Abstract
The effect of inflammation on the excitability and the level of substance P (SP) in cat mechanoreceptive C and Aδ dorsal root ganglion (DRG) neurons were studied in vivo using intracellular recording and immunocytochemical techniques.
Following injections of carrageenan (Carg) into the cat hindpaw, the percentage of C neurons exhibiting spontaneous activity increased from 7.2 to 20.7 % and the percentage of Aδ neurons increased from 6.9 to 18.6 %. In contrast to most cells from normal cats, which fired regularly below 10 Hz, many cells from Carg-treated cats fired at higher frequencies or in bursts.
Inflammation (Carg treatment) also depolarized membrane potentials, increased membrane input resistance, caused the disappearance of inward rectifying currents and lowered the mean current thresholds of tibial nerve-evoked responses in DRG neurons.
With inflammation, the percentage of C or Aδ neurons responding to low threshold mechanoreceptive stimuli increased (C neurons: normal, 13 %; inflamed, 41 %; Aδ neurons: normal, 13 %; inflamed, 39 %), while the percentage of C or Aδ neurons responding to high threshold mechanoreceptive stimuli remained unchanged.
Some receptive field (RF)-responsive cells were injected with Lucifer Yellow and their SP immunoreactivity was determined. Following Carg treatment, substantially higher percentages of RF-responsive cells were SP positive (C neurons: normal, 35.7 %; inflamed, 60 %; Aδ neurons: normal, 18.2 %; inflamed, 66.7 %).
These combined increases in the excitability of DRG neurons and SP-containing RF-responsive neurons could lead to sensitization of sensory neurons, thus contributing to the development of hyperalgesia.
Inflammation caused by injection of carrageenan (Carg) into the hindpaw has been used as a model for studying hyperalgesia accompanying inflammatory injuries. Behavioural studies show that thermal and mechanical pain thresholds begin to decline 2 h after Carg injection and hyperalgesia reaches a peak 3–4 h later (Kocher et al. 1987; Satoh et al. 1992; Traub, 1996; Kilo et al. 1997). Since Carg treatment enhances c-fos expression in the spinal cord (Buritova et al. 1996), increases the excitability of spinal dorsal horn neurons (Ren et al. 1992; Ma & Woolf, 1996) and facilitates synaptic inputs (Baba et al. 1999), sensitization of neurons in the central nervous system is thought to contribute to hyperalgesia (Woolf & Thompson, 1991; Ren et al. 1992; Treede et al. 1992). Because Carg-induced inflammation activates silent (i.e. receptor field-unresponsive) nociceptors and potentiates the activity of primary afferents (Schaible & Schmidt, 1985, 1988), sensitization of sensory neurons also could contribute to hyperalgesia.
Substance P (SP) is an important neuromodulator in primary sensory neurons (Salt & Hill, 1983; Willis & Coggeshall, 1991; Otsuk & Yoshioka, 1993; Iversen, 1998; Cao et al. 1998). Peripheral inflammation increases SP expression in the dorsal root ganglion (DRG) (Noguchi et al. 1988; Donnerer et al. 1992; Galeazza et al. 1995; McCarson & Krause, 1995; Neumann et al. 1996; McCarson, 1999), and triggers SP release in the spinal dorsal horn (Duggan et al. 1987; Oku et al. 1987; Schaible et al. 1990; Garry & Hargreaves, 1992; Zhao et al. 1992). Although both SP expression and the responsiveness of nociceptors to receptive field (RF) stimuli undergo profound changes during inflammation (Schaible & Schmidt, 1985, 1988), the relationship between SP and RF-responsive neurons in inflamed tissues remains unclear. In normal cats, Leah et al. (1985) found that SP-containing C-fibre neurons were not responsive to peripheral noxious stimulation while a majority of DRG C-fibre neurons responding to peripheral stimuli did not contain SP. Lawson et al. (1997) showed that all SP-positive cells in normal guinea-pig DRG with identified receptive properties were nociceptive, although not all nociceptors were SP positive. Schmidt and others proposed that many nociceptors in cat or rat peripheral tissues were silent under physiological conditions, but became RF responsive following the induction of inflammation or neuropathy (Schaible & Schmidt, 1988; Habler et al. 1988; McMahon & Koltzenburg, 1990; Schmidt et al. 1994). It is, therefore, conceivable that the unresponsive DRG C-fibre neurons reported by Leah et al. (1985) were SP-containing RF-unresponsive nociceptors. If this is correct, the proportion of SP-containing RF-responsive neurons should increase following inflammation. To determine the properties of DRG neurons and to find the relationship between SP expression and the RF responsiveness of these cells in the inflammatory state, we recorded DRG neurons in vivo and determined the SP content in identified RF-responsive DRG cells.
METHODS
Animal preparation
Experiments were performed on 62 adult cats of either sex weighing 2–4 kg. Cats were first anaesthetized with an intraperitoneal injection of sodium pentobarbitone (40 mg kg−1). A catheter was then inserted into the superficial vein of the right forelimb for further intravenous application of sodium pentobarbitone. When needed, a dose of 10 mg kg−1 sodium pentobarbitone was administered during the course of an experiment. Blood pressure was continuously monitored with a sphygmomanometer in the left carotid artery. The depth of anaesthesia of the animal was judged to be adequate when the pupils were closed and the blood pressure remained stable after strong paw pinches or C-strength electrical stimulation. A cannula was inserted into the trachea to allow artificial respiration and the end-tidal CO2 level was kept at 3.5–5.0 %. The animal was then placed on a stereotaxic frame and the spinal cord was immobilized with clamps. Gallamine triethiodide (4 mg kg−1, i.v.) was applied to paralyse the animal before experiments. The rectal temperature was maintained at 36–38°C using a thermostatically regulated heater. Glucose-containing (5 %) saline was infused into the right superficial vein at a rate of 0.1 ml min−1. An experiment was discontinued when the blood pressure dropped below 80 mmHg.
