20 μl min−1 while the bath was maintained at 37°C and continuously perfused at 3 ml min−1 in the same direction as the flow of luminal perfusate.
Measurement of intracellular pH (pHi)
Intracellular pH in the duct cells was estimated by microfluorometry as described previously (Ishiguro et al. 1996a) using the pH-sensitive fluoroprobe BCECF. After cannulating the duct for luminal perfusion, the duct cells were loaded with BCECF for 10 min by adding the acetoxymethyl ester BCECF-AM (2 μm) to the bathing solution. Perfusion of both bath and lumen was stopped during the 10 min loading period. Experiments were begun after the duct had been perfused with the control solution for 15 min. Small regions of the duct epithelium (10–20 cells) were illuminated alternately at excitation wavelengths of 430 and 480 nm. Values of pHi were calculated from the fluorescence ratio (F480/F430) measured at 530 nm. The system was calibrated using the high-K+-nigericin technique (Thomas et al. 1979).
Materials
BCECF-AM and dihydro-4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (H2DIDS) were obtained from Molecular Probes; bafilomycin A1, forskolin and atropine sulphate from Sigma; and N-methyl-N-isobutylamiloride (MIA) from Research Biochemicals International.
Statistics
Data are presented as the means ±s.e.m. unless otherwise indicated. Tests for statistically significant differences were made with Student's t test for paired or unpaired data.
RESULTS
Effects of CO2 and HCO3− on intracellular pH in unstimulated ducts
To investigate the CO2 permeability and HCO3− transport characteristics of the luminal and basolateral membranes of unstimulated pancreatic duct cells, changes in pHi were observed following the addition of HCO3−/CO2 first to the luminal perfusate and then to the bath (Fig. 1A). Initially both the duct lumen and the bath were perfused with a HCO3−-free, Hepes-buffered solution. When the luminal perfusate was switched to a HCO3−-buffered solution containing 25 mm HCO3−-5 % CO2, pHi decreased from 7.25 ± 0.06 (n= 5) to 7.04 ± 0.08 over a period of 30 s. The cytosol remained acidified during the following 4 min period of luminal perfusion with the HCO3−-buffered solution, but when HCO3−/CO2 was removed pHi quickly returned to its control value.
Figure 1. Effects of basolateral or luminal HCO3−/CO2 on intracellular pH.
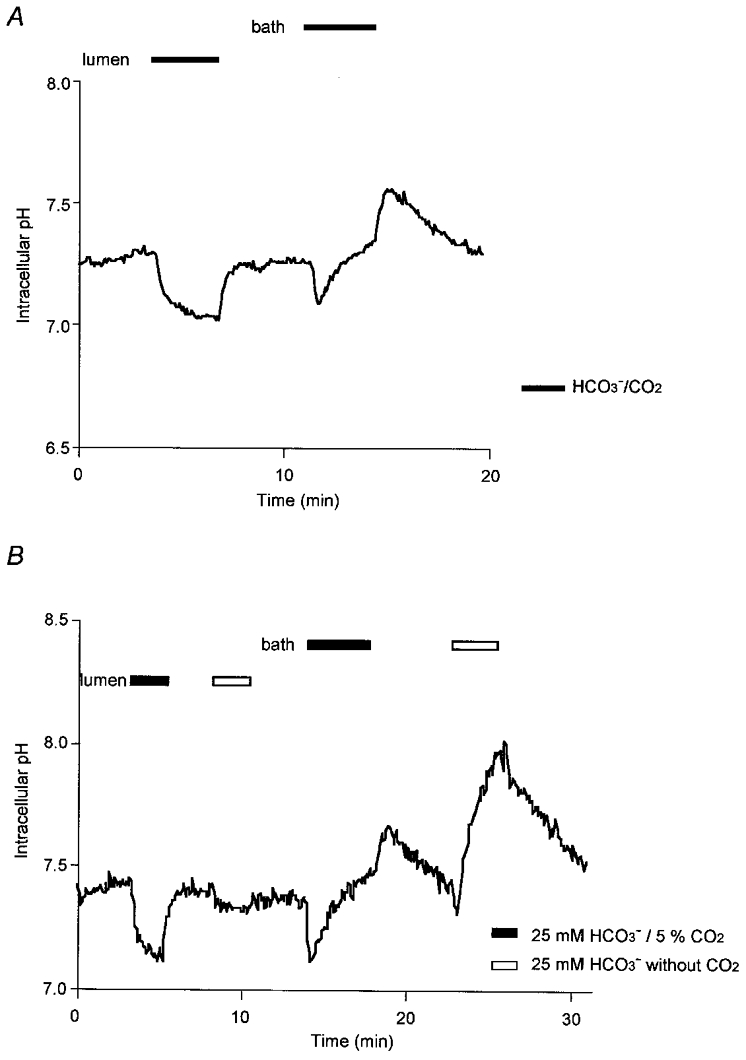
Initially the bath and lumen of guinea-pig pancreatic duct segments were separately perfused with the standard HCO3−/CO2-free Hepes-buffered solution. Thereafter, in A(representative of 5 experiments), first the luminal perfusate and then the bath solution was switched to a HCO3−-buffered solution containing 25 mm HCO3−-5 % CO2 (filled bar). In B (representative of 4 experiments), the luminal and bath solutions were switched first to 25 mm HCO3−-5 % CO2 (filled bar) and then to 25 mm HCO3−-0 % CO2 (open bar).
