Abstract
The rabbit Na+-glucose cotransporter (rbSGLT1) was expressed in Xenopus laevis oocytes and urea transport in rbSGLT1 and non-injected (control) oocytes was studied using [14C]urea as a tracer. The level of rbSGLT1 expression in these batches of oocytes was monitored by measuring the uptake of α-methyl-d-[14C]glucopyranoside ([14C]αMDG).
In rbSGLT1-expressing oocytes, there was a 4-fold increase in urea transport in the absence of sugar relative to that in control oocytes. Urea uptake was not Na+ dependent and was linear with both time of incubation (5–120 min) and increasing urea concentration (50 μm to 100 mm) in the bathing medium. rbSGLT1 urea uptake was blocked by the rbSGLT1-specific inhibitor phlorizin (Ki 1 μm) in 100 mm NaCl buffer, but was not affected in 100 mm choline chloride buffer. Phloretin inhibited rbSGLT1 urea uptake with a low affinity (Ki > 1 mm) in the presence and absence of Na+. The uptake of 55 μm urea through rbSGLT1 was not blocked by 100 mm urea analogues including thiourea, 1,3-dimethyl urea, 1,1-dimethyl urea and acetamide.
The activation energies (Ea) of urea transport for control and rbSGLT1-expressing oocytes were 14 ± 3 and 6 ± 1 kcal mol−1, respectively. The low Ea for urea transport through rbSGLT1 is comparable to the Ea of passive water transport through rbSGLT1.
Urea transport through rbSGLT1 was further increased when the cotransporter was activated by the addition of sugar to the external medium. The rate of sugar-dependent urea uptake was directly proportional to the rate of Na+-glucose-H2O cotransport such that the amount of urea transport was approximately proportional to the molar concentration ratio of urea to H2O (55 μm/55 m).
The low affinity Na+-glucose (pSGLT3), the Na+-iodide (rNIS) and the Na+-Cl−-GABA (hGAT1) cotransporters expressed in oocytes demonstrated similar urea transport properties.
These observations suggest that cotransporters behave as urea channels in the absence of substrates. Furthermore, under substrate-transporting conditions, the same cotransporters serve as urea cotransporters. This could account for urea transport in cells that appear not to have urea uniporters or channels.
Urea is a major metabolic product of mammalian biopathways and its transport plays a vital role in nitrogen elimination and osmotic homeostasis. Urea, which is transported via the blood to the kidneys for excretion, is integral to the urinary concentration mechanism in the kidney. Although there has been some evidence that urea is transported actively (Kawamura et al. 1976; Kato & Sands, 1998), it is generally considered that urea is passively transported across biological membranes by diffusion. In recent years, specific transporters for urea have been identified in the renal medulla and cloned (for a review see Tsukaguchi et al. 1998). Subsequent studies have shown that some water channels, such as aquaporins AQP3, AQP7 and AQP9, are permeable to urea (for a review see Borgnia et al. 1999). In both the facilitated urea transporters and aquaporins, urea is passively transported down its concentration gradient.
To date, no urea uniporters or channels have been identified in the renal proximal tubule, where about 40% of the urea filtered by the glomerulus is reabsorbed. However, given that the proximal tubule contains a significant number of cotransporters that behave as water channels and pumps (Zeuthen, 1991, 1994; Zeuthen et al. 1996, 1997; Loo et al. 1996, 1999; Meinild et al. 1998, 2000), we investigated whether cotransporters can also transport urea. We tested transporters with demonstrated water transport properties, such as the pig low affinity Na+-glucose (pSGLT3), rat Na+-iodide (rNIS) and human Na+-Cl−-GABA (hGAT1) transporters, but primarily focused on the rabbit Na+-glucose cotransporter (rbSGLT1). Our findings indicate that cotransporters act as urea channels in the absence of substrate and as urea cotransporters in the presence of substrate. These results may have further implications for the mechanism of urea transport in the renal proximal tubule and other tissue cells.