The recording procedure has been described before (Song & Zhao, 1994; Tao et al. 1997). Briefly, following a laminectomy of the spinal cord from segments L1 to S1, the cord was transected at the L1–L2 level. Bilateral DRGs at L7 were exposed. The dura and the connective tissue capsule were removed from the ganglion. Agar (2.5 %) was poured over the exposed spinal cord and DRGs. A small opening was made at the recorded ganglion and a warm (32–34°C) Krebs solution was continuously perfused (3–5 ml min−1). The solution contained (mm): NaCl 117.0, KCl 4.7, MgCl2 1.2, CaCl2 2.5, NaH2PO4 1.2, NaHCO3 25.0, and glucose 11.5. The pH of the solution was maintained at 7.3–7.4 by bubbling it with 95 %O2 and 5 %CO2 gas. The tibial or sciatic nerves were dissected out for electrical stimulation. A pool was made with flaps of skin and warm mineral oil was poured over the tibial or sciatic nerve. For induction of peripheral inflammation (Castro-Lopes et al. 1994; Tsuruoka et al. 1997), a total of 2–3 ml (20–30 mg kg−1) of Carg (3 %, Sigma) solution was injected subcutaneously at various locations in the cat hindpaw 2 h before recordings began. In control animals, the same amount of saline (0.9 % NaCl) solution was injected instead. After the conclusion of the experiments, the animals were killed with an overdose of sodium pentobarbitone followed by an i.v. injection of 10 ml saturated KCl. All experiments were performed according to the IASP guidelines which were approved by the Research Centre on Animals in Shanghai Brain Research Institute, Chinese Academy of Sciences.
Identification of conduction velocity of DRG neurons
Intracellular recordings were made from the somata of DRGs using a microelectrode filled with 4 m potassium acetate or Lucifer Yellow (30–50 mg ml−1 dissolved in 0.5 m LiCl solution). The typical resistance of the potassium acetate electrodes was 30–50 MΩ and of the Lucifer Yellow electrodes 80–160 MΩ. Once a stable recording was established, the tibial nerve was stimulated with 8 mA and 0.5 ms pulses (a supramaximal strength for C-afferent fibre activation) to evoke action potentials in the somata. Axonal conduction velocity (CV) was calculated from the latency of action potentials recorded in the DRG and the distance between the recording electrode at the DRG soma and the stimulating electrode at the tibial nerve. From the CV histogram, we could divide the CVs of neurons into three groups: > 20.0 m s−1, 2.0–20.0 m s−1 and < 2.0 m s−1, which correspond to Aβ-, Aδ- and C-afferent neurons as reported by others (Schmidt et al. 1994; Tao et al. 1997). Only Aδ and C neurons were studied.
The input resistance (Rin=ΔVmΔI−1) of a neuron was determined by passing a hyperpolarizing current of 0.05 or 0.1 nA through the microelectrode (i.e. ΔI= 0.05 or 0.1 nA) and recording changes in the membrane potential (ΔVm). In some experiments, action potentials were evoked by passing depolarizing currents through microelectrodes. An Axoclamp-2A amplifier (Axon Instruments) operated in the bridge mode was used to record spontaneous discharges and evoked responses. Data were stored on computer disks. The frequencies and patterns of spontaneous activities were analysed off-line with SMUP software (provided by Shanghai Medical University, P. R. of China).
Identification of receptive field of neurons
After measuring the CV, we searched for the mechanical RF of the recorded neuron. For noxious mechanical pinch, the skin of the hindlimb was pinched with a serrated forceps of ∼2.5 N force for 1–2 s, a strength that produced moderate pain when applied to human skin (Tao et al. 1997). For innocuous stimulation, repeated broad 1–2 s strokes of ∼0.2 N force were applied to the cutaneous surface of the hindlimb with a camel hair paint brush. According to the responses to the mechanical stimuli, the recorded neurons were categorized into: (1) low threshold mechanical (LTM) neurons which responded to brush stimuli, (2) high threshold mechanical (HTM) neurons which responded to noxious pinch stimuli, and (3) non-responding (silent) neurons which responded neither to brush nor pinch. Since noxious heat was not tested here, the HTM neurons could include polymodal nociceptors that responded to both noxious mechanical and heat stimulation. We adopted the term ‘silent neurons’ used by Schaible & Schmidt (1985, 1988) to emphasize the unresponsiveness of these neurons to our RF stimuli. Since the stimuli were not exhaustive, it should be noted that these neurons could well be responsive to stimuli not tested, e.g. heat or vibration.
Some RF-responsive cells were labelled with Lucifer Yellow (LY), which was applied electrophoretically with hyperpolarizing 3–4 nA, 350 ms rectangular pulses at 1 Hz for 3–5 min (Smithson et al. 1984; Leah et al. 1985; Tao et al. 1997). The total currents passed for the LY injection were 3.0–5.3 nA min−1. These values were much lower than those (16–60 nA min−1) used by Scharfman et al. (1989), who found that high levels of intracellular dye, including LY, might mask immunoreactivity of transmitters, e.g. GABA. It therefore is unlikely that the SP immunoreactivity in our experiments was affected by LY injections. To ensure that LY was indeed injected into the cell, electrical stimuli were again applied to the same cell after the dye injection. If action potentials could no longer be evoked, the cell was rejected from the study. Initially, we labelled only one cell in each DRG. In later experiments, two neurons at opposite ends of the ganglia were injected. To exclude inappropriately marked cells, the depth and the position of each LY-injected cell was recorded and compared with those obtained in immunocytochemical experiments. Those ganglia that had more than expected LY-labelled cells or had labelled cells at unexpected locations were not included in the analyses.
Following injections of LY, animals were killed with an overdose of sodium pentobarbitone. DRGs were dissected out, fixed with 4 % paraformaldehyde for 8–10 h and left overnight in a sucrose (20 %)-containing phosphate buffer solution (PBS) at 4°C. Serial frozen sections of 30 μm thickness were then cut and mounted on slides previously coated with gelatin (0.1 %, by weight gelatin, in 0.0002 m CrK(SO4)2.12H2O). This section thickness was chosen because it gave the most consistent results. Since the diameters of some small cells were < 30 μm, the shapes of the LY-labelled cells were carefully recorded to minimize the possibilities of false identification of SP labelling. Dye-labelled neurons were located and photographed under a fluorescence microscope with a camera lucida attachment. The location and neuronal profiles were drawn in detail.
Identification of SP immunoreactivity in LY-labelled RF neurons
Sections containing LY-labelled neurons were incubated in 0.3 % H2O2 for 10 min to block endogenous peroxidases. After being washed in cold PBS for 30 min, the sections were incubated at 4°C in a PBS solution containing antiserum to SP (Peninsula Lab. Inc., 1:5000), 5 % goat serum and 0.1 % Triton X-100. Forty-eight hours later, sections were washed with PBS three times for 30 min and incubated in goat anti-rabbit IgG (Vector, 1:200) at 37°C. After 2 h, sections were washed again and incubated in ABC-peroxidase complex (Vector, 1:100) for another 2 h at 37°C. Sections were then washed with Tris-HCl buffer (50 mmol l−1, pH 7.2) and reacted with diaminobenzidine (0.05 mg ml−1) and 0.01 % H2O2. The specificity of the antisera were tested by replacing the primary antisera with normal rabbit sera or by omitting the primary or secondary antibodies in the experiment. No immunoreactivity was detected under these conditions. After the immunocytochemical procedure, the sections were air-dried, dehydrated, cleared and coverslipped. The SP-like immunoreactivity (SP-LI) in LY-labelled neurons was evaluated under a microscope.