The sustained acidification observed during exposure of the luminal membrane to HCO3−/CO2 can best be explained by the continuous diffusion of CO2 from the lumen into the cell. This will occur as a result of the steep PCO2 gradient that is maintained between the lumen and the bath − the PCO2 in the cells attaining a steady-state value somewhere between that of the lumen (equilibrated with 5 % CO2) and the bath (0 % CO2). The diffusion of CO2 into the cells across the luminal membrane will continue to acidify the cytosol so long as there is an efflux pathway for HCO3−, and this most probably occurs via the anion exchangers that are present in the luminal and basolateral membranes (see below). Therefore the steady-state pH that is achieved during this period will reflect a balance between the rate of acidification due to CO2 entry and HCO3− efflux, and the rate of compensatory H+ extrusion via the Na+-H+ exchanger in the basolateral membrane, which is stimulated by the lower pHi.
In the second part of the experiment, the basolateral membrane was exposed to HCO3−/CO2 by switching the bath perfusate to the HCO3−-buffered solution. Intracellular pH decreased transiently but then gradually increased to a value which was significantly higher than before HCO3−/CO2 exposure (7.42 ± 0.03 in 3 min, P < 0.05). When HCO3−/CO2 was removed from the perfusate, there was a further transient increase in pHi followed by a gradual return towards the resting value.
In this case, pHi recovered quickly from the acidifying effect of CO2 diffusion into the cells and increased to a higher value. This could be explained either by enhanced H+ extrusion across the basolateral membrane, which is unlikely given that it did not occur when CO2 entered across the luminal membrane, or by active HCO3− uptake into the cells, necessarily across the basolateral membrane. This would be consistent with our previous data (Ishiguro et al. 1996a, 1998), which established the presence of a Na+-HCO3− cotransporter in the basolateral membrane.
To distinguish between the separate effects of CO2 and HCO3− on pHi, a solution was prepared which contained 25 mm HCO3− but which was equilibrated with 100 % O2 (pH ∼8.2) and thus was nominally free of CO2. When this solution was applied to the lumen (first open bar, Fig. 1B), pHi decreased by only 0.06 ± 0.01 units (n= 4), equivalent to only 26 % of the acidification caused by the preceding application of both HCO3− and CO2 (first filled bar). This supports our hypothesis that the initial decrease in pHi on exposure to luminal HCO3−/CO2 is due to CO2 entry by diffusion. The small residual decrease could have been due to the presence of a small amount of CO2 derived from the HCO3− in the solution.
Application of the 25 mm HCO3−-0 % CO2 solution to the bath (second open bar) also caused a much reduced transient acidification, but this was followed by a dramatic increase in pHi to 7.94 ± 0.05 in 3 min. This supports our hypothesis that the secondary increase in pHi, on exposure of the basolateral membrane to HCO3−/CO2, is due to HCO3− entry, exaggerated in these conditions by the absence of the acidifying effect of CO2 diffusion into the cell.
Dependence of HCO3− influx across the basolateral membrane on Na+ and Cl−
The transport mechanisms responsible for the influx of HCO3− across the basolateral membrane were examined further in ion substitution experiments. When the bath perfusate was switched from the standard Hepes-buffered solution to a Na+-free HCO3−-buffered solution (Fig. 2A), pHi decreased rapidly but then remained acidified throughout the 3 min period of Na+-free perfusion. Restoration of basolateral Na+ caused pHi to increase, indicating that the rise in pHi previously observed during exposure to basolateral HCO3−/CO2 (Fig. 1A) was dependent on basolateral Na+.
Figure 2. Dependence of HCO3− influx across the basolateral membrane on Na+ or Cl−.
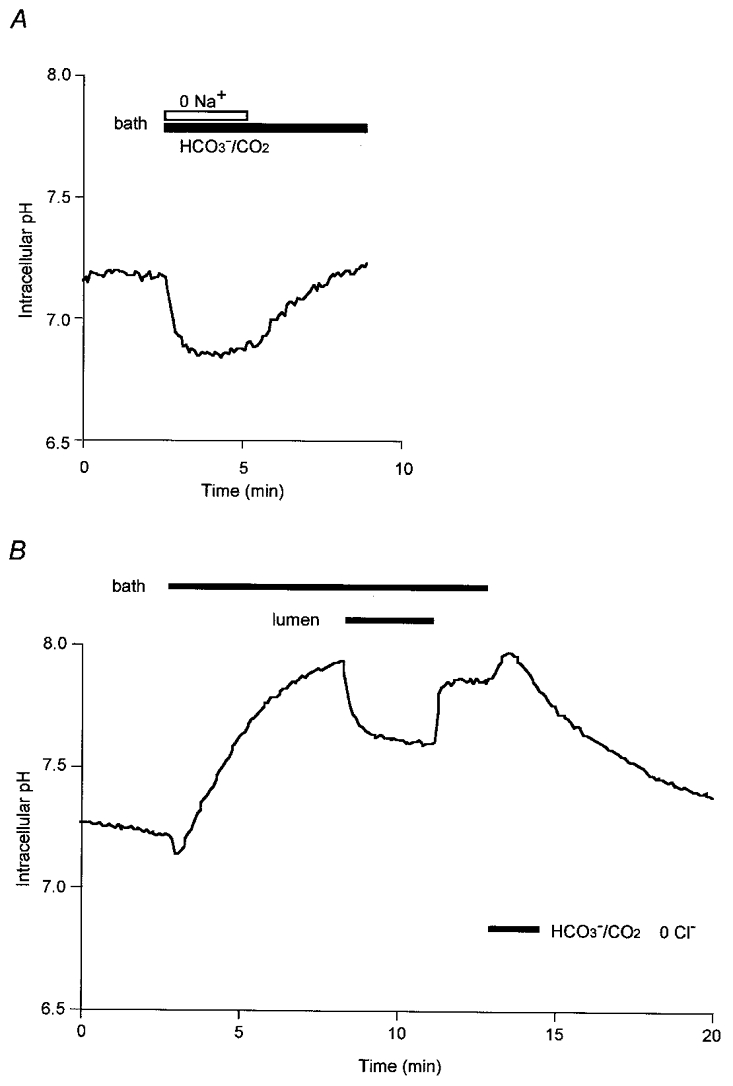
Initially the bath and lumen of the duct segments were separately perfused with the standard Hepes-buffered solution. Thereafter, in A (representative of 4 experiments), the bath solution was switched to a Na+-free HCO3−-buffered solution, while in B (also representative of 4 experiments), the bath solution and then the luminal perfusate were switched to a Cl−-free HCO3−-buffered solution.
