Abstract
Lawsonia intracellularis is an obligate intracellular organism that causes porcine proliferative enteropathy, a widespread infectious disease. Very little is known about the immune response and the epidemiologic features of the disease in the field. The aims of this study were to evaluate the duration and titers of antibody specific for L. intracellularis in gilts after an outbreak of proliferative hemorrhagic enteropathy (PHE), to evaluate maternal antibodies in piglets, and to evaluate seroconversion and fecal shedding in growing–finishing pigs. Thirty-six gilts in a herd that had recently experienced an outbreak of PHE, including 13 that had recovered, were bled 3 wk after the beginning of the outbreak and then every 3 wk until they became seronegative in 2 consecutive tests. Fourteen piglets from 5 gilts seropositive at farrowing and 5 piglets from 2 sows that remained seronegative were bled once or twice at the farrowing house and then every 3 wk until they reached market age. Fecal samples from these pigs were tested by polymerase chain reaction at 7 wk of age and then on the days of blood collection. After the PHE outbreak, the gilts had high serum antibody levels; the levels decreased over time, but antibody was still detectable for up to 3 mo in some animals. Four piglets from sows that were seropositive at farrowing had detectable passive antibodies up to 5 wk of age. Some nursery pigs started shedding L. intracellularis around 7 wk of age; peak shedding was observed between 13 and 16 wk. Antibody was not detected until 16 wk of age and was more often detected between 19 and 22 wk.
Introduction
Porcine proliferative enteropathy (PPE) is a widespread enteric infectious disease caused by the obligate intracellular bacterium Lawsonia intracellularis (1). PPE can have 2 different clinical presentations: chronic diarrhea and slow growth in growing–finishing pigs [porcine intestinal adenomatosis (PIA)] or death of gilts and finishing pigs close to market age with acute hemorrhagic diarrhea [proliferative hemorrhagic enteropathy (PHE)] (2). The importance of the subclinical form of the disease (3) or the existence of carrier pigs is unknown. Transmission of L. intracellularis is known to occur through the fecal–oral route, but the epidemiologic aspects of the disease within a herd and among herds are poorly understood.
Two of the antemortem methods available for diagnosis of PPE are the polymerase chain reaction (PCR) in fecal samples (4) and serologic tests (5). Previous studies (5,6) have shown that the indirect fluorescent antibody (IFA) test is much more sensitive (90% to 91%) than PCR in fecal samples (39% to 67%) for detecting experimental infection in pigs. However, the combination of these tests has higher sensitivity and negative predictive value than a single test (7). Recent data have shown that the immunoperoxidase monolayer assay (IPMA) has a sensitivity similar to that of the currently used IFA test (8). The IPMA showed high specificity (100%) and fair sensitivity (89%) in experimentally infected animals (9). The obvious next step is to evaluate the IPMA in field conditions.
Pigs infected with L. intracellularis are believed to have low and short-lived serum antibody titers (5). There is no information regarding titers and the duration of serum antibodies in gilts that have recovered from the acute form of PPE. Evidence of maternal antibodies in piglets has been reported in isolated cases (10,11). A recent epidemiologic study using the IFA test showed detectable antibody levels in growing–finishing pigs from 12 to 25 wk of age (7).
Information regarding the duration of serum antibodies in animals that have recovered from the PHE or the PIA form of PPE, transfer of passive immunity, and the presence or timing of seroconversion in growing–finishing pigs in field conditions is needed for a better understanding of the epidemiologic aspects of the disease. With this understanding, the use of antibiotics and, or, vaccines could be more strategic. Therefore, the objectives of this study were to evaluate the duration and titers of antibodies in gilts from a herd after an outbreak of PHE, to evaluate maternal antibodies in piglets, and to evaluate seroconversion and fecal shedding in growing–finishing pigs.
Materials and methods
Farm and history
This study was conducted in a recently repopulated, 1300-sow, commercial herd. The sow unit supplied several wean-to-finish units. Piglets were weaned at 17 d of age. The wean-to-finish sites typically had 3 or 4 barns, with 600 pigs in each barn, divided into 4 or 6 large pens. The pens had solid partitions, total slat floors, and deep pits.