Data analyses
Quantitative data (threshold, resting potentials and input resistance) were expressed as means ±s.e.m.a and their statistical significance was determined using Student’s two-tailed t test. The significance of frequency data (e.g. RF, SP-LI, spontaneously active neurons) was determined with the χ2 test.
RESULTS
Conduction velocity, current threshold and firing patterns
The cat hindpaw started to swell 2 h after Carg injection. The swelling reached a peak around 4 h and persisted for more than 8 h. Intracellular recordings of L7 DRG neurons were conducted during the 2–8 h post-injection period. A total of 452 neurons were recorded from L7 DRGs, 242 of which were from Carg-treated animals and 210 neurons were from control animals. All neurons displayed stable resting potentials ranging from −40 to −75 mV. Using the latency of evoked action potentials, we divided the recorded neurons into Aβ (CV>20 m s−1), Aδ (CV between 2 and 20 m s−1) and C (CV< 2 m s−1) neurons. Only Aδ and C afferent neurons were studied in detail. There was no significant difference in the frequency distribution histograms of CVs obtained from control and Carg-treated groups.
Current stimulations of the tibial nerve induced action potentials in both control and Carg-treated (inflamed) DRG neurons. Threshold currents for tibial nerve stimulation, defined as the smallest current required to evoke action potentials in the DRG soma, were much lower in Carg-treated than in control cats. Mean threshold currents for nerve stimulation in normal cats were 5.3 ± 0.4 mA (n= 40) for C neurons and 3.9 ± 0.3 mA (n= 23) for Aδ neurons. After inflammation, mean threshold currents were 3.3 ± 0.5 mA for C neurons (n= 49) and 2.1 ± 0.4 mA for Aδ neurons (n= 54; P < 0.05; Fig. 1).
Figure 1. Carrageenan-induced inflammation decreased the current stimulation thresholds of tibial nerves.
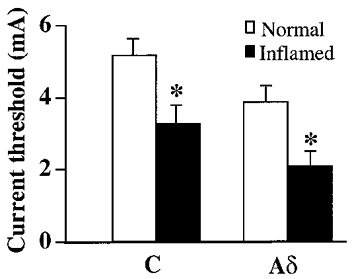
Under control conditions, the mean current threshold was 5.3 mA for C neurons and 3.9 mA for Aδ neurons. After inflammation, the mean current threshold was 3.3 mA for C neurons and 2.1 mA for Aδ neurons. The changes were significant (*P < 0.05).
We then studied the firing patterns of DRG neurons evoked by intracellular current injection. With close to threshold stimuli, the intracellular current injection evoked single action potentials in most neurons recorded from normal cats, but evoked multiple spikes in neurons from Carg-treated cats (Fig. 2A). To determine the percentage of cells exhibiting repetitive firings, suprathreshold rectangular current pulses (0.6 nA, 200 ms) were injected into the somata via recording microelectrodes. In the control group, the current injections evoked single action potentials in ∼95 % of the cells. Only 5.1 % (7/138) of C and 5.6 % (4/72) of Aδ neurons fired repetitively. After Carg treatment, the same current stimuli evoked repetitive action potentials in larger percentages of C (32.1 % (45/140), P < 0.001) and Aδ (17.7 % (18/102), P < 0.01) neurons (Fig. 2B).
Figure 2. Inflammation changed the waveforms of the evoked responses of C and Aδ neurons.
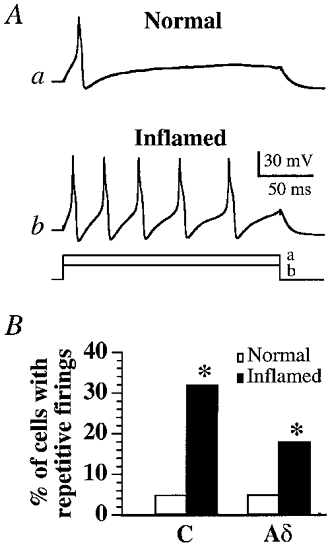
Intracellular current stimulation of a C neuron at an intensity of 0.32 nA induced single action potentials in a normal cat (Aa) while a weaker stimulus intensity (0.22 nA) could evoke repetitive firings in a C neuron from a Carg-treated cat (Ab). The bottom traces in A indicate the duration and the magnitude of the intracellular currents injected into these two cells. B, bar graph showing the percentages of C and Aδ neurons that exhibited repetitive discharges evoked by suprathreshold rectangular current pulses (0.6 nA, 200 ms). A significantly higher percentage (*P < 0.05) of C and Aδ neurons in Carg-treated cats fire repetitively.
Membrane properties
In addition to changes in the active membrane properties, the resting potential (RP) and membrane input resistance (Rin) changed noticeably following the Carg injection. Resting membrane potentials and Rins of various cells were recorded at different time points after Carg treatment (Fig. 3). Since the RPs and Rins of a neuron collected at different time points from normal rats during the course of experiments did not change significantly (P >0.05), the RPs and Rins obtained under these conditions were pooled together and taken as the zero point values (Fig. 3B). Mean resting potentials recorded 4–6 h after the injection were more depolarized (C neurons: normal, −53.0 ± 1.8 mV (n= 40); inflamed, −45.0 ± 2.6 mV (n= 29), P < 0.05; Aδ neurons: normal, −58.1 ± 1.9 mV (n= 23); inflamed, −51.8 ± 2.3 mV (n= 23), P < 0.05; Fig. 3A). The Rins were significantly higher following Carg treatment. The average Rin of Aδ neurons increased from 59.0 ± 12.1 MΩ (n= 23) in normal cats to 71.9 ± 9.8 MΩ (n= 20) (P < 0.05) 2–4 h after injection and to 72.5 ± 10.0 MΩ (n= 23) (P < 0.05) 4–6 h after injection. Increases in the Rins of C neurons lasted up to 8 h after Carg treatment (normal: 72.0 ± 9.2 MΩ (n= 40), inflamed at 2–4 h: 89.0 ± 9.5 MΩ (n= 20), P < 0.05; at 4–6 h: 95.0 ± 0.6 MΩ (n= 29), P < 0.05; at 6–8 h: 118.0 ± 14.9 MΩ (n= 22), P < 0.05; Fig. 3B).
Figure 3. The changes in resting membrane potentials (RP) and input resistances (Rin) of Aδ and C neurons after the injection of Carg.