When the bath superfusate was switched to a Cl−-free HCO3−-buffered solution (Fig. 2B), pHi transiently decreased and then increased to 7.93 ± 0.05 in 5 min (n= 4). This was a much higher value than that observed in the standard (Cl−-containing) HCO3−-buffered solution (Fig. 1A) and suggests that HCO3− influx via the basolateral membrane does not require the presence of basolateral Cl−. The fact that the rise in pHi was enhanced by the withdrawal of basolateral Cl− suggests that a basolateral Cl−-HCO3− exchanger (see below) may have contributed to the uptake of HCO3−. Subsequent application of the Cl−-free HCO3−-buffered solution to the lumen (Fig. 2B) caused a decrease in pHi to a new steady-state value. This would be expected since the rise in cytosolic PCO2 resulting from bilateral exposure to HCO3−/CO2 would inevitably shift pHi to a lower value.
Effects of higher concentrations of HCO3− on intracellular pH
To assess further the ability of the basolateral and luminal membranes to permit the entry of extracellular HCO3−, a larger transmembrane gradient of HCO3− was applied using a solution containing 125 mm HCO3−, 24 mm Cl−, 5 % CO2. In the experiment shown in Fig. 3A, the standard 25 mm HCO3− solution and the 125 mm HCO3− solution were each applied in turn, first to the luminal membrane and then to the basolateral membrane. Luminal application of the 125 mm HCO3− solution (first open bar) caused a rapid decrease in pHi of 0.20 ± 0.04 (n= 5) units, which was not significantly different from the decrease in pHi observed with the 25 mm HCO3− solution (first filled bar; 0.21 ± 0.03). No significant recovery of pHi was observed during luminal perfusion with the 125 mm HCO3− solution, indicating that little if any HCO3− entered the cell across the luminal membrane even with a large inward concentration gradient for HCO3−. In contrast, when the 125 mm HCO3− solution was applied to the basolateral membrane (Fig. 3A, second open bar), pHi increased rapidly to 8.09 ± 0.11. Although this change would have been partly due to HCO3− influx via the basolateral Cl−-HCO3− exchanger (driven by the reduction in basolateral Cl− concentration as in Fig. 2B), it indicates a marked difference in the capacity of the luminal and basolateral membranes to transport HCO3− into the cells. In particular, it suggests that the activity of the luminal anion exchanger may actually be suppressed under these conditions.
Figure 3. Effects of high extracellular HCO3− concentrations on intracellular pH.
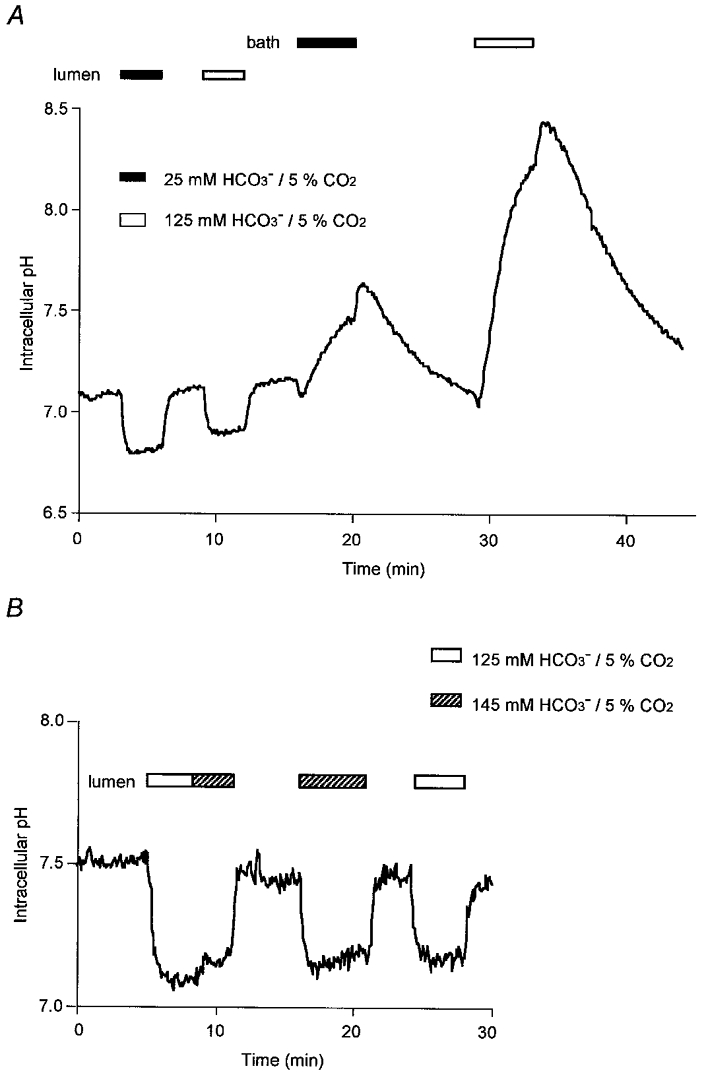
Initially the bath and lumen of the duct segments were separately perfused with the standard Hepes-buffered solution. Thereafter, in A (representative of 5 experiments), the luminal perfusate and then the bath solution was switched first to 25 mm HCO3−-5 % CO2 (filled bar) and then to 125 mm HCO3−-5 % CO2 (open bar). In B (representative of 5 experiments), the lumen was exposed either to 125 mm HCO3−-5 % CO2 (open bar) or to 145 mm HCO3−-5 % CO2 (hatched bar).