METHODS
The rabbit Na+-glucose (rbSGLT1), rat Na+-iodide (rNIS), human Na+-Cl−-GABA (hGAT1), and pig low affinity Na+-glucose (pSGLT3) cotransporters were overexpressed in Xenopus laevis oocytes as previously described (Hediger et al. 1987; Eskandari et al. 1997; Loo et al. 1999). Mature oocytes were collected from adult female frogs anaesthetized with 0.1% Tricaine. Frogs were killed with an overdose of anaesthetic after the final collection. These protocols were approved by the UCLA Animal Research Committee, and meet all the NIH guidelines for the humane treatment of animals used for research. Oocytes were treated with 0.4% collagenase-B (Boehringer-Mannheim, Indianapolis, IN, USA) for 1 h and defolliculated in a 150 mm K2HPO4 medium for 1 h. Oocytes were then incubated at 18°C in Barth’s medium. Oocytes were injected with 50 ng of capped cRNA (MegaScript, Ambion, Austin, TX, USA) or not injected (control) and 5–6 days were allowed for expression prior to use in experiments. Reagents were purchased from Sigma unless stated otherwise.
Sugar uptake was measured using α-methyl-d-glucopyranoside (αMDG), a non-metabolizable sugar analogue which is transported by rbSGLT1. The initial rates of urea and sugar uptake into oocytes were measured at 22°C (unless stated otherwise) using radioactive tracers (Ikeda et al. 1989). [14C]Urea (specific activity, 57 mCi mmol−1) and methyl-α-d-[U-14C]-glucopyranoside (specific activity, 293 mCi mmol−1) (Amersham Life Sciences, Elk Grove, IL, USA) uptake was usually carried out at final urea and αMDG concentrations of 55 and 50 μm, respectively. Radiotracer experiments were performed on oocytes obtained from the same donor frog, incubated in either 100 mm NaCl or 100 mm choline chloride medium. Experiments were repeated at least twice on oocytes isolated from different donor frogs. Uptake was expressed as a function of the amount (in pmol) per oocyte per minute (mean ±s.e.m.).
The urea permeability, Purea, was determined according to the equation: Purea=Jurea/[urea] X S where Jurea is the rate of urea flux (pmol oocyte−1 s−1), [urea] is the urea concentration (mol cm−3), and S is the oocyte membrane surface area (0.44 cm2; Zampighi et al. 1995).
The number of transporters (N) in the plasma membrane of oocytes expressing rbSGLT1 was determined from the maximal rate of sugar uptake, Jmax, and the turnover number of rbSGLT1 (25 s−1, Panayotova-Heiermann et al. 1994) using the relationship: N=Jmax×A/turnover number, where A is Avogadro’s number (6.02 × 1023 molecules mol−1). Jmax was estimated from the αMDG uptake at 50 μm and the K0.5 for αMDG uptake (0.2 mm) using the equation: J= (Jmax[αMDG])/(K0.5αMDG+[αMDG]), where K0.5 is the half-maximal concentration (mol l−1), [αMDG] is the αMDG concentration (mol l−1), and J is the rate of sugar uptake in oocytes (pmol oocyte−1 s−1).
RESULTS
Passive urea transport
Figure 1A illustrates the results from an experiment measuring the urea uptake in the absence of αMDG in the bathing medium by control and rbSGLT1-expressing oocytes. Urea uptake in oocytes expressing rbSGLT1 (7.6 ± 0.5 pmol oocyte−1 (30 min)−1) was 4-fold higher than in control oocytes (1.7 ± 0.03 pmol oocyte−1 (30 min)−1). Urea uptake in both control and SGLT1-expressing oocytes was linear with the time of incubation (5–120 min), and the rate of uptake was linear with urea concentration from 10 μm to 100 mm. In one experiment the uptake at 55 μm and 100 mm urea was 3.1 ± 0.2 and 5501 ± 65 pmol oocyte−1 h−1, respectively, in control oocytes (n= 4 oocytes), and 13.3 ± 1.3 and 25 500 ± 1000 pmol oocyte−1 h−1, respectively, in rbSGLT1-expressing oocytes (n= 6). The rate of urea uptake in control oocytes corresponds to a urea permeability, Purea, of 4 × 10−8 cm s−1. This is comparable to the urea permeabilities reported by others (e.g. Zhang & Verkman, 1991) after correcting for the increase in the oocyte plasma membrane area due to folding (Zampighi et al. 1995). Thus, the urea permeability due to rbSGLT1, which was obtained by subtraction of the background urea permeability of control oocytes from the total urea permeability of rbSGLT1-expressing oocytes, was 1.3 × 10−7 cm s−1. The increase in urea permeability was directly proportional to the level of SGLT1 expression. The level of expression was gauged in six oocytes by the magnitude of the maximum Na+-glucose current (Mackenzie et al. 1998) immediately before measuring the radioactive urea tracer uptake, and the urea flux (pmol oocyte−1 (30 min)−1) was directly proportional to the Na+-glucose current (nA) over a 3-fold range (regression coefficient, 0.7).