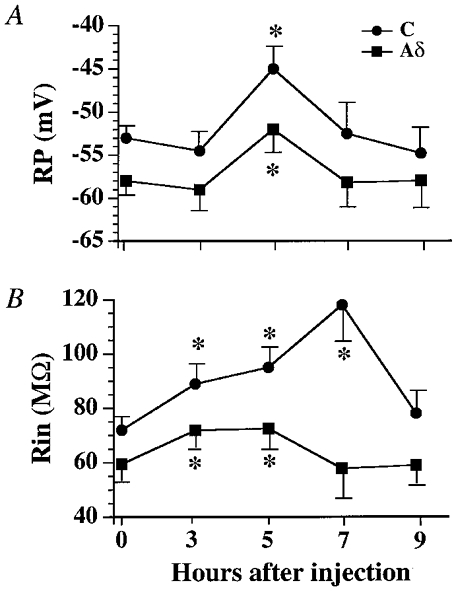
A, RPs became significantly depolarized 4–6 h after Carg injections (*P < 0.05). B, Rins were significantly elevated (*P < 0.05) between 2 and 6 h for Aδ neurons and 2 and 8 h for C neurons following the Carg treatment. The values at 0 h indicate the mean values for RPs or Rins obtained from normal cats. The values at 3, 5, 7 and 9 h were the mean RPs or Rins obtained from tested cells measured between 2 and 4 h, 4 and 6 h, 6 and 8 h, and 8 and 10 h after inflammation, respectively.
The membrane conductances of C neurons were also examined under current-clamp conditions. In the normal group (6 cats), injection of hyperpolarizing currents into LTM (n= 6) and HTM C (n= 6) neurons generated transient hyperpolarizations which decayed to steady-state levels within 25 ms (Fig. 4A). The voltage-current relationship exhibited a distinct inward rectification (Fig. 4B). In the Carg-treated group (8 cats), however, hyperpolarizing current stimuli produced steady electrotonic potentials 2–6 h after Carg. The depolarizing sags were completely absent and the voltage-current curve was linear in both LTM (n= 8) and HTM C (n= 4) neurons. With increasing Rins in these neurons, the same current stimuli would produce larger hyperpolarizations and rebounding action potentials.
Figure 4. Loss of inward rectification in electrotonic potentials in Carg-treated cat DRG neurons.
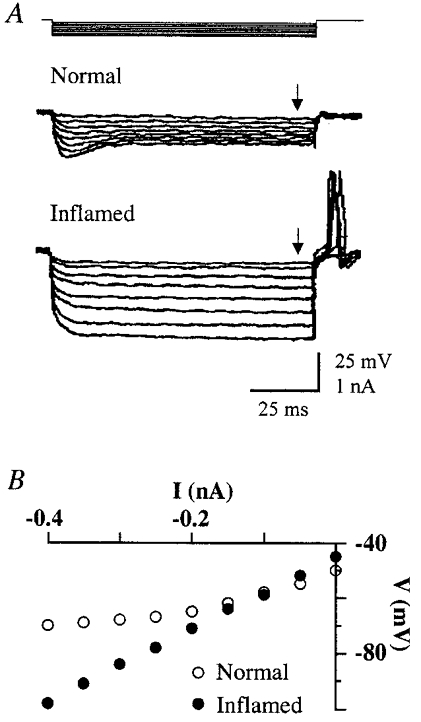
A, typical electrotonic potentials recorded from C neurons in normal and Carg-treated cats. The hyperpolarizing current steps of 200 ms duration used to evoke the responses are shown at the top. B, steady-state voltage-current curves from data given in A. (The arrows in A indicate the time points at which the measurements were taken.) Note the electrotonic potentials exhibit inward rectification at hyperpolarizing potentials in the normal cat but not in the Carg-treated cat.
Spontaneous activity
In normal cats, spontaneous discharges were observed in 7.2 % (10/138) of C neurons and 6.9 % (5/72) of Aδ neurons. In Carg-treated cats, a significantly higher percentage of C neurons (20.7 % (29/140), P < 0.005) and of Aδ neurons (18.6 % (19/102), P < 0.05) were spontaneously active (Fig. 5A). Furthermore, the Carg treatment altered the firing patterns of neurons. Three spontaneous firing patterns, i.e. regular, irregular and burst firing, were observed in DRG neurons (Table 1). In normal cats, most spontaneously active cells fired regularly at low frequencies (Fig. 5B, Table 1). In Carg-treated cats, more cells fired spontaneously at high frequencies (> 10 Hz; Fig. 5Ca) or in bursts (Fig. 5Cb; Table 1).
Figure 5. Inflammation changed the occurrence and patterns of the spontaneous firing of DRG neurons.
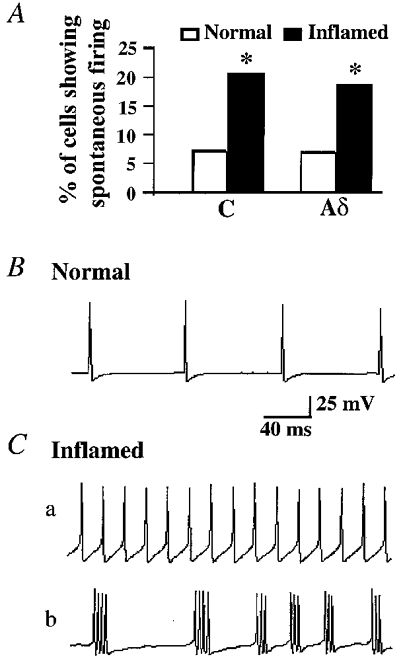
A, the percentage of C and Aδ cells exhibiting spontaneous firing increased significantly (*P < 0.05) following Carg treatment. B, an Aδ neuron recorded from the normal cat exhibited regular spontaneous firings. C, examples of spontaneous firing patterns recorded from DRG neurons in Carg-treated cats. Aδ neurons recorded from the Carg-treated cat fired at high frequency (80 Hz) (a) or in bursts (b).
Table 1.
Number of DRG cells with various spontaneous firing patterns in normal and Carg_treated cats
| Regulara | |||||||
|---|---|---|---|---|---|---|---|
| Cell type | Total | < 10 Hz | 10–30 Hz | 30–50 Hz | >50 Hz | Burstingb | Irregularc |
| C cells | |||||||
| Normal | 10 | 7 | 2 | — | — | 1 | — |
| Inflamed | 28 | 10 | 5 | 4 | 2 | 4 | 3 |
| Aδ cells | |||||||
| Normal | 5 | 4 | 1 | — | — | — | — |
| Inflamed | 19 | 5 | 2 | 4 | 1 | 5 | 2 |
Regular: cells fire with constant interspike intervals.
Bursting: cells fire with bursts of 3–5 action potentials.