At an even higher luminal HCO3− concentration (145 mm) there began to be some evidence of HCO3− entry across the luminal membrane (Fig. 3B). Initially the 125 mm HCO3− solution was applied to the lumen for 3 min (first open bar), during which period no obvious pHi increase was observed (as previously in Fig. 3A). The luminal concentration of HCO3− was then raised to 145 mm (first hatched bar) and this caused a slight rise in pHi over a 3 min period. Direct application of the 145 mm HCO3− solution to the lumen (second hatched bar) had a similar effect: pHi decreased quickly and then increased slowly by 0.10 ± 0.01 units over 5 min (n= 5). Direct application of the 125 mm HCO3− solution, on the other hand, caused a sustained reduction in pHi (second open bar). Thus HCO3− entry across the luminal membrane appears only to occur at very high luminal HCO3− concentrations.
Since the activities of the luminal membrane transporters inevitably change during stimulated secretion, a similar experiment was performed before and during application of 1 μm forskolin, which is known to mimic the effect of secretin in this preparation (Ishiguro et al. 1996b) by elevating intracellular cyclic AMP (Fig. 4). As before, the lumen was exposed to progressively higher HCO3− concentrations at constant PCO2 while the bath remained HCO3− free. Application of forskolin itself had little effect on pHi as reported previously (Ishiguro et al. 1996b). The pattern of changes in pHi when the lumen was exposed to the different HCO3− concentrations in the presence of forskolin was essentially the same as in the unstimulated state. Thus, whatever changes in transport occur in the luminal membrane during stimulation, there is still little evidence of passive HCO3− re-entry into the cells despite the steep concentration gradient.
Figure 4. Effect of forskolin stimulation on pHi changes with high luminal HCO3− concentrations.
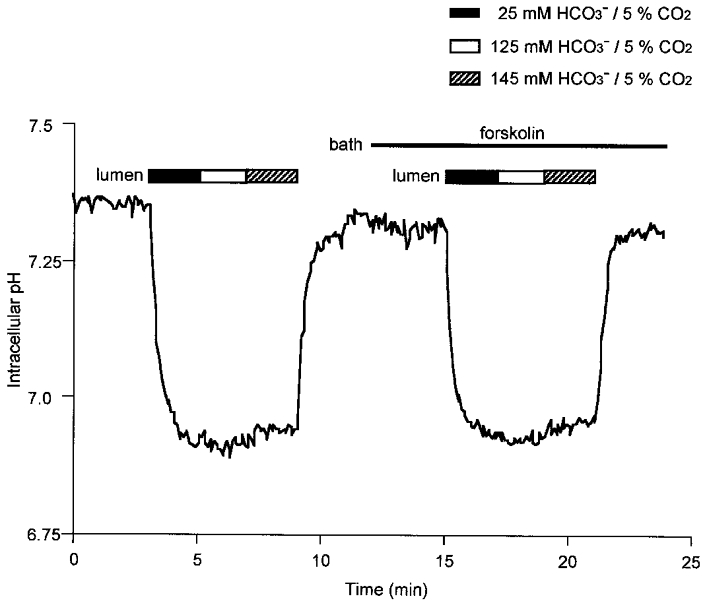
Initially the bath and lumen were separately perfused with the standard Hepes-buffered solution. Thereafter, the lumen was exposed to increasing concentrations of HCO3− equilibrated with 5 % CO2. This was then repeated during stimulation with 1 μm forskolin. Data are representative of 4 experiments.
Recovery of intracellular pH following an acid load
In order to localise the pH regulatory mechanisms responsible for HCO3− uptake and H+ extrusion in unstimulated ducts, we examined whether the recovery of pHi from an acid load was dependent on basolateral or luminal Na+. Acid loading was achieved by adding 20 mm NH4+ to the bath for 2 min, followed immediately by Na+ withdrawal from both the bath and lumen. We first examined the recovery of pHi in ducts in the absence of HCO3−/CO2 (Fig. 5A). While the bath and lumen were perfused with the Na+-free Hepes-buffered solution, there was no recovery of pHi. Restoration of luminal Na+ caused no change in pHi. However, restoration of basolateral Na+ caused pHi to return rapidly to the resting value. This indicates that a Na+-dependent, HCO3−-independent acid extruder, most probably an Na+-H+ exchanger, is located exclusively in the basolateral membrane.
Figure 5. Recovery of intracellular pH from an acid load.