Owing to a pseudorabies-positive status, the farm owner chose to depopulate the herd, following the US national eradication program guidelines. The facilities were emptied, cleaned, and disinfected. After 3 mo, the 1st group of 300 gilts, 160 d old, was received and allocated to individual crates in 3 barns. Feed medicated with 100 gr/ton of neomycin–terramycin (Neoterra 10/10; Pharmacia Animal Health, Kalamazoo, Michigan, USA) was used from arrival. Sudden death or slightly bloody diarrhea followed by death in 12 h began occurring in all 3 barns 70 to 80 d after the gilts' arrival, when they were 230 to 240 d old. Affected pigs were usually inappetent, although some continued to eat normally.
The outbreak persisted for 4 to 5 wk. Several feed and water medications were used during this time, without much success. There was a reduction in the number of new cases over time, but clinically affected animals did not usually respond and eventually died. At first, the level of neomycin–terramycin in the feed was increased to 200 gr/ton. After the confirmation of PHE, the medication was changed to 100 gr/ton of tylosin (Tylan 40; Elanco Animal Health, Indianapolis, Indiana, USA) in the feed and 2 mg/kg of body weight of injectable tylosin (Tylan 200; Elanco) twice a day in clinically affected animals. Water medication with 60 mg/L of tiamulin hydrogen fumarate (Denagard; Boehringer Ingelheim Vetmedica, Ames, Iowa, USA) was also used in 2 barns. About 10% of all animals in this 1st group of 300 gilts died in the outbreak.
Another group of 102- to 150-d-old gilts arrived at the farm at the end of the outbreak and gilts were allocated to different barns. They received 100 g/ton of tylosin (Tylan 40) in the feed from arrival and remained free of any clinical signs of PPE.
Postmortem examination and laboratory confirmation of PPE
Two severely clinically affected gilts were sent to the Veterinary Diagnostic Laboratory (VDL) at the University of Minnesota for postmortem examination and complementary tests 5 to 6 d after clinical signs and deaths had started in the herd. Samples were collected for bacteriologic, histopathologic, immunohistochemical, molecular diagnostic (PCR), parasitologic, toxicologic, and virologic studies.
Sample collection from the gilts
Thirty-six gilts, including 13 that had recovered, were bled 3 wk after the beginning of the outbreak and then every 3 wk until they became seronegative in 2 consecutive tests. All serum samples were tested by IPMA for serum IgG (8,9). Fecal samples from 7 of the 36 gilts were tested by PCR for L. intracellularis DNA (4) in the last 2 wk of gestation, 12 wk after the beginning of the outbreak.
Sample collection from the piglets
Fourteen piglets from 5 gilts seropositive at farrowing and 5 piglets from 2 sows that remained seronegative were ear-tagged and bled by jugular venipuncture once or twice at the farrowing house and then every 3 wk until they reached market age. Serum samples were tested by IPMA, which detects specific IgG against L. intracellularis. Fecal samples from these pigs were tested by PCR for L. intracellularis starting at 7 wk of age and then every 3 wk until they reached market age. The feed-grade antimicrobials used for growing-finishing pigs were 250 gr/ton of chlortetracycline-sulfamethazine-penicillin (Aureo SP 250; Alpharma, Fort Lee, New Jersey, USA) from weaning (17 d) to 7 wk of age, 25 gr/ton of carbadox (Mecadox; Phibro Animal Health, Fairfield, New Jersey, USA) from 7 to 11 wk, and 30 gr/ton of bacitracin methylene disalicylate (BMD; Alpharma) from 11 wk until market age.
Results
Confirmation of PPE
The 2 gilts sent alive to the VDL died during transport but were still fresh at arrival. They were in good body condition but extremely pale, and the hindquarters were soiled reddish-black. The mesentery of the terminal jejunum and entire ileum of both gilts was thick, gelatinous, and yellowish, owing to edema. The terminal jejunum and entire ileum of both gilts had a reticulated serosa, a markedly thickened wall, with a fibrinonecrotic membrane on the mucosal surface, and large blood clots in the lumen. The colon contents were pasty and dark red to black (melena). No other gross lesions were noted.