Irregular: cells fire with variable interspike intervals.
To determine the origin of the spontaneous activity of DRG neurons in Carg-treated cats, neuronal activity was measured following sectioning of nerves at proximal and/or distal ends of the ganglia (C neuron, n= 6; Aδ neuron, n= 2; Fig. 6). Transection of dorsal roots did not affect the spontaneous activity of DRG neurons (Fig. 6B, arrow a). On the other hand, the activity of the neurons ceased completely within 15–20 s after the tibial or sciatic nerve was sectioned (Fig. 6B, arrow b). Since the resting potentials of recorded cells remained stable for > 30 min after transection, and intracellular current stimulation still elicited spikes in these cells, sectioning of nerve fibres did not abolish the intrinsic excitability of the cells. Thus, the spontaneous discharge of DRG neurons in Carg-treated cats appears to initiate at peripheral nerve terminals or/and axons.
Figure 6. Spontaneous activity initiated at peripheral afferent terminals.
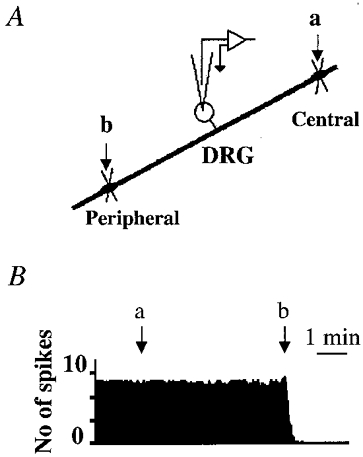
A, schematic diagram illustrating the locations of the section. B, after the central terminals of the recorded DRG C neuron in the Carg-treated cat were sectioned (arrow a) there was no change in the spontaneous activity. However, when the peripheral nerves were sectioned (arrow b) the spontaneous firing ceased completely.
Mechanical receptive fields and SP immunoreactivity
While > 90 % of the spontaneously active cells were mechanical RF responsive in both normal and Carg-treated cats, most (>75 %) of the RF-responsive cells did not fire spontaneously. After Carg treatment, the percentages of RF-responsive C neurons increased from 32.6 % (45/138 cells) to 58.6 % (82/140 cells; P < 0.05) and RF-responsive Aδ neurons increased from 34.7 % (25/72 cells) to 54.9 % (56/102 cells) (P < 0.05; Fig. 7B). Thus, inflammation activated 26.0 % of RF-unresponsive C neurons and 20.2 % of RF-unresponsive Aδ neurons. To determine whether inflammation altered the nature of the responsiveness of neurons, percentages of LTM and HTM neurons in control and Carg-injected cats were analysed (Fig. 7A and C). Among the 138 C neurons recorded from normal cats, 18 (13.0 %) were LTM neurons and 27 (19.6 %) were HTM neurons (Fig. 7C). Of the 72 recorded Aδ neurons, 9 (12.5 %) were LTM neurons and 16 (22.2 %) were HTM neurons. After inflammation, 57/140 recorded C neurons (40.7 %) were LTM neurons and 25/140 neurons (17.9 %) were HTM neurons. Of the Aδ neurons 40/102 (39 %) were LTM neurons and 16/102 (15.7 %) were HTM neurons. Thus, inflammation did not alter the percentage of the HTM neurons (P > 0.05), but significantly increased the percentage of the LTM neurons (P < 0.01; Fig. 7).
Figure 7. Inflammation changed the characteristics and responsiveness of peripheral mechanical receptive fields of C and Aδ neurons.
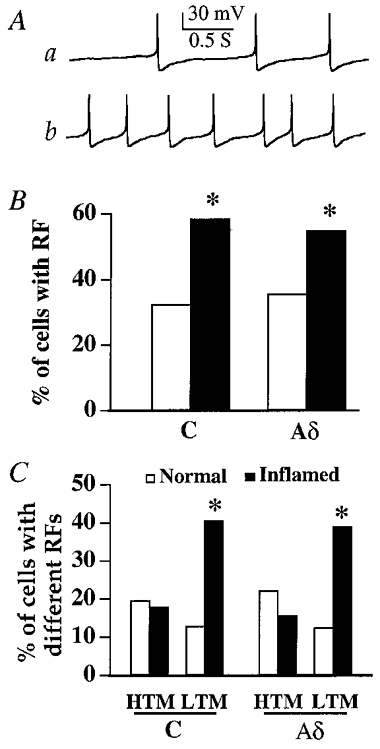
A, examples of action potentials evoked by both brush (a) and pinch (b) stimuli in a C neuron of a Carg-treated cat. This neuron is a LTM type. B, the percentages of C and Aδ neurons that had RFs increased significantly (*P < 0.05) after inflammation. C, the RF-responsive neurons were divided into LTM and HTM neurons. Following Carg treatment, the percentages of HTM C and Aδ neurons did not change. On the other hand, the percentages of LTM C and Aδ neurons were significantly higher (*P < 0.05).
Among the cells which were found to have RFs, 100 cells were injected with LY. These ganglia were then fixed and reacted for SP (Fig. 8A and B). For C neurons, 35.7 % of neurons (15/42) from control cats and 60.0 % (21/35) of the neurons from the Carg-treated cats were SP-LI positive. For Aδ neurons, only 18.2 % (2/11) of the neurons from control cats, but 66.7 % of neurons (8/12 cells) from the Carg-treated cats were SP-LI positive. Thus, the number of SP-LI-positive C and Aδ cells that responded to the stimulation of RFs increased significantly (P < 0.05 for both types of C and Aδ neurons) following Carg-induced inflammation (Fig. 8C).
Figure 8. Substance P expression in RF-responsive cells increased with inflammation.
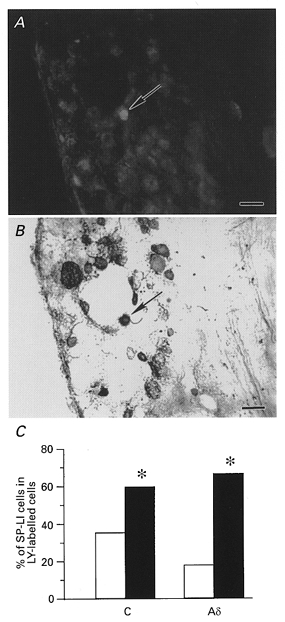
A, a LTM C neuron from a cat with inflamed cells was labelled with LY (indicated by arrow). B, the same neuron exhibited SP immunoreactivity (indicated by arrow). Scale bars in A and B, 50 μm. C, bar graph showing that a significantly higher percentage of LY-labelled C and Aδ DRG neurons were SP immunoreactive after inflammation (*P < 0.05 for both types of neurons). □, normal; ▪, inflamed cells.