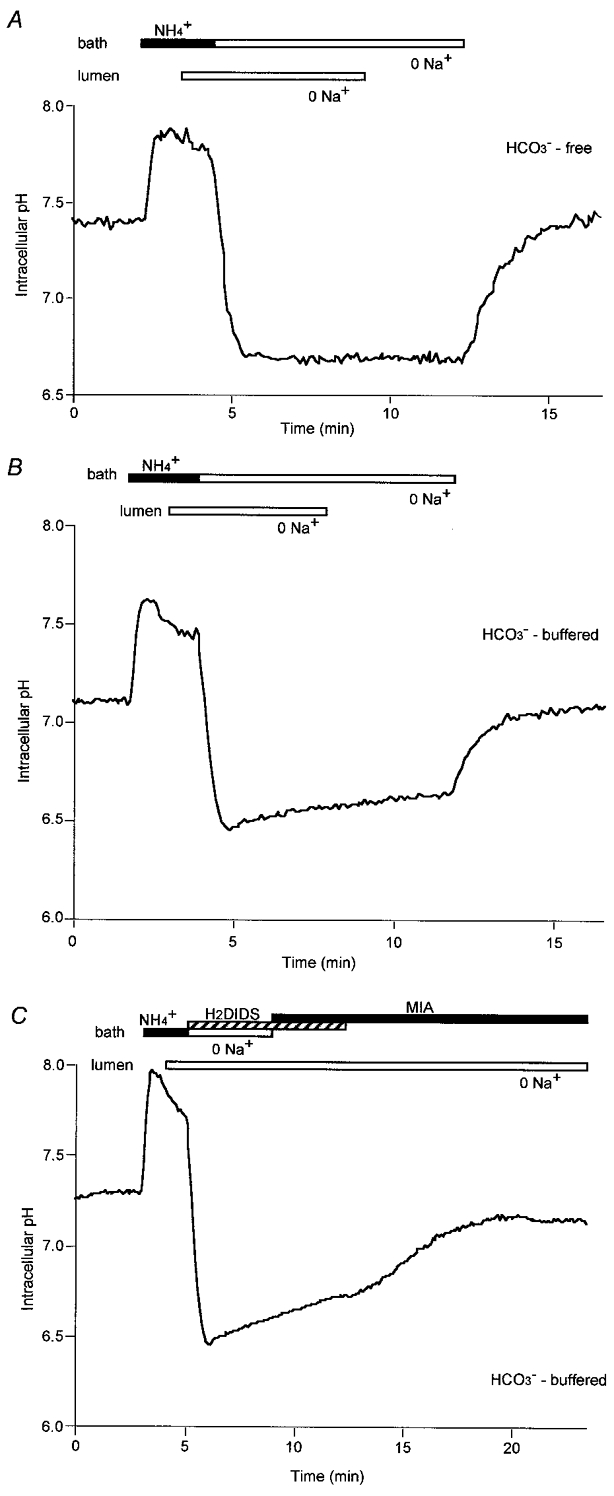
Duct cells were acid-loaded by adding 20 mm NH4+ to the bath for 2 min followed by Na+ removal (from the bath and lumen) in the absence (A) and presence (B and C) of HCO3−/CO2. In C,H2DIDS (0.5 mm) and MIA (10 μm) were added to the bath as indicated to block basolateral Na+-HCO3− cotransport and Na+-H+ exchange, respectively. Data are representative of at least 4 experiments.
The same experiment was repeated in the bilateral presence of HCO3−/CO2 and rather similar observations were made (Fig. 5B). However, in these conditions, a slow but significant recovery of pHi was observed in the absence of Na+ from both the lumen and the bath (0.13 ± 0.02 units in 4 min, n= 5). The mechanism responsible for this phenomenon is unclear since it was not inhibited by bafilomycin A1 (data not shown) and therefore cannot be attributed to the vesicular-type H+-ATPase previously reported in guinea-pig duct cells (De Ondarza & Hootman, 1997).
While restoration of Na+ to the lumen did not increase the rate of recovery above this slow basal level, restoration of basolateral Na+ caused pHi to return rapidly to its resting value. This suggests that not only the Na+-H+ exchanger but also the Na+-HCO3− cotransporter, which we have described previously (Ishiguro et al. 1996a), are located solely in the basolateral membrane.
To test this hypothesis, the effects of methylisobutylamiloride (MIA), an inhibitor of Na+-H+ exchange, and the disulphonic stilbene H2DIDS, an inhibitor of anion transport, were examined using a similar protocol (Fig. 5C). In this experiment, the increase in the rate of recovery of pHi when basolateral Na+ was restored after acidification (Fig. 5B) was blocked by the presence of 10 μm MIA and 0.5 mm H2DIDS in the bath. When H2DIDS, which acts as a reversible inhibitor of the basolateral Na+-HCO3− cotransporter, was withdrawn, there was a gradual recovery of pHi in the continued presence of MIA. This is consistent with there being a significant role for HCO3− uptake via the cotransporter in the regulation of pHi.
Effects of Cl− removal on intracellular pH in the presence of HCO3−
Studies on rat pancreatic ducts suggest that HCO3− secretion across the luminal membrane may involve the parallel operation of a Cl−-HCO3− exchanger and a cAMP-activated Cl− channel, the cystic fibrosis transmembrane conductance regulator (CFTR) (Gray et al. 1988, 1993; Novak & Greger, 1988). In our work, using guinea-pig pancreatic ducts, we have demonstrated that HCO3− transport across the luminal membrane is mediated by Cl−-HCO3− exchange under resting conditions but seems to involve an alternative mechanism during stimulation with secretin (Ishiguro et al. 1996b, 1998). To determine the localisation of Cl−-HCO3− exchangers in the guinea-pig ducts, we have investigated the effects on pHi of removing Cl− from the bath or lumen in the bilateral presence of HCO3−/CO2.