The histologic changes observed in the intestines were essentially as described by McOrist and Gebhart (2). Epithelial hyperplasia and absence of goblet cells associated with hemorrhage in the lamina propria were observed in sections of ileum and terminal jejunum from both gilts. Large numbers of bacteria were demonstrable in the cytoplasm of the hyperplastic glandular epithelial cells in sections stained with the monoclonal antibody specific for L. intracellularis (12). PCR tests for L. intracellularis (4) in ileal mucosa were positive in both animals.
Aerobic and anaerobic cultures of samples of ileum, colon, and mesenteric lymph nodes did not demonstrate growth of any pathogenic organism. Virus isolation, PCR, and immunohistochemical studies were negative for porcine respiratory and reproductive syndrome (PRRS) virus. Direct immunofluorescence studies of tonsils and virus isolation tests were also negative for pseudorabies virus. The fecal samples were negative for parasite ova and oocysts by direct microscopic examination. Mineral analysis of the liver did not show any abnormality. From the clinical and macroscopic findings and the laboratory results, PHE was confirmed.
Samples from the gilts
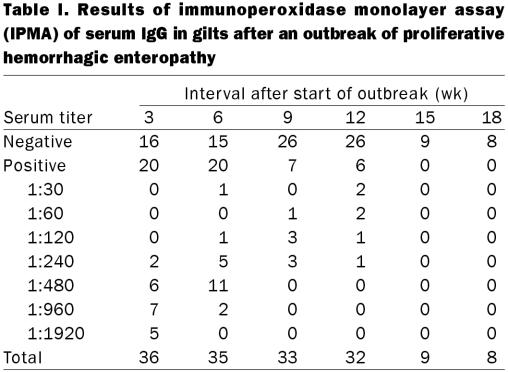
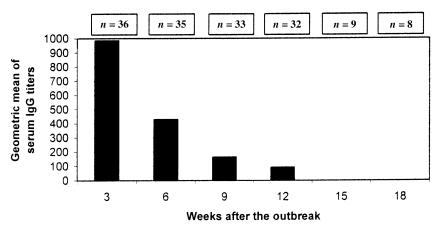
The results of IPMA testing for 18 wk after the outbreak are shown in Table I. Three wk after the beginning of the outbreak, 20 of the 36 gilts, including the 13 that recovered, had antibody titers against L. intracellularis ranging from 1:240 to 1:1920. The titers and the number of seropositive gilts gradually decreased over time (Figure 1), but 6 animals were still positive 12 wk after the outbreak. Coincidentally, these 6 gilts had high antibody titers, ranging from 1:960 to 1:1920, at the 1st bleeding, 3 wk after the beginning of the outbreak. In 4 of these 6 gilts, clinical signs of PHE had been observed, but the animals recovered.
Table I.
Figure 1. Geometric mean of serum IgG titers of gilts after an outbreak of proliferative hemorrhagic enteropathy.
The fecal samples collected from 7 gilts close to farrowing, 12 wk after the beginning of the outbreak, were PCR negative for L. intracellularis. Four still had serum antibody titers against L. intracellularis, whereas the other 3 animals were seronegative throughout the study.
Samples from the piglets
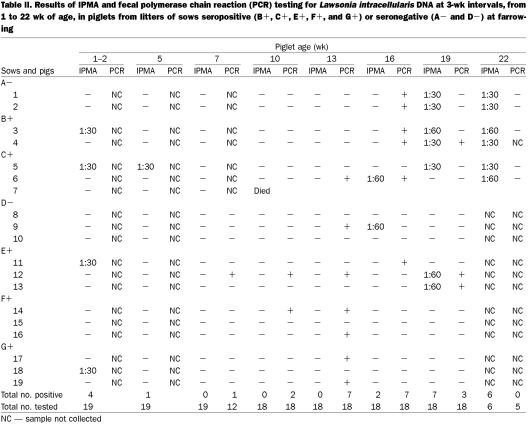
Four piglets (nos. 3, 5, 11, and 18) from gilts seropositive at farrowing were seropositive for L. intracellularis before weaning, and 1 of these (no. 5) was positive up to 5 wk of age (Table II). After that, serum antibodies were detected again only starting at 16 wk of age (in pigs 6 and 9), with a peak at 19 and 22 wk of age, when 7 and 6 pigs, respectively, were seropositive (Figure 2). One animal (no. 7) died at 8 wk of age from a cause not related to PPE. Nine of 18 pigs seroconverted between 16 and 22 wk of age but with low antibody titers (1:30 and 1:60). Antibody was detected in 2 non-consecutive bleedings in pig 6 (at 16 and 22 wk of age), in 2 consecutive bleedings in 5 pigs (nos. 1 through 5), and only once in the other 3 animals that seroconverted (nos. 9, 12, and 13).