Figure 1. Passive urea transport by rbSGLT1.
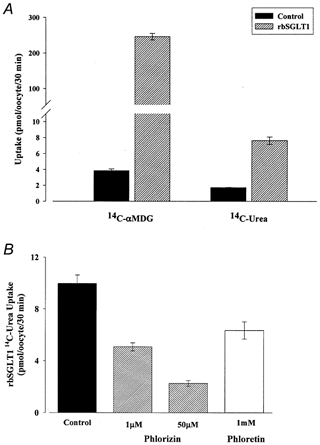
A, 50 μm[14C]αMDG and 55 μm[14C]urea uptake in control and rabbit SGLT1 (rbSGLT1)-expressing oocytes. Oocytes obtained from the same frog were either injected with 50 ng of rbSGLT1 cRNA or not injected (Control). Uptake was studied in oocytes after 5 days incubation in Barth’s medium. For transport experiments, oocytes were incubated in 100 mm NaCl and transport of sugar or urea was measured as a function of the amount (in pmol) per oocyte per 30 min. Each bar in the graph represents the mean αMDG or urea uptake by 4–5 oocytes. Left, ∼250 pmol of αMDG is transported by rbSGLT1-expressing oocytes, 65-fold higher than by control oocytes. Right, ∼8 pmol of urea is transported by rbSGLT1-expressing oocytes, 4-fold higher than by control oocytes. B, inhibition of rbSGLT1 urea transport by phlorizin and phloretin. Uptake of 55 μm[14C]urea was studied after 6 days incubation in non-injected oocytes and in rbSGLT1-injected oocytes. Urea flux was measured in the presence of 100 mm NaCl buffer and in the absence of sugar. Uptake was calculated as the amount of urea transported (in pmol) per oocyte per 30 min. Each bar represents the mean urea uptake by 4–6 rbSGLT1 oocytes from the same batch either in the absence (Control) or in the presence of phlorizin or phloretin. All values have been corrected for endogenous urea uptake. Addition of 1 or 50 μm phlorizin or 1 mm phloretin to the transport buffer had no effect on non-injected oocytes. In rbSGLT1 oocytes, the presence of 1 μm phlorizin in the transport buffer resulted in a 50% decrease in urea transport. Addition of 50 μm phlorizin further inhibited urea transport to ∼20% of that by control oocytes. Identical experiments performed with 1 mm phloretin also reduced rbSGLT1 urea uptake by ∼35% compared to control.
The rate of αMDG uptake (246 ± 9 pmol oocyte−1 (30 min)−1) in the same batch of oocytes as shown in Fig. 1A was 65-fold higher than in control oocytes (3.8 ± 0.2 pmol oocyte−1 (30 min)−1), corresponding to values obtained previously (Hediger et al. 1987; Ikeda et al. 1989). Using these fluxes we estimate that for the oocytes used in Fig. 1A, approximately 2 × 1010 copies of rbSGLT1 were located in the plasma membrane. Given this density of cotransporters, we obtained a Purea of 7 × 10−18 cm s−1 per rbSGLT1 cotransporter.