Removal of Cl− from the bath solution by replacement with glucuronate (Fig. 6A) caused a rapid increase in pHi of 0.24 ± 0.03 units (n= 5) over a 4 min period. Removal of Cl− from the luminal solution caused a slower and much smaller increase in pHi (0.07 ± 0.01 units). In Fig. 6B, the bath and lumen were initially both switched to the Cl−-free solution and then Cl− was restored first to the luminal solution and then to the bath. Restoring Cl− to the lumen caused pHi to decrease very slowly (by 0.06 ± 0.01 in 6 min, n= 4) whereas the subsequent restoration of Cl− to the bath caused pHi to decrease much more rapidly (by 0.28 ± 0.04 in 5 min) to the basal value. Taken together the data in Fig. 6 appear to suggest that the activity of the Cl−-HCO3− exchangers in the basolateral membrane of unstimulated pancreatic duct cells is considerably greater than that in the luminal membrane.
Figure 6. Effects of Cl− removal on intracellular pH in the presence of HCO3−.
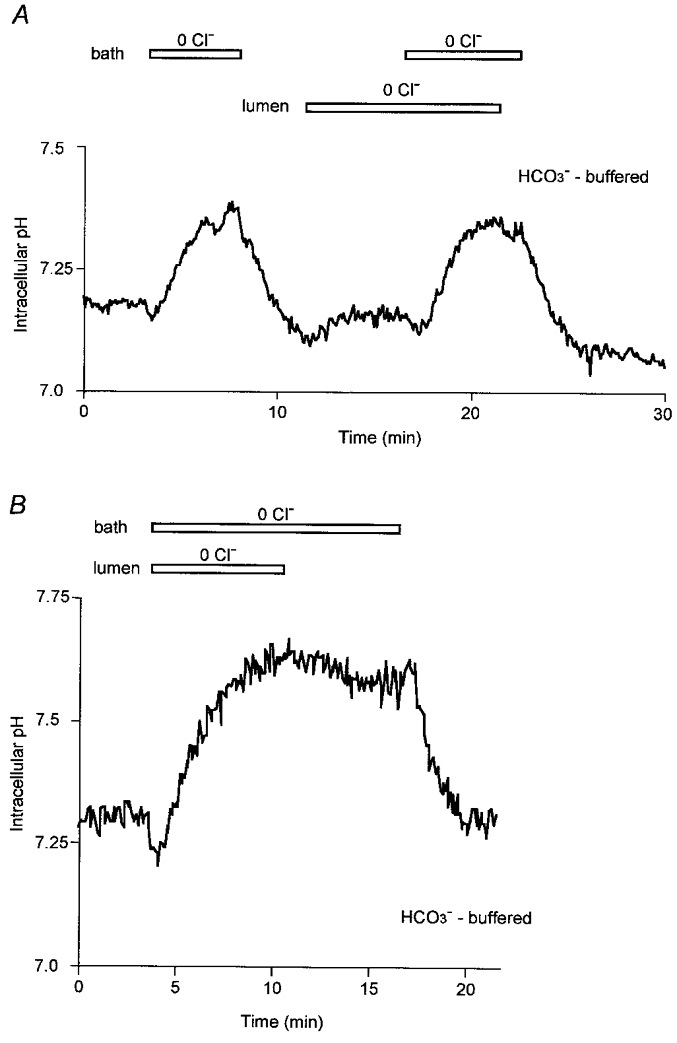
Initially the bath and lumen of the duct segments were separately perfused with the standard HCO3−-buffered solution. Thereafter, the luminal perfusate and/or the bath solution was switched to a Cl−-free, HCO3−-buffered solution (Cl− replaced with glucuronate) (open bar). Experiments in A and B are each representative of 5 experiments.
DISCUSSION
HCO3−/CO2 permeability of the basolateral and luminal membranes
Transport of HCO3− and Cl− across pancreatic duct epithelium has previously been examined by perfusing the main pancreatic duct of the cat in vivo (Case et al. 1969; Case & Scratcherd, 1970; Reber et al. 1986). These studies revealed that the main duct allowed very little exchange of Cl− for HCO3−, even at the slowest perfusion rates, and therefore must be relatively impermeable to HCO3−. Our present paper is the first to study the permeability of small, intra- and interlobular ducts where there is active HCO3− secretion (Case & Argent, 1993). By measuring pHi in microperfused, interlobular duct segments isolated from guinea-pig pancreas, we have demonstrated that (1) both basolateral and luminal membranes are permeable to extracellular CO2, (2) basolaterally applied HCO3− easily gains access to the cell, whereas (3) intraluminally applied HCO3− does not. Regardless of the mechanisms involved, this polarity is a basic property of the pancreatic duct epithelium which is necessary to achieve vectorial transport of HCO3− from blood to duct lumen.
Only a few previous reports have compared the permeability of the basolateral and luminal membranes of epithelial tissues to HCO3−/CO2. In perfused gastric glands (Waisbren et al. 1994), the luminal membranes of both parietal and chief cells were impermeable to HCO3− and to CO2 while the basolateral membranes were permeable to both. Not surprisingly the permeability characteristics of pancreatic duct cells are somewhat different.