DISCUSSION
After Carg-induced inflammation in the cat hindpaw, DRG neurons undergo the following changes: (1) firing frequencies are enhanced, (2) more cells become responsive to receptive field stimulation, and (3) an increasing number of RF-responsive cells display SP immunoreactivity. Since changes in the resting membrane potential and membrane-input resistance of DRG cells coincide with the time course of the behavioural effects of Carg (Kocher et al. 1987; Traub, 1996; Tsuruoka et al. 1997) and c-fos expression in the dorsal horn following Carg treatment (Buritova et al. 1996), the above plastic changes in the membrane characteristics of DRG neurons are likely to underlie the development of hyperalgesia. Because spontaneous discharges of DRG neurons in Carg-treated cats ceased immediately and completely after the tibial nerve was sectioned (Fig. 6), the increased activity of DRG neurons we observed depends on peripheral inputs. We also demonstrate that more LTM C and Aδ DRG cells respond to receptive field stimulation in Carg-treated cats than those in normal cats (Fig. 7). Although the exact mechanism underlying this increase is unclear, inflammatory mediators (e.g. histamine, serotonin, bradykinin, prostaglandin E2, SP and nerve growth factors), which are released from peripheral tissue, macrophages and lymphocytes following tissue damage, are likely to be responsible for this change. These mediators are known to excite and/or sensitize peripheral terminals (Schaible & Schmidt, 1988; Lembeck et al. 1992; Otsuka & Yoshioka, 1993) and promote spontaneous activity in DRG neurons (Schaible & Schmidt, 1985). Under long-term inflammatory conditions (> 2 days), others found that the duration of DRG neuron action potentials are shortened (Djouhri & Lawson, 1999) and A-type K+ currents are suppressed (Yoshimura & de Groat, 1999). The mechanisms underlying the short-term changes (2–8 h) in membrane properties of DRG neurons studied here may differ from those studied before. The excitability of DRGs is nevertheless enhanced. As DRG neurons become more depolarized (Fig. 3A), and susceptible to intracellular current stimulation (Fig. 2B) in inflammatory states, the sensitivity of neurons to peripheral inputs would be further augmented and thus, in turn, lead to sensitization of DRG neurons.
It is interesting that inflammation results in a reduction of hyperpolarization-activated inward rectifier and an increase in Rin (Fig. 4). Since the change in Rin lasts only up to 6 h while inflammation often persists for a longer period, the conductance changes seem to relate to the development but not the maintenance of hyperalgesia. Although the ionic mechanism of the inward rectifier has yet to be identified, hyperpolarization-activated currents (Ih) and inward rectifying K+ currents are likely to be involved (Scroggs et al. 1994; Wang et al. 1997). The mechanism underlying the inactivation of the inward rectifier following inflammatory injury is not known. One possibility could be increases in the release of peptides, e.g. SP and calcitonin gene-related peptide (CGRP), from the somata of DRG neurons. Several studies seem to be consistent with this possibility. We show here that more RF-responsive cells are SP immunoreactive (Fig. 8) following Carg treatment. Others show that the release of SP and CGRP and the content of these peptides increase substantially 2–6 h after the development of inflammation (Galeazza et al. 1995; McCarson & Krause, 1995; Honor et al. 1999; McCarson, 1999). Furthermore, SP is released directly from the somata of DRG neurons (Huang & Neher, 1996), induces membrane depolarization and increases membrane input resistance of isolated DRG neurons (Dray & Pinnock, 1982; Parker & Grillner, 1996; Z. Wang & Z.-Q. Zhao, unpublished observations). In central neurons, SP increases neuronal excitability by reducing an inward rectifying conductance (Stanfield et al. 1985). Future studies of the modulatory actions of SP or CGRP in the DRG are thus required to determine if these peptides indeed cause the inactivation of an inward rectifier and thus increase the activity of DRG neurons in the inflammatory state.
Another finding is that inflammation increases the RF responsiveness of cells. Surprisingly, Carg treatment appears to increase only the percentage of the LTM cells, but not the percentage of HTM cells. The mechanism underlying this change is unclear. One possible explanation may be that inflammation lowers the threshold of neurons to mechanical stimuli and makes them respond to normally unresponsive stimuli. Thus, silent neurons become activated with high threshold stimuli and HTM cells activated with low threshold stimuli. This then leads to the increased percentage of LTM cells. We further showed that Carg-induced inflammation enhances the percentage of mechanical RF-responsive cells that express SP. In normal cats, 35.7 % of C neurons and 18.2 % of Aδ neurons are RF responsive. After inflammation, RF-responsive C neurons are increased by 25.8 % (Fig. 7B, Table 2). The percentage of RF-responsive C cells that are SP positive is enhanced by a similar extent (24.3 %) (Fig. 8C, Table 2). This result is consistent with the idea that inflammation converts SP-containing mechanically silent, to RF-responsive, neurons. Such converted neurons could be the same type of neurons recorded by Leah et al. (1985) who found that a large percentage of the SP-containing C-fibre cells in normal conditions were not RF responsive. Our results also support the proposal that mechanically silent neurons can be activated by peripheral mechanical stimulation under pathophysiological conditions following the induction of experimental arthritis or tissue inflammation (Schaible & Schmidt, 1985, 1988; Schmidt et al. 1994). The observations obtained from Aδ neurons are somewhat different, however. Inflammation increases RF-responsive Aδ neurons by 20.2 % (Fig. 7B, Table 2). However, much higher percentages (48.5 %) of RF-responsive Aδ cells express SP after Carg treatment (Fig. 8C, Table 2). The conversion of SP-positive Aδ neurons from RF-unresponsive to RF-responsive neurons cannot account for all these increases. De novo expression of SP in Aδ neurons probably occurs. If this is true, the upregulation of SP expression has to develop within 2–6 h of Carg application, a process that is too quick to involve transcription. However, this result is consistent with the notion that peripheral inflammation upregulates SP translation.
Table 2.
Changes in C and Aδ neurons that had mechanical receptive fields (RFs) and substance P-like immunoreactivity (SP-LI) after inflammation
| Normal | Inflamed | Changes | |
|---|---|---|---|
| RF-responsive cells | |||
| C cells | 45/138a (32.6 %)b | 82/140 (58.6 %) | 26%c |
| Aδ cells | 25/72 (34.7 %) | 56/102 (54.9 %) | 20.2% |
| SP-LI-positive cells | |||
| C cells | 15/42d (35.7 %)e | 21/35 (60 %) | 24.3% |
| Aδ cells | 2/11 (18.2 %) | 8/12 (66.7 %) | 48.5% |
Numerator: number of cells that have RFs; denominator: total number of cells tested.