The presence of urea analogues did not affect urea uptake into control and rbSGLT1-expressing oocytes. Uptake of 55 μm urea into control and rbSGLT1-expressing oocytes was insensitive to the addition of excess concentrations (100 mm) of the urea analogues. For example, in one experiment where the rbSGLT1-specific uptake of 55 μm urea was 9.8 ± 1.1 pmol oocyte−1 (30 min)−1 (n= 5), the uptake in the presence of 100 mm thiourea, N-methylurea, 1,3-dimethylurea, acetamide and 1,1-dimethylurea was 8.6 ± 0.9 (n= 5), 9.5 ± 0.9 (n= 5), 8.9 ± 1.0 (n= 5), 9.5 ± 1.9 (n= 5) and 9.0 ± 1.7 pmol oocyte−1 (30 min)−1 (n= 5), respectively. This is consistent with the linearity of urea uptake up to 100 mm urea.
Urea uptake by rbSGLT1 was independent of Na+; for example, in two pairs of experiments the specific uptake was 14.2 ± 0.7 (n= 5) and 10.1 ± 0.5 pmol oocyte−1 (30 min)−1 (n= 6) in Na+, and 15.6 ± 0.6 (n= 6) and 10.3 ± 0.7 pmol oocyte−1 (30 min)−1 (n= 6) in choline. However, urea uptake was inhibited by phlorizin, a specific inhibitor of Na+-dependent glucose transport by rbSGLT1 (Fig. 1B). The addition of 1 μm phlorizin reduced the rbSGLT1 uptake of 55 μm urea by ∼50%. Increasing the concentration of phlorizin to 50 μm reduced urea uptake to 23% of that in the absence of phlorizin (Fig. 1B, Control). The apparent phlorizin inhibition constant, Ki, was 1 μm in the presence of 100 mm external Na+, but was greater than 1 mm in choline. Phloretin also inhibited urea uptake by rbSGLT1. The Ki for phloretin was greater than 1 mm in the absence and presence of Na+. Neither phlorizin nor phloretin inhibited urea uptake into non-injected oocytes.
The specificity of the increase in urea permeability of rbSGLT1-expressing oocytes was examined by measuring the uptake of radiolabelled mannitol, glycerol, sulphate, chloride and l-alanine. With these probes there was no observable increase in permeability. For example, in one experiment where the 55 μm urea uptake increased from 1.7 ± 0.3 pmol oocyte−1 (30 min)−1 (n= 5) in control oocytes to 7.6 ± 0.5 pmol oocyte−1 (30 min)−1 (n= 5) in rbSGLT1-expressing oocytes, the uptake of 55 μm mannitol was 1.4 ± 0.1 pmol oocyte−1 (30 min)−1 (n= 5) in control and 1.8 ± 0.2 pmol oocyte−1 (30 min)−1 (n= 5) in rbSGLT1-expressing oocytes. In the case of 55 μml-alanine the uptake was 24 ± 1 (n= 6) and 22 ± 1 pmol oocyte−1 (30 min)−1 (n= 6), respectively.
The temperature sensitivity of urea uptake was determined over the range 14–30°C and the corresponding Arrhenius plots are shown in Fig. 2. In control oocytes, the activation energy, Ea, for urea transport was 14 ± 3 kcal mol−1, a value comparable to that reported previously for the efflux of urea (10 kcal mol−1, Zhang & Verkman, 1991) and to that of passive water diffusion through the plasma membrane (12 kcal mol−1, Loo et al. 1996; 10 kcal mol−1, Zhang & Verkman, 1991). The Ea for urea transport in rbSGLT1 oocytes was 6 ± 1 kcal mol−1.
Figure 2. Arrhenius plots of urea uptake by rbSGLT1 (•) and control (non-injected, ○) oocytes.