Membrane localization of H+-HCO3− transporters
Our present data demonstrate the presence of an Na+-H+ exchanger, an Na+-HCO3− cotransporter and an unidentified Na+-independent acid-extrusion mechanism in the basolateral membrane. Anion exchangers are found in both basolateral and luminal membranes. These results are largely similar to those of a previous study of the rat pancreatic duct (Zhao et al. 1994). However, the observation that the Cl−-HCO3− exchanger activity in the basolateral membrane of unstimulated guinea-pig duct cells was greater than that in the luminal membrane was unexpected. We have previously demonstrated that the luminal exchanger plays a role in the spontaneous secretion of HCO3− and fluid in guinea-pig ducts (Ishiguro et al. 1998). This is achieved by the passive exchange of intracellular HCO3− with luminal Cl− via the anion exchanger in the luminal membrane, while Cl− ions recycle via a luminal Cl− conductance. A Cl−-HCO3− exchanger in the basolateral membrane would provide an alternative efflux pathway for intracellular HCO3− which would tend to dissipate the accumulation of HCO3− across the basolateral membrane whilst favouring the accumulation of Cl− instead. We would therefore predict that the fluid secreted by unstimulated ducts might be richer in Cl− than HCO3−.
HCO3− transport across the basolateral membrane
Two mechanisms have been postulated to account for HCO3− accumulation across the basolateral membrane: forward (blood to cell) transport of HCO3−, or backward (cell to blood) transport of H+ derived from carbonic acid (Case & Argent, 1993). The latter concept has been supported by several studies in perfused pancreas preparations, which have shown that a variety of buffer anions can substitute for HCO3−.
However, in this study, when solutions containing 25 or 125 mm HCO3− were applied to the basolateral membrane, extracellular HCO3− entered the cells rapidly. Since this was equally true in the nominal absence of CO2, these results support the hypothesis that HCO3− can be transported actively into the cell. The results are also consistent with a previous study in the isolated perfused cat pancreas in which Ammar et al. (1987) altered the pH of the arterial perfusate either at a constant PCO2 or at a constant HCO3− concentration. They found that HCO3− secretion was independent of arterial pH but critically dependent upon the arterial HCO3− concentration. In our study, the influx of HCO3− across the basolateral membrane was largely dependent on basolateral Na+ and was inhibited by H2DIDS, and thus is most probably mediated by Na+-HCO3− cotransport as we have suggested previously (Ishiguro et al. 1996a, 1998).
HCO3− transport across the luminal membrane
In the present study we have demonstrated that luminally applied HCO3− does not appear to enter the ductal cells until the luminal concentration of HCO3− is elevated above 125 mm. Given the presence of an anion exchanger in the luminal membrane this is surprising. When the lumen is perfused with solutions containing 125 mm HCO3− and 24 mm Cl−, the concentration gradients for HCO3− and Cl− across the luminal membrane should strongly favour the exchange of intracellular Cl− with luminal HCO3−, and a consequent rise in pHi as HCO3− enters the cells. The fact that this does not happen suggests that the luminal membrane Cl−-HCO3− exchanger may be inhibited under these conditions, perhaps as a result of the high luminal pH or the high concentration of luminal HCO3−. Interestingly, the need for this type of feedback inhibition of the exchanger has already been indicated by computer simulation studies of the rat pancreatic duct (Sohma et al. 1996, 1997). Most importantly, this finding again casts doubt on the ability of the exchanger to contribute to net HCO3− secretion during secretin stimulation where, in the guinea-pig, the luminal HCO3− concentration is already high. Also, as we have previously demonstrated (Ishiguro et al. 1998), secretin-stimulated HCO3− secretion in the guinea-pig appears to be mediated mainly by a transport mechanism which has, at most, a minimal requirement for Cl−.
An anion channel with a significant HCO3− conductance could account for Cl−-independent HCO3− secretion across the luminal membrane. The present finding, that HCO3− begins to enter the cells at luminal concentrations above 125 mm, suggests that there may be an anion conductance in the luminal membrane that is permeable to HCO3−. Although electrophysiological data are not yet available for the guinea-pig pancreatic duct, a luminal membrane potential of approximately −60 mV would be close to the reversal potential for HCO3− at luminal concentrations of this magnitude (assuming that the intracellular HCO3− concentration was approximately 10 mm since pHi was approximately 7.0). Thus, an increase in luminal HCO3− concentration from 125 to 145 mm might begin to allow HCO3− ions to enter the cells from the lumen and hence cause the small alkalinization observed in Fig. 3B. Provided that the membrane potential does not depolarise significantly during stimulation, there could therefore be a small luminally directed electrochemical gradient for HCO3− that would drive HCO3− secretion via an anion channel.
In summary, we have demonstrated that, while the basolateral membrane of interlobular duct cells from guinea-pig pancreas has powerful mechanisms for HCO3− uptake, the luminal membrane presents a significant barrier to the re-entry of secreted HCO3− ions from the lumen. Given the likely Cl− and HCO3− gradients across the luminal membrane during stimulated secretion, this can only be explained by a marked inhibition of the luminal anion exchanger. If this is the case, what is the pathway for HCO3− secretion across the luminal membrane? There is a possibility that HCO3− is secreted via a luminal anion channel. If the membrane potential is large enough, there could be a small electrochemical gradient favouring HCO3− efflux to the lumen even when the luminal HCO3− concentration exceeds 125 mm. However, if future electrophysiological measurements in the guinea-pig ducts show evidence of significant depolarisation during stimulation, as occurs in the rat (Noval & Pahl, 1993), alternative mechanisms will have to be considered.