Table II.
Figure 2. Serum antibody titers of piglets, as detected by the immunoperoxidase monolayer assay.
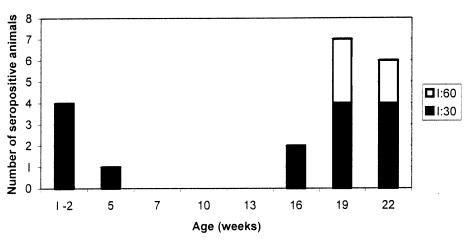
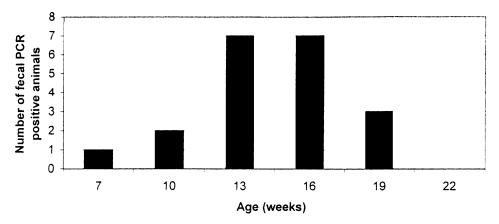
Fecal shedding was first detected by PCR much earlier than seroconversion, at 7 wk of age (in pig 12), and a larger number of animals had PCR-positive fecal samples at 13 and 16 wk of age (Table II, Figure 3). Fecal shedding was detected by PCR in 13 of 18 pigs between 7 and 19 wk of age. Pig 12 was PCR positive in fecal samples at 7, 10, 13, and 19 wk of age but had detectable serum antibodies only at 19 wk. Four animals (nos. 4, 6, 14, and 19) had PCR-positive fecal samples in 2 consecutive tests, and the remaining 8 pigs were positive only once. Besides pig 12, 7 pigs were both seropositive and fecal PCR positive. Unlike pig 12, seroconversion and fecal shedding in these 7 pigs was concomitant. In 5 pigs (nos. 11, 14, 16, 17, and 19) with detectable fecal shedding, serum antibodies were never detected.
Figure 3. Results of polymerase chain reaction (PCR) for Lawsonia intracellularis DNA in fecal samples collected from the piglets at 7 to 22 wk of age.
The herd was positive for PRRS before the depopulation. All gilts were vaccinated with PRRS modified-live vaccine (PrimePac PRRS; Schering-Plough Animal Health, Union, New Jersey, USA) at arrival. Blood from all 18 growing pigs tested positive by PRRS enzyme-linked immunosorbent assay (HerdCheck PRRS; IDEXX Laboratories, Westbrook, Maine, USA), with sp/ratios ranging from 1.0 to 2.67 between 10 and 19 wk of age. Despite their being seropositive, clinical disease was not observed.
Discussion
Pigs infected with L. intracellularis were believed to have low and short-lived serum antibody titers; the longest duration of detectable serum antibodies was 6 wk after experimental inoculation of pigs with a pure culture of L. intracellularis (5). However, that study was terminated just after the last bleeding, at 6 wk postinoculation. In the present study, following a PHE outbreak, gilts developed high serum antibody titers that lasted for up to 3 mo in some animals (Table I, Figure 1). The presence of specific serum antibodies (IgG) should be interpreted as reflecting previous exposure to L. intracellularis; it does not imply protection. In other words, the presence of serum antibodies indicates stimulation of the immune system by L. intracellularis antigen. As L. intracellularis is an obligate intracellular organism that resides in the cytoplasm of enterocytes, we would not expect serum IgG levels to be directly involved in protection. IgA in the local mucosa and cell-mediated immune responses are probably more involved in protection against L. intracellularis infection. These responses, however, are much more difficult to measure.
The low antibody titers detected in the first weeks of life in 4 piglets from serologically positive sows (Table II, Figure 2) indicate the maternal origin of the antibodies. Two other studies (10,11) have also shown serum antibodies in piglets between 1 and 3 wk of age. In 1 of these studies (11), the seropositive piglets were from litters of sows seropositive at farrowing. The findings of non- consistent seropositivity among piglet littermates in our study may indicate ingestion and, or, absorption of different amounts of colostrum, limitation of the sensitivity of the IPMA (89%), or a combination. More studies have to be done to determine the presence of IgA and IgG antibodies specific for L. intracellularis in the colostrum of seropositive sows and to correlate those findings with immunity in piglets.