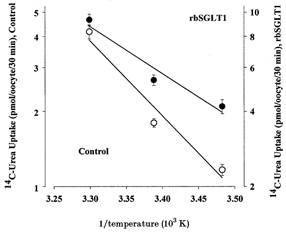
Urea uptake was measured in oocytes incubated in 55 μm[14C]urea for 30 min at 14, 22 and 30°C. The experiment was performed on 29 oocytes obtained from the same batch and after 5 days expression. Each data point with error bars indicates the mean urea uptake by 4–6 oocytes (±s.e.m.). The lines were drawn by linear regression. For control oocytes, a slope of −3.0 ± 0.6 corresponds to an activation energy, Ea (Ea=−2.3R X slope, where R is the gas constant), of 14 ± 3 kcal mol−1. For rbSGLT1-expressing oocytes, the slope of −1.3 ± 0.3 corresponds to an Ea of 6 ± 1 kcal mol−1.
Urea uptake was also higher in oocytes expressing the low affinity Na+-glucose (pSGLT3), the Na+-iodide (rNIS) and the Na+-Cl−-GABA (hGAT1) cotransporters (Fig. 3). The values for urea uptake obtained for rNIS- and hGAT1-expressing oocytes were 3-fold higher (8.5 ± 0.5 pmol oocyte−1 (30 min)−1) than when compared to that of control oocytes (3.2 ± 0.2 pmol oocyte−1 (30 min)−1). Urea uptake by pSGLT3 was also significantly higher than in control oocytes (P≤ 0.05; Fig. 3, right). The increase in urea transport was blocked by specific inhibitors, i.e. 100 μm phlorizin blocked pSGLT3 urea uptake by 82%, and 20 μm SKF 89976A reduced hGAT1 urea uptake by 20% (not shown).
Figure 3. Urea transport by different cotransporters.
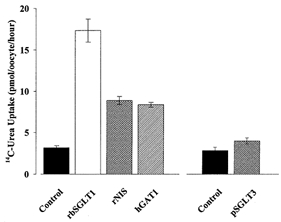
Uptake of 55 μm[14C]urea was measured for 30 min in oocytes after 5 days of expression of different cotransporters. Oocytes from the same batch were injected with 50 ng cRNA of rabbit Na+-glucose cotransporter (rbSGLT1), rat Na+-iodide cotransporter (rNIS), human Na+-Cl−-GABA transporter (hGAT1) or pig low affinity sugar transporter (pSGLT3), or were not injected (Control). Each bar represents the mean uptake by 4–6 oocytes and was tested for significance (P≤ 0.05).
Urea transport in the presence of substrates
The addition of substrates to the bathing medium further increased urea transport by cotransporters. To minimize complications due to the voltage dependence of cotransport activity, we used substrate concentrations below the K0.5 for transport. This decreased the depolarization of the membrane potential and the concomitant reduction in the rate of Na+-substrate cotransport. Figure 4 summarizes the results of one set of experiments with rbSGLT1, hGAT1 and pSGLT3. Urea transport by rbSGLT1 increased from 4.0 ± 0.3 to 8 ± 0.5 pmol oocyte−1 h−1 in the presence of 0.1 mmαMDG. Phlorizin (100 μm) inhibited this urea transport by rbSGLT1. For pSGLT3, the urea uptake increased from 1.1 ± 0.3 to 2.2 ± 0.01 pmol oocyte−1 h−1 in the presence of 1 mmαMDG. Addition of 5 mmαMDG increased the urea uptake by pSGLT3 from 4.0 ± 0.4 to 6.2 ± 0.4 pmol oocyte−1 (30 min)−1. Urea transport measured in the presence of 1 μm GABA by hGAT1 also increased from 7 ± 0.5 to 14 ± 1 pmol oocyte−1 h−1.
Figure 4. Urea transport in the presence (+) and absence (-) of substrate by oocytes expressing rbSGLT1, hGAT1 or pSGLT3.
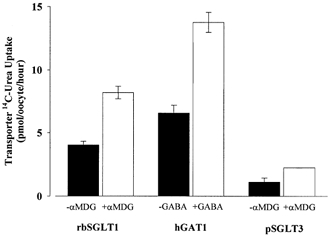
Urea transport was measured for 1 h in 100 mm NaCl buffer and ±100 μm aMDG for rbSGLT1, ±1 μm GABA for hGAT1, or ±1 mm aMDG for pSGLT3 oocytes. Each bar represents the mean urea uptake by 4–6 oocytes after 5 days expression and has been corrected for the uptake by non-injected oocytes. In the presence of the respective substrate, urea transport through rbSGLT1, hGAT1 and pSGLT3 increases by ∼50%.