Percentage of cells that are RF responsive.
Difference in percentage between normal and Carg-treated cats.
Numerator: number of cells that are LY labelled (i.e. RF responsive) and SP immunoreactive; denominator: total number of LY labelled cells tested.
Percentage of LY-labelled cells that show SP immunoreactivity.
Although detailed mechanisms underlying the changes in the properties of the DRG following inflammation await to be clarified, our studies emphasize the fact that concerted changes in the excitability of DRG neurons and SP expression occurs within hours of the development of inflammation. These changes could lead to the activation of silent mechanoreceptive cat C and Aδ sensory neurons and thus may underlie the development of hyperalgesia.
Acknowledgments
This work is supported by NNFSC (39870281), National Basic Research Program (G1999054000) of China. The support of the National Institutes of Health (NS30045, NS23061) and the Human Frontier Science Program (RG0073) to L.-Y.M.H. is acknowledged.
References
- Baba H, Doubell TP, Woolf CJ. Peripheral inflammation facilitates Aβ fiber-mediated synaptic input to the substantia gelatinosa of the adult rat spinal cord. Journal of Neuroscience. 1999;19:859–867. doi: 10.1523/JNEUROSCI.19-02-00859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buritova J, Honor P, Chapman V, Besson JM. Enhanced effects of co-administered dexamethasone and diclofenac on inflammatory pain and associated spinal c-Fos expression in the rat. Pain. 1996;64:559–568. doi: 10.1016/0304-3959(95)00167-0. [DOI] [PubMed] [Google Scholar]
- Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- Castro-Lopes JM, Tavares I, Tolle TR, Coimbra A. Carrageenan-induced inflammation of the hind foot provokes a rise of GABA-immunoreactive cells in the rat spinal cord that is prevented by peripheral neurectomy or neonatal capsaicin treatment. Pain. 1994;56:193–201. doi: 10.1016/0304-3959(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Lawson SN. Changes in somatic action potential shape in guinea-pig nociceptive primary afferent neurons during inflammationin vivo. The Journal of Physiology. 1999;520:565–576. doi: 10.1111/j.1469-7793.1999.t01-1-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnerer J, Schuligoi R, Stein C. Increased content and transport of substance P and calcitonin gene-related peptide in sensory nerves innervating inflamed tissue: Evidence for a regulatory function of nerve growth factorin vivo. Neuroscience. 1992;49:693–698. doi: 10.1016/0306-4522(92)90237-v. [DOI] [PubMed] [Google Scholar]
- Dray A, Pinnock RD. Effects of substance P on adult rat sensory ganglion neuronsin vitro. Neuroscience Letters. 1982;33:61–66. doi: 10.1016/0304-3940(82)90130-6. [DOI] [PubMed] [Google Scholar]
- Duggan AW, Morton CR, Zhao ZQ, Hendry IA. Noxious heating of the skin releases immunoreactive substance P in the substantia gelatinosa of the cat: a study with antibody microprobes. Brain Research. 1987;403:345–349. doi: 10.1016/0006-8993(87)90073-4. [DOI] [PubMed] [Google Scholar]
- Galeazza MT, Garry MG, Yost HJ, Strait KA, Hargreaves KM, Seybold VS. Plasticity in the synthesis and storage of substance P and calcitonin gene-related peptide in primary afferent neurons during peripheral inflammation. Neuroscience. 1995;66:443–458. doi: 10.1016/0306-4522(94)00545-g. [DOI] [PubMed] [Google Scholar]
- Garry MG, Hargreaves KM. Enhanced release of immunoreactive CGRP and substance P from spinal dorsal horn slices occurs during carrageenan inflammation. Brain Research. 1992;582:139–142. doi: 10.1016/0006-8993(92)90328-7. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Janig W, Koltzenburg M. A novel type of unmyelinated-chemosensitive nociceptor in the acutely inflamed bladder. Agents and Actions Supplements. 1988;25:219–221. doi: 10.1007/BF01965016. [DOI] [PubMed] [Google Scholar]
- Honor P, Menning PM, Rogers SD, Nichols ML, Basbaum AI, Besson JM, Mantyh PW. Spinal substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. Journal of Neuroscience. 1999;19:7670–7678. doi: 10.1523/JNEUROSCI.19-17-07670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LY, Neher E. Ca2+-dependent exocytosis in the somata of dorsal root ganglion neurons. Neuron. 1996;17:135–145. doi: 10.1016/s0896-6273(00)80287-1. [DOI] [PubMed] [Google Scholar]
- Iversen L. Substance P equals pain substance? Nature. 1998;392:334–335. doi: 10.1038/32776. [DOI] [PubMed] [Google Scholar]
- Kilo S, Harding-Rose C, Hargreaves KM, Flores CM. Peripheral CGRP release as a marker for neurogenic inflammation: a model system for the study of neuropeptide secretion in rat paw skin. Pain. 1997;73:201–207. doi: 10.1016/S0304-3959(97)00108-5. [DOI] [PubMed] [Google Scholar]
- Kocher L, Anton F, Reeh PW, Handwerker HO. The effect of carrageenan-induced inflammation on the sensitivity of unmyelinated skin nociceptors in the rat. Pain. 1987;29:363–373. doi: 10.1016/0304-3959(87)90051-0. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Crepps BA, Perl ER. Relationship of substance P to afferent characteristics of dorsal root ganglion neurons in guinea-pig. The Journal of Physiology. 1997;505:177–191. doi: 10.1111/j.1469-7793.1997.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leah JD, Cameron AA, Snow PJ. Neuropeptides in physiologically identified mammalian sensory neurons. Neuroscience Letters. 1985;56:257–263. doi: 10.1016/0304-3940(85)90252-6. [DOI] [PubMed] [Google Scholar]
- Lembeck F, Donnerer J, Tsuchiya M, Nagahisa A. The non-peptide tachykinin antagonist, CP-96,345, is a potent inhibitor of neurogenic inflammation. British Journal of Pharmacology. 1992;105:527–530. doi: 10.1111/j.1476-5381.1992.tb09013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Progressive tactile hypersensitivity: an inflammation-induced incremental increase in the excitability of the spinal cord. Pain. 1996;67:97–106. doi: 10.1016/0304-3959(96)03105-3. [DOI] [PubMed] [Google Scholar]
- McCarson KE. Central and peripheral expression of neurokinin-1 and neurokinin-3 receptor and substance P-encoding messenger RNAs: peripheral regulation during formalin-induced inflammation and lack of neurokinin receptor expression in primary afferent sensory neurons. Neuroscience. 1999;93:361–370. doi: 10.1016/s0306-4522(99)00102-5. [DOI] [PubMed] [Google Scholar]