Acknowledgments
This study was supported by the Ministry of Education, Science, and Culture and the Ministry of Health and Welfare (Japan). We thank Mr T. Saji for his technical assistance.
References
- Ammar EM, Hutson D, Scratcherd T. Absence of relationship between arterial pH and pancreatic bicarbonate secretion in the isolated perfused cat pancreas. The Journal of Physiology. 1987;388:495–504. doi: 10.1113/jphysiol.1987.sp016627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case RM, Argent BE. Pancreatic duct cell secretion: control and mechanisms of transport. In: Go VLW, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA, editors. The Pancreas: Biology, Pathophysiology, and Disease. 2. New York: Raven Press; 1993. pp. 301–350. [Google Scholar]
- Case RM, Harper AA, Scratcherd T. The secretion of electrolytes and enzymes by the pancreas of the anaesthetized cat. The Journal of Physiology. 1969;201:335–348. doi: 10.1113/jphysiol.1969.sp008759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case RM, Scratcherd T. On the permeability of the pancreatic duct membrane. Biochimica et Biophysica Acta. 1970;219:493–495. doi: 10.1016/0005-2736(70)90230-0. [DOI] [PubMed] [Google Scholar]
- De Ondarza J, Hootman SR. Confocal microscopic analysis of intracellular pH regulation in isolated guinea pig pancreatic ducts. American Journal of Physiology. 1997;272:G124–134. doi: 10.1152/ajpgi.1997.272.1.G124. [DOI] [PubMed] [Google Scholar]
- Gray MA, Greenwell JR, Argent BE. Secretin-regulated chloride channels on the apical plasma membrane of pancreatic duct cells. Journal of Membrane Biology. 1988;105:131–142. doi: 10.1007/BF02009166. [DOI] [PubMed] [Google Scholar]
- Gray MA, Plant S, Argent BE. cAMP-regulated whole cell chloride currents in pancreatic duct cells. American Journal of Physiology. 1993;264:C591–602. doi: 10.1152/ajpcell.1993.264.3.C591. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Naruse S, Kitagawa M, Hayakawa T, Case RM, Steward MC. Luminal ATP stimulates fluid and HCO3− secretion in guinea-pig pancreatic duct. The Journal of Physiology. 1999;591:551–558. doi: 10.1111/j.1469-7793.1999.0551m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Naruse S, Steward MC, Kitagawa M, Ko SBH, Hayakawa T, Case RM. Fluid secretion in interlobular ducts isolated from guinea-pig pancreas. The Journal of Physiology. 1998;511:407–422. doi: 10.1111/j.1469-7793.1998.407bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Lindsay AR G, Case RM. Accumulation of intracellular HCO3− by Na+-HCO3− cotransport in interlobular ducts from guinea-pig pancreas. The Journal of Physiology. 1996a;495:169–178. doi: 10.1113/jphysiol.1996.sp021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Wilson RW, Case RM. Bicarbonate secretion in interlobular ducts from guinea-pig pancreas. The Journal of Physiology. 1996b;495:179–191. doi: 10.1113/jphysiol.1996.sp021583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak I, Greger R. Properties of luminal membrane of isolated rat pancreatic ducts: effect of cyclic AMP and blockers of chloride transport. Pflügers Archiv. 1988;411:546–553. doi: 10.1007/BF00582376. [DOI] [PubMed] [Google Scholar]
- Novak I, Pahl C. Effect of secretin and inhibitors of HCO3−/H+ transport on the membrane voltage of rat pancreatic duct cells. Pflügers Archiv. 1993;425:272–279. doi: 10.1007/BF00374178. [DOI] [PubMed] [Google Scholar]
- Padfield PJ, Garner A, Case RM. Patterns of pancreatic secretion in the anaesthetised guinea pig following stimulation with secretin, cholecystokinin octapeptide, or bombesin. Pancreas. 1989;4:204–209. doi: 10.1097/00006676-198904000-00009. [DOI] [PubMed] [Google Scholar]
- Reber HA, Adler G, Wedgwood KR. Studies in the perfused pancreatic duct in the cat. In: Go VLW, Gardner JD, Brooks FP, Lebenthal E, DiMagno EP, Scheele GA, editors. The Exocrine Pancreas: Biology, Pathobiology, and Diseases. New York: Raven Press; 1986. pp. 255–273. [Google Scholar]
- Sewell WA, Young JA. Secretion of electrolytes by the pancreas of the anaesthetized rat. The Journal of Physiology. 1975;252:379–396. doi: 10.1113/jphysiol.1975.sp011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohma Y, Gray MA, Imai Y, Argent BE. A mathematical model of the pancreatic duct epithelium. Journal of Membrane Biology. 1996;154:53–67. doi: 10.1007/s002329900132. [DOI] [PubMed] [Google Scholar]
- Sohma Y, Gray MA, Imai Y, Argent BE. The pancreatic ductal tree – A new hypothesis for the secretion of a bicarbonate-rich fluid. Pediatric Pulmonology. 1997;14:277. (suppl.) [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilising spectroscopic probes generated. in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Waisbren SJ, Geibel JP, Modlin IM, Boron WF. Unusual permeability properties of gastric glands. Nature. 1994;368:332–335. doi: 10.1038/368332a0. [DOI] [PubMed] [Google Scholar]
- Zhao H, Star RA, Muallem S. Membrane localisation of H+ and HCO3− transporters in the rat pancreatic duct. Journal of General Physiology. 1994;104:57–85. doi: 10.1085/jgp.104.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]