We determined the stoichiometry between urea and Na+-glucose cotransport by rbSGLT1 by measuring the amount of [14C]urea uptake (55 μm) in the presence and absence of 0.2 mmαMDG. We also determined the amount of αMDG transported by rbSGLT1 by measuring the amount of Na+-dependent [14C]αMDG (0.2 mm) uptake in the same batch of rbSGLT1-expressing oocytes. The substrate-dependent urea uptake by rbSGLT1 was 1.6 ± 0.4 pmol oocyte−1 (30 min)−1 (n= 10) and the Na+-dependent αMDG uptake was 776 ± 28 pmol oocyte−1 (30 min)−1 (n= 10). The results from this experiment show that the urea to αMDG transport ratio of rbSGLT1 is approximately 2.1 × 10−3 urea/αMDG.
DISCUSSION
Recent studies have indicated that cotransporters can mediate the transport of water across cell membranes in addition to their usual substrates; for example, the rabbit Na+-glucose cotransporter (rbSGLT1) acts as a water channel and cotransports 260 water molecules along with 2 Na+ and 1 sugar molecule (Loo et al. 1996). In the present study, we have found that cotransporters can also mediate the transport of urea via two distinct pathways: a passive channel and an active cotransport pathway.
Cotransporters can passively transport urea in the absence of a cosubstrate. Cotransporters also have urea transport properties in common with those of urea uniporters and aquaporins known to transport urea. Passive urea transport by rbSGLT1-expressing oocytes: (1) was non-saturable up to 100 mm of urea; (2) was independent of cations, occurring in both NaCl and choline chloride medium; (3) was inhibited by the presence of phloretin (1 mm); and (4) in the presence of Na+, was highly sensitive to phlorizin (Ki 1 μm), a specific inhibitor that blocks conformational changes of the protein allowing for sugar and water transport. In addition, the Ea of passive urea transport through rbSGLT1 is low (≤ 6 ± 1 kcal mol−1) compared to that of control oocytes (14 ± 3 kcal mol−1). This low activation energy corresponds to that of passive water transport through rbSGLT1 (5 kcal mol−1, Meinild et al. 1998; Loo et al. 1999) and aquaporins (≤ 4 kcal mol−1, Preston et al. 1992) suggesting that the urea pathway is a channel. Given the similarities in kinetic properties and activation energies to passive water transport through cotransporters and aquaporins and their ability to discriminate amongst substrates, it would appear that cotransporters contain a selective aqueous pathway that is shared by water and urea.
Cotransporters also have the unique ability of coupling urea transport to substrate transport. In the presence of substrates, urea transport is remarkably similar to substrate-coupled water transport by cotransporters. Urea uptake by rbSGLT1 increased significantly beyond that of passive urea transport and was inhibited by phlorizin. Furthermore, urea is transported in the ratio of its concentration in water. Given that ∼260 water molecules are transported for every 2 Na+/sugar and that ∼2.1 × 10−3 urea molecules are transported per sugar molecule, the urea/water coupling coefficient for rbSGLT1 is 8.1 × 10−6 (range, 3 × 10−6 to 40 × 10−6 urea/H2O). The ratio of urea to water transported is roughly equivalent to the molar concentration ratio in the bulk phase (55 μm urea/55 m H2O).