In contrast to the high serum antibody titers observed in gilts after the outbreak, growing–finishing pigs showed low titers that usually lasted for only 3 wk. The period of seropositivity for most pigs was between 19 and 22 wk of age (Table II, Figure 2). In another serologic follow-up study (7), the pigs seroconverted earlier, around 12 and 16 wk of age. This difference could be related to several variables, such as a different antimicrobial program, different pig flow, or different types of floors.
Under the conditions of our study, fecal shedding was detected mainly at 13 and 16 wk of age and seroconversion at 19 and 22 wk. Thirteen pigs were positive by PCR in fecal samples between 7 and 22 wk of age; only 9 seroconverted. From these results, it appears that fecal shedding is the first finding in infected animals in field conditions, and the level of infection in some of these animals probably does not promote sufficient systemic humoral immune response to be detected by serologic testing. Thus, serologic testing may underestimate the prevalence of infection among growing–finishing pigs within a herd. However, it gives an idea about the timing of peak fecal shedding, which was 3 to 6 wk prior to seroconversion in the present study.
The long duration of fecal shedding in pig 12 suggests that some animals start shedding L. intracellularis early; thus, the concentration of bacteria in the environment and, consequently, the exposure of penmates builds up over time. Unfortunately, the location of tagged pigs in the wean-to-finish pens was not recorded. Also, clinical scores based on body condition and consistency of feces were not obtained, and therefore associations of seroconversion and fecal shedding with clinical signs could not be made.
Transmission of the bacteria from the breeding herd to the growing pigs could not be evaluated in this study owing to the single collection of fecal samples from only a few sows close to farrowing and the late start of fecal collection from the growing pigs. Occurrence of PIA in growing pigs after an outbreak of PHE in the breeding herd has been reported in 2 other studies (13,14). At that time, the 2 distinct clinical presentations (PIA and PHE) were not known to be caused by the same bacterium. In the 2 reports, there was not much information about facilities separation, personnel movement, and animal flow. Transmission of the bacteria between barns by fomites like rubber boots was very likely. In the present study, since the farm was a 2-site production system with different personnel at each site, the possibility of transmission by fomites was greatly reduced. Despite the negative PCR results for the seropositive sows close to farrowing, sow-to-piglet transmission is still a real possibility. The hypothesis of runt piglets becoming infected at the farrowing house and carrying the infection over time to the nursery needs to be studied. Fecal shedding has been reported as early as 3 wk of age in piglets (15) and between 25 and 32 d of age in pigs (16). Low levels of antibiotics and growth promoters in the feed of nursery pigs have a potential effect on the epidemiologic features of PPE. Just and associates (7) have suggested that the infectious dose of L. intracellularis must build up in the environment for several weeks or the in-feed medication delays the spread of infection in the nursery. Lanza and colleagues (17) demonstrated a negative association between the use of growth promoters and the presence of infection as determined by fecal shedding detected by PCR.
In conclusion, gilts that had recovered from PHE had high serum antibody levels for up to 3 mo. Piglets from sows that were seropositive at farrowing had detectable passive antibodies up to 5 wk of age. One nursery pig started shedding bacteria as early as 7 wk of age, but fecal shedding peaked around 13 and 16 wk and was usually followed by seroconversion between 19 and 22 wk of age. These results provide additional information for the interpretation of PPE serologic data and time of exposure of L. intracellularis in field conditions. With a better understanding about the time of exposure, strategies for antibiotic and, or, vaccine control can be improved.
Footnotes
Acknowledgments
We thank Debra Swanson for technical assistance and Dr. John Deen for critical review of the manuscript. Dr. Guedes was supported by the Brazilian government sponsoring agency Conselho Nacional de Desenvolvimento Cientifico e Tecnologico and the Universidade Federal de Minas Gerais.
Address correspondence and reprint requests to Dr. Roberto M.C. Guedes, 1971 Commonwealth Avenue, Room 205, Veterinary Science Building, Saint Paul, Minnesota 55108, USA, tel: 612-625-8110, fax: 612-625-5203, e-mail: gued0001@tc.umn.edu
Received February 19, 2002. Accepted April 19, 2002.