- McCarson KE, Krause JE. The formalin-induced expression of tachykinin peptide and neurokinin receptor messenger RNAs in rat sensory ganglia and spinal cord is modulated by opiate preadministration. Neuroscience. 1995;64:729–739. doi: 10.1016/0306-4522(94)00442-8. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Koltzenburg M. Novel classes of nociceptors: beyond-Sherrington. Trends in Neurosciences. 1990;13:199–201. doi: 10.1016/0166-2236(90)90159-8. [DOI] [PubMed] [Google Scholar]
- Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360–364. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Morita Y, Kiyama H, Ono K, Tohyama M. A noxious stimulus induces the preprotachykinin-A gene expression in the rat dorsal root ganglion: a quantitative study using in situ hybridization histochemistry. Brain Research. 1988;464:31–35. doi: 10.1016/0169-328x(88)90015-0. [DOI] [PubMed] [Google Scholar]
- Oku R, Satoh M, Takagi H. Release of substance P from the spinal dorsal horn is enhanced in polyarthritic rats. Neuroscience Letters. 1987;74:315–319. doi: 10.1016/0304-3940(87)90316-8. [DOI] [PubMed] [Google Scholar]
- Otsuka M, Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiological Reviews. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- Parker D, Grillner S. Tachykinin-mediated modulation of sensory neurons, interneurons and synaptic transmission in the lamprey spinal cord. Journal of Neurophysiology. 1996;76:4031–4039. doi: 10.1152/jn.1996.76.6.4031. [DOI] [PubMed] [Google Scholar]
- Ren K, Hylden JL, Williams GM, Ruda MA, Dubner R. The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuron activity in rats with unilateral inflammation. Pain. 1992;50:331–344. doi: 10.1016/0304-3959(92)90039-E. [DOI] [PubMed] [Google Scholar]
- Salt TE, Hill RG. Neurotransmitter candidates of somatosensory primary fibers. Neuroscience. 1983;10:1083–1103. doi: 10.1016/0306-4522(83)90101-x. [DOI] [PubMed] [Google Scholar]
- Satoh M, Kuraishi Y, Kawamura M. Effects of intrathecal antibodies to substance P, calcitonin gene-related peptide and galanin on repeated cold stress-induced hyperalgesia: comparison with carrageenan-induced hyperalgesia. Pain. 1992;49:273–278. doi: 10.1016/0304-3959(92)90151-Z. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Jarrott B, Hope PJ, Duggan AW. Release of immunoreactive substance P in the spinal cord during development of acute arthritis in the knee joint of the cat: a study with antibody microprobes. Brain Research. 1990;529:214–223. doi: 10.1016/0006-8993(90)90830-5. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Schmidt RF. Effect of an experimental arthritis on the sensory properties of fine articular afferent units. Journal of Neurophysiology. 1985;54:1109–1122. doi: 10.1152/jn.1985.54.5.1109. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Schmidt RF. Time course of mechanosensitivity changes in articular afferents during a developing experimental arthritis. Journal of Neurophysiology. 1988;60:2180–2195. doi: 10.1152/jn.1988.60.6.2180. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Kunkel DD, Schwartzkroin PA. Intracellular dyes mask immunoreactivity of hippocampal interneurons. Neuroscience Letters. 1989;96:23–28. doi: 10.1016/0304-3940(89)90237-1. [DOI] [PubMed] [Google Scholar]
- Schmidt RF, Schaible HG, McBlinger K, Hanesch U, Pawlak M. Silent and active nociceptors: structure, functions and clinical implications. In: Gebhart GF, Hammind DL, Jensen TS, editors. Progress in Pain Research and Management; Proceedings of the 7th World Congress on Pain; Seattle, USA. 1994. pp. 213–250. IASP Press. [Google Scholar]
- Scroggs RS, Todorovic SM, Anderson EG, Fox AP. Variation in IH, IR and ILEAK between acutely isolated adult rat dorsal root ganglion neurons of different size. Journal of Neurophysiology. 1994;71:271–279. doi: 10.1152/jn.1994.71.1.271. [DOI] [PubMed] [Google Scholar]
- Smithson KG, Cobbett P, MacVicar BA, Hatton GI. A reliable method for immunocytochemical identification of Lucifer Yellow, peptide-containing mammalian central neurons. Journal of Neuroscience Methods. 1984;10:59–69. doi: 10.1016/0165-0270(84)90080-3. [DOI] [PubMed] [Google Scholar]
- Song XJ, Zhao Z-Q. Interaction between substance P and excitatory amino acid receptors in modulation of nociceptive responses of cat spinal dorsal horn neurons. Neuroscience Letters. 1994;168:49–52. doi: 10.1016/0304-3940(94)90413-8. [DOI] [PubMed] [Google Scholar]
- Stanfield PR, Nakajima Y, Yamaguchi K. Substance P raises neuronal membrane excitability by reducing inward rectification. Nature. 1985;315:498–501. doi: 10.1038/315498a0. [DOI] [PubMed] [Google Scholar]
- Tao YX, Shu YS, Wang GD, Zhao Z-Q. SP and CGRP in electrophysiological identified C and Aδ fiber neurons of the dorsal root ganglia of catsin vivo. Chinese Journal of Neuroanatomy. 1997;13:15–18. [Google Scholar]
- Traub RJ. The spinal contribution of substance P to the generation and maintenance of inflammatory hyperalgesia in the rat. Pain. 1996;67:151–161. doi: 10.1016/0304-3959(96)03076-X. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanism of cutaneous hyperalgesia. Progress in Neurobiology. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Tsuruoka M, Hiruma Y, Willis WD. The subnucleus reticularis dorsalis is involved in antinociception produced by a low dose of naloxone during carrageenan-induced inflammation. Brain Research. 1997;762:264–268. doi: 10.1016/s0006-8993(97)00509-x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Van Den Berg RJ, Ypey DL. Hyperpolarization-activated current in the growth cone and soma of neonatal rat dorsal root ganglion neurons in culture. Journal of Neurophysiology. 1997;78:177–186. doi: 10.1152/jn.1997.78.1.177. [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. 2. New York: Plenum Press; 1991. [Google Scholar]
- Woolf CJ, Thompson SWN. The induction and maintenance of central sensitization is dependent on NMDA receptor activation: implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. Journal of Neuroscience. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ-, Yang HQ, Zhang KM, Zhuang XX. Release and depletion of substance P by capsaicin in substantia gelatinosa studied with the antibody microprobe technique and immunohistochemistry. Neuropeptides. 1992;23:161–167. doi: 10.1016/0143-4179(92)90118-g. [DOI] [PubMed] [Google Scholar]