Can the present observations be due to indirect effects of cotransporter expression in oocytes? In our view this is unlikely for the following reasons. (1) SGLT1 and the other cotransporters we have studied in the oocyte expression system are well behaved. For example, in our extensive studies of SGLT1 in oocytes using radioactive tracers, electrophysiological and electron microscopic techniques, we have found that the properties of Na+-glucose cotransport are as expected from our studies in other expression systems (Sf9 cells, COS cells) and in the native tissue. (2) The kinetics of Na+-glucose cotransport in oocytes are independent of the level of expression (e.g. Hirsch et al. 1996). (3) Freeze-fracture electron microscopic studies show a homogeneous population of monomeric SGLT1 proteins in the oocyte plasma membrane, even at widely different levels of expression (Zampighi et al. 1995; Eskandari et al. 1998). (4) Very specific blockers for the cotransporters, phlorizin for SGLT1 and SGLT3, and SKF 89976A for GAT1, inhibit urea uptake. (5) Only urea transport is correlated to cotransporter expression. Similar increases in permeability were not observed for other molecules such as mannitol and l-alanine.
We previously suggested that water is cotransported by a non-osmotic mechanism; specifically, we hypothesized that water is actively transported as a result of the conformational changes the protein undergoes during the transport of Na+ and sugar (Zeuthen, 1991, 1994; Zeuthen et al. 1996, 1997; Loo et al. 1996, 1999; Meinild et al. 1998, 2000). We now propose that urea is also cotransported by a similar mechanism. The finding that urea transport is coupled to substrate transport provides strong evidence against an osmotic mechanism for substrate-coupled water flow. In addition, the ratio of urea to water transported by rbSGLT1 is approximately equal to its concentration ratio, a proportion that would be significantly less if urea is transported by solvent drag. Our results suggest that urea and water are both transported as a result of protein conformational changes occurring during the cotransport cycle.
The glomeruli in the human kidney filter approximately 54 g of urea from the blood plasma every day. About 12–14 g of the filtered urea is reabsorbed, 40% of which occurs in the proximal tubule. So far, no urea uniporters or channels have been found in the proximal tubule. We suggest that cotransporters, including the Na+-glucose cotransporter, behave as urea channels and urea cotransporters, and may account for urea reabsorption in the proximal tubule. The cotransporters may also have a role in the movement of urea across the plasma membranes in the intestine, thyroid gland and brain.
Acknowledgments
We thank H. Valmonte for assistance with the experiments. This research was supported by NIH grants DK19567, DK44602 and GM52094, and USDA grant 99-35304-7975.
References
- Borgnia M, Nielsen S, Engel A, Agre P. Cellular and molecular biology of the aquaporin water channels. Annual Review of Biochemistry. 1999;68:425–458. doi: 10.1146/annurev.biochem.68.1.425. [DOI] [PubMed] [Google Scholar]
- Eskandari S, Loo DDF, Dai G, Levy O, Wright EM, Carrasco N. Thyroid Na+/I− symporter. Mechanism, stoichiometry, and specificity. Journal of Biological Chemistry. 1997;272:27230–27238. doi: 10.1074/jbc.272.43.27230. [DOI] [PubMed] [Google Scholar]
- Eskandari S, Wright EM, Kreman M, Starace DM, Zampighi GA. Structural analysis of cloned plasma membrane proteins by freeze-fracture electron microscopy. Proceedings of the National Academy of Sciences of the USA. 1998;95:11235–11240. doi: 10.1073/pnas.95.19.11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger MA, Ikeda T, Coady M, Gundersen CB, Wright EM. Expression of size-selected mRNA encoding the intestinal Na+/glucose cotransporter in Xenopus laevis oocytes. Proceedings of the National Academy of Sciences of the USA. 1987;84:2634–2637. doi: 10.1073/pnas.84.9.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JR, Loo DDF, Wright EM. Regulation of Na+/glucose cotansporter expression by protein kinases in Xenopus laevisoocytes. Journal of Biological Chemistry. 1996;271:14740–14746. doi: 10.1074/jbc.271.25.14740. [DOI] [PubMed] [Google Scholar]