References
- 1.McOrist S, Gebhart CJ, Boid R, Barns SM. Characterization of Lawsonia intracellularis gen. nov, sp nov, the obligately intracellular bacterium of porcine proliferative enteropathy. Int J Syst Bacteriol 1995;45:520–525. [DOI] [PubMed]
- 2.McOrist S, Gebhart CJ. Porcine proliferative enteropathies. In: Straw BE, D'Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State Univ Pr, 1999:521–534.
- 3.Ward GE, Winkelman NL. Recognizing the three forms of proliferative enteritis in swine. Vet Med Food Anim Pract 1990; 2:197–203.
- 4.Jones GF, Ward GE, Murtaugh MP, Lin G, Gebhart CJ. Enhanced detection of intracellular organism of swine proliferative enteritis, Ileal symbiont intracellularis, in feces by polymerase chain reaction. J Clin Microbiol 1993;31:2611–2615. [DOI] [PMC free article] [PubMed]
- 5.Knittel JP, Jordan DM, Schwartz KJ, et al. Evaluation of ante-mortem polymerase chain reaction and serologic methods for detection of Lawsonia intracellularis exposed pigs. Am J Vet Res 1998;59:722–726. [PubMed]
- 6.Guedes RMC, Gebhart CJ, Winkelman NA, Mackie-Nuss RAC, Marsteller TA, Deen J. Comparison of different methods for diagnosis of porcine proliferative enteropathy. Can J Vet Res 2002; 66:99–107. [PMC free article] [PubMed]
- 7.Just SD, Thoen CO, Thacker BJ, Thompson JU. Monitoring of Lawsonia intracellularis by indirect serum immunofluorescence assay in a commercial swine production system. J Swine Health Prod 2001;9:57–61.
- 8.Guedes RMC, Gebhart CJ, Winkelman NA, Mackie-Nuss R. Comparative study of an indirect immunofluorescent test and the immunoperoxidase monolayer assay for diagnosing porcine proliferative enteropathy. J Vet Diagn Invest (in press). [DOI] [PubMed]
- 9.Guedes RMC, Gebhart CJ, Deen J, Winkelman NL. Validation of an immunoperoxidase monolayer assay as a serologic test for porcine proliferative enteropathy. J Vet Diagn Invest (in press). [DOI] [PubMed]
- 10.Holyoake PK, Cutler RS, Caple IW, Monckton RP. Enzyme-linked immunosorbent assay for measuring Ileal symbiont intracellularis-specific immunoglobulin G response in sera of pigs. J Clin Microbiol 1994;32:1980–1985. [DOI] [PMC free article] [PubMed]
- 11.Wendt M, Bonitz A, McOrist S. Prevalence of Lawsonia intracellularis in German breeding herds. Proc Int Pig Vet Soc Cong 2000;16:27.
- 12.McOrist S, Boid R, Lawson GHK, McConell I. Monoclonal antibodies to intracellular Campylobacter-like organisms of the porcine proliferative enteropathies. Vet Rec 1987;121:421–422. [DOI] [PubMed]
- 13.Rowland AC, Rowntree PGM. A hemorrhagic bowel syndrome associated with intestinal adenomatosis in the pig. Vet Rec 1972;91:235–241. [DOI] [PubMed]
- 14.Love RJ, Love DN, Edwards MJ. Proliferative haemorrhagic enteropathy in pigs. Vet Rec 1977;100:65–68. [DOI] [PubMed]
- 15.Lopez J, Rodriguez J, Valle R, Alvarez M, Gomez M. A longitudinal study of porcine proliferative enteropathy in a commercial pig farm in Yucatan, Mexico. Proc Int Pig Vet Soc Cong 2000;16:68.
- 16.Moreno AM, Baccaro MR, Coutinho LL. Porcine proliferative enteritis: anatomopathological and epidemiological aspects. Proc Int Pig Vet Soc Cong 2000;16:63.
- 17.Lanza I, Pozo J, Munoz M, Rubio P, Carmenes P. Epidemiological study of porcine proliferative enteropathy in Spain. Proc Int Pig Vet Soc Cong 1996;14:259.