- Ikeda TS, Hwang E-S, Coady MJ, Hirayama BA, Hediger MA, Wright EM. Characterization of a Na+/glucose cotransporter cloned from rabbit small intestine. Journal of Membrane Biology. 1989;110:87–95. doi: 10.1007/BF01870995. [DOI] [PubMed] [Google Scholar]
- Kato A, Sands JM. Evidence for sodium-dependent active urea secretion in the deepest sub segment of the rat inner medullary collecting duct. Journal of Clinical Investigation. 1998;101:423–428. doi: 10.1172/JCI1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S, Kokko JP. Urea secretion by the straight segment of the proximal tubule. Journal of Clinical Investigation. 1976;58:604–612. doi: 10.1172/JCI108507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo DDF, Hirayama BA, Meinild A-K, Chandy G, Zeuthen T, Wright EM. Passive water and ion transport by cotransporters. Journal of Physiology. 1999;518:195–202. doi: 10.1111/j.1469-7793.1999.0195r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo DDF, Zeuthen T, Chandy G, Wright EM. Cotransport of water by the Na+/glucose cotransporter. Proceedings of the National Academy of Sciences of the USA. 1996;93:13367–13370. doi: 10.1073/pnas.93.23.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie B, Loo DDF, Wright EM. Relationships between Na+/glucose cotransporter (SGLT1) currents and fluxes. Journal of Membrane Biology. 1998;162:101–106. doi: 10.1007/s002329900347. [DOI] [PubMed] [Google Scholar]
- Meinild A-K, Klaerke DA, Loo DDF, Wright EM, Zeuthen T. The human Na+-glucose cotransporter is a molecular water pump. Journal of Physiology. 1998;508:15–21. doi: 10.1111/j.1469-7793.1998.015br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinild A-K, Loo DDF, Pajor AM, Zeuthen T, Wright EM. Water transport by the renal Na+-dicarboxylate cotransporter. American Journal of Physiology. 2000;278:F777–783. doi: 10.1152/ajprenal.2000.278.5.F777. [DOI] [PubMed] [Google Scholar]
- Panayotova-Heiermann M, Loo DDF, Lostao MP, Wright EM. Sodium/D-glucose cotransporter charge movements involve polar residues. Journal of Biological Chemistry. 1994;269:21016–21020. [PubMed] [Google Scholar]
- Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopusoocytes expressing red cell CHIP28 protein. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Tsukaguchi H, Shayakul C, Berger UV, Hediger MA. Urea transporters in kidney: molecular analysis and contribution to the urinary concentrating process. American Journal of Physiology. 1998;275:F319–324. doi: 10.1152/ajprenal.1998.275.3.F319. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Kreman M, Boorer KJ, Loo DDF, Bezanilla F, Chandy G, Hall JE, Wright EM. A method for determining the unitary functional capacity of cloned channels and transporters expressed in Xenopus laevis oocytes. Journal of Membrane Biology. 1995;148:65–78. doi: 10.1007/BF00234157. [DOI] [PubMed] [Google Scholar]
- Zeuthen T. Secondary active transport of water across ventricular cell membranes of choroid plexus epithelium of Necturus maculosus. Journal of Physiology. 1991;444:153–173. doi: 10.1113/jphysiol.1991.sp018871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen T. Cotransport of K+, Cl−, and H2O by membrane proteins from choroid plexus epithelium of Necturus maculosus. Journal of Physiology. 1994;478:203–219. doi: 10.1113/jphysiol.1994.sp020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen T, Hamann S, la Cour M. Cotransport of H+, lactate and H2O by membrane proteins in retinal pigment epithelium of bullfrog. Journal of Physiology. 1996;497:3–17. doi: 10.1113/jphysiol.1996.sp021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen T, Meinild A-K, Klaerke DA, Loo DDF, Wright EM, Belhage B, Litman T. Water transport by the Na+/glucose cotransporter under isotonic conditions. Biology of the Cell. 1997;89:307–312. doi: 10.1016/s0248-4900(97)83383-7. [DOI] [PubMed] [Google Scholar]
- Zhang R, Verkman AS. Water and urea permeability properties of Xenopus oocytes: expression of mRNA from toad urinary bladder. American Journal of Physiology. 1991;260:C26–34. doi: 10.1152/ajpcell.1991.260.1.C26. [DOI] [PubMed] [Google Scholar]


